Abstract
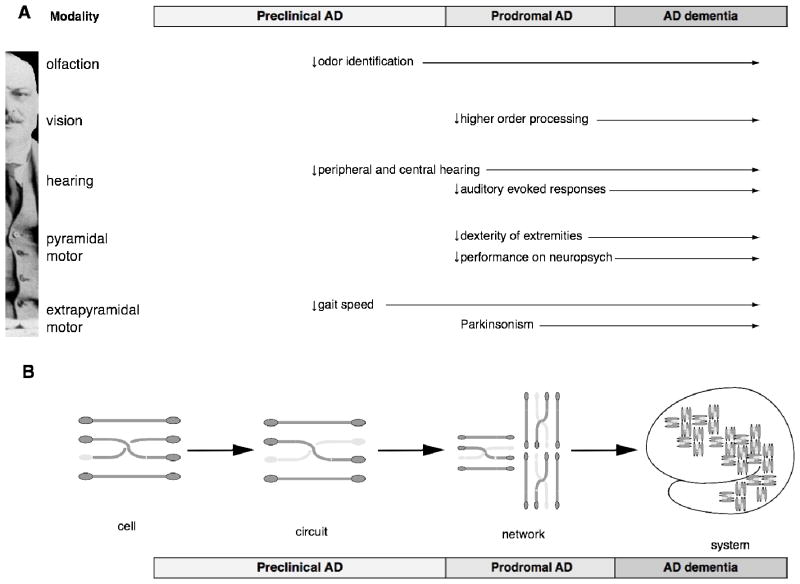
Recent evidence indicates that sensory and motor changes may precede the cognitive symptoms of Alzheimer’s disease (AD) by several years and may signify increased risk of developing AD. Traditionally, sensory and motor dysfunctions in aging and AD have been studied separately. To ascertain the evidence supporting the relationship between age-related changes in sensory and motor systems and the development of AD and to facilitate communication between several disciplines, the National Institute on Aging held an exploratory workshop titled “Sensory and Motor Dysfunctions in Aging and Alzheimer’s Disease”. The scientific sessions of the workshop focused on age-related and neuropathological changes in the olfactory, visual, auditory, and motor systems, followed by extensive discussion and hypothesis generation related to the possible links among sensory, cognitive, and motor domains in aging and AD. Based on the data presented and discussed at this workshop, it is clear that sensory and motor regions of the CNS are affected by Alzheimer pathology and that interventions targeting amelioration of sensory-motor deficits in AD may enhance patient function as AD progresses.
I. Introduction
With advancing age, we may notice ourselves walking a little more slowly, or having a bit of difficulty navigating our environment; or hearing less well; or not sensing the ambient aroma as acutely. Often we think of these sensory or motor changes as signs of aging; rarely do we think of them as early signs of Alzheimer’s disease (AD). For AD research, the defining phenotypic impairment is progressive loss of cognitive function, which we often consider as the first function to be lost in patients. However, clinical research has led to the recognition that changes in sensory and motor systems are present in many people at the early stages of AD. In particular, several longitudinal studies indicate that changes in olfaction, hearing, and even walking speed may precede the onset of cognitive impairments and dementia by 5–15 years, and are strong risk factors for AD dementia (1–5).
These clinical findings, together with the recognition that AD pathology develops over many years, raise the exciting possibility that specific sensory or motor changes may be early non-invasive biomarkers for AD; or even more provocatively, that treating these sensory or motor symptoms may help to prevent or treat AD dementia. While attempts have been made to explore these possibilities, it has quickly become obvious that current clinical measures of sensory or motor changes are not specific to AD. For instance, people may develop these sensory or motor impairments in association with other types of neurologic disorders, such as Parkinson’s disease (PD) (6) or distinct non-Alzheimer types of dementia (7); or they may be caused by non-neurologic impairments of the nose, eye, ear, or muscles (8). In fact, the majority of older adults with sensory or motor impairments do not seem to exhibit progression to the cognitive symptoms of AD. Neither do all AD patients begin with some or any of these sensory or motor changes. Consequently, the significance of these sensory or motor dysfunctions for the pathogenesis and diagnosis of AD has remained largely elusive, if not often controversial. To unravel the relationships between age-related sensory and motor dysfunctions and AD and harness their potential, new ideas, perspectives, and investigations are in order.
A number of recent advances in AD research necessitate a reconsideration of the role for sensory and motor dysfunction in aging and AD. First, the recently revised diagnostic criteria and guidelines for AD have expanded the conceptual framework of the disease to include a “preclinical” stage, which occurs years before the onset of the noticeable cognitive symptoms with the appearance of the underlying AD pathophysiological disease process, in particular the accumulation of the amyloid β (Aβ) protein (9). The specific markers, in particular functional markers, of this “preclinical” stage, have yet to be defined. Thus, it is timely to consider that the existence of non-cognitive functional changes, such as sensory or motor changes, may exemplify this “preclinical” stage and help to identify people 10 or 15 years before they are clinically diagnosed with AD. Second, findings reported from neuropathological assessments of patients diagnosed with AD seem to corroborate this possibility. For instance, the deposition of the Aβ peptide, one of the key hallmarks of AD pathology, may first appear in sensory association areas, well before its appearance in regions involving memory, such as entorhinal and hippocampal areas, and also before the cognitive clinical symptoms of AD (10). It may therefore be worth investigating whether combining AD pathology with specific sensory/motor changes would improve predictions of the emergence of the cognitive impairments and progression to AD dementia. Thirdly, genome-wide association studies have now established multiple susceptibility genes for non-Mendelian forms of AD, many of which have proposed molecular effects on the production, aggregation, or clearance of Aβ (11) and other AD-related molecules such as ApoE (12). Although these genes were identified based on diagnosis by cognitive symptoms of AD, it will be interesting to examine whether people with the AD susceptibility genes also develop the sensory or motor changes well before they progress to cognitive impairments and dementia (13). More importantly, will a combination of the presence of AD susceptibility genes with the sensory and motor changes increase the sensitivity and specificity to predict the emergence of cognitive impairments and progression to dementia?
To advance our comprehensive understanding of the pathology and clinical manifestations of AD and to explore the relevance of sensory and motor impairments in aging to AD, the National Institute on Aging (NIA) convened a 2-day workshop titled “Sensory and Motor Dysfunctions in Aging and AD” in the summer of 2010. The invited participants included many individuals who have contributed in leading ways to AD research as well as investigators in the fields of sensory and motor neuroscience and behavior who have been interested in but may not have been most directly involved with AD research. To recapture the spirit and the presentations at this workshop as well as to highlight the potential new directions of research related to sensory/motor systems and AD, the workshop organizers and participants worked together to generate this synthesized review.
The review is comprised of four similarly structured sections that present an analysis of the current evidence related to age-related changes in the olfactory, visual, auditory, and motor systems in the context of AD. Each section evaluates the relationship of the sensory or motor modality to AD at the clinical and epidemiological, the neuroanatomical and pathophysiological, the genetic, the neuroimaging and neurophysiology, and the cellular and molecular levels. Each section begins with a summary of key findings and ends with suggestions for future research directions. More detailed meta-articles for each modality are included in the accompanying appendices, which include a summary of the normal physiology of each sensory or motor modality. In the Discussion section, a conceptual framework is presented regarding the interface of sensory and motor dysfunction of aging and AD, followed by a discussion of several testable hypotheses to evaluate the etiological significance and clinical utility of the diagnostic precision and therapeutic efficacy offered by sensory and motor systems in preclinical and clinical AD.
II. Olfactory System in Aging and AD
II-1. Summary of Key Findings
Olfactory impairment is prevalent in AD patients and present in some cognitively normal older adults.
Olfactory dysfunction alone may predict progression towards AD dementia in a clinical setting and towards prodromal AD in preclinical subjects at a population level.
AD pathology is present in the peripheral and central olfactory neural network in most AD patients as well as some cognitively normal older adults, and the pathology is related to impaired odor identification.
Cognitively normal older adults who are genetically at risk for AD may have worse olfactory function than non-carriers.
Olfactory impairment is associated with reduced hippocampal volume by structural MRI.
Both odor-evoked fMRI and ERPs are preferentially affected in AD patients as well as individuals genetically at risk for AD.
In transgenic mice overexpressing Aβ or tau, the presence of AD pathology in the olfactory system strongly correlates with olfactory perceptual impairments.
II-2. Epidemiological and Clinical Studies
Studies have shown that olfactory function declines significantly with age (8, 14). While only about 6% of people between ages of 50 – 59 show impairments in odor identification, the percentage of individuals aged 60 – 69 triples. By age 80 years old or above, more than 60% of individuals show impairment in odor identification. Moreover, three quarters of the people with impairments of odor identification did not report that their sense of smell was compromised (15). While initially interpreted as a consequence of aging, subsequent studies support the notion that preclinical or prodromal AD or PD may contribute to this olfactory decline (16–18).
The connection between olfactory impairment and AD, initially reported in 1987 (19), has been confirmed in numerous studies that assessed odor identification, detection threshold, and odor recognition memory in patients diagnosed with the dementia phase of AD (6, 20, 21). More importantly, many studies have shown that olfactory impairment is more prevalent in individuals who go on to develop the dementia phase of AD and may precede the onset of cognitive impairment by several years (22–26). In particular, in patients with mild cognitive impairment (MCI), olfactory impairment was a strong predictor for conversion to AD dementia in a clinical setting (23). Among older adults with no cognitive impairment in the Rush Memory and Aging cohort, difficulty in odor identification was also predictive of subsequent development of MCI (26). Moreover, older individuals with odor identification score within the normal range were highly unlikely to go to on to develop AD in a five year period.
However, olfactory impairment alone is not adequate to predict which individuals will decline cognitively, especially in the general population (24). Other factors may contribute to olfactory impairment in older adults, including peripheral, e.g., conductive nasal disease, sinus diseases, allergies, and central, e.g., head trauma and incipient PD, etiologies (6, 15, 27, 28). While current methods of assessing olfactory function cannot satisfactorily differentiate olfactory impairments due to AD from other causes, new olfactory measures designed from insights about the neuropathology, genetics, neuroimaging, neurophysiology, and cellular and molecular mechanisms of AD may improve their sensitivity and specificity. Additionally, combining olfactory assessments with other biomarkers for AD may enhance their diagnostic accuracy (1). Since olfactory testing is not expensive and is non-invasive and appears to capture a unique proportion of the variance in the preclinical and prodromal stages of AD (1), it remains an important component of the battery of genetic, molecular, and clinical biomarkers that comprise an AD risk profile to detect, and perhaps follow the trajectory of AD, in its preclinical and prodromal phases.
II-3. Neuropathological Studies
The canonical AD pathology of neuronal loss in the setting of amyloid plaques and neurofibrillary tangles (NFT) afflicts many components of the olfactory neural system (29). Accumulation of NFT in the entorhinal cortex is an early site of AD pathology and correlates best with the initial appearance of the cognitive symptoms in AD (30, 31). The olfactory pathway is the most direct path between the entorhinal/hippocampal region and the external environment, i.e., olfactory epithelium (OE). Even though the entorhinal areas receive direct connections from the olfactory bulb, relatively little is known about the mechanisms underlying the entorhinal-mediated olfactory processing and whether the presence of AD pathology in entorhinal regions directly affects olfactory function (32). However, a significant relationship has been demonstrated between odor identification and a composite measure of plaques and tangles in multiple brain areas implicated in AD, including the entorhinal cortex (EC), in a cohort of individuals who died without cognitive impairment, and between odor identification and volume loss on quantitative MRI in the right amygdala and bilateral entorhinal and perirhinal cortices (33). Together, these results suggest that olfactory function may reflect incipient pathology before individuals show clinical evidence of AD (29, 34, 35).
Accumulations of neuropil threads and NFTs have been found in the olfactory bulbs (OBs) of patients diagnosed with definite AD, in the OBs of many patients diagnosed with probable AD or MCI, and in the OBs of some cognitively normal older adults (36). In addition, the frequencies of tau deposits in the OB and the EC were highly correlated (29, 36), indicating that both neuropil threads and NFTs appear in the olfactory system as early as they do in the EC. While a recent animal model study reported the correlation of olfactory dysfunction with Aβ burden in OB and piriform cortex (37), further research is needed to establish the relationship between the appearance of AD pathology in the olfactory neural network and olfactory function in humans. As we gain more insight into the physiology of the processing olfactory input from the olfactory bulb to cortical regions, it will be important to investigate the mechanisms by which AD pathology may influence the structure and function of the olfactory neural network, in particular, during the preclinical stages of AD.
In the OE, both Aβ and tauopathies have been shown to be highly enriched in postmortem tissues of individuals diagnosed with AD in comparison to cognitively normal older adults or young subjects; and the presence of OE AD pathology correlates with the presence of AD brain pathology (38–40). Like the EC and OB, AD pathology can also be found in the OE of a small fraction of the cognitively normal older adults (34, 38, 41). Until the advent of amyloid imaging and the unmasking of amyloid plaques in up to 30% of individuals with normal cognition (42), this lack of complete correlation between the presence of AD pathologies in OE and clinical diagnosis of AD dementia had diminished the interest and effort of further diagnostic and mechanistic research in the past two decades. In light of the introduction of the “preclinical” stage for AD, future research is warranted to assess whether AD pathologies in the OE in cognitively normal older adults is also indicative of the preclinical stage of AD and whether pathological expression of Aβ may contribute to olfactory neuron loss and olfactory impairment. The accessibility of OE for biopsy (40), despite its recognized limitation (43), has potential value for pathological and mechanistic studies. Further studies are needed to determine whether molecular markers can specifically differentiate AD from PD or other types of pathological processes among individuals with olfactory dysfunction will be of particular value.
In summary, AD pathology can be found at every level of the neural pathway processing olfactory information, and AD pathology is associated with impaired odor identification. Moreover, AD pathology is found in the olfactory neural network of cognitively normal older adults. However, little is known about the impact of AD pathology on the function of cells in the olfactory pathway. Further research to define the relationships between AD pathology and olfactory function in cognitively normal subjects will determine whether olfactory dysfunction may be a functional marker of the preclinical stage of AD and will facilitate the development of the next generation of olfactory tests to increase the sensitivity and specificity of olfactory testing for predicting the progression of AD.
II-4. Genetics
Although olfactory impairments are highly prevalent in older adults, in particular among older adults aged above 80 years old, whether age-related olfactory dysfunction is hereditary and whether alterations in genes or the epigenetic landscape associated with these olfactory impairments remain rich areas for investigation. Several studies demonstrate that olfactory impairment is more prevalent in individuals genetically at risk for AD. Olfactory function in first degree relatives of patients with AD (44) or in individuals with a family history of dementia (45) is considerably lower than their age-matched cognitively normal controls. Cognitively normal older adults carrying the APOE ε4 allele, the most robust genetic risk allele for the late onset AD (11), also may have much worse performance on a number of olfactory functions, including odor identification (46), odor threshold (47), and odor memory (48), than the age-matched non carriers.
Genetic risk factors for AD may significantly enhance the power of olfactory tests to predict the progression to cognitive impairment and dementia. In a community-based longitudinal study of memory and aging in King County, WA, the presence of both olfactory impairment and at least one APOE ε4 allele substantially increased the odds ratio (OR) for predicting cognitive decline in comparison to considering either olfactory impairment or carrying at least one APOE ε4 allele alone (22). With the identification of new AD susceptibility genes (11), it will be important to ascertain whether and how combining olfactory testing with AD genetic risk alleles in cognitively normal older adults may enhance the prediction of future progression to AD cognitive symptoms. Likewise, such combinations of genetic markers with olfactory functional markers may help identify older individuals at risk of developing AD and increase the specificity of potential therapeutic treatments.
II-5. Neuroimaging and Neurophysiology
Although it is not yet known whether the volumes of the olfactory pathway as measured by structural magnetic resonance imaging (MRI) are altered in patients with olfactory impairment, it has been shown that odor identification can predict left hippocampal volume in AD patients measured by structural MRI (49). In a clinical study, combining olfactory testing with structural MRI measures of hippocampus and entorhinal cortex led to strong predictive accuracy of conversion from MCI to AD dementia, which was appreciably higher than either measure alone (1). Functional MRI studies reveal that in AD patients, activation of several olfactory central processing areas, including piriform cortex, EC and hippocampus are altered in response to odor stimuli or odor-dependent tasks (50–52). A recent fMRI investigation of brain response during recognition memory for odor stimuli showed significant disruption in functional connectivity in non-demented APOE ε4 carriers in comparison to individuals without the ε4 allele (53). However, the utility of these olfactory-dependent fMRI measures, especially when combined with genetic risk factors, in predicting the clinical and pathological trajectory of AD remains to be investigated.
While neuroimaging studies are best suited to reveal neuroanatomical regions associated with olfactory impairment and AD, neurophysiological approaches, such as event related potentials (ERP), are exquisitely sensitive to temporal changes in the response of the brain to external stimuli. Sensory or perceptual elicited responses tend to be represented by early components of the ERP waveform; while cognitive and memory dependent responses are typically indicated by later components. Early odor-evoked ERPs distinguish patients with AD dementia from cognitively normal controls (54). In cognitively normal older adults, longer latencies for the cognitive component of odor-evoked ERPs can differentiate APOE ε4 carrying individuals from non-carriers (55). Further studies are needed to determine whether odor-evoked ERPs predict the clinical and pathological progression of AD, at least to the similar degree as cognitive-task dependent ERPs (54).
