Abstract
Motor unit number estimation (MUNE) of the rodent hindlimb has been used mainly for following the progression of motor neuron disorders. By performing MUNE in the tail, however, progression of axonal neuropathy could also be assessed, as both proximal and distal regions would be available for study. In this investigation, three raters performed a modified multipoint stimulation MUNE technique in the tails of 14 healthy adult rats. The technique was straightforward to perform, with a relatively narrow range of motor unit number estimates of 40 ± 16 (standard deviation) for the proximal tail and 21 ± 11 for the distal tail. Intrarater reliability coefficients were 0.31 (P = 0.033) and 0.32 (P = 0.028) for the proximal and distal tail, respectively. Interrater reliability coefficients were 0.22 (P = 0.086) and 0.44 (P = 0.004). These reliability assessments, along with the relatively low motor unit estimates and narrow range of values, support the idea that rat tail MUNE may have utility in the evaluation of rodent models of neuromuscular disease, including length-dependent neuropathy.
Keywords: amyotrophic lateral sclerosis, motor neuron disease, motor unit number estimate, neuropathy, tail
Motor unit number estimation (MUNE) is a technique for quantifying the number of functional motor units in a muscle or group of muscles. A variety of MUNE methods exist, including incremental MUNE,13 multipoint stimulation (MPS),11 statistical MUNE,6,7 and spike-triggered averaging.14 MUNE has been employed primarily as measure of disease progression in amyotrophic lateral sclerosis (ALS),3,4,9,16 but it has also been applied to patients with spinal muscular atrophy,5 a remote history of polio,21,22 and both demyelinating and axonal neuropathies.10,12 Although the vast majority of MUNE studies have been performed in humans, the technique has also been applied to the superoxide dismutase transgenic mouse model of ALS.17,18,20 In most rodent studies, rather than attempting to study a single muscle, the entire distal hindlimb is evaluated via stimulation of the sciatic nerve proximally and recording over the flexor and extensor compartments of the leg with ring electrodes. MUNE in ALS mice has been shown to be correlated with time of disease onset, since the presence of fewer motor units predicts earlier onset of clinical weakness.18 In addition, there appears to be a high degree of concordance in MUNE between the incremental and multipoint methods.19
Although MUNE in rodent models has been primarily employed in the study of motor neuron diseases, it also has potential research value in the study of neuropathy. Most axonal neuropathies show distance-dependent clinical and neurophysiological abnormalities; in humans, this distance dependence is most often documented by the study of both upper and lower extremity nerves. However, distance dependence is difficult to demonstrate in rodents, primarily due to their small size and the technical difficulty associated with recording from the upper limbs. The rodent tail, on the other hand, provides an ideal location to study both proximal and distal sites. For this reason, we explored a straightforward technique for performing MUNE on the rat tail, recording both proximally and distally along its length, and here we report normal values and reliability.
