Abstract
Objective
The Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT) and follow-up study (ADAPT-FS) examined effects of naproxen and celecoxib on cognition in the elderly. We report here results describing trajectories of cognitive evaluation test scores.
Methods
2356 participants completed baseline and at least one follow-up cognitive evaluation between 2001-2004. Study treatments were discontinued in 2004, but participants were followed until 2007. 1537 participants were re-evaluated in 2010-2011. Outcomes include seven cognitive evaluations administered yearly in-person in ADAPT and three of these evaluations that were administered by telephone near the end of ADAPT and again in ADAPT-FS.
Results
There were no important differences over time by treatment group on any ADAPT cognitive measure, a global composite, or the three cognitive measures re-assessed in ADAPT-FS by telephone.
Conclusions
Treatment for 1 – 3 years with naproxen or celecoxib did not protect against cognitive decline in older adults with a family history of AD.
Keywords: prevention, clinical trial, Alzheimer's disease, cognitive function, non steroidal anti-inflammatory drug, naproxen, celecoxib
Background
Several lines of evidence from molecular and epidemiologic studies suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) might protect against cognitive decline and impairment in the elderly (1-6), although not all studies have shown benefit (7-9). Motivated by these findings, the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) was initiated to test whether naproxen (a non-selective cyclooxygense [COX] inhibitor) or celecoxib (a selective COX-2 inhibitor) could delay the onset of dementia among cognitively intact older adults with a family history of Alzheimer's dementia. A secondary aim was to examine whether the treatments could attenuate age-related decline on quantitative measures of cognitive function (10). This secondary aim was motivated by the supposition that the quantitative cognitive measures would provide more power to detect treatment effects than the dichotomous outcome of dementia.
ADAPT study treatments were stopped after a median of 14.8 months following randomization due to concerns about the cardiovascular safety of sustained NSAID use in the elderly (11). Reports over the treatment interval indicated that neither celecoxib nor naproxen prevented onset of dementia (12) or slowed cognitive decline over time (13). In fact, these initial findings suggested that the treatments might have increased the risk for dementia and exacerbated decline on global measures of cognition.
The ADAPT Follow-up Study (ADAPT-FS) was carried out to evaluate the cognitive function of ADAPT participants nearly six years after the end of study treatment. A recent report from ADAPT-FS confirmed that neither naproxen nor celecoxib prevented onset of AD over this extended follow-up (14). Here we describe the long term effects of naproxen and celecoxib on cognitive performance over time, including previously unreported data accumulated during in-person visits in the 1.5 years after the end of study treatments in ADAPT and the ADAPT and ADAPT-FS telephone evaluations.
Methods
Description of ADAPT and ADAPT-FS
ADAPT has been described elsewhere (15). Briefly, participants enrolled between March 2001 and December 2004 and were assigned in parallel to three treatment groups in a 1:1:1.5 ratio: 1) naproxen sodium (220 mg b.i.d.); 2) celecoxib (200 mg b.i.d.); or 3) placebo. Participants were recruited at six field sites and were age 70 years or older and had a history of at least one first-degree relative with Alzheimer-like dementia. Before enrollment, they completed a cognitive screening test intended to identify and exclude those with dementia or other cognitive disorders. The ADAPT protocol was approved by the Institutional Review Boards (IRBs) at each field site and the coordinating center.
Participants were seen in-person annually and were contacted by telephone between in-person visits. In December 2004, enrollment and treatment administration were suspended following announcement from the Adenoma Prevention with Celecoxib (APC) trial that celecoxib used in two doses (one of which was identical to that used in ADAPT) produced increased risks of cardiovascular death, myocardial infarction, and related events (16). Treatments were stopped permanently in March 2005, but double-masked follow-up of ADAPT participants continued until February 2007.
Nearly three years after the termination of ADAPT, a follow up study, ADAPT-FS, collected information on cognitive status of ADAPT participants who were alive, had not refused further contact during ADAPT, and had not received a diagnosis of dementia during ADAPT. Eligible participants were contacted by phone between February 2010 and February 2011. The procedures for ADAPT-FS have been published (14). Participants provided oral consent for the telephone assessment and written consent for any subsequent in-person assessment. The ADAPT-FS protocol was also approved by the IRBs at the coordinating center and each of the six field sites.
Cognitive measures
ADAPT psychometrists administered an in-person Cognitive Assessment Battery (CAB) at baseline and at annual follow-up visits. The CAB included the Modified Mini-Mental State Examination (3MS-E) (17), Hopkins Verbal Learning Test – Revised (HVLT-R) (18), informant-rated Dementia Severity Rating Scale (DSRS) (19), Digit span tests (both forward and backward) (20), a generative verbal fluency test (21), narratives from the Rivermead Behavioral Memory Test (22), the Brief Visuospatial Memory Test –Revised (BVMT-R) (23), self-rating of memory functions (24), and the Geriatric Depression Scale (25). All of these measures except the self- and informant-rated scales for cognition and depression are considered here. We also calculated a global summary score from these seven measures as the unweighted mean of the standardized scores (z-scores) of the seven assessments using baseline norms.
In the final year of ADAPT and once during ADAPT-FS, a Telephone Assessment Battery (TAB) was administered. This battery included the Telephone Interview for Cognitive Status (TICS) (26), a test of generative verbal fluency (21), and the narratives subset of the Rivermead Behavioral Memory Test (RBMT) (22). For all cognitive assessments in the CAB and TAB included here, higher scores indicated better cognitive functioning.