II-6. Cellular Mechanisms and Model Systems
Much of the efforts to elucidate the cellular and molecular underpinnings of AD pathogenesis using cellular and animal models have focused on the memory and cognitive systems involving the hippocampus and entorhinal cortex; and few have examined the olfactory system. Extant studies of the olfactory system are correlational analyses of a) transgenic models overexpressing AD-linked genes broadly throughout the brain, b) characterization of engineered lines that express AD genes specifically in neuronal subtypes within the neural network that processes olfactory information, and c) investigation of the regenerative capacity of the adult olfactory neurons.
In transgenic mice overexpressing either Aβ or tau throughout the brain, sensory impairments of olfactory habituation or discrimination have been observed (37, 56–58). Importantly, these deficits appear at a very young age (3–6 months old), well before the onset of memory or other cognitive deficits. The deposition of AD pathology in the olfactory bulb in these transgenic animals are consistent with the observations that AD pathology can be observed in human olfactory bulb from cognitively normal older adults as well as individuals diagnosed with AD (29). At a structural level, a recent study demonstrated that overexpression of a pathogenic allele of APP, Aβ40 alone or Aβ42 alone, but not the wild type or synthetic mutant isoform of APP, disrupted the structural connectivity of the peripheral olfactory neural circuit in mice in the absence of amyloid plaques (56), resulting in olfactory deficits. More recently, a line with overexpression of a synthetic chimeric mouse-human APP, which results in accelerated olfactory sensory neuron death, was found to have structural connectivity and behavioral anomalies (58).
Recent findings suggest that pathology resulting from the overexpression of tau (59–61) or Aβ (62) specifically in EC neurons can propagate to synaptically connected downstream neurons . Future studies using olfactory-neuron specific transgenes may help to test the possibility of propagation from OB to the piriform cortex or to EC neurons. Recent work demonstrates that treatments which promote Aβ degradation can rescue both olfactory system physiology and odor perception in transgenic mice over-expressing Aβ (37, 63, 64). It will be important to determine whether reversing the olfactory impairments may prevent the occurrence of cognitive impairments emanating from downstream circuits in these transgenic models.
Another unique property of the olfactory system is that both the olfactory epithelium and the olfactory bulb have regenerative capacity that persists throughout adulthood, even in old age (65). In mice that are genetically deficient of APOE, the rate of olfactory nerve regeneration in the OE is significantly delayed, along with multiple measures of olfactory function (66–68). Although the synaptic integrity of the OB is also compromised in the APOE deficient mice (69), it is not yet known whether its regenerative capacity is also decreased or delayed. Interestingly, estrogen treatment has been shown to stimulate the regeneration of OE and synaptogenesis of OB in an APOE-dependent manner (70).
II-7. Key Research Directions
Olfactory dysfunction is a promising biomarker of early pathophysiological events of AD. Prospective studies of olfactory tests designed purposefully for detection of preclinical AD based on insights summarized above are needed in both the clinical setting and at the population level to determine their sensitivity and specificity for predicting the progression of cognitive impairments in AD, either alone or in combination with olfactory epithelial biopsy, genetic, neuroimaging, molecular and other biomarkers. Further analysis is needed to determine whether an olfactory outcome can be used as an intermediate outcome in a clinical trial of preclinical and prodromal AD.
Mechanistic studies leveraging the many advantages of the olfactory neural circuit as a model system for early AD pathogenesis will ascertain whether neural system failure in general and olfactory functional impairment specifically is caused by reversible neuronal dysfunction or irreversible neurodegenerative changes. Moreover, mouse genetic engineering to introduce AD pathogenetic features to different levels of the olfactory system will determine the relative contributions of changes in the epithelium, olfactory bulb, and piriform, entorhinal, and perirhinal cortices to olfactory dysfunction. This knowledge can be translated to design clinical screening instruments with improved sensitivity and specificity.
III. Visual System in Aging and AD
III -1. Summary of Key Findings
Subpopulations of patients with AD have concomitant eye diseases; and particular visual functions are selectively impaired in subgroups of AD patients.
Improvement of visual environment or stimulus may augment some cognitive functions in AD patients.
AD pathology is present in the peripheral and central pathways of the visual neural system and is present in visual association cortical areas in some cognitively normal older adults.
AD and age-related macular degeneration (AMD) share genetic risk factors, including ApoE and complement factor H, based on genetic/genomic association studies.
Structural and functional changes of the retina and peripheral nerve fibers can be detected in patients with AD.
Impairments of electrophysiological and psychophysical measures of central visual function are present in subpopulations of AD patients.
In transgenic mice overexpressing Aβ or APP, the presence of AD pathology in the retina is associated structural and functional disturbances.
Anti-Aβ treatment in mouse models of AMD or glaucoma ameliorates the underlying structural or functional changes associated with retina and retinal ganglion cells (RGCs).
III-2. Epidemiological and Clinical Studies
Cataracts, AMD, glaucoma, and diabetic retinopathy have significant impact on visual function in aging (71). Cataracts are the leading cause of low vision; and AMD is the leading cause of irreversible vision loss in older adults worldwide (72). Multiple lines of evidence suggest that cataracts are associated with AD (73), and specific subtypes of age-related cataract (ARC) and Alzheimer disease (AD) are related genetically and etiologically (74, 75).
In the mammalian lens, APP, Aβ, and components of the Aβ biogenesis pathway are expressed and processed in equatorial epithelia and cortical fiber cells (76, 77). Cytosolic Aβ deposition is also indicated to be present in lens fiber cells in AD patients and contribute to the pathogenesis of supranuclear cataract and age-related lens degeneration (73). Aβ binds aB-crystallin and promotes lens protein aggregation (73). Supporting this, Tg2576 transgenic mice expressing a pathogenic human APP isoform (78) have significantly increased rate of cataracts. In Down syndrome (DS, trisomy 21), an additional APP allele results in cerebral accumulation of Aβ, early onset neuropathology, and age-dependent cognitive defects (79, 80). DS patients develop supranuclear opacification accompanied by accelerated supranuclear Aβ accumulation, amyloid pathology and fiber cell cytoplasmic Aβ aggregates, which is identical to lens pathology in AD patients, suggesting Aβ accumulation is the key pathological determinant in supranuclear cataract in both DS and AD (74). Consistently, mice carrying the Down syndrome critical region including a complete copy of human APP have increased expression of human and mouse endogenous APP and develop age-related lens degeneration (81). A recent study provided further genetic evidence that ARC and AD are related etiologically. δ-Catenin is genetically and biologically associated with cortical cataract and future Alzheimer-related structural and functional brain changes (75). However, conflicting evidence argued that β-amyloid has no contribution to cortical cataract in donors with or without AD(82), suggesting that further study is needed to delineate the association of AD and cataract.
Type I and Type II diabetes are associated with AD (83–86), and diabetes is the direct cause of diabetic retinopathy (DR). Nearly all individuals who have had type I diabetes for more than 15 years develop DR. Approximately 50–80% of type II diabetic patients also develop retinopathy after 20 years of diabetes (87). Therefore, DR may be clinically associated with AD. It has been reported that, like what’s reported in CSF of AD patients (88), there is significant decrease in the Aβ42 level and a significant increase in the tau level in patients with diabetic retinopathy (89), suggesting potential roles of β-amyloid and tau in DR.
Both AMD and AD are neurodegenerative diseases in which aging is the principal risk factor (90). There is a significant association between the late stages of AMD and cognitive impairment (91–93). In one study, individuals with advanced AMD had an increased 2-year risk of developing AD in comparison to those with better vision, even though the risk was relatively small (91, 93). Interestingly, persons with severe cognitive impairment as measured by word fluency were also more likely to have early AMD than people with better test performance, although no association with other measures of cognitive impairment was reported (94). However, a recent study with a large cohort constructed from English National Health Service showed that the risk of AD or dementia following AMD was not elevated. The likelihood of being admitted for AMD following AD or dementia was very low, suggesting that, although AD and AMD may share environmental risk factors and histopathologic features, their coexistence at the individual level is no different from that expected by chance (95).
Glaucoma was also observed at a higher incidence rate in AD patients than in cognitively normal controls (96). Patients clinically diagnosed with both dementia and glaucoma had faster and more aggressive progression of glaucomatous optic neuropathy than patients with glaucoma alone; the optic nerve appeared to be less resistant to elevated intraocular pressure levels in glaucoma patients with AD than glaucoma alone patients (97). However, an epidemiological study from Denmark showed that there was no increased risk of developing AD in patients with glaucoma (98), suggesting that glaucoma per se may not be a risk factor for AD. Conversely, intracranial pressure is lower in patients with normal-tension glaucoma when compared with patients with primary open-angle glaucoma and nonglaucomatous control subjects. Decreased CSF production and turnover in Alzheimer disease patients may contribute to higher risk of developing glaucoma in AD patients (99). Further study is needed to confirm this hypothesis.
Acetyl-cholinesterase inhibitors (AChEIs), which delay the cognitive decline of AD (100), have a protective effect on the development of glaucoma. Topical rivastigmine lowered intraocular pressure (IOP) in rabbits (101). Oral administration of donepezil in normotensive Alzheimer patients resulted in decreased IOP and pupil diameter (102). A pilot study in normotensive glaucoma patients showed that donepezil improved visual field, ONH blood flow, regional cerebral blood flow, although IOP was not significantly changed. No deterioration of normotensive glaucoma morbidity was found in any of the measured parameters after 12 months of treatment (103). These studies suggest that cholinergic dysfunction may be a common pathological changes in AD and glaucoma, and AChEI may have dual benefit for treatment of both AD and glaucoma.
In addition to eye diseases, many visual system functions have been shown to decline with age, including visual acuity (104), color discrimination (105), contrast sensitivity at high spatial frequencies (106), depth perceptions (107), motion perception (108, 109), visually guided body motion (110), visual processing speed (104), and prolonged dark adaptation (111). Certain aspects of these visual functions have been reported to be more selectively impaired in subgroups of AD patients than the age-matched cognitively normal controls. For instance, in one study more than 50% of AD patients failed to discriminate blue color correctly while less than 25% of cognitively normal controls would make the same mistake; there was little difference between AD patients and controls in discriminating red or green colors (112). Wijk et al. (113) also reported more specific difficulty by AD patient in discriminating blue and green colors. However, other investigators have reported general color vision deficits in AD patients (114). Similarly, performance on monocular and binocular depth perception tests was significantly poorer in AD patients than in cognitively normal controls (115). AD patients also showed impaired contrast sensitivity to both high and low spatial frequencies; whereas cognitively normal older adults only had reduced contrast sensitivity at high spatial frequencies (116). It has been demonstrated in a longitudinal study that AD patients show changes in sensitivity to abruptly changing, low spatial frequencies in as little as six months, suggesting a selective change in the large cell (magnocellular) neural system responsible for detecting large moving objects (117). Consistent with this finding are reports of visual motion sensitivity also showing pronounced threshold elevations in a subpopulation of older adults as well as in AD patients (109, 118, 119). In addition, the ability to distinguish the shape of a set of moving dots in a 3-D structure and the self-movement direction simulated in radial dot motion was significantly impaired in at least a subpopulation of AD patients, demonstrating the potential functional impact of AD on visual motion perception (120, 121). Nevertheless, whether these visual functional impairments occur before or after the onset of cognitive impairment in AD patients will require further longitudinal studies.
In considering the evidence supporting the associations between visual dysfunctions and cognition, it is conceivable that diminished visual information, either caused by AD pathology or other pathologies, may directly impact vision-dependent cognitive outcomes. Deficits in the visual cognition of AD patients have been widely reported (122–132). Recent work by Cronin-Golomb, Gilmore and their colleagues has provided some evidence that the performance of AD patients in tasks such as letter identification, word reading, picture naming, face discrimination, and digit cancellation (133) can be improved, sometimes to normal levels, simply by increasing the contrast of stimuli to compensate for their contrast sensitivity deficit (134–136). In addition, enhancing the contrast or strength of visual stimuli may improve advanced AD patients’ activities of daily living, such as pill finding (137), playing Bingo (138), and eating (139). The positive impact of stimulus enhancement has been shown in patients who had been screened for visual acuity and major age-related vision problems, such as cataracts, glaucoma, and macular degeneration (133–138). It is likely that the stimulus enhancement would not help patients with severe vision pathology. Although we do not know whether enhancing the visual environment may have any impact on the underlying pathological events of AD, this line of studies suggests that aspects of the functional impairments experienced by some AD patients may be at least partially corrected by non-pharmacological approaches such as enhancing relevant visual signals. The line of research also suggests that cognitive evaluation of AD patients with tests that require vision may underestimate the competence of the patients unless vision – fair tests are used that compensate for vision deficits.
III-3. Neuropathological Studies
Aβ pathology has been shown to be present in most, if not all, segments of the visual pathway. In the frontal eye structures, Aβ deposition can be found inside the lens fiber cells as well as in the primary aqueous humor and vitreous bodies of AD patients. The molecular chaperone alpha B-crystallin, important for maintaining the transparency of the lens, can bind with Aβ, which may promote lens protein aggregation, thus contributing to the pathogenesis of supranuclear cataract and age-related lens degeneration (73). The levels of the soluble derivative of APP (sAPP) are particularly high in the vitreous and low in the aqueous humor. Both Aβ40 and Aβ42 levels are approximately two-fold greater in the vitreous than in the aqueous body (140). In fact, Aβ peptide is found in 40% of the aqueous humor of patients with glaucoma (141) at a concentration comparable to that in the cerebrospinal fluid (73). However, the pathogenic significance of the presence of AD-related pathologies in the frontal eye structure remains to be elucidated. Whether the pathogenesis of AD and glaucoma or cataract share common features in biochemistry and etiology requires further investigations.
The peripheral nerve components of the visual pathway, in particular the RGCs and the optic nerve fiber layer (NFL), develop cell death and axon degeneration in patients with AD. More than 25 years ago, Hinton et al. reported widespread axonal degeneration in optic nerves of 8 out of 10 AD patients, as well as a reduction of the number of RGCs, and thinning of optic NFL in 3 of the 4 AD patients examined, relative to age-matched controls (142). With regard to RGC loss in AD, both macular and peripheral retina are dominantly affected in the fovea as well as the superior and inferior quadrants, and the overall neuronal loss amounts to 36.4% (143, 144). In the early post-mortem histiological studies, it was suggested that this pattern of RGC loss in AD is quite distinct from the RGC loss in glaucoma, which selectively affects the mid-peripheral retina with no or little involvement of the macula (145–147). However, growing evidence using new imaging technologies, e.g. frequency domain optical coherence tomography (fdOCT) and spectral domain optical coherence tomography (SD-OCT), demonstrate early glaucomatous damage to the macula (148–153). Studies on the similarity and difference between macular changes in AD and glaucoma by these advanced imaging technologies are required to further delineate the connection of pathological course of AD-related retinal changes and glaucoma. In addition, AD patients exhibit a thinning of the optic disc rim with increased cup volume and a reduction in the rim area to disc area ratio; whereas glaucoma patients endure a more pronounced pallor and focal loss of the neural rim (154).
With regard to changes in NFL in AD, significant axonal degeneration has been found in retrobulbar optic nerves; and the axonal degeneration was more dominant in the posterior segment of the nerve, raising the possibility of retrograde axonal degeneration and subsequent RGC loss elicited by pathological events in subcortical and cortical visual areas (155). Together, these findings suggest that RGC loss and NFL changes in AD may be unique and not the result of advanced undiagnosed glaucoma (147). However, it is important to point out that the presence of AD pathologies in the pre-cortical visual pathways is not uniform for all AD patients as some histological studies with small sample sizes failed to see statistically significant reductions in RGC number or NFL changes (156, 157). Moreover, AD pathology, such as Aβ deposition, has been found in the drusen, extracellular deposits beneath the retinal pigment epithelium or within the optic nerve head, in patients with AMD but with no AD dementia (90, 158–161). Thus, further investigations are required to ascertain whether these controversial findings reflect the underlying heterogeneity of AD and the general relevance of these visual components in the pathogenesis of AD dementia.
The presence of AD pathologies in subcortical visual center, primary visual cortex, visual association cortex, and high order visual association cortex of the inferior temporal gyrus has been extensively reported (162–165). However, the temporal sequence of AD pathological deposition in these visual areas and the associated clinical significance has been debated. The current canonical view states that the neuropathology of AD is typically first seen in the limbic and peri-limbic cortices with subsequent extension to the higher order posterior association areas, and then to successively lower-order association areas, and finally primary sensory and motor regions (166–168). Yet, several histology studies indicate that the posterior cortical visual areas can be substantially affected with AD pathologies in the early stages of AD or prior to the onset of dementia (164, 169). One possible factor that may help to reconcile these findings is that the conventional clinical diagnostic criteria of AD used for neuropathological analyses rely heavily on verbal amnestic criteria, which may tend to promote findings of sentinel histopathological markers in hippocampal, subicular, and entorhinal areas. In contrast, when visuospatial criteria (170) are used as the basis for neuropathological analyses, the earliest AD histopathological markers are found at the transition zone between striate and pre-striate visual cortex (171). In fact, posterior cortical atrophy (PCA), a syndrome with insidious onset of visual dysfunction, is now commonly considered as a variant of AD (172–174).