METHODS
Animals and Set-Up
A total of 14 male Wistar rats weighing approximately 300 g obtained from Charles River Laboratories, Wilmington, Massachusetts) were acclimated for 1 week on a 12:12 light/dark cycle (lights on at 07:00) and allowed food and water ad libitum. All electrophysiological measurements were taken while the animals were anesthetized under 2.5% isoflurane in oxygen at room temperature. Animals were taped in a prone position on a surgical board, and tail temperature was carefully maintained at 32°C using a thermostatically controlled heating blanket (Med1Online, Golden, Colorado). All procedures were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Tail MUNE
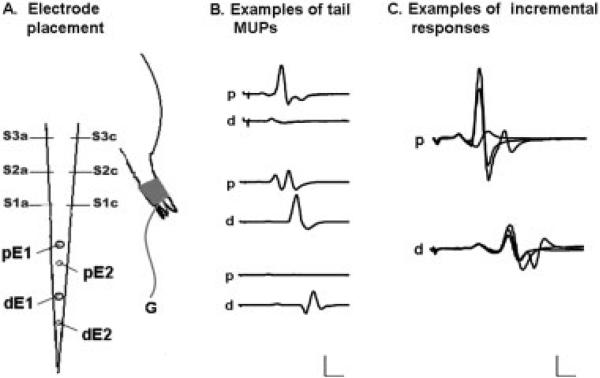
Multipoint MUNE was performed by modifying previously reported methods to apply to the rat tail.18,23 All recording and stimulation was performed with shielded monopolar needle electrodes (Model #74612−100/25; AmbuNeuroline, Madison, Wisconsin). For proximal measurements, stimulation was performed via two shielded monopolar needle electrodes (S1a and S1c; Fig. 1a) initially placed laterally 8 cm from the base of the tail. A monopolar needle recording electrode (pE1) was placed 3 cm distal to the stimulating electrode, and a reference electrode (pE2) was placed another centimeter distally (Fig. 1a). A pre-gelled, self-adhesive ground disk electrode was placed on the plantar surface of the right hindpaw (Fig. 1a). In addition, two other stimulation sites were marked, one 5 cm from the base of the tail (S2a and S2c) and the other 2 cm from the base of the tail (S3a and S3c), such that the stimulation sites were each 3 cm apart. Stimulation was performed at the most distal site first and proceeded proximally through all three sites.
FIGURE 1.
Drawing showing tail MUNE and representative traces. (A) An active needle electrode (S1c) was placed in the lateral portion of the tail, and an inactive needle electrode (S1a) was placed on the opposite side. The proximal active recording electrode (pE1) was placed 3 cm distal, and a reference needle electrode (pE2) was placed 1 cm distal to pE1. For the distal tail, the stimulating needles were the same as for the proximal tail, but the recording electrodes (dE1 and dE2) were placed 6 and 7 cm, respectively, to S1a and S1c. S2a, S2c, S3a, and S3c represent the more proximal points of stimulating electrode placement. A pre-gelled ground electrode (G) was placed on the plantar surface of the right hindpaw. (B) Proximal and distal tail traces from the same animal show distinct motor unit potentials in the proximal and distal tail (top and bottom 2 traces) as well as the same unit innervating both proximal and distal tail muscles (middle 2 traces). Gain was set at 200 μV, 1 mV, and 500 μV, respectively; sweep was set at 2 ms. (C) Representative proximal and distal tail traces showing 3 incremental motor unit potentials (p = proximal, d = distal). Gain was set at 200 μV per division and sweep at 1 ms per division.
For distal tail recording, the stimulating needle electrodes were placed in the same positions as those described for the proximal tail. However, for recording, dE1 was placed 6 cm distal to the stimulating electrode (as compared with 3 cm for the proximal tail recordings) and dE2 was placed 2 cm distal to dE1 (Fig. 1a). The proximal and distal recording needles were left in place throughout each MUNE measurement session. Placement of the electrodes was marked with indelible ink so that MUNE could be performed in the identical location during future sessions.
Stimulation and recording was performed with an electromyographic (EMG) monitoring system (TECA Synergy T2; Viasys, Madison, WI) with band-pass filters set between 100 and 3000 Hz. Stimuli were 0.1-ms duration monophasic pulses of constant current delivered though a stimulator set to fine intensity control (±0.01 mA accuracy). Prior to commencing the actual study, we sought to confirm that we would not simply be collecting the same motor unit potentials (MUPs) at both recording sites. Thus, in 2 rats, we recorded simultaneously from both proximal and distal tail. Although it appeared that at times an identical MUP was being detected at both locations, at most times it appeared that unique MUPs were being detected. This supported the validity that the MUNEs at the two different recording sites would not be calculated from an identical collection of MUPs (see Fig. 1b).