ADAPT and ADAPT-FS participants whose CAB or TAB results fell below specified criteria, or those who were otherwise thought by a study clinician to require further evaluation, were asked to have an in-person Dementia Evaluation Visit (DEV). The CAB, TAB and DEV protocols have been described elsewhere (15, 27, 28). The DEV involved a more extensive neuropsychological assessment, a detailed medical history, neurological examination and global mental status examination, collateral interviews and, when appropriate, laboratory testing and neuroimaging. Participants continued to have annual cognitive assessment visits in ADAPT after dementia diagnoses for as long as they were willing and able (until the end of study).
Data analyses
Analyses included all available data with participants included in the treatment group to which they were assigned. By design, naproxen and celecoxib were both compared with placebo and not with one another. Estimates of the change from baseline to each follow-up time point and the change from the ADAPT TAB assessments to the ADAPT-FS TAB assessments were calculated for each applicable assessment and compared using t-tests and linear regression adjusting for clinic and age strata. Longitudinal analyses of change from baseline to all follow-up points were conducted using generalized estimating equation (GEE) regression assuming a exchangeable covariance structure for the within-person replicate measurements with robust standard errors and controlling for the randomization stratification variables (field site and age group). The GEE model provided estimates of the mean difference between the active treatment groups and those given placebo across all ADAPT follow-up times. Additionally, we calculated odds ratios using logistic regression for each treatment compared with placebo using the outcome of a decline from baseline of either a specified number of points on the 3MS-E (5, 6, 7, 8, 9, or 10 points) or a specified effect size (0.50, 0.75, 1.00, or 1.25 SDs) on the global summary score at any time during follow-up.
A similar, but post-hoc, longitudinal GEE analysis was performed to examine the cognitive trajectories in those who did versus did not develop dementia (not by treatment group) over the course of in-person follow-up.
Sensitivity analyses for the GEE models of treatment effect were performed by testing for treatment interactions with the following variables 1) before versus after study-wide treatment termination; 2) by end-of-study dementia diagnostic status; 3) by end-of-study vital status; or 4) by presence of one or more APOE ε4 alleles. Although we attempted to exclude from enrollment people with cognitive impairment, 8 participants with dementia and 57 participants with cognitive impairment but not dementia (CIND) were not detected by the enrollment cognitive screener. We also constructed the GEE models after first excluding participants with prevalent dementia at baseline or after excluding those participants who had either prevalent dementia or CIND.
Results
Study population
Out of 2528 participants enrolled in ADAPT, 1537 also participated in ADAPT-FS. These ADAPT participants were highly educated and mostly Caucasian with a median age at randomization in ADAPT of 75. The participants who completed the ADAPT-FS cognitive assessment were similar to the original ADAPT sample. Detailed descriptions of baseline characteristics for all ADAPT participants (15, 27, 28) as well as for the participants who did complete versus did not complete cognitive assessment in ADAPT-FS (14) have been previously reported. Table 1 reviews baseline characteristics at the time of original randomization of the 2356 participants who completed at least one follow-up assessment in ADAPT or ADAPT-FS. At the time of ADAPT-FS enrollment, their median age was 82 years, and a large majority (85%) of them still lived in their own homes.
Table 1.
Characteristics at randomization of ADAPT participants with follow-up cognitive assessment
| Total | Celecoxib | Naproxen | Placebo | |
|---|---|---|---|---|
| N | 2356 | 677 | 667 | 1012 |
| Characteristic | ||||
| Age, median | 74.5 | 74.5 | 74.5 | 74.5 |
| Age, % | ||||
| 70-74 | 55.7 | 55.7 | 56.1 | 55.5 |
| 75-79 | 31.3 | 30.7 | 31.6 | 31.5 |
| 80-84 | 11.2 | 11.8 | 10.2 | 11.4 |