III-4. Genetics
APOE polymorphisms differentially modify risk for AMD and AD (92, 175–180). In sporadic AD, the ApoE ε4 allele is the strongest risk factor (175–177, 180, 181), whereas the ApoE ε2 allele confers a reduced risk (175, 181). In contrast, ApoE ε2 allele is linked to an elevated risk for AMD, ApoE4 allele appears to have lower risk for the disease (92, 178, 179, 182). In addition, inflammatory processes seem to be involved in both AMD and AD. Polymorphism of complement factor H (CFH) is the major genetic risk factor for AMD (183–186), has been associated with increased risk of AD (180). CFH has been detected in amyloid plaques in the brains of AD patients (187), and increased levels of CFH have been detected in the plasma of AD patients (188). However, a recent study of the relationship between complement-factor-related AMD genetic risk factors and AD (189) revealed modestly significant associations between CFH, the age-related maculopathy susceptibility protein 2, and the complement component 3 SNPs with AD, but these relationships were in different direction to that observed in AMD. In addition, the multilocus genetic model that predicts around a half of the sibling risk for AMD does not predict risk for AD. These results suggest that while activation of the alternative complement pathway is central to AMD pathogenesis, it is less involved in AD (189). Future efforts may identify other discrete risk factors and biomarkers that could be combined with these genetic risk factors to enhance the predictive value and specificity of the onset and progression of AD as well as AMD. Recently, a genome-wide association study of the Framingham Eye Study cohort showed that δ-catenin, a protein that interacts with the presenilins, is genetically and biologically associated with age-related cataract and future AD-related structural and functional brain changes (75), offering another possible genetic link between age-related eye disorders and AD.
III-5. Neuroimaging and Neurophysiology
Structural and functional changes of the peripheral and central visual systems in aging have been reported (as assessed by methods discussed in the visual systems appendix). In patients with mild to moderate probable AD, there is a significant reduction of two retinal hemodynamic parameters in comparison to age-matched controls (190). In addition, significant thinning of NFL thickness has been reported in AD patients, as well as patients with MCI (190–194). Moreover, the morphological changes of NFL thickness in AD patients significantly correlated with the delay of implicit times of pattern electroretinogram (194). The mean total macular volume was also significantly reduced in AD patients, and the reduction correlated with the severity of cognitive impairment measured by mini-mental state examination (MMSE) (191). However, whether any of these structural or functional changes in the retina would serve as potential diagnostic biomarkers for early diagnosis of AD may require longitudinal studies of older adults transitioning from normal aging to AD or older adults genetically at risk for AD.
The most extensively studied central visual function in AD is motion processing, in particular as determined by radial optic flow. Psychophysical analysis has revealed that AD patients exhibit exquisite deficits in differentiating movement directions inferred from optic flow (195–197). Further neurophysiological studies suggest that both the sensory/perceptual components and the cognitive components involved in motion perception under optic flow are affected in early AD patients as both the earlier and later components of ERP signals are diminished under a variety of experimental paradigms (198–201). In addition, these neurophysiological deficits highly correlate with navigational impairments in AD patients (202). AD patients with severe cognitive impairments but less navigational impairments show nearly normal early ERP signals (203), suggesting that the visual perceptual components of these patients may remain intact and supporting the existence of functional heterogeneity in the AD patient population. Conversely, a small proportion of cognitively normal older adults also exhibit navigational impairments as well as the associated changes in the early components of ERP signals (202). Whether the navigational impairments in these cognitive normal older adults may be a pre-clinical sign of AD may require further longitudinal assessments, possibly in combination with genetic risk factors. In addition, further studies are required to delineate the relationship between motion processing impairments in AD and what may be a fundamental insensitivity to the temporal dynamics of visual stimuli (204, 205), possibly linked to underlying retinal pathology (192, 206), and genetic risk factors for AD.
A variety of neuroimaging studies support the conclusion that central visual dysfunction is an important part of functional impairment in AD. PET measurement of regional cerebral blood flow suggests great involvement of dorsal stream visual motion areas than of ventral object oriented areas (207, 208). Studies have shown that hypoactivation in the posterior visual association areas measured by functional MRI (fMRI) is strongly associated with the navigational impairments or visuospatial disorientation in patients with AD (168, 209–211). Whether the hypo-activation in the visual association cortex directly relates to the presence of AD pathologies in this area, or is a consequence of system-wide disturbances that may precede pathological changes, may best be determined by coupling with metabolic and ligand specific imaging modalities.
III-6. Cellular Mechanisms and Model Systems
Mechanistic studies of the visual system using model organisms have thus far primarily focused on three questions in transgenic mice overexpressing APP, Aβ or tau: Can AD-like pathology be detected in visual pathways? If so, is the AD pathology associated with specific visual impairments? If so, would removal of this AD pathology reverse visual impairments?
The lenses harvested from AD and Down Syndrome-related mouse models show characteristic patterns of supranuclear opacification accompanied by accelerated supranuclear Aβ accumulation (81), similar to the pathological events observed in Down syndrome (DS) patients and the lens pathology in AD patients (74). Individuals with DS develop AD neuropathology in their third and fourth decades, and they have a significantly elevated risk for dementia later in life (212). The postmortem retinas of various AD mouse models also contain Aβ plaques, revealed by the plaque-labeling fluorochrome, curcumin (213). The plaques are associated with functional defects (214–217), consistent with clinical observations. Systemic administration of curcumin to APPswe/PS1Δ9 transgenic mice allows in vivo detection of Aβ plaques in the retina using non-invasive optic imaging, earlier than the appearance of Aβ plaques in the brain (213), suggesting that retinal Aβ plaques may also be an early, non-invasive diagnostic biomarker for AD. Recently, the presence of pathogenic APP and/or amyloid plaques in a mouse models of AD was shown to alter cortical plasticity in the primary visual and association areas (218, 219).
Anti-Aβ treatment in an AMD mouse model reduced the amount of Aβ in the retina and the electroretinogram deficit was abrogated (220, 221), suggesting that Aβ may be a therapeutic target for visual defects in both AD (222) and AMD. Investigations of glaucoma mouse models support the link between AD and glaucoma pathologies (223–225). Targeting Aβ formation and aggregation pathways effectively reduced glaucomatous RGC apoptosis in vivo, suggesting that, although RGC loss in AD has its unique characteristics and may not be the result of advanced undiagnosed glaucoma (147), the Aβ pathway may contribute to glaucoma-induced RGC apoptosis (224). Further investigations into the impact of targeting Aβ on visual dysfunction in glaucoma related animal models may provide important insights into the molecular link between glaucoma and AD.
III-7. Key Research Directions
Longitudinal studies of cognitively normal subjects who are followed until conversion to symptomatic AD in both clinical settings and at a population level, are needed to characterize the temporal relationships between visual dysfunctions and cognitive impairments in AD. These studies will test the clinical utility of following visual dysfunction, by determining its sensitivity and specificity for predicting the progression of cognitive impairments in AD, either alone or in combination with genetic, non-invasive retinal imaging, neuroimaging, or other biomarkers.
Mechanistic studies in model organisms and humans are needed to ascertain whether functional visual impairment in AD is caused by neurodegenerative or disordered structural plasticity changes in the visual system and to determine the relative contributions of these changes in the lens, retina, retinal nerve fiber, primary visual cortex, and visual association cortex as a consequence of AD pathogenesis with its associated brain dysfunction.
IV. Auditory System in Aging and AD
IV-1. Summary of Key Findings
Hearing loss is highly prevalent in older adults, and it is the most prevalent sensory loss in older adults.
Impairments in speech perception in noise, often an indication of central auditory dysfunction, are also quite common in older adults.
Both hearing loss and central auditory dysfunction are associated with a high risk of conversion to dementia 5 – 10 years later.
AD pathology is found in the central areas of the auditory neural pathway in most AD patients, but not in the peripheral ear and cochlear structures.
Combining CSF Aβ levels with auditory evoked responses may enhance the predictive accuracy of conversion to AD dementia.
Greater peripheral hearing loss is associated with poorer performance on both verbal and non-verbal cognitive tests.
In older adults, the frontal and temporo-parietal cortices are recruited to facilitate auditory speech processing as revealed by fMRI studies.
In transgenic mice overexpressing APP, the presence of AD pathology in primary auditory cortex is associated with changes in auditory evoked responses.
IV-2. Epidemiological and Clinical Studies
The prevalence of age-related hearing loss in older adults, referred to as presbycusis, doubles with each increasing age decade (226). About 40–45% of adults over the age of 65 show some degree of hearing impairment, with this figure rising to 83% in the population over the age of 70 (227). These data make hearing loss the third most prevalent chronic medical condition among older adults, exceeded only by arthritis and hypertension (228). The most common complaint among older adults with regard to their hearing is difficulty in understanding speech, with such complaints doubling with each decade over the age of 60 (229). This problem is especially exacerbated in the presence of background noise. Indeed, one of the hallmarks of age-related central hearing impairment is a difficulty in speech recognition in a noisy background, even when speech recognition is relatively good in a quiet background (230, 231). Older adults are especially susceptible to interference from the presence of competing speakers (“cocktail party effect”), due in part to poor frequency resolution and encoding of sounds by the impaired cochlea and in part to higher-level cognitive factors (232–234).
Some small longitudinal studies have shown that deficits in central auditory processing in the absence of severe peripheral hearing loss, as assessed by measures discussed in the auditory system appendix, were associated with high incidences of cognitive decline and Alzheimer’s dementia (2, 235–237). These studies have demonstrated that individuals with central auditory dysfunction were at a significantly increased risk for incident dementia with hazard ratios ranging from 9.9 (95%CI=3.6 – 26.7) to 23.3 (95%CI=6.6 – 82.7) (236, 237). Because behavioral measures of central auditory processing include an individual’s ability to understand speech embedded in noise, competing speech, or under dichotic listening conditions, central processing within the primary auditory cortex and higher order association areas is required for the participant to identify and understand the presented signal. Further research examining the basis of this association has demonstrated that measures of central auditory function are strongly associated with measures of executive function (237), which in turn have been implicated as an early marker for dementia (238–242). There are likely shared cognitive processes underlying both central auditory function and executive functioning given that both tasks require participants to selectively attend to one stream of information while inhibiting non-relevant information (243). Further studies may be required to ascertain the relationship between central auditory function and executive functions, in particular testing whether deficits in central auditory processing and executive functions may result from the same neurodegenerative processes that precede dementia (237).
Recently, a prospective study utilizing data from the Baltimore Longitudinal Study of Aging has demonstrated that peripheral hearing loss as measured with pure tone audiometry is independently associated with incident dementia. In this study (3), a cohort of 639 adults without prevalent dementia or MCI at baseline was followed for a median of 11.9 years. Hearing loss at baseline was associated with the risk of incident all-cause dementia after adjustment for known confounders (1.27x increased risk/10dB of hearing loss; 95% CI = 1.06 – 1.50). In another prospective study of 1,984 community-dwelling older adults, findings were similar with hearing loss being independently associated with accelerated rates of cognitive decline on both verbal and non-verbal measures of cognition over 6 years (244). Most recently, a study of the 1,057 surviving men of the Caerphilly cohort also confirmed the association of pure-tone average threshold with dementia and cognitive decline over a 17-year period (245). These findings have profound implications for the relationship between hearing loss and cognitive impairments in Alzheimer’s disease and dementia. A shared neuropathologic etiology is a possibility (246). Alternatively, hearing loss may be associated with dementia through a causal pathway mediated by social isolation and/or cognitive load. Communication impairments caused by hearing loss can lead to social isolation in older adults (247), and epidemiologic (248) and neuroanatomic studies (249) have demonstrated associations between social isolation and dementia. As indicated previously, the negative effect of hearing loss on cognitive performance is suggested by studies demonstrating that under conditions where auditory perception is difficult (i.e. hearing loss) greater cognitive resources are dedicated to auditory perceptual processing to the detriment of other cognitive processes such as working memory (250–252). Further mechanistic and interventional studies will be required to test these possible relationships between hearing loss and AD.
IV-3. Neuropathological Studies
The presence of AD pathologies in the auditory system has not been extensively studied. In the few reported studies, the peripheral auditory structures (including the cochlea and the associated hair cells), unlike the counterparts in the peripheral olfactory and visual pathways, do not contain plaques and tangles in patients with AD, although mild degeneration of hair cells, neural processes, and spiral ganglion cells in the basal membrane of the cochlea has been reported (253, 254). However, both plaques and tangles have been found in many major components of the central auditory pathways. Specifically, Ohm and Braak first reported in 1989 that considerable plaque formation can be found in the central nucleus and the dorsomedial nucleus of the inferior colliculus, and to a lesser degree, in the deep layers of the dorsal cortex of the inferior colliculus in the midbrain among all the confirmed AD patients. In addition, NFTs were occasionally found in the dorsal cochlear nucleus, the periolivary region, the ventral nucleus of the lateral lemniscus of the brainstem, and in the central nucleus of the inferior colliculus in the midbrain (162). The presence of plaques and tangles in the inferior colliculus was later confirmed (254). It is important to note that these pathological changes were detected in advanced AD patients. In addition, senile plaques and NFTs were found in medial geniculate nucleus in the thalamus as well as the primary auditory and auditory association cortices for all the AD patients examined (254). Although sensorineural hearing loss and central hearing deficits have been reported in AD patients, it remains to be studied whether the presence of AD pathologies in the central auditory pathways are related to hearing loss in those adults. Moreover, whether the pathologies may be present in pre-symptomatic AD patients with hearing loss and thus contributing to their hearing impairment has not yet been investigated.
IV-4. Genetics
Genetic influences have been considered to play an important role in the development of age-related hearing loss (255). For example, data from a comparative study of hearing acuity for 179 monozygotic and 150 dizygotic adult twin pairs ranging in age from 52 to 60 years submitted to biometric modeling showed that 65–70% of the variance in the middle and high frequency ranges could be accounted for by genetic factors (256). However. only a few studies have examined the gene risk factors for age-related hearing loss, or presbycusis. Thus far, only three genes, KCNQ4 (encoding a voltage-gated potassium channel), NAT2 (N-acetyltransferase), and GRM7 (glutamate metabotrophic receptor 7) are considered susceptibility genes for presbycusis that have been verified by studies in at least two different populations (257–260). Whether people carrying risk alleles of these genes for presbycusis would be more susceptible to the development of AD has not yet been investigated. Nevertheless, one recent population-based study of older adults (85 years old or above) found that six subjects with the APOE ε4/4 genotype had the highest levels of hearing loss, those with the APOE ε3/4 or ε2/4 genotype had intermediate levels of hearing loss, and those without the APOE ε4 allele had the lowest levels of hearing loss (261). In addition, in this study the APOE ε4 allele was associated with a 2.0-fold increased risk of hearing impairment compared with those without the APOE ε4 allele, suggesting that the APOE ε4 allele contributes to the development of age-related hearing loss. As the APOE ε4 allele is also the strongest genetic risk factor for developing AD, it will be important to examine whether a combination of hearing loss and APOE ε4 would predict conversion to AD for older adults with normal cognition but some level of hearing loss at the baseline.
IV-5. Neuroimaging and Neurophysiology
Auditory evoked potentials (AEPs) are commonly used to assess neurophysiological integrity of ascending auditory pathway and the primary auditory cortex. AEPs include auditory brainstem responses (ABRs), which consists of five waves (I–V) generated by the peripheral portion of cranial nerve VIII, the central portion of cranial nerve VIII, cochlear nucleus, superior olivary complex/lateral lemniscus, and inferior colliculus, respectively. AEPs also include middle latency responses (MLRs), which are the earliest auditory cortical responses and typically occur at 30 to 50 ms after the stimulus onset with P30 and P50 indicating the two major evoked potential peaks. The third type of AEPs typically occurs in the late latency range, thus called late latency responses (LLRs) and they also measure functions of the auditory cortex and the associated cortices. The P1 peak occurs at 100 ms after the onset of stimulus; other LLRs include P2, P3 as well as N1, which occurs between P1 and P2.
In older adults, the amplitude and latency of these AEPs are highly influenced by the complexity of the sound signals as well as the degree of hearing loss that each individual has. For older adults with or without hearing loss, the P1 and N1 latencies are delayed in comparison to young adults with normal hearing (262). Moreover, the N1 latency delay was absent when a simpler sound signal was used; and the latency delays for both P1 and N1 were absent at a slower rate of sound presentation but magnified at a faster rate of sound presentation (262), demonstrating the intricate inter-relationships between the function of the central auditory system and the degree of sound complexity. The effects of aging and hearing loss on ABRs have been largely inconsistent (263), possibly due to the variations in the choice of sound stimuli and the definition of hearing loss. Nevertheless, age-related hearing loss is associated with decreases in the amplitude and delays in the latency of all waves in ABRs (264). Few studies thus far looked into how the AERs are affected in people with both AD and hearing loss or people with hearing loss and genetically at risk for AD. One recent clinical study of 53 MCI patients showed that an index combining delayed latencies and reduced amplitudes of AERs with CSF Aβ levels achieved higher values of sensitivity and specificity in the discrimination between AD converters and MCI stable patients, relative to the separate use of CSF Aβ levels and AERs (265). More systematic studies of AERs in AD patients or patients at risk for AD in combination with other AD-related biomarkers, similar to the ones discussed in the olfactory and visual sections, may be helpful to evaluate the relationships among hearing loss, AERs, and AD.
Functional and structural neuroimaging studies have offered some important insights about hearing loss and cognitive function in older adults. For instance, the degree of hearing loss has been associated with decreases in fMRI signals across all major neural components along the auditory ascending pathway, including the primary auditory cortex as well as the decrease in gray matter volume of the primary auditory cortex (266). Further neuroimaging studies have demonstrated a compensatory recruitment of regions in the frontal and temporoparietal cortex to maintain auditory speech processing in older adults (267), and this pattern of neural compensation may explain the general preservation of language comprehension that is seen even in individuals with advanced dementia (268). The cognitive load induced by hearing loss could, therefore, result in a smaller pool of resources being available for other cognitive tasks (269) with a consequent depletion of cognitive reserve and earlier expression of dementia symptomatology. Such a hypothesis is generally consistent with cross-sectional and longitudinal studies demonstrating that greater hearing loss is primarily associated with poorer performance on those cognitive tests (verbal and non-verbal) that would be expected to overwhelm available resources (e.g. tests of memory and executive function) rather than cognitive tests focused on less complex speeded tasks (244, 270, 271). While we do not know yet whether this proposed mechanism may underlie the connection between hearing loss and future development of cognitive impairments in patients at risk for AD, further studies to ascertain the impact of rehabilitating auditory function in older adults with hearing loss on their long-term prospect of cognitive function may provide important insight about the significance of restoring or maintaining auditory function on delaying AD dementia. Moreover, it may be of great value to study how the auditory pathways and the associated higher level cortical areas are affected in AD patients or older adults genetically at risk for AD, using fMRI, structural MRI as well as PET imaging technologies specifically designed for AD studies.
