Abstract
We examined clinical outcomes with proton pump inhibitors (PPI) use within CYP2C19 genotype groups during clopidogrel treatment following acute myocardial infarction (AMI). 2062 patients were genotyped for CYP2C19*2 and *17 variants in TRIUMPH. 12 month clinical outcomes were analyzed among patients discharged on clopidogrel within CYP2C19*2 carrier, CYP2C19*17 carrier, and CYP2C19*1 homozygote genotype groups. PPI use was not associated with a difference in mortality. Among clopidogrel-treated Caucasians following AMI, PPI use was associated with a significantly higher rate of cardiac rehospitalization (HR 1.62, 95% CI 1.19-2.19; p = 0.002) compared with no PPI use. PPI users who were carriers of the CYP2C19*17 variant experienced significantly higher rates of cardiac rehospitalization (HR 2.05, 95% CI 1.26-3.33; p = 0.003), carriers of the CYP2C19*2 variant had a trend toward increased 1-year cardiac rehospitalization (HR 1.69, 95% CI 0.95-2.99; P=0.07) while no significant differences were observed among CYP2C19*1 homozygotes. These results indicate that the risks associated with PPI use among clopidogrel-treated Caucasian post-MI patients are impacted by CYP2C19 genotype, and suggest knowledge of genotype may be useful for personalizing PPI use among patients following AMI to reduce rehospitalization.
Keywords: cardiovascular diseases, clopidogrel, genetic variation, myocardial infarction, pharmacogenetics, CYP2C19
In the United States, over 900,000 individuals have an acute myocardial infarction (AMI) each year.1 Dual antiplatelet therapy with aspirin and an adenosine diphosphate (ADP) P2Y12 receptor antagonists is recommended for the secondary prevention of ischemic events following AMI.2 However, adverse ischemic events following AMI remain frequent.3 Although clopidogrel is the most commonly prescribed ADP P2Y12 receptor antagonist in the management of AMI,4 the degree of platelet inhibition with clopidogrel varies significantly between individuals.5 High-on treatment platelet reactivity in patients on clopidogrel is an independent risk factor for increased ischemic events following AMI.5 The proposed mechanisms responsible for impaired response to clopidogrel are multifactorial.6 However, variability in the metabolism of clopidogrel to its active metabolite has been implicated as a possible mechanism leading to clinical failure of dual antiplatelet therapy following AMI.7
Clopidogrel is a prodrug that is metabolized to its active metabolite by enzymes of the hepatic cytochrome P450 (CYP450) system. A number of factors influence the metabolism of clopidogrel, including drug-drug interactions and genetic polymorphisms (SNPs) in CYP450 isoenzymes, the most important of which is CYP2C19.5 Specific variants of CYP2C19, particularly CYP2C19*2 and CYP2C19*17, have been associated with marked differences in production of clopidogrel's active metabolite and resultant platelet inhibition.8 Previous investigations have shown that, in clopidogrel-treated AMI patients, individuals with the loss-of-function CYP2C19*2 allele have less platelet inhibition and a higher rate of adverse cardiac events, whereas carriers of the gain-of function CYP2C19*17 allele have the highest platelet inhibition and a potential increase in bleeding events.8
Proton pump inhibitors (PPIs) are also metabolized using CYP2C19.9 The pharmacodynamic effect of PPIs on clopidogrel is influenced by the drug-drug interaction that occurs at the CYP2C19 isoenzyme.6, 10 Although the clinical consequences of this drug-drug interaction remain controversial,7 PPI use has been associated with a reduction in platelet inhibition by clopidogrel in some studies.11
The clinical implications of the interplay between genetic variation in CYP2C19 and the drug-drug interactions involving PPIs and clopidogrel are less well understood.6, 12 We, therefore, posed the question: in post-MI patients discharged on clopidogrel, is there a particular CYP2C19 genotype group in which adding a PPI to clopidogrel treatment will increase adverse cardiac events? We investigated this question in the large, prospective, multicenter Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients' Health status (TRIUMPH) cohort.13 We specifically evaluated 1-year mortality, cardiac rehospitalization and bleeding within CYP2C19 genotype groups in Caucasian and African-American patients discharged on clopidogrel following an AMI.
Methods
Subjects and Follow-up
From April 11, 2005 to December 31, 2008, 4340 patients with AMI were prospectively enrolled into the TRIUMPH observational cohort study from 24 medical centers in the United States, as previously described.13-15 All patients were required to have a type 1 AMI evidenced by an elevated troponin level and documented clinical ischemia (i.e. diagnostic ST changes on an ECG or ischemic signs/symptoms).13 2979 TRIUMPH patients consented to genetic testing.15 Of these, 2955 (99.2%) were discharged alive and were included in the present analyses. The final sample was restricted to Caucasian (n = 1632) and African American (n = 430) patients discharged on clopidogrel following AMI (total N = 2062). Subjects discharged on PPI following AMI and/or at 1 or more follow-up interview were included in the PPI group.
Each patient was prospectively interviewed during the initial hospitalization to ascertain socio-demographic (including self-identified race), economic and health status characteristics. Detailed chart abstractions were performed of the initial hospitalization to obtain patients' medical history, laboratory results, disease severity, inpatient care, and medications (including baseline, during the hospitalization, and discharge). TRIUMPH received Institutional Review Board approval at all participating sites and written informed consent was obtained from each participant.