A maximum compound motor action potential (CMAP) was recorded at the first stimulation site for both proximal and distal recording sites. The modified multipoint stimulation technique was then applied. Specifically, current was increased incrementally (starting from a subthreshold value) until the first unique and consistent, all-or-none motor response was obtained. This waveform was digitally recorded. Current was then increased until the first jump in amplitude was identified; this waveform was then saved. Current was increased again until another jump in amplitude occurred; this last waveform was then saved (Fig. 1c, proximal). If alternation of motor units occurred, the unit with the smallest incremental increase was chosen as long as the unit appeared consistently. The same procedure was repeated at the distal tail recording site. This procedure was then repeated for the next two stimulation sites. Response amplitudes for both the CMAP and the incremental responses were measured from largest negative peak to largest positive peak. MUPs smaller than 25 μV (identified rarely) were excluded from the analysis. In addition, increments in amplitude of greater than 1000 μV were also excluded, as with repeated effort it was usually possible to find a smaller increment. Sensitivity of the recording trace was initially set to 100 μV but was increased, as necessary, as the stimulus increased, so that entire responses were always visible on the screen.
When advancing from the first stimulation site to the second, MUPs with identical morphology to those seen with distal stimulation were sometimes identified. If this occurred, the anode and cathode were swapped, and the stimulation was re-attempted. This almost always resulted in a group of new MUPs being identified. After completion of the second site, if this problem was encountered at the third site, the electrodes were swapped back again. We did not specifically attempt to compare MUP morphology between the first and third stimulation sites.
For calculation of MUNE, the largest CMAP recorded from any of the stimulation sites was used (this was often, but not always, that obtained from the most distal stimulation site). In order to calculate the MUNE, the peak-to-peak amplitudes from the third trace from each recording site were added together and the total divided by 9. The largest supramaximal CMAP peak-to-peak amplitude was then divided by this value. In addition to calculating MUNE in both the proximal and distal tail, a proximal-to-distal ratio was determined by dividing the proximal tail MUNE by the distal tail MUNE for each rat.
This modified multipoint technique was conducted by one rater (rater 1) a total of three times in order to evaluate intrarater reliability. In addition, the same tests were conducted by two additional raters (raters 2 and 3) on the same group of animals, blinded to the first rater's results and to each other's, in order to evaluate interrater reliability. Each of these measurement sessions were conducted on different days and with each rater replacing the stimulating and recording electrodes based on the ink marks described earlier.
Data Analysis
Mean CMAP and MUP amplitudes were calculated for both intra- and interrater proximal and distal tail measurements. Intraclass correlation coefficients were calculated for both intra- and interrater reliability on all variables: proximal tail MUNE, distal tail MUNE, and proximal-to-distal ratio, across all three sets of measurements using SPSS, version 15.0 (SPSS, Inc., Chicago, Illinois). For calculation of the interrater reliability measurements only the first set of data obtained from rater 1 was utilized. In order to calculate overall MUNEs in the proximal and distal tail, all three sets of rater 1's estimates, along with rater 2 and rater 3's estimates, were used to obtain a weighted average. In addition, one-way analyses of variance (ANOVAs), as well as the coefficients of variation, were calculated for the same parameters. Tests were considered significant at α = 0.05. All numbers are reported as mean ± standard deviation unless otherwise stated.
RESULTS
General
Averaging the data for all three raters, mean CMAP amplitude was 8.29 ± 2.99 mV for the proximal recording site and 4.07 ± 1.59 mV for the distal recording site. For MUP amplitude, the mean was 442 ± 194 μV for the proximal recording site and 430 ± 159 μV for the distal recording site. Because MUP amplitude was similar between the two sites, and the proximal CMAP amplitude was approximately twice that obtained with distal recording, the average MUNE for the proximal recording site was about twice that of the distal site: 40 ± 16 vs. 21 ± 11, giving an average proximal-to-distal MUNE ratio of 1.9. The coefficients of variation for MUNE in the proximal tail ranged among the raters from 21% to 42% in the proximal tail and 31% to 55% for the distal tail (see last column of Tables 1 and 2).
Table 1.