IV-6. Cellular Mechanisms and Model Systems
Thus far, only one study has examined changes in AEPs in mice carrying mutated human amyloid precursor protein and presenilin-1 transgenes (272). Interestingly, the AEPs in the middle latency range, which are indicative of primary auditory cortical function, are most affected in these transgenic mice; and these changes are associated with the appearance of Aβ pathology in the primary auditory cortex. However, the effects on hearing in these transgenic mice were not examined in this study. Future systematic analysis of the structure and function of the auditory system in AD-relevant transgenic models may be helpful to decipher the relevance of AD pathologies in age-related hearing loss in older adults.
IV-7. Key Research Directions
The prevalence of hearing loss in patients with AD is unknown. Whether peripheral and central auditory dysfunctions are promising biomarkers of early pathophysiological events of AD remains to be tested. Prospective studies, starting with cognitively normal subjects who are then followed until conversion to symptomatic AD in both clinical settings and at a population level, are needed to characterize and demonstrate the clinical utility of peripheral and central auditory dysfunction, including its sensitivity and specificity for predicting the progression of cognitive impairments in AD, either alone or in combination with genetic, neurophysiological, neuroimaging, or other biomarkers.
Interventional studies of hearing rehabilitative treatment on cognition and dementia are needed to definitively assess the relationship between hearing loss and AD.
Mechanistic studies in humans and model organisms are needed to ascertain whether some of the auditory dysfunctions in cognitively normal older adults may be caused by neurodegenerative and neuroplastic processes related to AD pathology.
V. Motor Systems in Aging and AD
V-1. Summary of Key Findings
Motor impairment is highly prevalent in older adults.
Many pyramidal and extrapyramidal (or parkinsonian) motor impairments affect a substantial portion of AD patients, even at an early stage of the disease, and progressively worsen along with cognitive impairment.
Motor impairment may precede the onset of cognitive impairment for AD by a decade and longer.
AD pathology can be found in motor neurons of the pyramidal motor pathways and in extrapyramidal motor pathways in AD patients, as well as in some cognitively intact older adults; the presence of AD pathology in monoaminergic nuclei, including the locus coeruleus and substantia nigra, correlates with the presence of some motor signs.
A number of genes (p16, IL-18, COMT) have been associated with alterations in motor function in non-demented older adults; and the AD risk genes, PS1 and APOE ε4, have been associated with several motor symptoms in AD.
In healthy older adults, the primary motor cortex exhibits hypoexcitability; whereas in AD patients, the primary motor cortex exhibits hyperexcitability.
Many cellular and molecular pathways involved in aging and longevity have been implicated in both AD and PD, suggesting a convergence of pathways for therapeutic targeting.
In transgenic models of AD, overexpression of genes involved in AD pathology (PS1, APP, Aβ, tau, and APOE) is associated with significant motor neuron impairments at the cellular and behavioral levels.
V-2. Epidemiological and Clinical Studies
It has been estimated that by the age of 80 years up to 50% of older adults may have some element of motor impairment (273). While the relationships between many of these motor functions and AD have not been fully investigated, a recent study suggests that both the level and the rate of declining grip strength in older adults are associated with an increased risk for developing AD, similar to parkinsonian signs (4, 274). In addition, studies reported that older adults with amnestic MCI showed worse performance on factors reflecting rhythmicity and variability of gait compared to older adults with non-amnestic MCI or healthy older controls (275–277). Furthermore, prominent and distinct abnormalities of both simple and complex eye movements have been reported in patients with AD (278, 279) and can be used to differentiate the clinical syndrome in AD from other types of dementia as well as to predict the underlying pathology (280). Finger tapping speed has also been shown to decline after the onset of dementia (281, 282). Whether such motoric declines could be a valuable early marker for AD requires further study.
Due to their relevance to PD, extrapyramidal motor functions have been extensively studied. In a community population of older adults, the prevalence of parkinsonian-type motor dysfunction is fairly high, estimated to be about 15% for people 65 to 74 years of age, 30% for those 75 to 84, and 50% for those 85 and older (283), and are associated with risk of disability (284). Although many of these motor syndromes and signs have been described in various AD populations, for this review we will focus on those that are the most commonly of interest in clinical practice and management of AD, as well as being of potential value for what they may tell us about the biology of AD itself. For example, in a population-based study (282), seven motor measures (present or absent) were assessed: bradykinesia, rest tremor, limb rigidity, tone increase, axial tone, deviation of gait and posture from normal and an aggregate measure called “parkinsonism,” (defined as the presence of 2/3 signs among rest tremor, limb rigidity or bradykinesia). Using this approach, gait and postural changes of any kind were present in nearly 50% among those with dementia (all types combined), and the classical parkinsonism was present in about 12% of dementia patients. Further, among community dwelling persons with AD, parkinsonian signs are an important predictor of mortality (285). Because motor signs, in particular gait impairment, may be common in non-Alzheimer dementias (vascular, dementia with Lewy bodies, and PD with dementia) compared with AD (5, 7, 286), these results may be used to frame the upper prevalence bounds for identifying motor signs in dementia patients in general practice. More specific estimates for motor signs in AD have been reported in a population-based sample of older adults living in New York City. Motor signs, assessed using a modified United Parkinson’s Disease Rating Scale (UPDRS) (287), were present in 18.5% of octogenarian AD patients at first diagnosis. The predominant signs were in the domains of posture/gait (combined in the study) (11%), bradykinesia (6%), speech/facial expression (5%) and rigidity (5%). Of note, this was a prospective study beginning with non-cognitively impaired participants. About 12% of these normal volunteers were noted to have motor signs. Further, after a follow-up period of 3.6 years, the motor signs increase overall (22%) in the AD patients. The relative proportion of these signs remained the same across the longitudinal study suggesting that these signs are present or develop before AD begins and are progressive. Others have shown in prospective studies of initially cognitively intact older volunteers that motor signs, and in particular gait speed, are likely changing years prior to manifestation of AD symptoms (4, 281, 282, 288, 289). A recent longitudinal study examining motor signs in initially cognitively healthy adults observed the emergence of accelerated rates of motor decline (gait speed slowing) twelve years preceding the emergence of MCI, and slowing of manual tapping speed at or near the time when cognitive impairment was observed (281). Although other parkinsonian signs such as tremor (290, 291), myoclonus (292), and muscle weakness (293) also have been associated with AD, the timing of these motor changes in relation to cognitive impairment have not been widely studied.
Dementia itself may indirectly influence motor function. During cognitive-motor “dual tasking” paradigms, which require the participants to walk and perform a secondary interference task (294), motor performance may be compromised. The advantage of using the dual-task approach is that attentional demands are experimentally manipulated. It has been reported that allocation of attention to concurrent and competing tasks depends on executive processes that are responsible for cortical control of locomotion, and the frontal subcortical regions are especially vulnerable to effects of vascular disease in aging (295, 296). Hence, it is possible to make inferences about the causal effect of attention resources on gait and mobility performance (295). Whether cognitive training may also enhance motor function remains to be an important area for additional research.
V-3. Neuropathological Studies
Aβ deposition and neurofibrillary tangles have been widely reported to be found in many cortical areas, including the primary motor cortex and supplementary motor areas (273), not only in AD patients (166) but also in cognitively intact older adults (297). However, it is unclear the degree to which these pathological markers directly affect the function and associated motor behaviors mediated through the motor neurons in these brain regions. It is also worth noting that for a number of motor neuron degenerative diseases, such as amyotrophic lateral sclerosis (ALS), progressive supranuclear palsy (PSP), corticobasal degeneration (CBGD), and Lewy body dementia (LBD), a burden of AD pathologies is fairly common in cortical motor areas (297), further supporting the possibility that the presence of AD pathology in cortical motor areas could contribute to motor neuron degeneration.
For the extrapyramidal motor pathway, the presence of AD pathology appears to be predominantly in the midbrain substantia nigra (SN) area, although progressive and early degeneration is also present in other monamine neurons, such as the locus coeruleus (LC) (298). An early study examined sections of basal ganglia, subthalamic nucleus, and SN for NFT and neuropil threads (NPT) and in the SN for neuronal numbers in patients with AD (299). Although NFT and NPT were present in all these regions in AD patients, they were more prevalent and in higher densities in the SN region. In addition, the numbers of NFTs and NPTs in the SN were positively correlated with rigidity, tremor, and bradykinesia in these AD patients without the concomitant presence of Lewy bodies. There were no correlations between NFTs and NPTs in the basal ganglia or subthalamic nucleus and extrapyramidal signs in AD. More recently, a study of 86 cases from the Religious Orders Study also reported that overall 78% of the older adults with or without dementia had NFT pathologies in the SN regardless of the status of dementia (300), and the SN pathologies were specifically correlated with motor signs related to a cluster of gait, balance and posture signs (arising from a chair, shuffling gait, body bradykinesia, turning, posture, and postural stability). But none of the motor signs were associated with measures of amyloid burden, infarcts, or Lewy bodies. The presence of AD pathologies in both the medial and lateral SN suggests that the dopaminergic afferent pathway to the putamen and to the caudate nucleus of the basal ganglia/striatum may both be affected. However, the significance of this clinical-pathological association may be confounded by the presence of multiple pathologies in AD patients. The presence of SN alpha-synuclein aggregates and SN neuronal loss may interfere with the correlation between AD NFT pathology with extrapyrimadal motor impairments because the SN synucleinopathy and neuronal loss also strongly correlate with these motor signs (301). Several recent studies suggest that a number of common neuropathologies, including nigral synuclein and neuronal loss, cerebrovascular pathologies, and AD pathology, may all make separate contributions to the severity of parkinsonism in older adults with and without dementia (273, 302). Further research may thus be necessary to dissect the differences in the contributions of these common neuropathologies to late-life motor and cognitive impairments.
Although AD/NFT pathology in other extrapyramidal brain regions is not strongly associated with the extrapyramidal motor impairments, other types of neuropathological changes in non-SN motor regions have been implicated in the extrapyramidal motor signs in AD. Early studies of the neurochemistry of motor signs in AD suggested that there were deficits in monoaminergic systems as assessed by CSF in those AD patients who developed extrapyramidal signs (303) or myoclonus (304). In addition, common white matter pathologies may play an important role in motor signs with AD. White matter pathologies commonly include myelin pallor, decreased axons, blood-brain barrier breakdown, spongiosis, and dilated perivascular spaces (305). Although these changes are not considered part of the classic AD pathology spectrum, they are nevertheless very common with aging (306), and have not been accounted for in most post-mortem studies of AD. Nevertheless, these white matter changes are commonly found in regions through which the corticospinal tract traverses, and have been consistently associated with motor change and thus likely play a significant role in older AD patients with gait or generalized slowing (307–309).
V-4. Genetics
Two studies in 2007 offered initial evidence to suggest genetic factors affecting physical function, in particular gait speed, in older adults. One of the studies examined 938 older adults and found that a single nucleotide polymorphism (SNP) of the genetic region encoding the p16INK4a, a protein important for regulating cell senescence, aging and tumor suppression, was associated with reduced gait speed and physical function (310). Another study, on the other hand, identified a SNP in the proinflammatory cytokine interleukin-18 (IL-18) gene was associated with improved walking speed after examining 1,671 older adults from two studies (311). More recently, the Met158/Val158 allele in the COMT gene, which has been implicated in regulating cognitive processes and regulating tonic dopamine release in the striatum, was also shown to be associated with faster gait speed based on a study of 278 non-demented older adults (312). However, whether any of these genetic factors may relate to the gait speed changes associated with AD has not been investigated.
The discovery of genetic variants of AD has further shaped the recognition and understanding of motor signs associated with AD (313). A recent review of the clinical phenotype of nine kindreds with early onset AD caused by different presenilin 1 point mutations, showed that some of these cases were frequently linked to several motor syndromes including spastic paraparesis, Parkinsonism, and myoclonus (314). The clear genetic link to AD in these early-onset autosomal dominant variants presents one of the strongest arguments that motor signs can be a part of the fundamental clinical phenotype of AD, rather than a secondary phenomenon simply associated with common aging of the nervous system. In another study of community-dwelling older adults, an increased rate of motor decline was associated with the presence of at least one copy of the APOE ε4 allele, and this association appeared to increase with age (13). As more genetic risk factors have been associated with AD, it will be important to ascertain whether any of these newly identified AD risk genes may be associated with motor impairment in AD. Furthermore, although most of the genetic studies have been predominantly focused on gait speed, further analysis on the association of these genetic risk factors with other types of motor impairment in aging and AD may be required to gain a more complete understanding of the genetic associations among motor dysfunction, aging, and AD.
V-5. Neuroimaging and Neurophysiology
Transcranial magnetic stimulation is a powerful, non-invasive tool to disrupt neural activity in focal brain regions, even repetitively, thus allowing the assessment of brain activity in the form of evoked potentials on a milli-second scale (315). In healthy older adults, the primary motor cortex exhibits reduced amplitudes of evoked potentials at a resting state but preserved excitation threshold when compared to younger adults (316). For AD patients, the primary motor cortex became hyper-excitable as the threshold for excitation was significantly lower than that in healthy older adults, while the amplitude and the latency remained largely comparable (317). However, this hyperexcitability was unlikely due to dysfunction of inhibitory intracortical cholinergic circuits because the cholinersterase inhibitor rivastigmine failed to reverse the hyperexcitability (317). Future studies that will determine whether preclinical AD, MCI patients, and people genetically at risk for AD have hyperexcitability in primary motor cortex as a candidate biomarker for AD pathogenesis.
Neuroimaging, both structural and functional, studies have been shown to be useful in assessing brain changes associated with specific oculomotor abnormalities in aging and neurodegenerative conditions (318, 319). However, because most of the current neuroimaging instruments are not portable, and movement-related artifacts degrade the resolution of these imaging techniques, functional neuroimaging cannot be performed when subjects are walking or have abnormal involuntary movements. Several neuroimaging studies find that white matter change may be a reflection of both vascular injury and secondary degeneration from cortical AD pathology (320–322), complementing population-based autopsy series which have shown that the co-existence of AD and vascular disease to be the rule, rather than the exception (323, 324). However, how these neuroimaging signatures may change in people at risk for AD or AD patients remains largely unknown. There is a clear relationship between white matter hyperintensity change on MRI usually attributed to small vessel ischemic disease and the rate of cognitive and motor slowing over time (308). Further studies using imaging may be helpful to identify widespread vascular disease and its concomitant role in motor change in AD as well.
V-6. Cellular Mechanisms and Model Systems
Aging of the motor system in model organisms has primarily focused on the SN in the context of the relevance to PD. As with AD, aging is the greatest risk factor for the development and progression of PD (325). Many cellular mechanisms commonly implicated in the aging process, including mitochondrial dysfunction, age-related declines in the activity of proteasomes, dysregulation of the cell cycle by histone deacetylases such as sirtuins, reduced efficiency of chaperones, imbalanced autophagic recycling, changes in inflammation, altered complement and microglia activation, impaired response to neurotrophic factors, progressive accumulation of iron in brain with age, dysregulation of the longevity-affecting insulin signaling pathway, as well as age-related deposition of α-synuclein, have all be linked to the pathogenesis of PD (325). Some of these aging pathways have also been associated with the pathogenesis of AD, suggesting the existence of several common cellular mechanisms underlying AD and PD for the aged.
Interestingly, the effects of AD pathologies on motor neuron function have been reported for AD transgenic models by a number of studies. Transgenic mice expressing mutant PS1 variants showed significant motor deficits as assessed by muscle action potentials and rotorod running tests (326). In transgenic mice overexpressing either human four-repeat tau, diverse mutant human tau isoforms that cause frontotemporal dementia, or various human APP or Aβ mutants, significant axonopathy and impairments in motor performance, such as beam walking, inverted grip hanging, or hindlimb clasping, have been reported across many studies (327). Moreover, in mice overexpressing the human APOE ε4 protein in the brain and spinal cord, neurons developed an age-dependent axonopathy and severe sensorimotor defects, such as reduced locomotor activity and swimming inability (327). Together, these findings all support the possibility that motor impairments observed in AD patients or people genetically at risk for AD may be attributable to underlying AD pathology, and also that systems other than the cholinergic one may partake in these degenerative dysfunctions. Future studies will be needed to assess whether treatments which are developed to prevent or lower levels of AD pathology would improve or prevent further declines of motor function as well as delay cognitive impairment.
V-7. Key Research Directions
The relative contributions from motor systems, in particular AD pathology in cortical motor regions, to neuropsychological tests that probe both cognitive and pyramidal motor functions and that comprise part of the clinical diagnosis of AD remain to be addressed.
Prospective studies, starting with cognitively normal subjects followed until conversion to AD dementia, and autopsy in both clinical settings and at a population level, are needed to characterize and demonstrate the clinical utility of combining motor functional changes with genetic, neurophysiological, neuroimaging, or other biomarkers for identifying individuals at risk and characterizing the progress of cognitive and motor impairment in AD.
Mechanistic studies, in both humans and model organisms, are necessary to understand the etiological relationship between cognitive and motor impairment in AD.
Interventional studies may be helpful to address whether improving motor functions in pre-clinical AD population will delay or reverse cognitive declines.