Follow-up interviews were scheduled on all survivors at 1, 6, and 12 months after the date of discharge for the index hospitalization, as previously described.13 If a patient agreed to additional blood collection, an in-home visit and interview was performed by a trained medical personnel at 1 and 6 months. At 12-months, clinical follow-up was performed by telephone interview at a single specialized center. For those patients not agreeing to additional blood collection, 1 and 6 month interviews were performed by telephone from the same single specialized center used for the 12 month interview. At each interview, all patients were asked to report all interval events (e.g., procedures, diagnostic tests, hospitalizations, and outpatient visits) since their last study contact, as well as current medications on the date of interview.
Clinical Outcomes
The primary outcome of the study was all-cause mortality. For every patient in the study, all-cause mortality was assessed using the Social Security Administration Death Master File (http://www.ntis.gov/products/ssadmf.aspx) and was queried to determine patients' vital status as of 12/31/2010. (Of note, this query was performed prior to new restrictions and expunging of some records from the database.) Secondary outcomes were ascertained from the follow-up interviews and included the individual endpoints of cardiac rehospitalization or bleeding. 157 Caucasians and 88 African-Americans were missing information pertaining to cardiac rehospitalization. 229 Caucasians and 105 African-Americans were missing information pertaining to bleeding. If a patient reported being hospitalized since the previous interview, records of that hospitalization were requested to adjudicate cardiovascular events, including MI, heart failure, or revascularization procedures. Chart abstractions were sent to 2 cardiologists for independent determination of the reason for hospitalization. If there was disagreement between the 2 cardiologists, the record was adjudicated by a third cardiologist, and, if disagreement persisted, up to 5 cardiologists independently reviewed the charts until consensus was obtained. Bleeding outcomes were documented in two ways. Major bleeding was adjudicated by three independent cardiologists. Minor (‘nuisance’ or BARC Type-1) bleeding was determined by interview.13 The bleeding outcome used for this study combined major or minor bleeding episodes.
Genotype Methods, Quality Control, and Classification
The methods used for genotyping and quality control are described in the Supplemental Methods. A dominant genetic model was used for CYP2C19 *2 and *17. Patients were classified as CYP2C19*1 in the absence of *2 and *17 variants. For CYP2C19 *17, the -3402 (rs11188072) and -806 (rs12248560) variants were genotyped and linkage was determined. Given that the two CYP2C19*17 variants were in tight linkage disequilibrium in our cohort, the analysis was restricted to the CYP2C19*17 variant (CYP2C19*17 -806; rs12248560) responsible for a C > T transition in the promoter that creates a consensus binding site for the GATA transcription factor family, resulting in increased CYP2C19 expression and activity.16, 17
Statistical Analyses
Analyses were performed separately in Caucasians and African-Americans to minimize the risk of false positive findings due to population stratification. In each group (Caucasians and African-Americans), baseline characteristics and clinical outcomes were compared between the PPI vs. no PPI groups and within each CYP2C19 genotype group (i.e. *1 homozygotes and *2 or *17 carriers). Categorical data are reported as counts and percentages and compared using chi-square or Fisher's exact tests as appropriate. Continuous data are reported as mean ± standard deviation (SD) and compared using t-tests. Hardy-Weinberg equilibrium was assessed using chi-square tests or Monte Carlo permutation with 10000 iterations, as appropriate. Follow-up began at the date of discharge from the index hospitalization. Survival function estimates (time until all-cause mortality, time until cardiac rehospitalization, and time to bleeding) were evaluated through Kaplan-Meier analysis. The log-rank test was used to identify differences in clinical outcomes between the PPI and no PPI groups overall, and within each CYP2C19 genotype group. Cox proportional hazards models were then used to adjust for sex, GRACE score (an extensively validated score of predicting post-MI death and recurrent MI (c-statistic 0.84) from admission clinical, ECG and biomarker variables),18, 19 and any patient characteristic that was significantly different between PPI users vs. non-users overall and within CYP2C19 genotype group. For primary effects, p-values < 0.05 were considered statistically significant; interaction p-values < 0.1 were considered statistically significant. Analyses were performed with SAS version 9.2 (SAS Institute, Inc., Cary, NC) and R version 2.11.1.
Results
2062 TRIUMPH patients (N = 1632 Caucasians; 430 African-Americans) were discharged on clopidogrel after MI. More than 80% of TRIUMPH patients discharged on clopidogrel had received in-hospital revascularization; the majority of these being percutaneous coronary intervention (PCI) (87.1% of Caucasians and 77.4% of African Americans). Over the study period, PPIs were used by 19% of Caucasians (N = 307) and 15% of African-Americans (N = 65). Baseline characteristics of TRIUMPH patients discharged on clopidogrel with and without the use of a PPI are shown in Table 1 (comparisons within each genotype group are shown in Supplemental Tables S1A-C). In both Caucasians and African-Americans, PPI users were older and had a higher mean GRACE risk score at discharge. In Caucasians, PPI users were more likely to be female (32.6% vs. 24.5%; p = 0.003) and have a higher rate of co-morbidities including hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation or flutter, history of prior PCI or coronary artery bypass grafting (CABG), and present with NSTEMI (vs. STEMI) compared with patients not using a PPI (p values ranging from 0.06 to <0.001; Table 1). In African-Americans, PPI users were more likely to have peripheral vascular disease (13.8% vs. 4.9%; p= 0.006) and a history of prior PCI (29.2% vs. 18.4%; p = 0.04). In both Caucasian and African-American patients, PPI users and non-users were discharged on similar secondary prevention medications, except for a lower rate of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in the PPI group compared with the no PPI group among African-Americans.