Intrarater reliability of MUNE on the rat tail.
| Recording location | Test | Mean (SD) | Range | r * | P † | Coefficient of variation (%) |
|---|---|---|---|---|---|---|
| Proximal tail | 1 | 33 (6.8) | 21−46 | – | – | 21 |
| 2 | 29 (11.3) | 15−54 | – | – | 39 | |
| 3 | 29 (9.9) | 14−48 | 0.31 | 0.033 | 34 | |
| Distal tail | 1 | 21 (7.2) | 12−43 | – | – | 34 |
| 2 | 20 (6.1) | 12−32 | – | – | 31 | |
| 3 | 19 (7.5) | 6−34 | 0.32 | 0.028 | 39 | |
| p/d tail ratio | 1 | 1.8 (0.63) | 0.6−2.8 | – | – | 35 |
| 2 | 1.5 (0.57) | 0.9−3.0 | – | – | 38 | |
| 3 | 1.8 (0.86) | 0.8−3.5 | 0.27 | 0.048 | 48 |
MUNE, motor unit number estimation; p/d, proximal to distal.
r value from intraclass correlations between same rater measurements.
P-value from intraclass correlation coefficients.
Table 2.
Interrater reliability of MUNE on the rat tail.
| Recording location | Rater | Mean (SD) | Range | r * | P † | Coefficient of variation (%) |
|---|---|---|---|---|---|---|
| Proximal tail | 1 | 33 (6.8) | 21−46 | – | – | 21 |
| 2 | 42 (17.8) | 26−81 | – | – | 42 | |
| 3 | 47 (16.0) | 17−81 | 0.22 | 0.086 | 34 | |
| Distal tail | 1 | 21 (7.2) | 12−43 | – | – | 34 |
| 2 | 16 (6.4) | 9−26 | – | – | 40 | |
| 3 | 28 (15.4) | 9−53 | 0.44 | 0.004 | 55 | |
| p/d tail ratio | 1 | 1.8 (0.63) | 0.6−2.8 | – | – | 35 |
| 2 | 2.9 (1.5) | 1.4−6.7 | – | – | 52 | |
| 3 | 2.3 (1.7) | 0.7−6.6 | 0.41 | 0.007 | 74 |
MUNE, motor unit number estimation; p/d, proximal to distal.
r value from intraclass correlations between different rater measurements.
P-value from intraclass correlation coefficients.
The study was relatively easy to perform, taking approximately 20−25 minutes per rat to obtain data from both sites following the aforementioned technique exactly. The CMAP did vary to some extent in amplitude depending on stimulation site, usually being highest with distal stimulation and decreasing with more proximal stimulation, as would be expected. However, this was not uniformly true and, in several cases, a slightly larger amplitude response was obtained with more proximal stimulation; again, the largest CMAP, regardless of site of stimulation, was always the one used to calculate MUNE. The CMAP also varied somewhat unexpectedly with stimulus intensity. Specifically, after an apparent maximal response was obtained, with further increases in stimulus intensity, the amplitude often decreased by 10−15%. Even with the slight variability in CMAPs as described earlier, the reproducibility between raters (proximal CMAP: r = 0.70, P < 0.001; distal CMAP: r = 0.50, P = 0.001) and within raters (proximal CMAP: r = 0.76, P < 0.001; distal CMAP: r = 0.56, P < 0.001) was good.
As expected, individual MUPs varied considerably in size and shape at both recording sites, as suggested by Figure 1b and c. With a stimulus duration of 0.1 ms, the first MUPs would generally reach threshold with approximately 2−3-mA stimulus intensities. Given the use of needle electrodes for both stimulating and recording, noise levels were very low. They allowed for reliable measurement of MUPs as small as 25μV (our lower limit of acceptability), although some MUPs had decidedly complex shapes. We observed alternation of MUPs, but given the low stimulus intensities we were able to identify consistent morphologies that allowed us to accurately choose the smallest increment greater than 25 μV in amplitude.