| > 85 | 1.8 | 1.8 | 2.1 | 1.6 |
| Sex, % | ||||
| Male | 54.2 | 52.4 | 54 | 55.5 |
| Female | 45.8 | 47.6 | 46 | 44.5 |
| Race/Ethnic origin, % | ||||
| White | 96.8 | 96 | 96.9 | 97.3 |
| African-American | 1.5 | 1.8 | 1.9 | 1.1 |
| Hispanic | 0.8 | 1.5 | 0.3 | 0.6 |
| Other | 0.8 | 0.6 | 0.7 | 0.9 |
| Did not answer | 0.1 | 0.1 | 0.1 | 0.1 |
| Education, % | ||||
| < High school | 3.9 | 3.5 | 4.6 | 3.7 |
| High school degree | 19.2 | 20.7 | 16.8 | 19.9 |
| College, no degree | 27.4 | 27.5 | 28.5 | 26.6 |
| College degree | 19.2 | 19.4 | 16.8 | 20.7 |
| Post graduate | 30.3 | 29 | 33.3 | 29.2 |
| Marital status, % | ||||
| Married | 72.1 | 70.6 | 74.8 | 71.2 |
| Widowed | 18.3 | 19.9 | 16.2 | 18.6 |
| Separated | 0.6 | 0.4 | 0.3 | 0.8 |
| Divorced | 6.4 | 6.4 | 5.7 | 6.9 |
| Never married | 2.6 | 2.7 | 3 | 2.4 |
| Not reported | 0 | 0 | 0 | 0 |
| Karnofsky score, % | ||||
| 100 | 83.3 | 84.5 | 82 | 83.4 |
| 90 | 14.7 | 13.3 | 16.9 | 14.2 |
| 80 | 1.8 | 2.1 | 0.9 | 2.2 |
| 60-70 | 0.2 | 0.1 | 0.1 | 0.2 |
| Cognitive score, median | ||||
| Adjusted 3MS-E | 95.0 | 95.0 | 95.0 | 95.0 |
| GVF | 25.0 | 24.0 | 24.0 | 25.0 |
| RBMT delayed recall | 6.5 | 6.5 | 6.0 | 6.5 |
| BVMT-R delayed recall | 8.0 | 8.0 | 8.0 | 8.0 |
| HVLT-R delayed recall | 9.0 | 9.0 | 9.0 | 9.0 |
| Digit Span- forward | 8.0 | 8.0 | 8.0 | 8.0 |
| Digit Span-backward | 7.0 | 7.0 | 6.0 | 7.0 |
BVMT-R, Brief Visuospatial Memory Test-Revised; HVLT-R, Hopkins Verbal Learning Test-Revised; RBMT, Rivermead Behavioral Memory Test; and 3MS-E, Modified Mini-Mental State Examination
Cognitive function over time
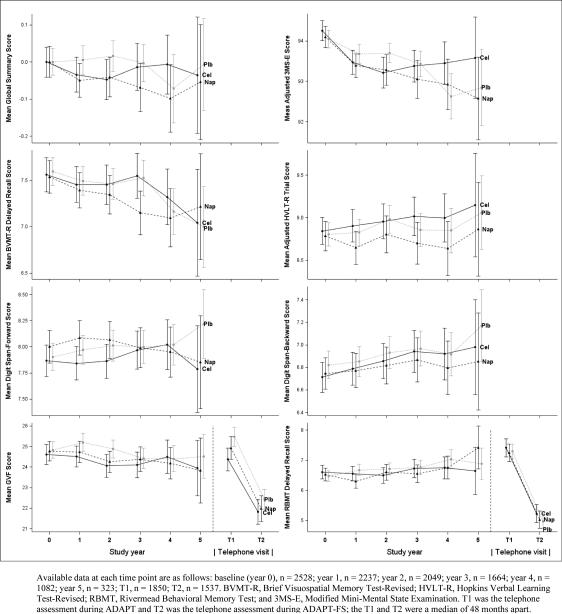
Figure 1 shows the raw mean scores with 95% confidence intervals for each assessment using the CAB and TAB by follow-up visit or telephone contact. Table 2 shows the number of participants with data available at each ADAPT follow-up visit CAB and their mean change scores with confidence intervals by treatment group. In general, the participants experienced very small declines over time in the global summary score, 3MS-E score, GVF, and BVMT-R. These declines did not differ by treatment group except for small differences favoring placebo over both naproxen and celecoxib in year two; however, this did not continue in later years for the global summary score, 3MS-E or GVF. Scores on the RBMT, HVLT and digit span tests did not decline over time and did not differ by treatment group in any year of follow-up.
Figure 1.
Raw scores for each of the 7 tests of cognitive function and the global summary over time
Table 4.
Mean change* from ADAPT telephone assessment to ADAPT-FS telephone assessment by treatment group
| Measure | Celecoxib | Naproxen | Placebo | p-value | |
|---|---|---|---|---|---|
| Mean change (95% CI) | Mean change (95% CI) | Mean change (95% CI) | Celecoxib vs Placebo | Naproxen vs Placebo | |
| TICS | −1.47 (−1.80, −1.14) | −1.66 (−1.98, −1.33) | −1.56 (−1.83, −1.30) | 0.66 | 0.64 |
| RBMT delayed recall | −2.48 (−2.81, −2.15) | −2.39 (−2.72, −2.07) | −2.41 (−2.67, −2.14) | 0.73 | 0.95 |
| GVF | −3.62 (−4.21, −3.03) | −3.20 (−3.78, −2.84) | −3.32 (−3.80, −2.84) | 0.43 | 0.76 |
CI: confidence interval; TICS: Telephone Interview for Cognitive Status; GVF: Generative Verbal Fluency; RBMT: Rivermead Behavioral Memory Test
Estimates are from linear regression adjusting for clinic and age strata. Negative values indicate decline in cognitive measure
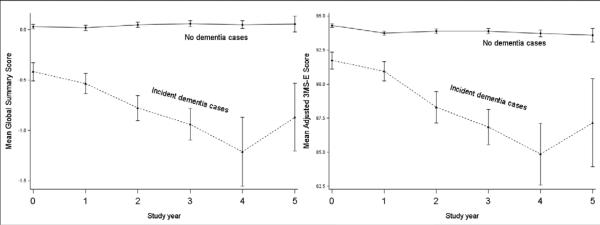
Figure 2 shows the global summary and 3MS-E scores over time for those who were diagnosed with dementia during ADAPT or ADAPT-FS versus all others. The global summary and 3MS-E scores for those who remained dementia-free shows remarkably little change over five years. The participants who received a dementia diagnosis at some point during follow-up scored significantly lower at baseline on the 3MS-E (-2.5 points [95%CI: -3.1, -1.8]; p < 0.0001) and the global summary score (-0.4 standardized points [-0.5, -0.3], p < 0.0001) than others. The difference was slightly smaller but remained highly significant after excluding from analysis those participants who had prevalent dementia or CIND at baseline (3MS-E: -1.9 [95%CI: -2.5, 1.3]; p < 0.0001; global summary: -0.3 [95% CI: -0.4, -0.2]; p < 0.0001).
Figure 2.