Table 1. Baseline Clinical Characteristics of Clopidogrel-Treated TRIUMPH Patients following Acute Myocardial Infarction with and without the use of a Proton Pump Inhibitor.
| Caucasians | African Americans | |||||
|---|---|---|---|---|---|---|
| PPI (n = 307 patients) | No PPI (n = 1325 patients) | p-value | PPI (n = 65 patients) | No PPI (n = 365 patients) | p-value | |
| Age | 60.4 ± 11.6 | 58.7 ± 12.0 | 0.02 | 60.4 ± 11.8 | 56.7 ± 11.8 | 0.02 |
| Male | 207 (67.4) | 1001 (75.5) | 0.003 | 34 (52.3) | 205 (56.2) | 0.56 |
| Hypertension | 202 (65.8) | 754 (56.9) | 0.004 | 51 (78.5) | 282 (77.3) | 0.83 |
| Dyslipidemia | 184 (59.9) | 634 (47.8) | 0.0001 | 33 (50.8) | 157 (43.0) | 0.25 |
| Diabetes Mellitus | 97 (31.6) | 300 (22.6) | 0.001 | 29 (44.6) | 140 (38.4) | 0.34 |
| Smoking History | 224 (73.2) | 972 (73.9) | 0.79 | 47 (72.3) | 266 (73.9) | 0.79 |
| Congestive Heart Failure | 19 (6.2) | 54 (4.1) | 0.11 | 9 (13.8) | 29 (7.9) | 0.12 |
| Chronic Kidney Disease | 16 (5.2) | 50 (3.8) | 0.25 | 6 (9.2) | 39 (10.7) | 0.72 |
| Atrial fibrillation/flutter | 22 (7.2) | 47 (3.5) | 0.005 | 0 (0.0) | 10 (2.7) | 0.37 |
| Peripheral Vascular Disease | 14 (4.6) | 45 (3.4) | 0.32 | 9 (13.8) | 18 (4.9) | 0.006 |
| Prior Myocardial Infarction | 61 (19.9) | 213 (16.1) | 0.11 | 18 (27.7) | 81 (22.2) | 0.33 |
| Prior PCI | 76 (24.8) | 265 (20.0) | 0.06 | 19 (29.2) | 67 (18.4) | 0.04 |
| Prior CABG | 55 (17.9) | 128 (9.7) | < 0.0001 | 5 (7.7) | 33 (9.0) | 1.00 |
| Prior Stroke | 12 (3.9) | 43 (3.2) | 0.56 | 5 (7.7) | 20 (5.5) | 0.56 |
| Prior TIA | 10 (3.3) | 25 (1.9) | 0.14 | 0 (0.0) | 8 (2.2) | 0.61 |
| GRACE Risk Score | 98.9 ± 27.8 | 94.7 ± 28.2 | 0.02 | 102.1 ± 29.8 | 92.8 ± 27.8 | 0.02 |
| Clinical Presentation of AMI | ||||||
| STEMI | 150 (48.9) | 740 (55.8) | 0.03 | 27 (41.5) | 155 (42.5) | 0.89 |
| NSTEMI | 153 (49.8) | 581 (43.8) | 0.06 | 38 (58.5) | 210 (57.5) | 0.89 |
| Index Hospital Revascularization | ||||||
| PCI | 263 (85.7) | 1158 (87.4) | 0.42 | 45 (69.2) | 288 (78.9) | 0.09 |
| CABG | 5 (1.6) | 38 (2.9) | 0.32 | 6 (9.2) | 10 (2.7) | 0.001 |
| Discharge Medications | ||||||
| Aspirin | 298 (97.1) | 1285 (97.0) | 0.94 | 62 (95.4) | 346 (94.8) | 0.84 |
| Clopidogrel | 307 (100.0) | 1325 (100.0) | 1.00 | 65 (100.0) | 365 (100.0) | 1.00 |
| Beta-blocker | 286 (93.2) | 1227 (92.6) | 0.74 | 61 (93.8) | 333 (91.2) | 0.48 |
| ACEI/ARB | 244 (79.5) | 1043 (78.7) | 0.77 | 41 (63.1) | 302 (82.7) | 0.0003 |
| Lipid lowering medication | 280 (91.2) | 1230 (92.8) | 0.33 | 59 (90.8) | 338 (92.6) | 0.61 |
Values are shown as absolute numbers (percentages) or mean ± SD.
ACE = angiotensin-converting enzyme; AMI = acute myocardial infarction; ARB = angiotensin receptor blocker; CABG = coronary artery bypass graft; NSTEMI = non-ST-elevation myocardial infarction; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction; TIA = Transient ischemic attack.
As shown in Table 2, omeprazole was the most frequently reported PPI used during the study period in both Caucasian and African-American PPI users (Caucasians [C]: 62%; African-Americans [AA]: 60%) followed by pantoprazole (C: 28%; AA: 36%), esomeprazole (C: 21%; AA: 25%), lansoprazole (C: 13%; AA: 14%), and rabeprazole (C: 4%; AA: 0%). Some patients used more than one PPI. No statistically significant differences were observed between genotype groups for the use of each individual PPI (frequency of use within each genotype group is shown in Supplemental Table S2).