VII. Discussion
In this section, we present a conceptual framework to connect dysfunctions in sensory and motor systems with AD (see figure), including a brief summary of the supporting evidence. Then, we address three major concerns regarding the diagnostic and etiological value of sensory and motor dysfunctions for the pathogenesis of AD and propose a number of testable hypotheses for future research. With the current emphasis of AD research on the preclinical disease phase and cost-effectiveness, we argue that a deeper understanding of the sensory and motor changes that occur in the context of AD offer a viable road forward towards early detection of AD and towards therapeutic interventions to improve the quality of life of AD patients.
Figure.
Models of the relationship between sensory/motor dysfunction and AD.
A) A Clinical model. Seminal observations of dysfunction of each sensory/motor modality are documented at the earliest reported stage during the clinical course of AD based on the studies referenced in this review. Parallel progression of these initial sensory or motor dysfunctions to advanced AD dementia raises the question of whether AD may be a heterogeneous disease in origin or a disease with heterogeneous clinical courses. A comprehensive assessment of all relevant sensory and motor measures from the preclinical stage of AD to the advanced dementia stage may help to test this possibility. Future research may also aim to extend these findings to earlier periods of the preclinical AD stage with better sensitivity and specificity by either improving the measurement methodology for detecting sensory and motor changes or by combining sensory/motor measures with other biomarkers, such as CSF, neuroimaging, and genetic risk factors for AD.
B) A cellular model. A schematic framework of disease progression where the light gray neurons represent dysfunctional neurons. In this model, dysfunction arises sporadically and then propagates in a pattern that is instructed by the connectivity of the involved neurons, circuits, networks, and systems. Since we speculate that disease initiation is sporadic, the involved circuits and networks at the earliest stages will likely differ between individual patients. This model may be tested by further research with AD-related model organisms to assess the mechanisms of disease progression from a sensory or motor domain to the cognitive domain and to investigate the effects of early intervention on early sensory or motor dysfunction and on prevention of progression to cognitive impairment.
The cognitive and behavioral manifestations of AD define the natural history of the clinical disease. The natural history of the molecular and cellular alterations caused by AD, such as the accumulation and aggregation of proteins and changes in synaptic number and plasticity, remain under intense investigation (196, 328). These efforts have tremendously increased our understanding of the pathological and clinical events of AD. However, disappointing results from recent therapeutic trials in the dementia stage indicate that a deeper understanding is required to reduce the burden of AD. Recent revisions of the clinical criteria and proposed revisions of the pathologic criteria for the diagnosis of AD reflect an appreciation that AD develops over decades and that its various clinical manifestations are a later stage, representing only a fraction of the total disease duration (9, 329, 330). Currently, disentangling changes due to the aging process and the pathogenesis of preclinical AD is very challenging.
As summarized in the preceding sections, a wide range of sensory and motor systems are commonly impaired in old age. Moreover, many deficits in olfactory, auditory, visual, and motor systems presage or appear concurrently with the cognitive symptoms that currently define the clinical picture of AD. In particular, for most neural systems examined, a case-control design revealed significant differences between young and old individuals or patients with AD relative to controls. In longitudinal studies cited earlier, significant differences in olfactory and auditory performance separated individuals who converted to the dementia phase of AD from individuals who did not. These findings raise a tantalizing possibility that certain sensory and motor deficits occur early in course of AD pathogenesis — preceding cognitive impairments — and thus might serve as a means for early detection and therapeutic intervention to forestall further functional deterioration and cognitive impairment.
From a neural system perspective, integrated sensory, motor, and cognitive systems provide the means for the organism to perceive and respond to its environment, i.e., deficits in any of these components can lead to impaired function at the clinical/behavioral level. Processing sensory input and generating motor output are among the most fundamental functions of the mammalian neural system. Organisms experience their environment through their sensory neural pathways, and motor systems provide the means by which organisms manifest behavioral responses to these stimuli. The cognitive component of these neural systems processes the incoming sensory input transmitted by the sensory end organs, and then formulates and directs the responses by specifically regulating the motor neural pathways. In addition, the cognitive component may also modulate the sensory neural pathways to refine the sensory input through mechanisms of top-down regulation (331–335). The interconnections among sensory, cognitive, and motor neural pathways suggest that when one neural pathway is impaired, the function of the other neural systems will be altered as well. For instance, an impairment in a sensory pathway may reduce the quality of input received by the cognitive domain thus compromising cognitive function, e.g. hippocampal function (336–339); an impairment in the cognitive domain could reduce its modulatory effect on the sensory pathway as well as diminish its control of motor output generation, leading to both altered sensory responses and motor output patterns; while an impairment in the motor pathway may impede the proper transmission of the commands from the cognitive domain, thus resulting in uncoordinated motor patterns and inefficient capture of environmental signals. Given this interdependence of sensory, motor, and cognitive systems, changes in sensory or motor function may either cause or reflect subtle deficits in the cognitive processes prior to the progression to more severe cognitive impairment conditions. Hence, effective treatment of any of these neural systems may decrease the overall burden of age-related diseases, such as AD.
The elemental nature of the neural networks that underlie sensory perception and motor output has motivated investigators to develop powerful tools to interrogate their function, some of which were recently standardized as the NIH toolbox (340). When instruments for measurement of sensory and motor function have been applied to aging individuals or those with impending or clinically diagnosed AD, important evidence of neural system dysfunction has emerged from each sensory modality and the motor system. Functionally, the compromise of sensory and motor systems seen in aging individuals diminishes their quality of life and increases risk for additional comorbidities, such as falls. Fortunately, a number of rehabilitation or intervention strategies for vision, hearing, and motor function have been developed to ameliorate the declines in some of the sensory and motor systems for older adults. These strategies afford the opportunity to address the question of whether treating these age-related sensory or motor declines would prevent or reduce the progression of AD.
Despite the collective body of evidence from epidemiological and clinical studies assembled in this review and our current understanding of the principles governing neural circuitry connections, the role of sensory and motor signs and mechanisms in the diagnosis and pathophysiology of AD has been understudied for a number of reasons. We discuss three major concerns about the importance of sensory and motor dysfunction in AD, and propose a number of testable hypotheses to promote future investigations to address the issues.
The first major concern stems from the fact that one sensory or motor dysfunction does not fully capture the entire population of AD patients, e.g. the sensitivity issue. Since memory loss and cognitive impairment are the most common functional changes among AD patients, the canonical view is that AD begins with degeneration of the cognitive system. Yet, some individuals with preclinical AD may first manifest visual symptoms, e.g. PCA, hearing loss, diminished sense of smell, or slowing of gait; while others do not present with sensory or motor declines before the onset of memory loss. In light of the revised diagnostic criteria for AD, where targeting cognitive impairments for AD treatment may be considered too late, we may want to consider alternative hypotheses regarding the relationship between sensory and motor dysfunctions and AD. One alternative view is that AD is a heterogeneous disease with many different initiation points in the central nervous system often with early clinical signs in the sensory and motor domains. Cognitively normal older adults with a particular type of sensory or motor dysfunction may represent a discrete subgroup of individuals at risk of developing AD. The methodology to probe sensory and motor systems is more sensitive to changes because our understanding of the underlying neural systems is more advanced. To test this hypothesis, strong support was provided by the group of workshop attendees for systematic prospective cohort studies to assess a battery of carefully chosen sensory, motor, and cognitive tests in a healthy, middle aged population as they age to determine the clinical course and the composition of early sensory and motor dysfunctions among those who eventually develop AD dementia. The development and refinement of means to quantify sensory and motor functional declines in our aging population remains an important goal of current research (340). These measures afford investigators reliable tools to test interventions that may reduce the impact of sensory and motor dysfunction. Furthermore, these measures may afford the clinician the opportunity to counsel and intervene in a preventive manner to reduce the impact of sensory and motor deficits on cognitive performance or activities of daily living (ADLs).
The second major concern states that sensory or motor dysfunctions are not specific to AD. This lack of specificity has long undercut the potential utility of these sensory or motor changes to serve as biomarkers for AD, especially preclinical and prodromal AD. As outlined above in this review, a number of sensory and motor dysfunctions increase the risk (or odds ratio) of developing AD substantially. Moreover, as discussed in the olfactory system section, findings from a number of recent studies suggest that functional assessment of sensory and motor neural systems may provide additional diagnostic accuracy of prodromal AD when coupled with molecular, genetic, or imaging biomarkers. The diagnosis and management of preclinical AD is a pressing concern with current projections of disease burden of AD over the next several decades. Current methodology to visualize amyloid deposition in the brain (341), quantify markers of neurodegeneration in the cerebral spinal fluid (342), and determine volume loss in the cortex (343) provide independent means to detect the pathogenesis of AD in its preclinical stage. However, individuals have significant variations to accommodate the pathological burden of AD, and the correlations between these biomarkers and concurrent neural system failure have not been consistent (344). In other words, individuals may harbor similar degrees of pathological burden with very different functional states. This variability for individuals to harbor AD pathology asymptomatically implies that the determination of the magnitude of pathologic markers solely may not accurately predict impending neural system failure. Functional measures may provide a means to detect impending neural system failure. Efforts to include sensory and motor assessments have begun in individual cohorts, but an examination of these measures of sensory and motors systems in larger multi-center cohorts, e.g., ADNI, the ADRC, or the DIAN cohorts, which afford the comparison with other biomarkers of AD pathology, will have sufficient power to test the hypothesis that specific sensory and motor signs in the setting of a positive biomarker screen portends neural system failure and symptom development. With further validation, functional measures could be incorporated into clinical trial designs to identify individuals on the brink of neural system failure and monitor the efficacy of the therapeutic intervention on the function of a neural system (345). Sensory and motor functional markers could be coupled with other molecular, genetic, and imaging biomarkers to generate an algorithm to predict conversion to the MCI phase and the dementia phase of AD (1).
The third major concern lies in the distribution patterns of AD neuropathology across sensory, motor, and cognitive neural pathways. As discussed in earlier sensory and motor sections, the distributions of AD pathologies are not consistent across all sensory or motor neural pathways. For example, while AD pathologies have been detected in the peripheral olfactory pathway, the eye, and the visual association areas, even prior to the onset of cognitive impairment and in the absence of pathologies in the cognitive brain regions, they are mostly lacking in the auditory pathway, especially the periphery, in the early phase of AD. Furthermore, little is known at the human level whether the presence of AD pathologies in sensory areas is associated with sensory functional declines. In contrast, there is a correlation between the presence of NFTs in the entorhinal cortex and hippocampus and the presence of cognitive impairment in all AD patients. This clinical-pathological correlation for the cognitive system, along with the relative lack of evidence for a clinical-pathological correlation in humans for the sensory and motor systems, has thus long favored the idea that cognitive system is the most vulnerable neural system in AD. However, as presented in the olfaction and vision sections, a number of animal model studies have demonstrated a strong correlation between the presence of AD pathologies in the sensory neural pathway and the functional changes of the sensory system. In addition, in motor systems, studies have shown that the presence of tau pathology in SN correlates with the presence of extrapyramidal motor signs in AD patients. Therefore, additional work is needed to examine the clinical-pathological correlations for sensory systems in AD. If AD is a heterogeneous disease with distinct sensory or motor endophenotypes at its preclinical stage, understanding the etiology of sensory and motor dysfunctions in preclinical AD is warranted to improve early diagnosis and to tailor therapeutic interventions that are specific to the underlying disease process and to modality specific rehabilitation to maintain functional capacity.
To further contemplate how dysfunctions in the sensory or motor pathway would eventually progress into cognitive impairments as observed in all AD patients, the workshop participants explored two general possibilities. The first hypothesis was whether the AD pathologic process initiated in one sensory or motor system for each individual could eventually propagate to other brain regions, especially the entorhinal cortex and hippocampus. Clinically, the evolution of cognitive deficits in MCI and AD patients suggests that neural system failure propagates through the brain as the disease progresses, starting in the entorhinal cortex and spreading through the hippocampal formation towards other cortical areas. From a pathogenic perspective, it has been proposed that the disease is comprised of two fundamental stages: an initiating stage where the pathological cascade is ignited and a propagation stage where the pathological cascade spreads to inflict additional neural circuits ultimately leading to neural system failure (346). This view is based on seminal pathological case series (347). A thorough examination of this hypothesis will require experiments in circuit-based or network-based animal models. For instance, a model of limited tau overexpression enriched in the entorhinal cortex, and demonstrated the propagation of tau pathology to distal hippocampal regions in the network (59, 60, 348). Moreover, vulnerable sensory or motor neural circuits may be fertile ground to test hypotheses related to initiation and propagation in animal models. These circuits offer a plethora of experimental tools for subtly perturbing vulnerable circuits with disease genes and for analyzing the structural and functional consequences of these perturbations in the olfactory neural network of mice (37, 56). A cell culture approach complements these in vivo approaches to investigate mechanisms of disease propagation as the spread of pathological tau conformations by nucleating species (349). One clinical implication of this formulation predicts that a functional survey of the sensory and motor neural systems will be more sensitive than surveying only one or subset of the systems. For instance, the elucidation of the changes at the circuit and network levels caused by AD — and the compensatory strategies employed by the brain to maintain function in response to these assaults — are necessary to bridge alterations at the molecular and cellular levels with clinical (cognitive and behavioral) observations. The complexity of the nervous system at the circuit and network levels is daunting, but new approaches in systems neuroscience in both animal models and humans are rendering this objective increasingly feasible.
The second hypothesis was whether altered sensory and motor input into the entorhinal cortex/hippocampus and association cortices contribute to the dysfunction arising from these areas. If the data from the proposed longitudinal studies confirm dysfunction in sensory and motor systems in the asymptomatic phase of AD, two mechanistic possibilities arise. One view is that the dysfunction occurs in parallel with the development of the disease in the association cortices and hippocampus. By contrast, the dysfunction in the sensory systems may represent a primary disturbance to the expected input into the association cortices and the hippocampus, which triggers pathological plasticity processes (339). For instance, altered neuronal activity was shown to reduce the transport and release of the neurotrophic factor BDNF by Christine Gall and Gary Lynch (350). A provocative proposal by Takao Hensch postulated that a reopening of the critical period resulting in enhanced plasticity may contribute to the development of AD pathology. Indeed, the expression of genes and synthesis of gene products implicated in the closing of the critical period are inverted in AD pathogenesis. Moreover, many epidemiological risk factors for AD such as head trauma and cerebrovascular insults evoke cortical plasticity responses to affect repair. Mechanistic investigations to test this and related hypotheses at the circuit and network levels will likely be performed in the next generation of animal models. These new animal models are based on the facility of genetic manipulation of sensory and motor circuits to express disease genes under spatial and temporal control and to deploy functional markers at strategic locations within these circuits and networks. Furthermore, these investigations can be extended to humans with increasing sophisticated functional neuroimaging techniques.
In summary, there is considerable evidence that the interrogation of sensory and motor systems in aging individuals reveals deficits. Combined with neuropsychological, imaging, and CSF outcomes, these indices of sensory and motor function may be employed to accurately identify asymptomatic individuals with impending neural system failure due to smoldering AD pathology. Moreover, these functional indices may serve as potential outcomes for clinical trials in the preclinical phase of the disease. A comprehensive understanding of the damage inflicted by AD comprising all levels of the functional hierarchy of the nervous system, from the molecular and cellular through the circuit and network to the cognitive, behavioral, and social levels, will provide mechanistic insights into its pathogenesis. It is likely that this comprehensive understanding will inform the development and refinement of much needed effective and affordable therapeutic strategies. Further investigation of genetically tractable sensory and motor circuits in animal models of aging and prodromal AD will enhance our knowledge of these disorders at circuit and networks levels. Taken together, we recommend continued investigations of sensory and motor systems in aged individuals and animal models. We anticipate that the results from these studies will contribute to preclinical detection of neurodegenerative disease and will lead to the development and implementation of effective preventive and therapeutic modalities for AD, thereby reducing the burden of this devastating disease.
Supplementary Material
Acknowledgments
We thank Dr. Wen Chen for her inspirational leadership to conceptualize and organize the Sensory and Motor Dysfunction in Aging and Alzheimer’s Disease workshop and for her tireless efforts to recruit the authors for each section, to write and harmonize the sections into the first draft of this manuscript, and for her constructive comments. We thank Drs. Dallas Anderson, Neil Buckholtz, Marcel Morrison-Bogorad, D. Stephen Snyder, and Molly V. Wagster for their support and contributions to the conceptualization and planning of the conference. We also thank Drs. Marc Diamond, Joseph M. Furman, Alvin W. Lyckman, Jr., Gary Lynch, and Patrick J. Sparto for their presentations and discussions at the workshop, which formed part of the basis for this review article.