Global summary and 3MS-E by dementia diagnosis (during ADAPT or ADAPT-FS)
Table 3 shows GEE estimates of the difference in mean change from baseline across all years of follow-up, confirming the findings from the yearly estimates. The difference in mean change on the GVF for celecoxib versus placebo is -0.40 (95% CI: -0.81, 0.00; p = 0.05) and for naproxen versus placebo is -0.39 (95% CI -0.80 to 0.02; p = 0.06), indicating slightly more decline in the active groups compared to placebo. Estimates for all other cognitive measures showed very little difference in change between the active groups and placebo (all p > 0.05).
Table 3.
Longitudinal effect of treatment on cognitive function for ADAPT visits only
| Measure | Celecoxib vs Placebo | Naproxen vs Placebo | ||
|---|---|---|---|---|
| Estimated* difference in mean change from baseline (95% CI) | p-value | Estimated* difference in mean change from baseline (95% CI) | p-value | |
| Global summary | −0.01 ( −0.04 to 0.03 ) | 0.72 | −0.03 ( −0.07 to 0.00 ) | 0.08 |
| Adjusted 3MS-E | −0.24 ( −0.52 to 0.05 ) | 0.11 | −0.27 ( −0.58 to 0.03 ) | 0.08 |
| GVF | −0.40 ( −0.81 to 0.00 ) | 0.05 | −0.39 ( −0.80 to 0.02 ) | 0.06 |
| RBMT delayed recall | −0.13 ( −0.34 to 0.08 ) | 0.22 | −0.17 ( −0.38 to 0.05 ) | 0.13 |
| BVMT-R delayed recall | 0.04 ( −0.12 to 0.21 ) | 0.60 | −0.10 ( −0.27 to 0.06 ) | 0.22 |
| HVLT-R delayed recall | 0.07 ( −0.10 to 0.24 ) | 0.44 | −0.11 ( −0.28 to 0.07 ) | 0.24 |
| Digit span forward | −0.02 ( −0.14 to 0.10 ) | 0.78 | −0.01 ( −0.13 to 0.12 ) | 0.93 |
| Digit span backward | 0.08 ( −0.05 to 0.20 ) | 0.22 | −0.03 ( −0.15 to 0.10 ) | 0.69 |
CI: confidence interval; BVMT-R: Brief Visuospatial Memory Test - Revised; GVF: generative verbal fluency; HVLT-R: Hopkins Verbal Learning Test - Revised; RBMT: Rivermead Behavioral Memory Test; 3MS-E: Modified Mini-Mental State Examination
Calculated using generalized estimating equations (GEE) to account for within person correlation in repeated measures and adjusted for clinic and age strata. Negative values indicate more decline in active as compared to placebo
As shown in Supplementary Table 1, odds ratios comparing each treatment group with placebo tended slightly toward more decline in the active groups compared with placebo for the global summary cutpoints and the 3MS-E cutpoints.
The ADAPT TAB and ADAPT-FS TAB were conducted a median (1st, 3rd quartile) of 48 months (44, 51) apart. The changes in TICS, RBMT and GVF between the ADAPT and ADAPT-FS TABs are shown in Table 4. In general, the TICS declined less than two points on average (out of maximum possible score of 41); the RBMT declined less than three points on average (out of maximum possible score of 21); and the GVF declined less than four points on average (out of maximum score in this population at baseline of 53). None of these changes differed by treatment group.
Sensitivity analyses
We conducted four tests for interactions (described in methods) for each of the eight cognitive measures (seven assessments plus global summary) to see how the two treatment effects varied in several subgroups of people or at different times. With a total of 64 interaction tests, we expected to see between three and four significant p-values (at the 0.05 level) by chance alone. However, we found no evidence for interactions between treatment group and a dummy variable indicating whether the visit occurred before or after the study-wide treatment termination date for the global summary, 3MS-E, RBMT, BVMT, HVLT or either digit span test. The treatment effect for naproxen versus placebo on the GVF was negative (favoring placebo) before the treatment termination and positive (favoring naproxen) after the treatment termination (interaction p = 0.05). Treatment effect estimates did not differ in those with and without end-of-study dementia diagnoses for any of the cognitive measures (all interaction p > 0.05). There was little evidence of a difference in either treatment effect by presence or absence of APOE ε4 with the possible exception of the HVLT-R. For the HVLT-R the average difference in decline of scores was larger in the celecoxib than placebo group for those participants with [.epsilon]4 versus without (interaction p = 0.03). Also, for the HVLT-R only, comparing those participants who died versus those who survived over both ADAPT and ADAPT-FS, the difference in the rate of decline was larger in the active groups compared to placebo (celecoxib interaction p = 0.05; naproxen interaction p = 0.06). Given the number of tests performed and the general lack of consistent findings, we do not believe that the “statistically significant” interaction tests are meaningful.
We also repeated the longitudinal analyses excluding 1) people who were found to have dementia at baseline and 2) people with either dementia or CIND at baseline and found the magnitude of the treatment effect estimates to be virtually unchanged after both sets of exclusions (data not shown).
Discussion
Despite encouraging evidence from prior molecular and epidemiologic studies (1), the findings from ADAPT and ADAPT-FS do not support the conclusion naproxen or celecoxib (given over a relatively short interval) afford protection against cognitive impairment in the elderly. In a previous report using data from ADAPT and ADAPT-FS, we focused on the effects of naproxen and celecoxib on the primary outcome of dementia onset (14). Here, we examined the long term effects of these NSAIDs on quantitative measures of cognitive decline. These analyses were motivated by the consideration that quantitative measures may provide a more sensitive index of change in cognitive functioning than a dichotomous diagnosis of dementia, and therefore, yield greater power to detect any neurocognitive benefits of naproxen and celecoxib. Moreover, these measures might have captured certain domains of cognitive function that are preferentially protected by the treatments. However, our findings offer little evidence that the drugs attenuated age-related cognitive decline overall or in any specific domain, or that the treatment effects differed by follow-up time, eventual dementia onset, mortality, or APOE genotype status.