Table 2. Type of Proton Pump Inhibitor Used During the Study Period.
| Caucasians PPI users | African-Americans PPI users | |
|---|---|---|
| Omeprazole | 62% | 60% |
| Pantoprazole | 28% | 36% |
| Esomeprazole | 21% | 25% |
| Lansoprazole | 13% | 14% |
| Rabeprazole | 4% | 0% |
Values are shown as percentages
PPI Use was Not Associated with Significant Differences in All-Cause Mortality in Either Race
The 1-year mortality rate for patients discharged on clopidogrel was 3.6% (N=59) for Caucasians and 7.2 % (N = 31) for African Americans. Unadjusted rates of all-cause mortality were not significantly different between PPI users and non-users among Caucasians (2.9% vs. 3.8%, respectively; p = 0.48) or African Americans (6.2% vs. 7.4%, respectively; p = 0.64). These findings were similar after multivariable adjustment using Cox proportional hazards models (not shown). Among Caucasian and African-American patients discharged on clopidogrel, there was no significant difference in 1-year mortality between PPI users and non-users within any particular CYP2C19 genotype group (data not shown).
PPI Use was Associated with Significantly Increased Cardiac Rehospitalization in Caucasians with the Greatest Increase in Risk observed among CYP2C19*17 Carriers
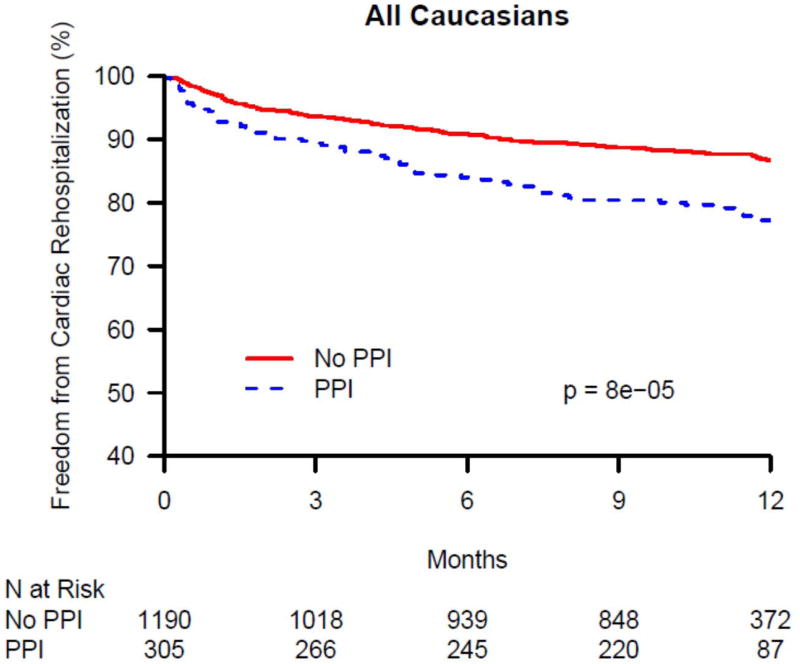
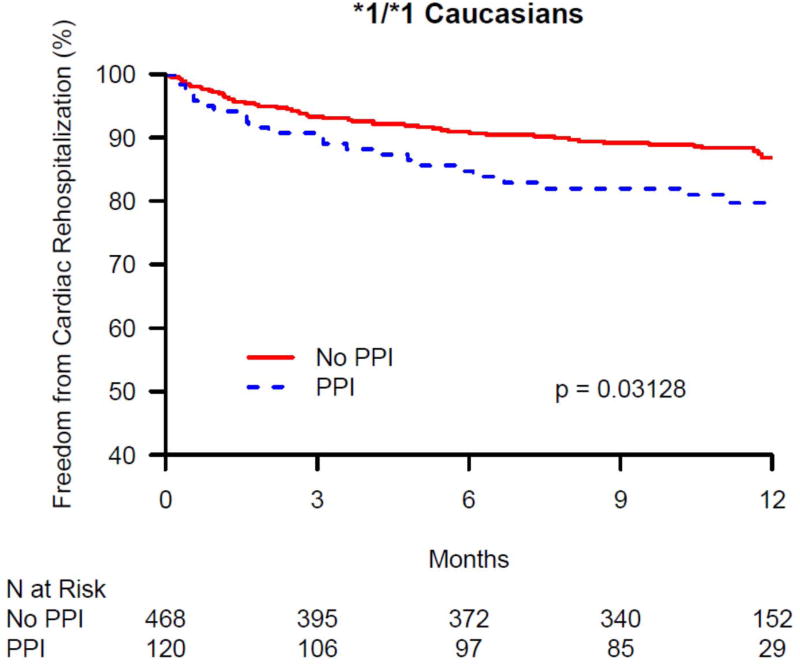
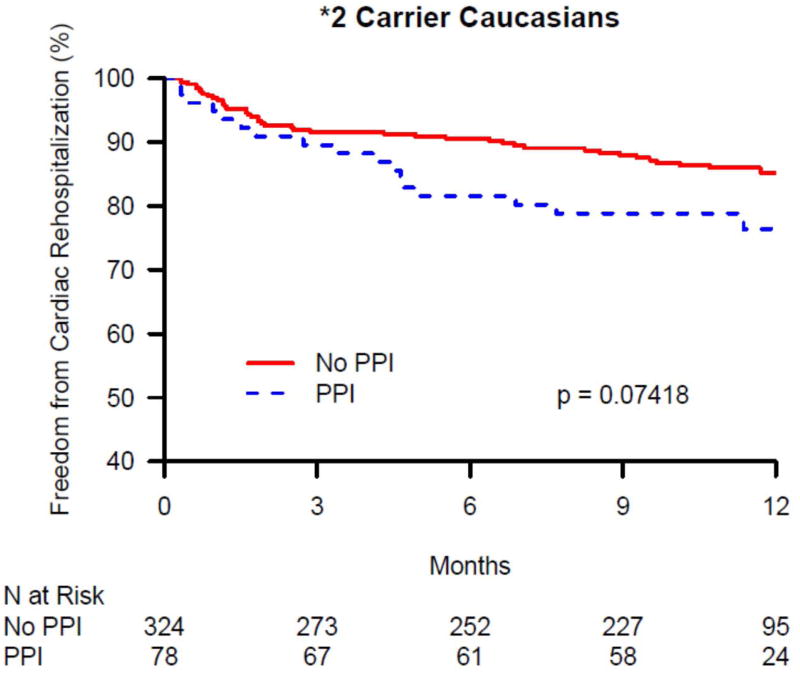
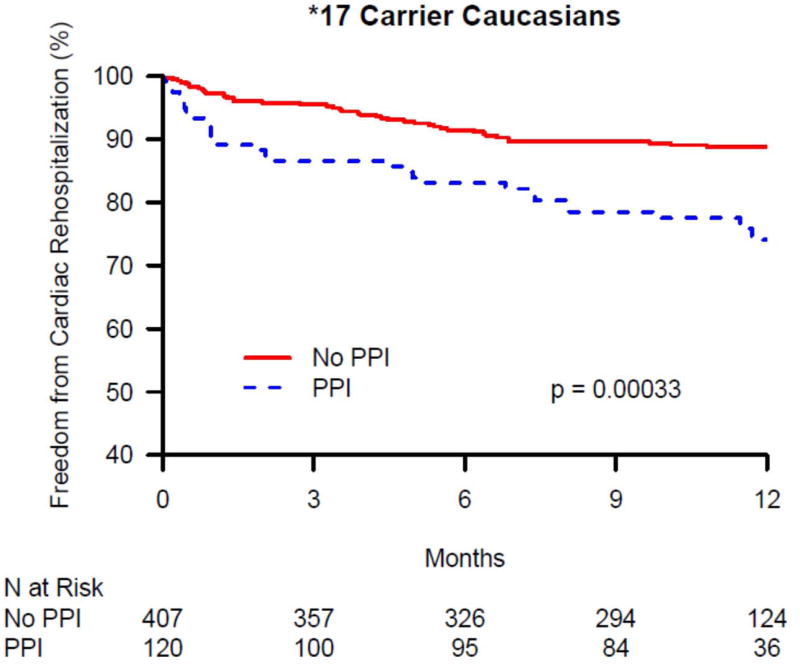
The 1-year cardiac rehospitalization rate was 13.6% in Caucasians (N = 201) and 15.2% in African-Americans (N = 52). There was no significant difference in 1-year cardiac rehospitalization between PPI users and non-users among African-Americans discharged on clopidogrel, overall, or within CYP2C19 genotype groups (data not shown). In contrast, there was a significant difference in 1-year cardiac rehospitalization between PPI users and non-users among Caucasians discharged on clopidogrel in the overall group and within CYP2C19 genotype groups (Table 3). As seen in Figure 1A, in