List of abbreviations
- ABR
auditory brain responses
- AD
Alzheimer’s disease
- AEP
auditory event potentials
- AMD
age-related macular degeneration
- CFH
complement factor H
- EC
entorhinal cortex
- ERP
event related potential
- fMRI
functional MRI
- IOP
intraocular pressure
- LC
locus coeruleus
- MCI
mild cognitive impairment
- MMSE
mini mental status examination
- MRI
magnetic resonance imaging
- NFL
optic nerve fiber layer
- NFT
neurofibrillary tangle
- OB
olfactory bulb
- OE
olfactory epithelium
- PCA
posterior cortical atrophy
- PD
Parkinson’s disease
- PET
positron emission tomography
- RGC
retinal ganglion cell
- SN
substantia nigra
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64(10):871–9. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2008;134(7):771–7. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–20. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60(4):539–44. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 5.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):929–35. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 1998;55(1):84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–8. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 8.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–12. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 9.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 11.Bertram L. Alzheimer’s genetics in the GWAS era: a continuing story of ‘replications and refutations’. Curr Neurol Neurosci Rep. 2011;11(3):246–53. doi: 10.1007/s11910-011-0193-z. [DOI] [PubMed] [Google Scholar]
- 12.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012;124(3):305–23. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23(1):63–9. doi: 10.1097/wad.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–3. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 15.Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 2011;121(4):873–8. doi: 10.1002/lary.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36(4):367–73. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep. 2006;6(5):379–86. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- 18.Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein R, Pankratz N, et al. Odor identification and cognitive function in the Beaver Dam Offspring Study. J Clin Exp Neuropsychol. 2013;35(7):669–76. doi: 10.1080/13803395.2013.809701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull. 1987;18(5):597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- 20.Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol Aging. 1990;11(4):465–9. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- 21.Nordin S, Murphy C. Odor memory in normal aging and Alzheimer’s disease. Ann N Y Acad Sci. 1998;855:686–93. doi: 10.1111/j.1749-6632.1998.tb10646.x. [DOI] [PubMed] [Google Scholar]
- 22.Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53(7):1480–7. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- 23.Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157(9):1399–405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- 24.Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56(8):1517–21. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26(2):61–7. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–8. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 27.Raynor LA, Pankow JS, Cruickshanks KJ, Schubert CR, Miller MB, Klein R, et al. Familial aggregation of olfactory impairment and odor identification in older adults. Laryngoscope. 2010;120(8):1614–8. doi: 10.1002/lary.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert CR, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Fischer ME. Association of Exercise With Lower Long-term Risk of Olfactory Impairment in Older Adults. JAMA Otolaryngol Head Neck Surg. 2013;139(10):1061–6. doi: 10.1001/jamaoto.2013.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport. 2001;12(2):285–8. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–7. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15(3):355–6. doi: 10.1016/0197-4580(94)90032-9. discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 32.Chapuis J, Cohen Y, He X, Zhang Z, Jin S, Xu F, et al. Lateral entorhinal modulation of piriform cortical activity and fine odor discrimination. J Neurosci. 2013;33(33):13449–59. doi: 10.1523/JNEUROSCI.1387-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segura B, Baggio HC, Solana E, Palacios EM, Vendrell P, Bargallo N, et al. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013;246:148–53. doi: 10.1016/j.bbr.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009;1170:730–5. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78(1):30–5. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, et al. Early olfactory involvement in Alzheimer’s disease. Can J Neurol Sci. 2003;30(1):20–5. doi: 10.1017/s0317167100002389. [DOI] [PubMed] [Google Scholar]
- 37.Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 2010;30(2):505–14. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67(4):462–9. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabaton M, Cammarata S, Mancardi GL, Cordone G, Perry G, Loeb C. Abnormal tau-reactive filaments in olfactory mucosa in biopsy specimens of patients with probable Alzheimer’s disease. Neurology. 1991;41(3):391–4. doi: 10.1212/wnl.41.3.391. [DOI] [PubMed] [Google Scholar]
- 40.Talamo BR, Rudel R, Kosik KS, Lee VM, Neff S, Adelman L, et al. Pathological changes in olfactory neurons in patients with Alzheimer’s disease. Nature. 1989;337(6209):736–9. doi: 10.1038/337736a0. [DOI] [PubMed] [Google Scholar]
- 41.Lee JH, Goedert M, Hill WD, Lee VM, Trojanowski JQ. Tau proteins are abnormally expressed in olfactory epithelium of Alzheimer patients and developmentally regulated in human fetal spinal cord. Exp Neurol. 1993;121(1):93–105. doi: 10.1006/exnr.1993.1074. [DOI] [PubMed] [Google Scholar]
- 42.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanza DC, Moran DT, Doty RL, Trojanowski JQ, Lee JH, Rowley JC, 3rd, et al. Endoscopic human olfactory biopsy technique: a preliminary report. Laryngoscope. 1993;103(7):815–9. doi: 10.1288/00005537-199307000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Serby M, Mohan C, Aryan M, Williams L, Mohs RC, Davis KL. Olfactory identification deficits in relatives of Alzheimer’s disease patients. Biol Psychiatry. 1996;39(5):375–7. doi: 10.1016/0006-3223(95)00472-6. [DOI] [PubMed] [Google Scholar]
- 45.Schiffman SS, Graham BG, Sattely-Miller EA, Zervakis J, Welsh-Bohmer K. Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer’s disease. Neurobiol Aging. 2002;23(3):397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 46.Calhoun-Haney R, Murphy C. Apolipoprotein epsilon4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cogn. 2005;58(2):178–82. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–31. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert PE, Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26(6):779–94. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 49.Murphy C, Jernigan TL, Fennema-Notestine C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: a structural MRI study. J Int Neuropsychol Soc. 2003;9(3):459–71. doi: 10.1017/S1355617703930116. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010;1357:184–94. doi: 10.1016/j.brainres.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy C, Cerf-Ducastel B, Calhoun-Haney R, Gilbert PE, Ferdon S. ERP, fMRI and functional connectivity studies of brain response to odor in normal aging and Alzheimer’s disease. Chem Senses. 2005;30 (Suppl 1):i170–1. doi: 10.1093/chemse/bjh168. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain. 2010;133(9):2714–26. doi: 10.1093/brain/awq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haase L, Wang M, Green E, Murphy C. Functional connectivity during recognition memory in individuals genetically at risk for Alzheimer’s disease. Hum Brain Mapp. 2013;34(3):530–42. doi: 10.1002/hbm.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan CD, Murphy C. Olfactory event-related potentials in Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8(6):753–63. doi: 10.1017/s1355617702860039. [DOI] [PubMed] [Google Scholar]
- 55.Murphy C, Solomon ES, Haase L, Wang M, Morgan CD. Olfaction in aging and Alzheimer’s disease: event-related potentials to a cross-modal odor-recognition memory task discriminate ApoE epsilon4+ and ApoE epsilon 4- individuals. Ann N Y Acad Sci. 2009;1170:647–57. doi: 10.1111/j.1749-6632.2009.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao L, Schrank BR, Rodriguez S, Benz EG, Moulia TW, Rickenbacher GT, et al. Abeta alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat Commun. 2012;3:1009. doi: 10.1038/ncomms2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macknin JB, Higuchi M, Lee VM, Trojanowski JQ, Doty RL. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein. Brain Res. 2004;1000(1–2):174–8. doi: 10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 58.Cheng N, Bai L, Steuer E, Belluscio L. Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer’s disease model. J Neurosci. 2013;33(30):12208–17. doi: 10.1523/JNEUROSCI.0291-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73(4):685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7(2):e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci. 2013;33(3):1024–37. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, et al. Transsynaptic progression of amyloid-beta-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68(3):428–41. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s beta-amyloidosis mouse model. J Neurosci. 2011;31(44):15962–71. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134(Pt 1):258–77. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graziadei PP, Monti Graziadei GA. Neurogenesis and plasticity of the olfactory sensory neurons. Ann N Y Acad Sci. 1985;457:127–42. doi: 10.1111/j.1749-6632.1985.tb20802.x. [DOI] [PubMed] [Google Scholar]
- 66.Nathan BP, Gairhe S, Nwosu I, Clark S, Struble RG. Reconstitution of the olfactory epithelium following injury in apoE-deficient mice. Exp Neurol. 2010;226(1):40–6. doi: 10.1016/j.expneurol.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan BP, Nisar R, Short J, Randall S, Grissom E, Griffin G, et al. Delayed olfactory nerve regeneration in ApoE-deficient mice. Brain Res. 2005;1041(1):87–94. doi: 10.1016/j.brainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150(1–2):1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- 69.Nwosu I, Gairhe S, Struble RG, Nathan BP. Impact of apoE deficiency during synaptic remodeling in the mouse olfactory bulb. Neurosci Lett. 2008;441(3):282–5. doi: 10.1016/j.neulet.2008.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nathan BP, Tonsor M, Struble RG. Acute responses to estradiol replacement in the olfactory system of apoE-deficient and wild-type mice. Brain Res. 2010;1343:66–74. doi: 10.1016/j.brainres.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gohdes DM, Balamurugan A, Larsen BA, Maylahn C. Age-related eye diseases: an emerging challenge for public health professionals. Prev Chronic Dis. 2005;2(3):A17. [PMC free article] [PubMed] [Google Scholar]
- 72.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet. 2003;361(9365):1258–65. doi: 10.1016/S0140-6736(03)12981-9. [DOI] [PubMed] [Google Scholar]
- 74.Moncaster JA, Pineda R, Moir RD, Lu S, Burton MA, Ghosh JG, et al. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One. 2010;5(5):e10659. doi: 10.1371/journal.pone.0010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jun G, Moncaster JA, Koutras C, Seshadri S, Buros J, McKee AC, et al. delta-Catenin is genetically and biologically associated with cortical cataract and future Alzheimer-related structural and functional brain changes. PLoS One. 2012;7(9):e43728. doi: 10.1371/journal.pone.0043728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frederikse PH, Zigler JS., Jr Presenilin expression in the ocular lens. Curr Eye Res. 1998;17(9):947–52. doi: 10.1076/ceyr.17.9.947.5135. [DOI] [PubMed] [Google Scholar]
- 77.Frederikse PH, Garland D, Zigler JS, Jr, Piatigorsky J. Oxidative stress increases production of beta-amyloid precursor protein and beta-amyloid (Abeta) in mammalian lenses, and Abeta has toxic effects on lens epithelial cells. J Biol Chem. 1996;271(17):10169–74. doi: 10.1074/jbc.271.17.10169. [DOI] [PubMed] [Google Scholar]
- 78.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 79.Lott IT, Head E. Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol Aging. 2005;26(3):383–9. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17(3):278–82. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 81.Frederikse PH, Ren XO. Lens defects and age-related fiber cell degeneration in a mouse model of increased AbetaPP gene dosage in Down syndrome. Am J Pathol. 2002;161(6):1985–90. doi: 10.1016/s0002-9440(10)64475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michael R, Otto C, Lenferink A, Gelpi E, Montenegro GA, Rosandic J, et al. Absence of amyloid-beta in lenses of Alzheimer patients: A confocal Raman microspectroscopic study. Exp Eye Res. 2014;119:44–53. doi: 10.1016/j.exer.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 83.Burdo JR, Chen Q, Calcutt NA, Schubert D. The pathological interaction between diabetes and presymptomatic Alzheimer’s disease. Neurobiol Aging. 2009;30(12):1910–7. doi: 10.1016/j.neurobiolaging.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 84.De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123(2):531–9. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao C, Liu Y, Li L, Holscher C. New animal models of Alzheimer’s disease that display insulin desensitization in the brain. Rev Neurosci. 2013;24(6):607–15. doi: 10.1515/revneuro-2013-0034. [DOI] [PubMed] [Google Scholar]
- 86.Jolivalt CG, Hurford R, Lee CA, Dumaop W, Rockenstein E, Masliah E. Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp Neurol. 2010;223(2):422–31. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 88.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289(16):2094–103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 89.Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49(2):106–8. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 90.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78(2):243–56. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Clemons TE, Rankin MW, McBee WL Age-Related Eye Disease Study Research G. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Arch Ophthalmol. 2006;124(4):537–43. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63(1):200–6. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pham TQ, Kifley A, Mitchell P, Wang JJ. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006;52(6):353–8. doi: 10.1159/000094984. [DOI] [PubMed] [Google Scholar]
- 94.Wong TY, Klein R, Nieto FJ, Moraes SA, Mosley TH, Couper DJ, et al. Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;134(6):828–35. doi: 10.1016/s0002-9394(02)01672-0. [DOI] [PubMed] [Google Scholar]
- 95.Keenan TD, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, Alzheimer disease, and dementia: record linkage study of hospital admissions. JAMA Ophthalmol. 2014;132(1):63–8. doi: 10.1001/jamaophthalmol.2013.5696. [DOI] [PubMed] [Google Scholar]
- 96.Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol. 2002;47(3):165–8. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- 97.Bayer AU, Ferrari F. Severe progression of glaucomatous optic neuropathy in patients with Alzheimer’s disease. Eye (Lond) 2002;16(2):209–12. doi: 10.1038/sj.eye.6700034. [DOI] [PubMed] [Google Scholar]
- 98.Kessing LV, Lopez AG, Andersen PK, Kessing SV. No increased risk of developing Alzheimer disease in patients with glaucoma. J Glaucoma. 2007;16(1):47–51. doi: 10.1097/IJG.0b013e31802b3527. [DOI] [PubMed] [Google Scholar]
- 99.Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP. Senescent changes in cerebrospinal fluid circulatory physiology and their role in the pathogenesis of normal-tension glaucoma. Am J Ophthalmol. 2013;156(1):5–14. e2. doi: 10.1016/j.ajo.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Rodrigues Simoes MC, Dias Viegas FP, Moreira MS, de Freitas Silva M, Riquiel MM, da Rosa PM, et al. Donepezil: an important prototype to the design of new drug candidates for Alzheimer’s disease. Mini Rev Med Chem. 2014;14(1):2–19. doi: 10.2174/1389557513666131119201353. [DOI] [PubMed] [Google Scholar]
- 101.Goldblum D, Garweg JG, Bohnke M. Topical rivastigmine, a selective acetylcholinesterase inhibitor, lowers intraocular pressure in rabbits. J Ocul Pharmacol Ther. 2000;16(1):29–35. doi: 10.1089/jop.2000.16.29. [DOI] [PubMed] [Google Scholar]
- 102.Estermann S, Daepp GC, Cattapan-Ludewig K, Berkhoff M, Frueh BE, Goldblum D. Effect of oral donepezil on intraocular pressure in normotensive Alzheimer patients. J Ocul Pharmacol Ther. 2006;22(1):62–7. doi: 10.1089/jop.2006.22.62. [DOI] [PubMed] [Google Scholar]
- 103.Yoshida Y, Sugiyama T, Utsunomiya K, Ogura Y, Ikeda T. A pilot study for the effects of donepezil therapy on cerebral and optic nerve head blood flow, visual field defect in normal-tension glaucoma. J Ocul Pharmacol Ther. 2010;26(2):187–92. doi: 10.1089/jop.2009.0117. [DOI] [PubMed] [Google Scholar]
- 104.Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin. 2003;21(3):709–28. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- 105.Schefrin BE, Shinomori K, Werner JS. Contributions of neural pathways to age-related losses in chromatic discrimination. J Opt Soc Am A Opt Image Sci Vis. 1995;12(6):1233–41. doi: 10.1364/josaa.12.001233. [DOI] [PubMed] [Google Scholar]
- 106.Greene HA, Madden DJ. Adult age differences in visual acuity, stereopsis, and contrast sensitivity. Am J Optom Physiol Opt. 1987;64(10):749–53. doi: 10.1097/00006324-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 107.Norman JF, Dawson TE, Butler AK. The effects of age upon the perception of depth and 3-D shape from differential motion and binocular disparity. Perception. 2000;29(11):1335–59. doi: 10.1068/p3111. [DOI] [PubMed] [Google Scholar]
- 108.Long GM, Crambert RF. The nature and basis of age-related changes in dynamic visual acuity. Psychol Aging. 1990;5(1):138–43. doi: 10.1037//0882-7974.5.1.138. [DOI] [PubMed] [Google Scholar]
- 109.Gilmore GC, Wenk HE, Naylor LA, Stuve TA. Motion perception and aging. Psychol Aging. 1992;7(4):654–60. doi: 10.1037//0882-7974.7.4.654. [DOI] [PubMed] [Google Scholar]
- 110.Ballard KJ, Robin DA, Woodworth G, Zimba LD. Age-related changes in motor control during articulator visuomotor tracking. J Speech Lang Hear Res. 2001;44(4):763–77. doi: 10.1044/1092-4388(2001/060). [DOI] [PubMed] [Google Scholar]
- 111.Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision Res. 1999;39(23):3975–82. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 112.Cronin-Golomb A, Sugiura R, Corkin S, Growdon JH. Incomplete achromatopsia in Alzheimer’s disease. Neurobiol Aging. 1993;14(5):471–7. doi: 10.1016/0197-4580(93)90105-k. [DOI] [PubMed] [Google Scholar]
- 113.Wijk H, Berg S, Sivik L, Steen B. Colour discrimination, colour naming and colour preferences among individuals with Alzheimer’s disease. Int J Geriatr Psychiatry. 1999;14(12):1000–5. [PubMed] [Google Scholar]
- 114.Pache M, Smeets CH, Gasio PF, Savaskan E, Flammer J, Wirz-Justice A, et al. Colour vision deficiencies in Alzheimer’s disease. Age Ageing. 2003;32(4):422–6. doi: 10.1093/ageing/32.4.422. [DOI] [PubMed] [Google Scholar]
- 115.Mendez MF, Cherrier MM, Meadows RS. Depth perception in Alzheimer’s disease. Percept Mot Skills. 1996;83(3 Pt 1):987–95. doi: 10.2466/pms.1996.83.3.987. [DOI] [PubMed] [Google Scholar]
- 116.Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, Banks KS. Visual dysfunction in Alzheimer’s disease: relation to normal aging. Ann Neurol. 1991;29(1):41–52. doi: 10.1002/ana.410290110. [DOI] [PubMed] [Google Scholar]