Intrarater Reliability
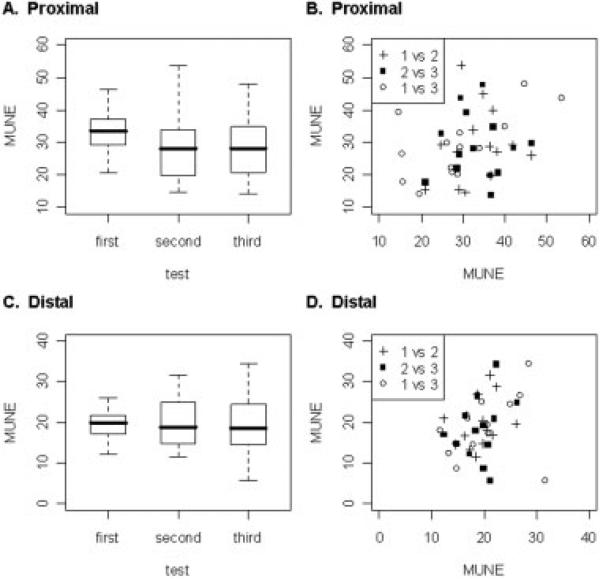
Overall, the average number of motor units estimated by rater 1 was 31 ± 16 for the proximal and 20 ± 11 for the distal tail over the three trials performed. The differences in the number of motor units estimated by the same rater in either the proximal and distal tail (Table 1 and Figure 2a and c) as a group, on repeated testing were not significant, despite the presence of only a modest correlation for both proximal and distal recording sites when comparing measurements on individual rats (r = 0.31, P = 0.033 for proximal, and r = 0.32, P = 0.028 for distal; Table 1 and Fig. 2b and d). Similarly, there were no significant differences in the proximal-to-distal ratio across any of the repeated measurements (see Fig. 4a) despite a correlation coefficient of 0.27, P = 0.048 (Table 1).
FIGURE 2.
Boxplots of the proximal and distal tail MUNE results for the same rater and scatterplots of the associated intrarater reliability in proximal and distal tail. (A) There were no significant differences in proximal tail MUNE for the same rater among any tests. (B) The intrarater correlation coefficient for proximal tail MUNE was r = 0.31, P = 0.033 (1 vs 2 = first versus second test, 2 vs 3 = second versus third test, and 1 vs 3 = first versus third test). (C) There were no significant differences in distal tail MUNE for the same rater among any tests. (D) The intrarater correlation coefficient for distal tail MUNE was r = 0.32, P = 0.028 (1 vs 2 = first versus second test, 2 vs 3 = second versus third test, and 1 vs 3 = first versus third test).
FIGURE 4.
Boxplots show that the average proximal-to-distal tail MUNE ratio for the same and different raters was greater than 1.0. (A) There were no significant differences in proximal-to-distal tail MUNE ratios for the same rater among any tests. (B) There were no significant differences in proximal-to-distal tail MUNE ratios among any raters.