the overall Caucasian group (i.e. not stratified by genotype group), the rate of cardiac rehospitalization was significantly higher in PPI users compared with non-users in both unadjusted and adjusted analyses (21.2% vs. 11.7%, p < 0.0001; adjusted HR 1.62, 95% CI 1.19-2.19; p = 0.002). Figures 1B – 1D show the difference in cardiac rehospitalization between PPI users and non-users in Caucasians discharged on clopidogrel within CYP2C19 genotype groups. Within the CYP2C19*1 homozygote genotype group, there was a significant increase in 1-year rate of cardiac rehospitalization in PPI users, compared to non-users (19.2% vs. 11.3%; p = 0.03; Figure 1B), in unadjusted analyses; however after adjusting for covariates, this difference was no longer significant (HR 1.51, 95% CI 0.90-2.54; p = 0.12; Table 3). Within the CYP2C19*2 genotype group, PPI users had a trend toward increased 1-year cardiac rehospitalization compared to non-users (21.8% vs. 13%; unadjusted p = 0.07; Figure 1C; adjusted HR 1.69, 95% CI 0.95-2.99; p = 0.07; Table 3). Notably, as seen in Figure 1D, the most significant association was seen within CYP2C19*17 carriers. Within this genotype group, PPI users had significantly more 1-year cardiac rehospitalization compared to non-users (23.3% vs. 10.3%; unadjusted p = 0.0003; adjusted HR 2.05, 95% CI 1.26-3.33; p = 0.003; Table 3).
Table 3. Adjusted Hazard Ratios (HR) for 1-year Cardiac Rehospitalization of Clopidogrel-treated Caucasians following AMI within CYP2C19 genetic groups and PPI use.
| Caucasians | ||
|---|---|---|
| HR (95% CI) | p-value | |
| All Caucasians | 1.62 (1.19-2.19) | 0.002 |
| CYP2C19 *1 homozygotes | 1.51 (0.90-2.54) | 0.12 |
| CYP2C19 *2 carriers | 1.69 (0.95-2.99) | 0.07 |
| CYP2C19 *17 carriers | 2.05 (1.26-3.33) | 0.003 |
Analyses adjusted for sex, GRACE risk score, and any patient characteristic that was significantly different between PPI users vs. non-users overall (Table 1) and within CYP2C19 genotype (Supplemental Tables 1A-C).
Figure 1.