- 117.Gilmore GC, Whitehouse PJ. Contrast sensitivity in Alzheimer’s disease: a 1-year longitudinal analysis. Optom Vis Sci. 1995;72(2):83–91. doi: 10.1097/00006324-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 118.Trick GL, Silverman SE. Visual sensitivity to motion: age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991;41(9):1437–40. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- 119.Gilmore GC, Wenk HE, Naylor LA, Koss E. Motion perception and Alzheimer’s disease. J Gerontol. 1994;49(2):P52–7. doi: 10.1093/geronj/49.2.p52. [DOI] [PubMed] [Google Scholar]
- 120.Rizzo M, Nawrot M. Perception of movement and shape in Alzheimer’s disease. Brain. 1998;121 ( Pt 12):2259–70. doi: 10.1093/brain/121.12.2259. [DOI] [PubMed] [Google Scholar]
- 121.Tetewsky SJ, Duffy CJ. Visual loss and getting lost in Alzheimer’s disease. Neurology. 1999;52(5):958–65. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- 122.Cronin-Golomb A, Corkin S, Growdon JH. Visual dysfunction predicts cognitive deficits in Alzheimer’s disease. Optom Vis Sci. 1995;72(3):168–76. doi: 10.1097/00006324-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 123.Cummings JL, Houlihan JP, Hill MA. The pattern of reading deterioration in dementia of the Alzheimer type: observations and implications. Brain Lang. 1986;29(2):315–23. doi: 10.1016/0093-934x(86)90051-9. [DOI] [PubMed] [Google Scholar]
- 124.Della Sala S, Muggia S, Spinnler H, Zuffi M. Cognitive modelling of face processing: evidence from Alzheimer patients. Neuropsychologia. 1995;33(6):675–87. doi: 10.1016/0028-3932(95)00009-r. [DOI] [PubMed] [Google Scholar]
- 125.Glosser G, Gallo J, Duda N, de Vries JJ, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(3):198–206. [PubMed] [Google Scholar]
- 126.Hodges JR, Salmon DP, Butters N. Recognition and naming of famous faces in Alzheimer’s disease: a cognitive analysis. Neuropsychologia. 1993;31(8):775–88. doi: 10.1016/0028-3932(93)90128-m. [DOI] [PubMed] [Google Scholar]
- 127.Kurylo DD, Corkin S, Growdon JH. Perceptual organization in Alzheimer’s disease. Psychol Aging. 1994;9(4):562–7. doi: 10.1037/0882-7974.9.4.562. [DOI] [PubMed] [Google Scholar]
- 128.Kurylo DD, Corkin S, Dolan RP, Rizzo JF, 3rd, Parker SW, Growdon JH. Broad-band visual capacities are not selectively impaired in Alzheimer’s disease. Neurobiol Aging. 1994;15(3):305–11. doi: 10.1016/0197-4580(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 129.Mendez MF, Turner J, Gilmore GC, Remler B, Tomsak RL. Balint’s syndrome in Alzheimer’s disease: visuospatial functions. Int J Neurosci. 1990;54(3–4):339–46. doi: 10.3109/00207459008986653. [DOI] [PubMed] [Google Scholar]
- 130.Ricker JH, Keenan PA, Jacobson MW. Visuoperceptual-spatial ability and visual memory in vascular dementia and dementia of the Alzheimer type. Neuropsychologia. 1994;32(10):1287–96. doi: 10.1016/0028-3932(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 131.Rizzo M, Anderson SW, Dawson J, Nawrot M. Vision and cognition in Alzheimer’s disease. Neuropsychologia. 2000;38(8):1157–69. doi: 10.1016/s0028-3932(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 132.Tippett LJ, Blackwood K, Farah MJ. Visual object and face processing in mild-to-moderate Alzheimer’s disease: from segmentation to imagination. Neuropsychologia. 2003;41(4):453–68. doi: 10.1016/s0028-3932(02)00140-9. [DOI] [PubMed] [Google Scholar]
- 133.Toner CK, Reese BE, Neargarder S, Riedel TM, Gilmore GC, Cronin-Golomb A. Vision-fair neuropsychological assessment in normal aging, Parkinson’s disease and Alzheimer’s disease. Psychol Aging. 2012;27(3):785–90. doi: 10.1037/a0026368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cronin-Golomb A, Gilmore GC, Neargarder S, Morrison SR, Laudate TM. Enhanced stimulus strength improves visual cognition in aging and Alzheimer’s disease. Cortex. 2007;43(7):952–66. doi: 10.1016/s0010-9452(08)70693-2. [DOI] [PubMed] [Google Scholar]
- 135.Gilmore GC, Cronin-Golomb A, Neargarder SA, Morrison SR. Enhanced stimulus contrast normalizes visual processing of rapidly presented letters in Alzheimer’s disease. Vision Res. 2005;45(8):1013–20. doi: 10.1016/j.visres.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 136.Gilmore GC, Groth KE, Thomas CW. Stimulus contrast and word reading speed in Alzheimer’s disease. Exp Aging Res. 2005;31(1):15–33. doi: 10.1080/03610730590882828. [DOI] [PubMed] [Google Scholar]
- 137.Seichepine DR, Neargarder S, McCallum ME, Tabor K, Riedel TM, Gilmore GC, et al. Luminance affects age-related deficits in object detection: implications for computerized psychological assessments. Psychol Aging. 2012;27(2):522–8. doi: 10.1037/a0025576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Laudate TM, Neargarder S, Dunne TE, Sullivan KD, Joshi P, Gilmore GC, et al. Bingo! Externally supported performance intervention for deficient visual search in normal aging, Parkinson’s disease, and Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19(1–2):102–21. doi: 10.1080/13825585.2011.621930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dunne TE, Neargarder SA, Cipolloni PB, Cronin-Golomb A. Visual contrast enhances food and liquid intake in advanced Alzheimer’s disease. Clin Nutr. 2004;23(4):533–8. doi: 10.1016/j.clnu.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 140.Prakasam A, Muthuswamy A, Ablonczy Z, Greig NH, Fauq A, Rao KJ, et al. Differential accumulation of secreted AbetaPP metabolites in ocular fluids. J Alzheimers Dis. 2010;20(4):1243–53. doi: 10.3233/JAD-2010-100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Janciauskiene S, Krakau T. Alzheimer’s peptide: a possible link between glaucoma, exfoliation syndrome and Alzheimer’s disease. Acta Ophthalmol Scand. 2001;79(3):328–9. doi: 10.1034/j.1600-0420.2001.790327.x. [DOI] [PubMed] [Google Scholar]
- 142.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315(8):485–7. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 143.Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. 1996;17(3):385–95. doi: 10.1016/0197-4580(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 144.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989;501(2):364–72. doi: 10.1016/0006-8993(89)90653-7. [DOI] [PubMed] [Google Scholar]
- 145.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 146.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100(1):135–46. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 147.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17(3):377–84. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 148.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hood DC, Raza AS, de Moraes CG, Johnson CA, Liebmann JM, Ritch R. The Nature of Macular Damage in Glaucoma as Revealed by Averaging Optical Coherence Tomography Data. Transl Vis Sci Technol. 2012;1(1):3. doi: 10.1167/tvst.1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee KS, Lee JR, Na JH, Kook MS. Usefulness of macular thickness derived from spectral-domain optical coherence tomography in the detection of glaucoma progression. Invest Ophthalmol Vis Sci. 2013;54(3):1941–9. doi: 10.1167/iovs.12-11160. [DOI] [PubMed] [Google Scholar]
- 151.Mardin CY. Structural diagnostics of course observation for glaucoma. Ophthalmologe. 2013;110(11):1036–44. doi: 10.1007/s00347-012-2672-2. [DOI] [PubMed] [Google Scholar]
- 152.Renard JP, Fenolland JR, El Chehab H, Francoz M, Marill AM, Messaoudi R, et al. Analysis of macular ganglion cell complex (GCC) with spectral-domain optical coherence tomography (SD-OCT) in glaucoma. J Fr Ophtalmol. 2013;36(4):299–309. doi: 10.1016/j.jfo.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 153.Hood DC, Slobodnick A, Raza AS, de Moraes CG, Teng CC, Ritch R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Vis Sci. 2014;55(2):632–49. doi: 10.1167/iovs.13-13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsai CS, Ritch R, Schwartz B, Lee SS, Miller NR, Chi T, et al. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch Ophthalmol. 1991;109(2):199–204. doi: 10.1001/archopht.1991.01080020045040. [DOI] [PubMed] [Google Scholar]
- 155.Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology. 1990;97(1):9–17. doi: 10.1016/s0161-6420(90)32621-0. [DOI] [PubMed] [Google Scholar]
- 156.Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann Neurol. 1993;33(3):248–57. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- 157.Davies DC, McCoubrie P, McDonald B, Jobst KA. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br J Ophthalmol. 1995;79(6):596–600. doi: 10.1136/bjo.79.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003;9:184–90. [PubMed] [Google Scholar]
- 159.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(18):11830–5. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Loffler KU, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci. 1995;36(1):24–31. [PubMed] [Google Scholar]
- 161.Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, et al. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115(10):2793–800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ohm TG, Braak H. Auditory brainstem nuclei in Alzheimer’s disease. Neurosci Lett. 1989;96(1):60–3. doi: 10.1016/0304-3940(89)90243-7. [DOI] [PubMed] [Google Scholar]
- 163.Gomez-Tortosa E, Irizarry MC, Gomez-Isla T, Hyman BT. Clinical and neuropathological correlates of dementia with Lewy bodies. Ann N Y Acad Sci. 2000;920:9–15. doi: 10.1111/j.1749-6632.2000.tb06899.x. [DOI] [PubMed] [Google Scholar]
- 164.Leuba G, Saini K. Pathology of subcortical visual centres in relation to cortical degeneration in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1995;21(5):410–22. doi: 10.1111/j.1365-2990.1995.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 165.Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7(6):1799–808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1(1):103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 167.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 8–84. [DOI] [PubMed] [Google Scholar]
- 168.Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease. A clinico-pathological study. Arch Psychiatr Nervenkr. 1976;223(1):15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- 169.McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(6):621–30. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- 170.Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175–80. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- 171.Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–74. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 172.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45(7):789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- 173.Cogan DG. Visual disturbances with focal progressive dementing disease. Am J Ophthalmol. 1985;100(1):68–72. doi: 10.1016/s0002-9394(14)74985-2. [DOI] [PubMed] [Google Scholar]
- 174.Henderson VW, Mack W, Williams BW. Spatial disorientation in Alzheimer’s disease. Arch Neurol. 1989;46(4):391–4. doi: 10.1001/archneur.1989.00520400045018. [DOI] [PubMed] [Google Scholar]
- 175.Chartier-Harlin MC, Parfitt M, Legrain S, Perez-Tur J, Brousseau T, Evans A, et al. Apolipoprotein E, epsilon 4 allele as a major risk factor for sporadic early and late-onset forms of Alzheimer’s disease: analysis of the 19q13.2 chromosomal region. Hum Mol Genet. 1994;3(4):569–74. doi: 10.1093/hmg/3.4.569. [DOI] [PubMed] [Google Scholar]
- 176.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 177.Kuusisto J, Koivisto K, Kervinen K, Mykkanen L, Helkala EL, Vanhanen M, et al. Association of apolipoprotein E phenotypes with late onset Alzheimer’s disease: population based study. BMJ. 1994;309(6955):636–8. doi: 10.1136/bmj.309.6955.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schmidt S, Klaver C, Saunders A, Postel E, De La Paz M, Agarwal A, et al. A pooled case-control study of the apolipoprotein E (APOE) gene in age-related maculopathy. Ophthalmic Genet. 2002;23(4):209–23. doi: 10.1076/opge.23.4.209.13883. [DOI] [PubMed] [Google Scholar]
- 179.Zareparsi S, Reddick AC, Branham KE, Moore KB, Jessup L, Thoms S, et al. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest Ophthalmol Vis Sci. 2004;45(5):1306–10. doi: 10.1167/iovs.03-1253. [DOI] [PubMed] [Google Scholar]
- 180.Zubenko GS, Stiffler S, Stabler S, Kopp U, Hughes HB, Cohen BM, et al. Association of the apolipoprotein E epsilon 4 allele with clinical subtypes of autopsy-confirmed Alzheimer’s disease. Am J Med Genet. 1994;54(3):199–205. doi: 10.1002/ajmg.1320540306. [DOI] [PubMed] [Google Scholar]
- 181.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–56. [PubMed] [Google Scholar]
- 182.Kovacs KA, Pamer Z, Kovacs A, Fekete S, Miseta A, Kovacs B, et al. Association of apolipoprotein E polymorphism with age-related macular degeneration and Alzheimer’s disease in south-western Hungary. Ideggyogy Sz. 2007;60(3–4):169–72. [PubMed] [Google Scholar]
- 183.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 184.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 186.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Strohmeyer R, Shen Y, Rogers J. Detection of complement alternative pathway mRNA and proteins in the Alzheimer’s disease brain. Brain Res Mol Brain Res. 2000;81(1–2):7–18. doi: 10.1016/s0169-328x(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 188.Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain. 2006;129(Pt 11):3042–50. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- 189.Proitsi P, Lupton MK, Dudbridge F, Tsolaki M, Hamilton G, Daniilidou M, et al. Alzheimer’s disease and age-related macular degeneration have different genetic models for complement gene variation. Neurobiol Aging. 2012;33(8):1843, e9–17. doi: 10.1016/j.neurobiolaging.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 190.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48(5):2285–9. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 191.Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26(1):18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 192.Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett. 2010;480(1):69–72. doi: 10.1016/j.neulet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 193.Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;420(2):97–9. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 194.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112(10):1860–7. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 195.Mapstone M, Logan D, Duffy CJ. Cue integration for the perception and control of self-movement in ageing and Alzheimer’s disease. Brain. 2006;129(Pt 11):2931–44. doi: 10.1093/brain/awl201. [DOI] [PubMed] [Google Scholar]
- 196.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mapstone M, Duffy CJ. Approaching objects cause confusion in patients with Alzheimer’s disease regarding their direction of self-movement. Brain. 2010;133(9):2690–701. doi: 10.1093/brain/awq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chapman RM, Nowlis GH, McCrary JW, Chapman JA, Sandoval TC, Guillily MD, et al. Brain event-related potentials: diagnosing early-stage Alzheimer’s disease. Neurobiol Aging. 2007;28(2):194–201. doi: 10.1016/j.neurobiolaging.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chapman RM, McCrary JW, Gardner MN, Sandoval TC, Guillily MD, Reilly LA, et al. Brain ERP components predict which individuals progress to Alzheimer’s disease and which do not. Neurobiol Aging. 2011;32(10):1742–55. doi: 10.1016/j.neurobiolaging.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mathalon DH, Bennett A, Askari N, Gray EM, Rosenbloom MJ, Ford JM. Response-monitoring dysfunction in aging and Alzheimer’s disease: an event-related potential study. Neurobiol Aging. 2003;24(5):675–85. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 201.Polich J, Corey-Bloom J. Alzheimer’s disease and P300: review and evaluation of task and modality. Curr Alzheimer Res. 2005;2(5):515–25. doi: 10.2174/156720505774932214. [DOI] [PubMed] [Google Scholar]
- 202.Kavcic V, Fernandez R, Logan D, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer’s disease. Brain. 2006;129(Pt 3):736–46. doi: 10.1093/brain/awh727. [DOI] [PubMed] [Google Scholar]
- 203.Fernandez R, Kavcic V, Duffy CJ. Neurophysiologic analyses of low- and high-level visual processing in Alzheimer disease. Neurology. 2007;68(24):2066–76. doi: 10.1212/01.wnl.0000264873.62313.81. [DOI] [PubMed] [Google Scholar]
- 204.Curran S, Wilson S, Musa S, Wattis J. Critical Flicker Fusion Threshold in patients with Alzheimer’s disease and vascular dementia. Int J Geriatr Psychiatry. 2004;19(6):575–81. doi: 10.1002/gps.1134. [DOI] [PubMed] [Google Scholar]
- 205.Valenti DA. Alzheimer’s disease: screening biomarkers using frequency doubling technology visual field. ISRN Neurol. 2013;2013:989583. doi: 10.1155/2013/989583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moschos MM, Markopoulos I, Chatziralli I, Rouvas A, Papageorgiou SG, Ladas I, et al. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr Alzheimer Res. 2012;9(7):782–8. doi: 10.2174/156720512802455340. [DOI] [PubMed] [Google Scholar]
- 207.Mentis MJ, Horwitz B, Grady CL, Alexander GE, VanMeter JW, Maisog JM, et al. Visual cortical dysfunction in Alzheimer’s disease evaluated with a temporally graded “stress test” during PET. Am J Psychiatry. 1996;153(1):32–40. doi: 10.1176/ajp.153.1.32. [DOI] [PubMed] [Google Scholar]
- 208.Beason-Held LL, Purpura KP, Van Meter JW, Azari NP, Mangot DJ, Optican LM, et al. PET reveals occipitotemporal pathway activation during elementary form perception in humans. Vis Neurosci. 1998;15(3):503–10. doi: 10.1017/s0952523898153117. [DOI] [PubMed] [Google Scholar]
- 209.Brun A, Englund E. Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology. 1981;5(5):549–64. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 210.Kiyosawa M, Bosley TM, Chawluk J, Jamieson D, Schatz NJ, Savino PJ, et al. Alzheimer’s disease with prominent visual symptoms. Clinical and metabolic evaluation. Ophthalmology. 1989;96(7):1077–85. doi: 10.1016/s0161-6420(89)32769-2. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 211.Pietrini P, Furey ML, Graff-Radford N, Freo U, Alexander GE, Grady CL, et al. Preferential metabolic involvement of visual cortical areas in a subtype of Alzheimer’s disease: clinical implications. Am J Psychiatry. 1996;153(10):1261–8. doi: 10.1176/ajp.153.10.1261. [DOI] [PubMed] [Google Scholar]
- 212.Wilcock DM, Griffin WS. Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation. 2013;10:84. doi: 10.1186/1742-2094-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54 (Suppl 1):S204–17. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chiu K, Chan TF, Wu A, Leung IY, So KF, Chang RC. Neurodegeneration of the retina in mouse models of Alzheimer’s disease: what can we learn from the retina? Age (Dordr) 2012;34(3):633–49. doi: 10.1007/s11357-011-9260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Leung CK, Chiu V, Weinreb RN, Liu S, Ye C, Yu M, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118(8):1558–62. doi: 10.1016/j.ophtha.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 216.Perez SE, Lumayag S, Kovacs B, Mufson EJ, Xu S. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2009;50(2):793–800. doi: 10.1167/iovs.08-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shimazawa M, Inokuchi Y, Okuno T, Nakajima Y, Sakaguchi G, Kato A, et al. Reduced retinal function in amyloid precursor protein-over-expressing transgenic mice via attenuating glutamate-N-methyl-d-aspartate receptor signaling. J Neurochem. 2008;107(1):279–90. doi: 10.1111/j.1471-4159.2008.05606.x. [DOI] [PubMed] [Google Scholar]
- 218.William CM, Andermann ML, Goldey GJ, Roumis DK, Reid RC, Shatz CJ, et al. Synaptic plasticity defect following visual deprivation in Alzheimer’s disease transgenic mice. J Neurosci. 2012 doi: 10.1523/JNEUROSCI.5369-11.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rudinskiy N, Hawkes JM, Betensky RA, Eguchi M, Yamaguchi S, Spires-Jones TL, et al. Orchestrated experience-driven Arc responses are disrupted in a mouse model of Alzheimer’s disease. Nat Neurosci. 2012;15(10):1422–9. doi: 10.1038/nn.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ding JD, Lin J, Mace BE, Herrmann R, Sullivan P, Bowes Rickman C. Targeting age-related macular degeneration with Alzheimer’s disease based immunotherapies: anti-amyloid-beta antibody attenuates pathologies in an age-related macular degeneration mouse model. Vision Res. 2008;48(3):339–45. doi: 10.1016/j.visres.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108(28):E279–87. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, et al. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26(20):5340–6. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Goldblum D, Kipfer-Kauer A, Sarra GM, Wolf S, Frueh BE. Distribution of amyloid precursor protein and amyloid-beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Invest Ophthalmol Vis Sci. 2007;48(11):5085–90. doi: 10.1167/iovs.06-1249. [DOI] [PubMed] [Google Scholar]