Interrater Reliability
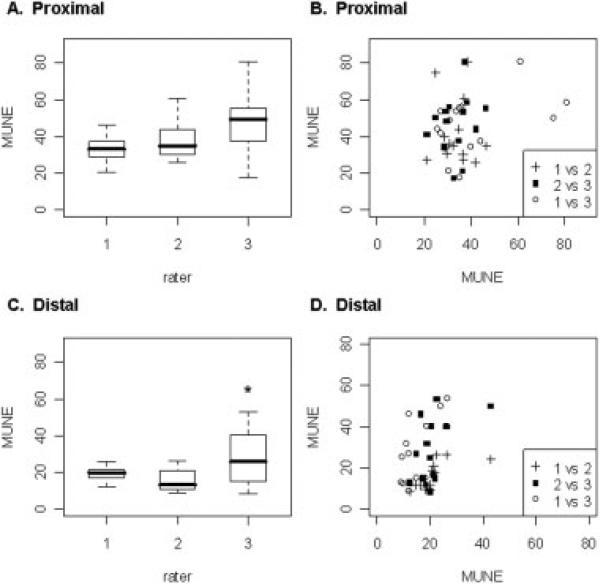
The interrater reliability for proximal tail MUNE was overall less than the intrarater reliability, with a correlation coefficient of 0.22, P = 0.086 (Table 2 and Fig. 3b). However, the reliability of distal tail MUNE, similar to intrarater, had a correlation coefficient of 0.44, P = 0.004 (Table 2 and Fig. 3d). This was true despite there being a significant difference in distal tail MUNE between raters, with the third rater estimating significantly more units than the second rater (Table 2 and Fig. 3c; 28 ± 15.4 vs. 16 ± 6.4; F(2,39) = 4.815, P = 0.014; Tukey's least significant difference test: P = 0.004). In addition, there were no significant differences in the proximal-to-distal tail MUNE ratio among the raters (Fig. 4b) with an interrater reliability correlation coefficient of 0.41, P = 0.007 (Table 2).
FIGURE 3.
Boxplots of proximal and distal tail MUNE results for different raters and scatterplots of the associated interrater reliability in proximal and distal tail. (A) There were no significant differences in proximal tail MUNE among any raters. (B) The interrater correlation coefficient for proximal tail MUNE was r = 0.22, P = 0.09 (NS) (1 vs 2 = first versus second rater, 2 vs 3 = second versus third rater, and 1 vs 3 = first versus third rater). (C) For the distal tail, rater 3 estimated significantly more motor units than rater 2 but not rater 1 (*P < 0.05). (D) The interrater correlation coefficient for distal tail MUNE was r = 0.44, P = 0.004 (1 vs 2 = first versus second rater, 2 vs 3 = second versus third rater, and 1 vs 3 = first versus third rater).
Comparison among the three raters also revealed a clear training effect. Rater 1 had been performing various forms of MUNE regularly for weeks on rats; rater 2 had substantial experience in performing MUNE in humans, but had only recently learned to perform the technique on rats; and rater 3 was a research assistant recently trained in the technique, with no previous experience whatsoever with MUNE (Fig. 3a and c).
DISCUSSION
The goal of this study was to characterize MUNE in the proximal and distal rat tail using a modified multipoint technique. We found that, in addition to the procedures being relatively easy to perform, they also provided consistent results in normal rats. This is most clearly demonstrated in Figures 2a and c and 3a and c, which show that the range of MUNE values was relatively small across all the animals for both the proximal and distal sites, thus yielding overall excellent reproducibility for the group of animals as a whole. This narrow range of values also reduced the importance of the reproducibility values themselves. Even so, although the intra- and interrater correlations were not impressive, significant relationships were found. Another noteworthy observation was the effect of training on the reliability of the measurements. The consistency of the results was directly related to each rater's experience, with rater 3 having had only limited practice and having a much wider range of values.
Our results suggest that the rat tail likely contains fewer motor units than muscles of the rat medial gastrocnemius, in which estimates ranging from 57 ± 11 to 117 ± 21, depending on the technique used, have been reported.1 Similarly, MUNE in the mouse leg, in which the flexor and extensor leg compartments are measured simultaneously, revealed much larger estimates, with a normal mean value of 310 ± 100.20 The smaller motor unit estimates observed in the rat tail may be advantageous, because MUNE reliability improves with decreasing numbers of motor units.2,8 Also supporting this premise, we found here that the interrater reliability of MUNE for the distal tail, where the estimates were about half those of the proximal tail, was considerably higher than that for the proximal tail.