Kaplan-Maier analysis of cardiac rehospitalization associated with and without the use of a proton pump inhibitors (PPI) in Caucasian clopidogrel-treated genotyped TRIUMPH patients following an acute myocardial infarction. Freedom from cardiac rehospitalization are compared between PPI users and non-users within (A) all genotyped Caucasians, (B) CYP2C19*1 homozygotes, (C) carriers of the CYP2C19*2 allele, and (D) carriers of the CYP2C19*17 allele. In Caucasians, PPI use was associated with significantly increased rates of cardiac rehospitalization (A) and was most significant among Caucasian carriers of the gain of function CYP2C19*17 allele (D).
Within the CYP2C19*17 genotype group, the association of PPI use and increased 1-year cardiac rehospitalization remained significant even if those subject also carrying the CYP2C19*2 allele (i.e. CYP2C19*17/*2 heterozygotes; N = 106) were excluded from the analysis (adjusted HR 2.02, 95% CI 1.17- 3.47; p = 0.01).
PPI Use was Associated with Significantly Increased Cardiac Rehospitalization Even After Exclusion of Elective Cardiac Rehospitalization
The causes for cardiac rehospitalization were analyzed to further assess the significant differences observed in clopidogrel-treated patients with and without PPI use. In the overall Caucasian group (i.e. not stratified by CYP2C19 genotype group), and in each CYP2C19 genotype group, approximately one-third of the rehospitalizations were for elective procedures (CABG or valve surgery, PCI, catheterization, and defibrillator or pacemaker implantation; 34.9% in overall group; 33% in CYP2C19 *1 homozygotes; 39% in CYP2C19 *2 carriers; 33% in CYP2C19 *17 carriers). After limiting cardiac rehospitalizations to non-elective admissions only (Supplemental Table S3), the rate of cardiac rehospitalization for PPI users remained significantly higher compared with non-users in clopidogrel-treated Caucasians (14.1% vs. 7.7%, p = 0.00004; HR 1.95, 95% CI 1.36-2.78; p = 0.0002) and within the clopidogrel-treated Caucasian CYP2C19*17 genotype group (18.3% vs. 6.4%, p = 0.00009; HR 2.48 (95% CI 1.39-4.42; p = 0.002).
PPI Use was not Associated with Significantly Increased Bleeding
The rates of bleeding following AMI among clopidogrel-treated patients were not significantly different when PPI users were compared with non-users in Caucasian (PPI use: 16.5% vs. no PPI use: 14.6%, p = 0.69) or African-American (PPI use: 8.2% vs. no PPI use: 11%, p = 0.51) TRIUMPH patients.
Discussion
The aim of our study was to answer a simple question: in post-MI patients discharged on clopidogrel, is there a particular CYP2C19 genotype group in which adding a PPI to clopidogrel treatment is associated with a higher rate of adverse cardiac events? In TRIUMPH, we found that PPI use was not associated with any differences in the rates of all-cause mortality in either race or within each CYP2C19 genotype group. Among African-Americans, the rates of cardiac rehospitalization were not significantly different in PPI users vs. nonusers in any of the CYP2C19 genotype groups. However, the use of a PPI was associated with significantly increased rates of cardiac rehospitalization in Caucasians, and the magnitude of this increased risk was most evident among Caucasian carriers of the CYP2C19*17 gain of function allele. This finding remained significant after (1) adjusting for sex, GRACE score and any baseline characteristic that differed within genotype group, (2) excluding CYP2C19*2/*17 heterozygotes and (3) limiting cardiac rehospitalizations to non-elective admissions. In TRIUMPH, 36% of Caucasians were carriers of the CYP2C19*17 genotype. Thus, our findings have the potential to impact the clinical management of a large proportion of Caucasians following an acute MI.
Two recent studies have provided evidence of inhibition of the CYP2C19 isoenzyme by PPIs, demonstrating a decreased plasma concentration of clopidogrel's active metabolite.20,21 A recent randomized cross-over study of healthy *1 homozygotes demonstrated that PPIs reduce the concentration of clopidogrel's active metabolite and reduce the degree of platelet inhibition.10 Currently, the evidence regarding the effect of CYP2C19 genetic variation on platelet inhibition in patients on a PPI and clopidogrel is limited. In a prospective double-blind cross-over randomized study, esomeprazole use significantly reduced platelet inhibition in clopidogrel-treated ACS patients who were *17 carriers or *1 homozygotes compared with placebo, but did not did not attenuate the antiplatelet effect in *2 carriers.22 This finding was also observed in an open-label cross-over study of healthy subjects, where platelet inhibition was significantly reduced by omeprazole and rabeprazole in *1 homozygotes but not in *2 carriers.23 Additional studies have also shown a less pronounced effect of PPI use on CYP2C19*2 carriers compared with *1 homozygotes.24, 25 These findings are supported by our study. In our study, the highest risk of cardiac rehospitalization was observed in *17 Caucasian carriers compared with the Caucasian *2 carriers and *1 homozygotes. The mechanism(s) responsible for the differential effect of various CYP2C19 variants on platelet reactivity in clopidogrel-treated patients on a PPI are not yet understood and will require further investigation.