- 224.Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104(33):13444–9. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xiong K, Cai H, Luo XG, Struble RG, Clough RW, Yan XX. Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina. Exp Brain Res. 2007;181(3):435–46. doi: 10.1007/s00221-007-0943-y. [DOI] [PubMed] [Google Scholar]
- 226.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171(20):1851–2. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148(9):879–86. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 228.Lethbridge-Cejku M, Schiller J, Bernadel L. Summary health statistics for U.S. adults: National Health Interview Survey 2002. Vital Health Stat 10. 2004;10:1–151. [PubMed] [Google Scholar]
- 229.van Rooij JC, Plomp R. Auditive and cognitive factors in speech perception by elderly listeners. III. Additional data and final discussion. J Acoust Soc Am. 1992;91(2):1028–33. doi: 10.1121/1.402628. [DOI] [PubMed] [Google Scholar]
- 230.Dubno JR, Dirks DD, Morgan DE. Effects of age and mild hearing loss on speech recognition in noise. J Acoust Soc Am. 1984;76(1):87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- 231.Humes LE. Speech understanding in the elderly. J Am Acad Audiol. 1996;7(3):161–7. [PubMed] [Google Scholar]
- 232.Best V, Gallun FJ, Mason CR, Kidd G, Jr, Shinn-Cunningham BG. The impact of noise and hearing loss on the processing of simultaneous sentences. Ear Hear. 2010;31(2):213–20. doi: 10.1097/AUD.0b013e3181c34ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Humes LE, Roberts L. Speech-recognition difficulties of the hearing-impaired elderly: the contributions of audibility. J Speech Hear Res. 1990;33(4):726–35. doi: 10.1044/jshr.3304.726. [DOI] [PubMed] [Google Scholar]
- 234.Tun PA, O’Kane G, Wingfield A. Distraction by competing speech in young and older adult listeners. Psychol Aging. 2002;17(3):453–67. doi: 10.1037//0882-7974.17.3.453. [DOI] [PubMed] [Google Scholar]
- 235.Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc. 2002;50(3):482–8. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- 236.Gates GA, Cobb JL, Linn RT, Rees T, Wolf PA, D’Agostino RB. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg. 1996;122(2):161–7. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- 237.Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2011;137(4):390–5. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58(9):853–8. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 239.Fabrigoule C, Rouch I, Taberly A, Letenneur L, Commenges D, Mazaux JM, et al. Cognitive process in preclinical phase of dementia. Brain. 1998;121 ( Pt 1):135–41. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- 240.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14(2):266–78. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE) Am J Geriatr Psychiatry. 2005;13(2):134–41. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- 242.Royall DR, Chiodo LK, Polk MJ. Misclassification is likely in the assessment of mild cognitive impairment. Neuroepidemiology. 2004;23(4):185–91. doi: 10.1159/000078504. [DOI] [PubMed] [Google Scholar]
- 243.Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23(8):635–66. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–9. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, Babisch W, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79(15):1583–90. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- 246.Baloyannis SJ, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer’s disease: a Golgi and electron microscope study. Acta Otolaryngol. 2009;129(4):416–8. doi: 10.1080/00016480802579074. [DOI] [PubMed] [Google Scholar]
- 247.Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40(3):320–6. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 248.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–6. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 249.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–12. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 250.Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Q J Exp Psychol. 1968;20(3):241–8. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- 251.Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am. 1995;97(1):593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- 252.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24(3):761–6. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sinha UK, Saadat D, Linthicum FH, Jr, Hollen KM, Miller CA. Temporal bone findings in Alzheimer’s disease. Laryngoscope. 1996;106(1 Pt 1):1–5. doi: 10.1097/00005537-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 254.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43(4):779–85. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- 255.Christensen K, Frederiksen H, Hoffman HJ. Genetic and environmental influences on self-reported reduced hearing in the old and oldest old. J Am Geriatr Soc. 2001;49(11):1512–7. doi: 10.1046/j.1532-5415.2001.4911245.x. [DOI] [PubMed] [Google Scholar]
- 256.Wingfield A, Panizzon M, Grant MD, Toomey R, Kremen WS, Franz CE, et al. A twin-study of genetic contributions to hearing acuity in late middle age. J Gerontol A Biol Sci Med Sci. 2007;62(11):1294–9. doi: 10.1093/gerona/62.11.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Van Eyken E, Van Laer L, Fransen E, Topsakal V, Lemkens N, Laureys W, et al. KCNQ4: a gene for age-related hearing impairment? Hum Mutat. 2006;27(10):1007–16. doi: 10.1002/humu.20375. [DOI] [PubMed] [Google Scholar]
- 258.Van Eyken E, Van Camp G, Fransen E, Topsakal V, Hendrickx JJ, Demeester K, et al. Contribution of the N-acetyltransferase 2 polymorphism NAT2*6A to age-related hearing impairment. J Med Genet. 2007;44(9):570–8. doi: 10.1136/jmg.2007.049205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Van Laer L, Huyghe JR, Hannula S, Van Eyken E, Stephan DA, Maki-Torkko E, et al. A genome-wide association study for age-related hearing impairment in the Saami. Eur J Hum Genet. 2010;18(6):685–93. doi: 10.1038/ejhg.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, Corneveaux JJ, et al. GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet. 2009;18(4):785–96. doi: 10.1093/hmg/ddn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kurniawan C, Westendorp RG, de Craen AJ, Gussekloo J, de Laat J, van Exel E. Gene dose of apolipoprotein E and age-related hearing loss. Neurobiol Aging. 2012;33(9):2230, e7–e12. doi: 10.1016/j.neurobiolaging.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 262.Tremblay K, Ross B. Effects of age and age-related hearing loss on the brain. J Commun Disord. 2007;40(4):305–12. doi: 10.1016/j.jcomdis.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 263.Rosenhall U, Pedersen K, Dotevall M. Effects of presbycusis and other types of hearing loss on auditory brainstem responses. Scand Audiol. 1986;15(4):179–85. doi: 10.3109/01050398609042141. [DOI] [PubMed] [Google Scholar]
- 264.Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, et al. Age-related changes in the auditory brainstem response. J Am Acad Audiol. 2012;23(1):18–35. doi: 10.3766/jaaa.23.1.3. quiz 74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Papaliagkas VT, Anogianakis G, Tsolaki MN, Koliakos G, Kimiskidis VK. Combination of P300 and CSF beta-amyloid(1-42) assays may provide a potential tool in the early diagnosis of Alzheimer’s disease. Curr Alzheimer Res. 2010;7(4):295–9. doi: 10.2174/156720510791162421. [DOI] [PubMed] [Google Scholar]
- 266.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638–43. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96(6):2830–9. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- 268.Rousseaux M, Seve A, Vallet M, Pasquier F, Mackowiak-Cordoliani MA. An analysis of communication in conversation in patients with dementia. Neuropsychologia. 2010;48(13):3884–90. doi: 10.1016/j.neuropsychologia.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 269.Kahneman D. Attention and effort. Prentice-Hall; 1973. [Google Scholar]
- 270.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131–6. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–70. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang J, Ikonen S, Gurevicius K, Van Groen T, Tanila H. Altered auditory-evoked potentials in mice carrying mutated human amyloid precursor protein and presenilin-1 transgenes. Neuroscience. 2003;116(2):511–7. doi: 10.1016/s0306-4522(02)00714-5. [DOI] [PubMed] [Google Scholar]
- 273.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. 2011;11(5):665–76. doi: 10.1586/ern.11.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1–2):66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- 275.Verghese J, Robbins M, Holtzer R, Zimmerman M, Wang C, Xue X, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–51. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Boyle PA, Wilson RS, Aggarwal NT, Arvanitakis Z, Kelly J, Bienias JL, et al. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005;65(12):1901–6. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- 277.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63(12):1763–9. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 278.Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain. 2008;131(Pt 5):1268–81. doi: 10.1093/brain/awn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mosimann UP, Felblinger J, Ballinari P, Hess CW, Muri RM. Visual exploration behaviour during clock reading in Alzheimer’s disease. Brain. 2004;127(Pt 2):431–8. doi: 10.1093/brain/awh051. [DOI] [PubMed] [Google Scholar]
- 280.Boxer AL, Garbutt S, Seeley WW, Jafari A, Heuer HW, Mirsky J, et al. Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer disease. Arch Neurol. 2012;69(4):509–17. doi: 10.1001/archneurol.2011.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–6. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50(5):1496–8. doi: 10.1212/wnl.50.5.1496. [DOI] [PubMed] [Google Scholar]
- 283.Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334(2):71–6. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 284.Murray AM, Bennett DA, Mendes de Leon CF, Beckett LA, Evans DA. A longitudinal study of parkinsonism and disability in a community population of older people. J Gerontol A Biol Sci Med Sci. 2004;59(8):864–70. doi: 10.1093/gerona/59.8.m864. [DOI] [PubMed] [Google Scholar]
- 285.Bennett DA, Beckett LA, Wilson RS, Murray AM, Evans DA. Parkinsonian signs and mortality from Alzheimer’s disease. Lancet. 1998;351(9116):1631. doi: 10.1016/S0140-6736(05)77686-8. [DOI] [PubMed] [Google Scholar]
- 286.Allan LM, Ballard CG, Burn DJ, Kenny RA. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc. 2005;53(10):1681–7. doi: 10.1111/j.1532-5415.2005.53552.x. [DOI] [PubMed] [Google Scholar]
- 287.Portet F, Scarmeas N, Cosentino S, Helzner EP, Stern Y. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120–6. doi: 10.1001/archneurol.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59(4):601–6. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 289.Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229–230:89–93. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 290.Bermejo-Pareja F, Louis ED, Benito-Leon J Neurological Disorders in Central Spain Study G. Risk of incident dementia in essential tremor: a population-based study. Mov Disord. 2007;22(11):1573–80. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 291.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73(8):621–5. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schmidt C, Redyk K, Meissner B, Krack L, von Ahsen N, Roeber S, et al. Clinical features of rapidly progressive Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;29(4):371–8. doi: 10.1159/000278692. [DOI] [PubMed] [Google Scholar]
- 293.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11):1339–44. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–42. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control. 2012;16(1):64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–23. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 297.Giannakopoulos P, Hof PR, Michel JP, Guimon J, Bouras C. Cerebral cortex pathology in aging and Alzheimer’s disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Res Brain Res Rev. 1997;25(2):217–45. doi: 10.1016/s0165-0173(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 298.Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45(1):38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 299.Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann Neurol. 1997;41(3):368–74. doi: 10.1002/ana.410410312. [DOI] [PubMed] [Google Scholar]
- 300.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59(1):166–73. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 301.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64(8):1397–403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 302.Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71(2):258–66. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kaye JA, May C, Daly E, Atack JR, Sweeney DJ, Luxenberg JS, et al. Cerebrospinal fluid monoamine markers are decreased in dementia of the Alzheimer type with extrapyramidal features. Neurology. 1988;38(4):554–7. doi: 10.1212/wnl.38.4.554. [DOI] [PubMed] [Google Scholar]
- 304.Kaye JA, May C, Atack JR, Daly E, Sweeney DL, Beal MF, et al. Cerebrospinal fluid neurochemistry in the myoclonic subtype of Alzheimer’s disease. Ann Neurol. 1988;24(5):647–50. doi: 10.1002/ana.410240509. [DOI] [PubMed] [Google Scholar]
- 305.Bowler JV. Modern concept of vascular cognitive impairment. Br Med Bull. 2007;83:291–305. doi: 10.1093/bmb/ldm021. [DOI] [PubMed] [Google Scholar]
- 306.Yue NC, Arnold AM, Longstreth WT, Jr, Elster AD, Jungreis CA, O’Leary DH, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202(1):33–9. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 307.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36(1):56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 308.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108–13. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57(6):990–4. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 310.Melzer D, Frayling TM, Murray A, Hurst AJ, Harries LW, Song H, et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech Ageing Dev. 2007;128(5–6):370–7. doi: 10.1016/j.mad.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Frayling TM, Rafiq S, Murray A, Hurst AJ, Weedon MN, Henley W, et al. An interleukin-18 polymorphism is associated with reduced serum concentrations and better physical functioning in older people. J Gerontol A Biol Sci Med Sci. 2007;62(1):73–8. doi: 10.1093/gerona/62.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31(3):523–31. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Menendez M. Pathological and clinical heterogeneity of presenilin 1 gene mutations. J Alzheimers Dis. 2004;6(5):475–82. doi: 10.3233/jad-2004-6503. [DOI] [PubMed] [Google Scholar]
- 314.Gomez-Tortosa E, Barquero S, Baron M, Gil-Neciga E, Castellanos F, Zurdo M, et al. Clinical-genetic correlations in familial Alzheimer’s disease caused by presenilin 1 mutations. J Alzheimers Dis. 2010;19(3):873–84. doi: 10.3233/JAD-2010-1292. [DOI] [PubMed] [Google Scholar]
- 315.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–50. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 316.Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, et al. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55(1):74–7. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 317.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(4):555–9. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–63. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mirsky JB, Heuer HW, Jafari A, Kramer JH, Schenk AK, Viskontas IV, et al. Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn Behav Neurol. 2011;24(2):50–8. doi: 10.1097/WNN.0b013e318223f6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Silbert LC, Dodge HH, Perkins LG, Sherbakov L, Lahna D, Erten-Lyons D, et al. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79(8):741–7. doi: 10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73(21):1722–8. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 324.Fernando MS, Ince PG, Function MRCC Ageing Neuropathology Study G. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226(1–2):13–7. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 325.Hindle JV. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing. 2010;39(2):156–61. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 326.Lazarov O, Morfini GA, Pigino G, Gadadhar A, Chen X, Robinson J, et al. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer’s disease-linked mutant presenilin 1. J Neurosci. 2007;27(26):7011–20. doi: 10.1523/JNEUROSCI.4272-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wirths O, Bayer TA. Motor impairment in Alzheimer’s disease and transgenic Alzheimer’s disease mouse models. Genes Brain Behav. 2008;7 (Suppl 1):1–5. doi: 10.1111/j.1601-183X.2007.00373.x. [DOI] [PubMed] [Google Scholar]
- 328.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3(77):77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Nunez-Parra A, Maurer RK, Krahe K, Smith RS, Araneda RC. Disruption of centrifugal inhibition to olfactory bulb granule cells impairs olfactory discrimination. Proceedings of the National Academy of Sciences; 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Progress in Neurobiology. 2007;82(3):109–21. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 333.Miri A, Azim E, Jessell TM. Edging toward Entelechy in Motor Control. Neuron. 2013;80(3):827–34. doi: 10.1016/j.neuron.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 334.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013;14(5):350–63. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76(6):1175–88. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proceedings of the National Academy of Sciences. 1997;94(8):4119–24. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, et al. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472(7342):217–20. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- 338.Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472(7342):213–6. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Cui B, Zhu L, She X, Wu M, Ma Q, Wang T, et al. Chronic noise exposure causes persistence of tau hyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Experimental Neurology. 2012;238(2):122–9. doi: 10.1016/j.expneurol.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 340.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 Suppl 3):S2–6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain. 2013;136(Pt 8):2510–26. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69(2):203–13. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68(8):1062–4. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 347.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 348.Harris JA, Koyama A, Maeda S, Ho K, Devidze N, Dubal DB, et al. Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS One. 2012;7(9):e45881. doi: 10.1371/journal.pone.0045881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11(3):155–9. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Chen LY, Rex CS, Pham DT, Lynch G, Gall CM. BDNF signaling during learning is regionally differentiated within hippocampus. J Neurosci. 2010;30(45):15097–101. doi: 10.1523/JNEUROSCI.3549-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.