Several non-randomized prospective studies previously examined the relationship between NSAID use and cognitive decline over time. The majority of these, including three population based studies (2, 5, 29), a volunteer based study (30), and an ancillary study to a hypertension trial (31), found that NSAIDs were associated with less decline on certain global cognitive or brief cognitive screening measures. Two of these studies found that the protective association was more apparent among younger elderly (5, 31), and one suggested the protection was greater for those with an APOE ε4 allele (5). However, the findings have not been entirely consistent, with one of the most recent non-randomized studies reporting a lack of association between NSAID use and global cognitive decline (8).
ADAPT was the first study to examine this relationship in a randomized experiment among cognitively intact elderly. In an earlier analysis that included ADAPT data collected only until the end of study treatment, we reported that both naproxen and celecoxib were associated with slightly greater decline on global cognitive measures. Here, we found that over longer term observation after treatments had been stopped, the possible adverse effects of these treatments were no longer apparent. This was true for the global as well as specific cognitive measures.
The current study has several limitations that merit discussion. First, the duration of treatment administration was considerably less than planned due to the premature termination of the study in response to growing concerns about the safety of NSAID use in the elderly (11). As we noted before when describing the primary outcome of dementia onset, the median length of treatment for participants in ADAPT was less than 1.5 years, which is far shorter than the planned 7 years. It is quite possible that this duration of treatment was insufficient to alter cognitive trajectories in any meaningful way. A second concern is that we observed very little overall decline in cognitive functioning among trial participants who did not go on to develop dementia (Figure 2 shows the remarkable stability of these scores); although, there were small declines in the four years between the ADAPT and ADAPT-FS telephone assessments. This sort of observation has now been found in several studies (e.g., see Figure 1 in Wilson, et al. (32)). Certainly, the lack of variability in decline among ADAPT participants could have made it even more difficult to detect meaningful effects of the treatments on these trajectories. Also, the median age of those enrolled in ADAPT was older than several of the most notable observational studies suggesting an inverse association between NSAID use and AD, and it is notable that the studies that failed to find such an association generally had older samples than the remainder.
Possible neurocognitive benefits of NSAIDs in the elderly have been a focus of much interest and considerable debate. Initial enthusiasm sparked by suggestive findings from several molecular and epidemiological studies has been dampened by the disappointing results from randomized trials for the treatment of dementia, secondary prevention trials of effects on progression of mild cognitive impairment and now, with these results from ADAPT/ADAPT-FS the primary prevention of dementia (for review see Szekely and Zandi (33)). In ADAPT/ADAPT-FS, it can now be stated that the treatments did not appear to prevent the onset of dementia nor to attenuate decline in global cognition or specific cognitive domains over ten years of follow-up. Although the fact that the NSAID treatments were stopped prematurely may limit the inference possible from ADAPT/ADAPT-FS, it is clear the results of the trial do not support the hypothesis that naproxen and celecoxib, at least with brief exposure, provide meaningful neurocognitive benefits in the elderly.
Supplementary Material
Table 2.
Mean changes from baseline in cognitive function by treatment group for ADAPT visits
| Measure | Celecoxib | Naproxen | Placebo | p-value* | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean change (95% CI) | N | Mean change (95% CI) | N | Mean change (95% CI) | Celecoxib vs Placebo | Naproxen vs Placebo | |
| Global summary | ||||||||
| 1 year | 651 | −0.04 (−0.07, −.01) | 633 | −0.06 (−0.09, −.03) | 951 | −0.03 (−0.05, −.00) | 0.57 | 0.14 |
| 2 years | 601 | −0.06 (−0.09, −.02) | 578 | −0.06 (−0.09, −.02) | 870 | −0.02 (−0.05, 0.00) | 0.09 | 0.10 |
| 3 years | 469 | −0.05 (−0.09, −.00) | 479 | −0.10 (−0.15, −.06) | 715 | −0.05 (−0.08, −.02) | 0.89 | 0.05 |
| 4 years | 310 | −0.05 (−0.10, 0.01) | 311 | −0.12 (−0.19, −.04) | 461 | −0.13 (−0.20, −.06) | 0.10 | 0.82 |