Previous data on the clinical significance of PPI use among clopidogrel-treated patients within CYP2C19 genotype groups are limited and controversial. A French registry analysis of AMI patients did not find any association between PPI use and adverse cardiac events in patients who were carriers or homozygotes for the CYP2C19*2 allele.26 A post-hoc subgroup analysis on the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel (TRITON)-TIMI 38 also did not find any evidence that PPI use was associated with an increase in adverse cardiac events among either *2 carriers or *1 homozygotes.11 Because these studies did not report pharmacodynamic data, the PPI effect on platelet reactivity and cardiovascular outcomes within CYP2C19 genotype groups could not be fully assessed. The Do Platelet Function Assays Predict Clinical Outcomes in Clopidogrel-Pretreated Patients Undergoing Elective PCI (POPULAR) study is the only prior study to date to analyze the PPI interaction with CYP2C19 variants on platelet reactivity and clinical outcomes.27 In this study that assessed patients after elective PCI, both *2 carriers and non-carriers had significantly higher rates of high on-treatment platelet reactivity with concurrent PPI use compared with no PPI use.27 However, PPI use was not associated with an increase in 12-month adverse cardiac events in either *2 carriers or non-carriers.27 An important limitation of this study, and all of the aforementioned studies, were that *17 carriers were not assessed.
Our study is the first analysis to assess clinical outcomes of PPI use in clopidogrel-treated patients within the CYP2C19*17 genotype group. Previously, no association was observed between PPI use and adverse cardiac events among carriers of the CYP2C19*2 allele.11, 26, 27 Our data supports these findings. The mechanism of this may, in part, be due to the low baseline activity of CYP2C19 in *2 carriers, where it is unlikely that a PPI would confer a further decrease in CYP2C19 activity to result in a clinically meaningful difference.6, 12 Given the increased CYP2C19 activity observed in CYP2C19*17 carriers, use of CYP2C19 inhibitors such as PPIs among CYP2C19*17 carriers may have greater potential to significantly lower enzymatic activity, resulting in reduced clopidogrel-related platelet inhibition and worse clinical outcomes. Although our data are biologically plausible, further studies are needed to confirm the mechanisms proposed by ourselves and others.
Several important limitations should be considered when interpreting our study. First, our study did not assess platelet function, thus we are not able to correlate the clinical findings of our study with measures of platelet reactivity. However, multiple studies have confirmed increased on-treatment platelet reactivity in CYP2C19*2 allele carriers28-30 and decreased on-treatment platelet reactivity in CYP2C19*17 allele carriers.30, 31 Second, compliance with clopidogrel and PPI was not assessed in our study, which could influence the clinical outcomes. However, classifying patients by discharge medication status is a well-recognized and often used approach, since most patients remain on their discharge regimen after hospitalization.32 Third, our findings regarding the CYP2C19*17 genotype group have not been independently replicated in a separate cohort, since our study is, to our knowledge, the first study to analyze the clinical outcomes with PPI use in the CYP2C19*17 genotype group. All of our results, however, are biologically plausible and supported by in vitro platelet reactivity studies; the fact that our findings in the CYP2C19*2 and *1/*1 genotype groups are consistent with recent clinical studies and trials11, 26, 27 gives further credibility to our observations in the CYP2C19*17 genotype group. Finally, despite using adjusted analyses to account for differences between the PPI and no PPI use groups in the overall population and within each CYP2C19 genotype group, it is still possible that unknown confounders may have contributed to the observed differences in clinical outcomes.
Conclusions
Among clopidogrel-treated Caucasians following AMI in the TRIUMPH registry, PPI use was associated with significantly increased cardiac rehospitalization. The increase in risk of rehospitalization among PPI users varied by CYP2C19 genotype, with the greatest increase in risk observed among carriers of the gain of function CYP2C19*17 allele. PPI use was not associated with any significant differences in all-cause mortality for clopidogrel-treated patients in either race. These results extend prior studies of the effect of PPI use on platelet function, including recent clinical trial data, and suggest the risks associated with PPI use in clopidogrel-treated Caucasian post-MI patients may be impacted by CYP2C19 genotype. If validated, personalized treatment with PPIs based on CYP2C19 genotype status should be considered as this may, ultimately, lead to improved post-MI outcomes.
Supplementary Material
Acknowledgments
Funding Support: This work and S. Cresci's effort are supported in part by the National Institutes of Health (Cresci R01 NR013396). TRIUMPH was sponsored by the National Institutes of Health: Washington University School of Medicine SCCOR Grant P50 HL077113.