In addition to being able to study proximal and distal recording sites, there are several other advantages to performing MUNE on the tail. First, the tail is easily accessible and can be tattooed in order to insure that repeated measurements are conducted in the exact same location, thereby markedly reducing error due to inaccurate electrode placement. Second, the tail is sufficiently long so as to allow the employment of a modified multipoint stimulation technique with relative ease; such an approach is considerably more challenging if stimulating the sciatic nerve and recording off the hindlimb. Finally, the relatively small caliber of the tail implies that needle electrodes may be able to detect the signatures of most nearby motor unit potentials. Prior to undertaking this study we attempted to abrade the rat tail to determine the feasibility of surface measurements. The procedure proved virtually impossible to implement as it produced obvious injury and resulted in poor-quality data.
The use of needle electrodes has both advantages and disadvantages. The major advantage of MUNE in the tail is remarkably low noise levels, not easily achievable with surface electrodes. One major disadvantage is that the individual MUPs tend to have more complex shapes, making it perhaps slightly more difficult to identify increments with increasing stimulation intensity. An additional disadvantage is the possibility that small shifts in needle position will result in changes in amplitude of the recorded MUPs. However, given the small size of the stimuli required to obtain the first 3 MUPs and the fact that the waveforms appeared very consistent, we suspect that such movement played a negligible role in these measurements. However, we often did observe a reduction in CMAP amplitude with increasing stimulation intensity, the cause of which was not obvious. Although movement of the tail here may have contributed, this seems unlikely, because reducing the stimulus intensity resulted in the CMAP amplitude again increasing. Perhaps one other more likely explanation is that these reductions were caused by the addition of distant volume-conducted potentials to the CMAP.
Clearly, the exact anatomy of the rat tail will impact MUNE in this region, but, unfortunately, this anatomy has not been especially well described. We know that muscles on the dorsal and lateral sides are innervated by the dorsal and ventral longitudinal nerves, respectively.15 Our own limited dissection of a rat tail revealed that these muscles are generally longitudinally oriented, and each descended more than 1 cm and possibly up to several centimeters. Thus, it is not surprising, as we have shown in Figure 1, that stimulation at a specific point may excite motor units that are simultaneously detectable at both proximal and distal sites.
In conclusion, the results of this study show that modified multipoint tail MUNE in the rat is a reliable and technically straightforward procedure. This technique may find value in motor neuron disorders research as well as in the study of length-dependent neuropathic conditions in which the earliest reductions in motor unit estimates would be expected to be observed first in the most distal nerve segments.
Acknowledgments
This work was supported in part by the National Institutes of Health (Grant F32 DK079550).
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ANOVA
analysis of variance
- CMAP
compound motor action potential
- MUNE
motor unit number estimation
- MUP
motor unit potential
REFERENCES
- 1.Arasaki K, Tamaki M, Hosoya Y, Kudo N. Validity of electromyograms and tension as a means of motor unit number estimation. Muscle Nerve. 1997;20:552–560. doi: 10.1002/(sici)1097-4598(199705)20:5<552::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Bromberg MB. Motor unit estimation: reproducibility of the spike-triggered averaging technique in normal and ALS subjects. Muscle Nerve. 1993;16:466–471. doi: 10.1002/mus.880160506. [DOI] [PubMed] [Google Scholar]