| 5 years | 94 | −0.11 (−0.23, −.00) | 88 | −0.10 (−0.21, 0.01) | 141 | −0.11 (−0.20, −.03) | 0.99 | 0.86 |
| Adjusted 3MS-E | ||||||||
| 1 year | 651 | −0.81 (−1.07, −0.55) | 633 | −0.70 (−0.97, −0.43) | 951 | −0.49 (−0.70, −0.29) | 0.06 | 0.22 |
| 2 years | 601 | −1.12 (−1.45, −0.79) | 578 | −0.90 (−1.22, −0.58) | 869 | −0.46 (−0.68, −0.23) | 0.00 | 0.02 |
| 3 years | 468 | −1.12 (−1.46, −0.77) | 479 | −1.19 (−1.59, −0.78) | 715 | −0.71 (−0.99, −0.44) | 0.07 | 0.05 |
| 4 years | 310 | −1.01 (−1.46, −0.56) | 311 | −1.28 (−1.84, −0.72) | 461 | −1.59 (−2.09, −1.09) | 0.11 | 0.43 |
| 5 years | 94 | −1.40 (−2.33, −0.48) | 88 | −1.89 (−2.79, −0.98) | 141 | −1.65 (−2.46, −0.83) | 0.70 | 0.70 |
| GVF | ||||||||
| 1 year | 651 | −0.09 (−0.50, 0.33) | 633 | −0.10 (−0.54, 0.35) | 951 | 0.31 (−0.05, 0.66) | 0.16 | 0.16 |
| 2 years | 601 | −0.52 (−0.98, −0.06) | 578 | −0.67 (−1.14, −0.19) | 868 | −0.03 (−0.39, 0.34) | 0.10 | 0.03 |
| 3 years | 469 | −0.62 (−1.11, −0.13) | 479 | −0.70 (−1.22, −0.18) | 715 | −0.51 (−0.94, −0.08) | 0.75 | 0.58 |
| 4 years | 310 | −0.18 (−0.83, 0.48) | 311 | −0.67 (−1.35, 0.01) | 460 | −0.28 (−0.81, 0.25) | 0.80 | 0.38 |
| 5 years | 94 | −1.72 (−2.94, −0.50) | 88 | −1.28 (−2.56, −0.01) | 141 | −1.04 (−2.01, −0.06) | 0.38 | 0.76 |
| RBMT delayed recall | ||||||||
| 1 year | 651 | −0.07 (−0.33, 0.19) | 633 | −0.24 (−0.48, 0.01) | 951 | 0.05 (−0.16, 0.25) | 0.49 | 0.09 |
| 2 years | 601 | −0.14 (−0.41, 0.12) | 578 | 0.06 (−0.21, 0.33) | 869 | 0.06 (−0.16, 0.28) | 0.25 | 1.00 |
| 3 years | 468 | 0.01 (−0.27, 0.28) | 478 | −0.09 (−0.33, 0.16) | 715 | 0.08 (−0.13, 0.29) | 0.68 | 0.31 |
| 4 years | 310 | 0.07 (−0.30, 0.44) | 310 | 0.24 (−0.18, 0.65) | 460 | 0.33 ( 0.00, 0.66) | 0.30 | 0.73 |
| 5 years | 94 | −0.05 (−0.86, 0.76) | 88 | 1.31 ( 0.53, 2.09) | 141 | 0.32 (−0.26, 0.91) | 0.45 | 0.04 |
| BVMT-R delayed recall | ||||||||
| 1 year | 651 | −0.14 (−0.32, 0.03) | 633 | −0.20 (−0.39, −0.02) | 951 | −0.21 (−0.34, −0.07) | 0.57 | 0.98 |
| 2 years | 600 | −0.19 (−0.39, −0.00) | 578 | −0.28 (−0.48, −0.08) | 867 | −0.26 (−0.42, −0.10) | 0.61 | 0.87 |
| 3 years | 465 | −0.18 (−0.41, 0.05) | 479 | −0.50 (−0.73, −0.28) | 714 | −0.27 (−0.45, −0.09) | 0.53 | 0.11 |
| 4 years | 309 | −0.41 (−0.72, −0.10) | 310 | −0.43 (−0.70, −0.15) | 459 | −0.62 (−0.87, −0.38) | 0.29 | 0.30 |
| 5 years | 94 | −0.86 (−1.38, −0.34) | 88 | −0.70 (−1.19, −0.22) | 141 | −0.99 (−1.42, −0.57) | 0.70 | 0.39 |
| HVLT-R delayed recall | ||||||||
| 1 year | 651 | 0.02 (−0.17, 0.20) | 633 | −0.12 (−0.32, 0.07) | 950 | −0.10 (−0.24, 0.05) | 0.34 | 0.83 |
| 2 years | 601 | 0.01 (−0.18, 0.20) | 578 | 0.04 (−0.17, 0.26) | 869 | 0.06 (−0.10, 0.22) | 0.72 | 0.88 |
| 3 years | 468 | 0.06 (−0.15, 0.27) | 478 | −0.05 (−0.27, 0.18) | 714 | 0.02 (−0.16, 0.19) | 0.75 | 0.66 |
| 4 years | 310 | −0.08 (−0.37, 0.22) | 310 | −0.16 (−0.47, 0.14) | 460 | −0.04 (−0.27, 0.19) | 0.85 | 0.52 |
| 5 years | 94 | 0.18 (−0.39, 0.76) | 88 | −0.13 (−0.64, 0.39) | 141 | 0.18 (−0.22, 0.58) | 0.99 | 0.36 |
| Digit span forward | 650 | −0.00 (−0.14, 0.13) | 633 | 0.06 (−0.08, 0.19) | 951 | 0.03 (−0.08, 0.14) | 0.73 | 0.73 |
| 1 year | 599 | 0.03 (−0.11, 0.17) | 576 | 0.02 (−0.12, 0.17) | 868 | 0.03 (−0.08, 0.15) | 0.96 | 0.90 |
| 2 years | 467 | 0.07 (−0.10, 0.23) | 479 | −0.14 (−0.31, 0.03) | 715 | −0.04 (−0.18, 0.09) | 0.31 | 0.40 |
| 3 years | 308 | 0.11 (−0.09, 0.32) | 310 | −0.15 (−0.35, 0.06) | 460 | −0.03 (−0.21, 0.15) | 0.30 | 0.41 |
| 4 years | 94 | −0.05 (−0.35, 0.24) | 88 | −0.17 (−0.54, 0.20) | 141 | 0.07 (−0.23, 0.37) | 0.58 | 0.32 |
| 5 years | ||||||||
| Digit span backward | ||||||||
| 1 year | 648 | 0.08 (−0.05, 0.21) | 633 | 0.00 (−0.13, 0.14) | 951 | −0.00 (−0.11, 0.11) | 0.34 | 0.94 |
| 2 years | 599 | 0.15 ( 0.00, 0.29) | 577 | 0.01 (−0.13, 0.15) | 866 | 0.05 (−0.07, 0.17) | 0.32 | 0.68 |
| 3 years | 466 | 0.24 ( 0.08, 0.41) | 479 | 0.05 (−0.12, 0.22) | 714 | 0.02 (−0.12, 0.15) | 0.04 | 0.76 |
| 4 years | 308 | 0.20 (−0.01, 0.41) | 310 | −0.05 (−0.26, 0.17) | 460 | −0.15 (−0.31, −0.00) | 0.01 | 0.41 |
| 5 years | 94 | 0.32 (−0.08, 0.71) | 88 | 0.00 (−0.39, 0.39) | 141 | 0.01 (−0.27, 0.29) | 0.19 | 0.98 |
CI: confidence interval; BVMT-R: Brief Visuospatial Memory Test - Revised; GVF: generative verbal fluency; HVLT-R: Hopkins Verbal Learning Test - Revised; RBMT: Rivermead Behavioral Memory Test; 3MS-E: Modified Mini-Mental State Examination
T test for differences: active compared to placebo. Negative values indicate decline in cognitive measure during follow-up visit
Acknowledgments
Funding: National Institute on Aging (NIA) U01 AG15477, 2U01 AG015477-06A2; N. Bud Grossman Center for Memory Research and Care, Minnesota Medical Foundation
Study data:
ADAPT and ADAPT-FS datasets are available upon request. All requests must be vetted by the Study Officers and must include an analysis plan. If the request is approved, the requestor must have IRB approval to receive data. Requests can be submitted to the address provided on the ADAPT or ADAPT-FS websites:
http://jhuccs1.us/adapt/default.htm
http://jhuccs1.us/adapt-fs/default.htm
Study Officers:
John Breitner, MD, MPH (Study Chair), Centre for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute and McGill University, Montreal
Laura Baker, PhD, Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle
Lea Drye, PhD, Johns Hopkins Bloomberg School of Public Health, Baltimore
Denis Evans, MD, Rush University Medical Center, Chicago
Constantine Lyketsos, MD, MHS, Johns Hopkins School of Medicine, Baltimore
Laurie Ryan, PhD, National Institute on Aging, Bethesda
Peter Zandi, PhD, Johns Hopkins Bloomberg School of Public Health, Baltimore
Research group: Resource centers
Chairman's Office, Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle: Laura Baker, PhD (Director); John Breitner, MD, MPH (Co-Director, affiliated with Centre for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute and McGill University, Montreal); Hector Hernandez Saucedo (Lead Coordinator); Jane Anau; Brenna Cholerton, PhD; Kirise Kramer
Coordinating Center, Johns Hopkins Bloomberg School of Public Health, Baltimore: Peter Zandi, PhD (Director); Lea Drye, PhD (Associate Director); Anne Shanklin Casper, MA, CCRC (Lead Coordinator); Curtis Meinert, PhD; Barbara Martin, PhD; Gabrielle Jenkins, Lee McCaffrey, MA; Jill Meinert; Vijay Vaidya, MPH; Alka Ahuja, MS; Pat May, MS
Project Office, National Institute on Aging, Bethesda: Laurie Ryan, PhD (Project Officer)
N. Bud Grossman Center for Memory Research and Care: Karen Ashe, MD, PhD
Field sites
Johns Hopkins School of Medicine, Baltimore: Constantine G. Lyketsos, MD, MHS (Director); Martin Steinberg, MD (Associate Director); Jason Brandt, PhD (Neuropsychologist); Julia J. Pedroso, RN, MA (Lead Coordinator); Alyssa Bergey, MA; Carol Gogel, RN; Lynn Smith, MA; Jennifer Kraus
Boston University School of Medicine, Boston: Robert A. Stern, PhD (Director); Robert C. Green, MD (Previous Director); Brandon Gavett, PhD (Neuropsychologist); Jane Mwicigi, MBChB, MPH (Lead Coordinator); Lorraine Baldwin; Theresa McGowan; Patricia Johnson; Wendy Qiu, MD; Jamie Frederick; Sumati Raghavan, MBBS; Carol Rossi, RN; Alan Mandell, MD; Daniella Dinizo, MPH; Mary Tara Roth, RN, MSN, MPH
University of Rochester School of Medicine, Rochester: Anton Porsteinsson, MD (Director); M. Saleem Ismail, MD (Previous Director); Miriam Weber, PhD (Neuropsychologist); Connie Brand, RN (Lead Coordinator); Jennifer Richard; Kelly Stear; Sue Schepp; Kelly Cosman; Kimberly Martin, RN
Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle: Suzanne Craft, PhD (Director); Laura Baker, PhD (Neuropsychologist); Deborah Dahl, RN, BSN (Lead Coordinator); Grace Garrett; Jamie Tidwell; Stephen Thielke, MD, MSPH; Lauren Smith; Matthew Arbuckle; William Strong; Juliet Ladenberg; Maureen Callaghan, MD; Stennis Watson, MD; Jeannine Skinner; Kaysha Bowton
Sun Health Research Institute, Sun City, AZ: Marwan Sabbagh, MD (Director); Christine Belden, PsyD (Neuropsychologist); Carolyn Liebsack, RN, BSN, CCRC (Lead Coordinator); Kathryn Davis; Lauren Arnieri; Michael Malek-Ahmadi; Lisa Nicholson, RN; Sandra Jacobson, MD; Elliot Schwartz
Roskamp Institute Memory Clinic, Tampa: Michael Mullan, MBBS, PhD (Director); Cheryl Luis, PhD, ABPP-CN (Neuropsychologist); Julia Parrish, LPN, CCRC (Lead Coordinator); Marlee Faircloth; Terry Ervin; Janette Girard; Deborah Burke, MD; Andrew Keegan, MD
Collaborator:
Denis Evans, MD, Rush-Presbyterian-St. Luke's Medical Center, Chicago
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szekely CA, Town T, Zandi PP. NSAIDs for the chemoprevention of Alzheimer's disease. Subcell Biochem. 2007;42:229–248. doi: 10.1007/1-4020-5688-5_11. [DOI] [PubMed] [Google Scholar]
- 2.Rozzini R, Ferrucci L, Losonczy K, Havlik RJ, Guralnik JM. Protective effect of chronic NSAID use on cognitive decline in older persons. J Am Geriatr Soc. 1996;44:1025–1029. doi: 10.1111/j.1532-5415.1996.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 3.Jonker C, Comijs HC, Smit JH. Does aspirin or other NSAIDs reduce the risk of cognitive decline in elderly persons? Results from a population-based study. Neurobiol Aging. 2003;24:583–588. doi: 10.1016/s0197-4580(02)00188-4. [DOI] [PubMed] [Google Scholar]
- 4.Gray SL, Hanlon JT, Landerman LR, Artz M, Schmader KE, Fillenbaum GG. Is antioxidant use protective of cognitive function in the community-dwelling elderly? Am J Geriatr Pharmacother. 2003;1:3–10. doi: 10.1016/s1543-5946(03)80011-9. [DOI] [PubMed] [Google Scholar]