- 3.Bromberg MB, Forshew DA, Nau KL, Bromberg J, Simmons Z, Fries TJ. Motor unit number estimation, isometric strength, and electromyographic measures in amyotrophic lateral sclerosis. Muscle Nerve. 1993;16:1213–1219. doi: 10.1002/mus.880161111. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg MB, Larson WL. Relationships between motor-unit number estimates and isometric strength in distal muscles in ALS/MND. J Neurol Sci. 1996;139(suppl):38–42. doi: 10.1016/0022-510x(96)00074-3. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg MB, Swoboda KJ. Motor unit number estimation in infants and children with spinal muscular atrophy. Muscle Nerve. 2002;25:445–447. doi: 10.1002/mus.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daube JR. Estimating the number of motor units in a muscle. J Clin Neurophysiol. 1995;12:585–594. doi: 10.1097/00004691-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Daube JR. Motor unit number estimates—from A to Z. J Neurol Sci. 2006;242:23–35. doi: 10.1016/j.jns.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Felice KJ. Thenar motor unit number estimates using the multiple point stimulation technique: reproducibility studies in ALS patients and normal subjects. Muscle Nerve. 1995;18:1412–1416. doi: 10.1002/mus.880181211. [DOI] [PubMed] [Google Scholar]
- 9.Felice KJ. A longitudinal study comparing thenar motor unit number estimates to other quantitative tests in patients with amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:179–185. doi: 10.1002/(sici)1097-4598(199702)20:2<179::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Jillapalli D, Bradshaw DY, Shefner JM. Motor unit number estimation in the evaluation of focal conduction block. Muscle Nerve. 2003;27:676–681. doi: 10.1002/mus.10367. [DOI] [PubMed] [Google Scholar]
- 11.Kadrie HA, Yates SK, Milner-Brown HS, Brown WF. Multiple point electrical stimulation of ulnar and median nerves. J Neurol Neurosurg Psychiatry. 1976;39:973–985. doi: 10.1136/jnnp.39.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawson VH, Gordon Smith A, Bromberg MB. Assessment of axonal loss in Charcot–Marie–Tooth neuropathies. Exp Neurol. 2003;184:753–757. doi: 10.1016/S0014-4886(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 13.McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121–131. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendell LM, Henneman E. Terminals of single Ia fibers: distribution within a pool of 300 homonymous motor neurons. Science. 1968;160:96–98. doi: 10.1126/science.160.3823.96. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi T, Goto I. Serial in vivo determinations of nerve conduction velocity in rat tails. Physiological and pathological changes. Electroencephalogr Clin Neurophysiol. 1973;35:125–131. doi: 10.1016/0013-4694(73)90168-5. [DOI] [PubMed] [Google Scholar]
- 16.Olney RK, Yuen EC, Engstrom JW. Statistical motor unit number estimation: reproducibility and sources of error in patients with amyotrophic lateral sclerosis. Muscle Nerve. 2000;23:193–197. doi: 10.1002/(sici)1097-4598(200002)23:2<193::aid-mus8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Shefner JM, Brown RH, Jr, Cole D, Chaturvedi P, Schoenfeld D, Pastuszak K, Matthews R, et al. Effect of neurophilin ligands on motor units in mice with SOD1 ALS mutations. Neurology. 2001;57:1857–1861. doi: 10.1212/wnl.57.10.1857. [DOI] [PubMed] [Google Scholar]
- 18.Shefner JM, Cudkowicz M, Brown RH., Jr Motor unit number estimation predicts disease onset and survival in a transgenic mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2006;34:603–607. doi: 10.1002/mus.20628. [DOI] [PubMed] [Google Scholar]
- 19.Shefner JM, Cudkowicz ME, Brown RH., Jr Comparison of incremental with multipoint MUNE methods in transgenic ALS mice. Muscle Nerve. 2002;25:39–42. doi: 10.1002/mus.10000. [DOI] [PubMed] [Google Scholar]
- 20.Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ, et al. Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology. 1999;53:1239–1246. doi: 10.1212/wnl.53.6.1239. [DOI] [PubMed] [Google Scholar]
- 21.Sorenson EJ, Daube JR, Windebank AJ. Electrophysiological findings in a cohort of old polio survivors. J Peripher Nerv Syst. 2006;11:241–246. doi: 10.1111/j.1529-8027.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Sorenson EJ, Daube JR, Windebank AJ. Motor unit number estimates correlate with strength in polio survivors. Muscle Nerve. 2006;34:608–613. doi: 10.1002/mus.20632. [DOI] [PubMed] [Google Scholar]
- 23.Wang FC, Delwaide PJ. Number and relative size of thenar motor units estimated by an adapted multiple point stimulation method. Muscle Nerve. 1995;18:969–979. doi: 10.1002/mus.880180908. [DOI] [PubMed] [Google Scholar]