- 5.Hayden KM, Zandi PP, Khachaturian AS, et al. Does NSAID use modify cognitive trajectories in the elderly? The Cache County study. Neurology. 2007;69:275–282. doi: 10.1212/01.wnl.0000265223.25679.2a. [DOI] [PubMed] [Google Scholar]
- 6.Bertozzi B, Barbisoni P, Franzoni S, Frisoni GB, Rozzinia R, Trabucchi M. Association of chronic non-steroidal anti-inflammatory drugs use and cognitive decline in non-demented elderly patients admitted to a geriatric evaluation and rehabilitation unit. Arch Gerontol Geriatr. 1996;23:71–79. doi: 10.1016/0167-4943(96)00709-1. [DOI] [PubMed] [Google Scholar]
- 7.Saag KG, Rubenstein LM, Chrischilles EA, Wallace RB. Nonsteroidal antiinflammatory drugs and cognitive decline in the elderly. J Rheumatol. 1995;22:2142–2147. [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Grodstein F, Bienias JL, et al. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008;70:2219–2225. doi: 10.1212/01.wnl.0000313813.48505.86. [DOI] [PubMed] [Google Scholar]
- 9.May FE, Moore MT, Stewart RB, Hale WE. Lack of association of nonsteroidal anti-inflammatory drug use and cognitive decline in the elderly. Gerontology. 1992;38:275–279. doi: 10.1159/000213340. [DOI] [PubMed] [Google Scholar]
- 10.Meinert CL, McCaffrey LD, Breitner JC. Alzheimer's Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5:93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitner JC, Martin BK, Meinert CL. The suspension of treatments in ADAPT: concerns beyond the cardiovascular safety of celecoxib or naproxen. PLoS Clin Trials. 2006;1:e41. doi: 10.1371/journal.pctr.0010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 13.Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Results of a follow-up study to the randomized Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT). Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2012.11.012. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinert CL, McCaffrey LD, Breitner JC. Alzheimer's Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5:93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ADAPT Steering Committee Statement from the Steering Committee of the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) for communication to the FDA Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee. 2005 Feb 18; 2005. [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. Journal of ClinicalPsychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 18.Brandt J. The Hopkins Verbal Learning Test: development of a new verbal memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- 19.Clark CM, Ewbank DC. Performance of the Dementia Severity Rating Scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Disease and Associated Disorders. 1996;10:31–39. [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. Psychological Corporation; New York: 1981. [Google Scholar]
- 21.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 22.Wilson BA, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test of everyday memory behavior. J Clin Exp Psychol. 1989;11:855–887. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- 23.Benedict RH, Schrectlen D, Groninger L, Dobrashki M, Shpritz B. Revision of the Brief Visuospatial Memory tests: Studies of normal performance, reliability and validity. PsychologicalAssessment. 1996;8:153. [Google Scholar]
- 24.Squire LR, Wetzel CD, Slater PC. Memory complaint after electroconvulsive therapy: assessment with a new self-rating instrument. Biol Psychiatry. 1979;14:801. [PubMed] [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatry Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 26.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 27.Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 28.Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grodstein F, Skarupski KA, Bienias JL, Wilson RS, Bennett DA, Evans DA. Anti-inflammatory agents and cognitive decline in a bi-racial population. Neuroepidemiology. 2008;30:45–50. doi: 10.1159/000115749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldstein SR, Wendell CR, Seliger SL, Ferrucci L, Metter EJ, Zonderman AB. Nonsteroidal anti-inflammatory drugs, aspirin, and cognitive function in the Baltimore longitudinal study of aging. J Am Geriatr Soc. 2010;58:38–43. doi: 10.1111/j.1532-5415.2009.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince M, Rabe-Hesketh S, Brennan P. Do antiarthritic drugs decrease the risk for cognitive decline? An analysis based on data from the MRC treatment trial of hypertension in older adults. Neurology. 1998;50:374–379. doi: 10.1212/wnl.50.2.374. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer's disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010;9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.