Footnotes
Conflicts of Interest: Jeremiah P. Depta: None
Petra A. Lenzini: None
David E. Lanfear: None
Tracy Y. Wang: Research Grants: Lilly USA (Significant), Daiichi Sankyo (Significant), and Gilead Science (Significant); Consultant: Astra Zeneca (Modest), American College of Cardiology Foundation (Significant)
John A. Spertus: Research Grants: Eli Lilly, EveHeart, Genentech, Gilead; Consultant: St. Jude Medical (modest), United Healthcare (modest), Amgen (modest), Gilead (modest), Genentech (modest), Janssen (modest), Novartis (modest); Copyrights/Patents: Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, Peripheral Artery Questionnaire, US Patents: 7,643,969; 7,853,456; 12/965,656; 13/615,401
Richard G. Bach: Research Grants: AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Merck/Schering-Plough; Consultant (CEC Activity only): Roche (Significant), Pfizer (Modest)
Sharon Cresci: None
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt DL. Role of antiplatelet therapy across the spectrum of patients with coronary artery disease. Am J Cardiol. 2009;103:11A–19A. doi: 10.1016/j.amjcard.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Depta JP, Bhatt DL. Aspirin and platelet adenosine diphosphate receptor antagonists in acute coronary syndromes and percutaneous coronary intervention: role in therapy and strategies to overcome resistance. Am J Cardiovasc Drugs. 2008;8:91–112. doi: 10.1007/BF03256587. [DOI] [PubMed] [Google Scholar]
- 4.Somma KA, Bhatt DL, Fonarow GC, Cannon CP, Cox M, Laskey W, et al. Guideline adherence after ST-segment elevation versus non-ST segment elevation myocardial infarction. Am J Cardiovasc Drugs. 2012;5:654–661. doi: 10.1161/CIRCOUTCOMES.111.963959. [DOI] [PubMed] [Google Scholar]
- 5.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Depta JP, Bhatt DL. Antiplatelet therapy and proton pump inhibition: cause for concern? Curr Opin Cardiol. 2012;27:642–650. doi: 10.1097/HCO.0b013e32835830b6. [DOI] [PubMed] [Google Scholar]
- 7.Depta JP, Bhatt DL. Omeprazole and clopidogrel: Should clinicians be worried? Cleve Clin J Med. 2010;77:113–116. doi: 10.3949/ccjm.77a.09173. [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 9.Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64:935–951. doi: 10.1007/s00228-008-0538-y. [DOI] [PubMed] [Google Scholar]
- 10.Frelinger AL, 3rd, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59:1304–1311. doi: 10.1016/j.jacc.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 11.O'Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989–997. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 12.O'Donoghue ML. CYP2C19 genotype and proton pump inhibitors in clopidogrel-treated patients: does it take two to tango? Circulation. 2011;123:468–470. doi: 10.1161/CIRCULATIONAHA.110.006866. [DOI] [PubMed] [Google Scholar]
- 13.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cresci S, Wu J, Province MA, Spertus JA, Steffes M, McGill JB, et al. Peroxisome proliferator-activated receptor pathway gene polymorphism associated with extent of coronary artery disease in patients with type 2 diabetes in the bypass angioplasty revascularization investigation 2 diabetes trial. Circulation. 2011;124:1426–1434. doi: 10.1161/CIRCULATIONAHA.111.029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanfear DE, Jones PG, Cresci S, Tang F, Rathore SS, Spertus JA. Factors influencing patient willingness to participate in genetic research after a myocardial infarction. Genome medicine. 2011;3:39. doi: 10.1186/gm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, An N, Wang H, Gao Y, Liu D, Bian T, et al. Evaluation of the effects of 20 nonsynonymous single nucleotide polymorphisms of CYP2C19 on S-mephenytoin 4′-hydroxylation and omeprazole 5′-hydroxylation. Drug Metab Dispos. 2011;39:830–837. doi: 10.1124/dmd.110.037549. [DOI] [PubMed] [Google Scholar]
- 18.Yan AT, Yan RT, Tan M, Eagle KA, Granger CB, Dabbous OH, et al. In-hospital revascularization and one-year outcome of acute coronary syndrome patients stratified by the GRACE risk score. Am J Cardiol. 2005;96:913–916. doi: 10.1016/j.amjcard.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 19.Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153:29–35. doi: 10.1016/j.ahj.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Ohbuchi M, Noguchi K, Kawamura A, Usui T. Different effects of proton pump inhibitors and famotidine on the clopidogrel metabolic activation by recombinant CYP2B6, CYP2C19 and CYP3A4. Xenobiotica. 2012;42:633–640. doi: 10.3109/00498254.2011.653655. [DOI] [PubMed] [Google Scholar]
- 21.Zvyaga T, Chang SY, Chen C, Yang Z, Vuppugalla R, Hurley J, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos. 2012;40:1698–1711. doi: 10.1124/dmd.112.045575. [DOI] [PubMed] [Google Scholar]
- 22.Fernando H, Bassler N, Habersberger J, Sheffield LJ, Sharma R, Dart AM, et al. Randomized double-blind placebo-controlled crossover study to determine the effects of esomeprazole on inhibition of platelet function by clopidogrel. J Thromb Haemost. 2011;9:1582–1589. doi: 10.1111/j.1538-7836.2011.04414.x. [DOI] [PubMed] [Google Scholar]
- 23.Furuta T, Iwaki T, Umemura K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol. 2010;70:383–392. doi: 10.1111/j.1365-2125.2010.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulot JS, Wuerzner G, Bachelot-Loza C, Azizi M, Blanchard A, Peyrard S, et al. Effect of an increased clopidogrel maintenance dose or lansoprazole co-administration on the antiplatelet response to clopidogrel in CYP2C19-genotyped healthy subjects. J Thromb Haemost. 2010;8:610–613. doi: 10.1111/j.1538-7836.2009.03729.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Dang DS, Chen YF, Yan M, Shi GB, Zhao QC. The influence of omeprazole on platelet inhibition of clopidogrel in various CYP2C19 mutant alleles. Genet Test Mol Biomarkers. 2012;16:1293–1297. doi: 10.1089/gtmb.2012.0119. [DOI] [PubMed] [Google Scholar]
- 26.Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011;123:474–482. doi: 10.1161/CIRCULATIONAHA.110.965640. [DOI] [PubMed] [Google Scholar]
- 27.Harmsze AM, van Werkum JW, Souverein PC, Breet NJ, Bouman HJ, Hackeng CM, et al. Combined influence of proton-pump inhibitors, calcium-channel blockers and CYP2C19*2 on on-treatment platelet reactivity and on the occurrence of atherothrombotic events after percutaneous coronary intervention. J Thromb Haemost. 2011;9:1892–1901. doi: 10.1111/j.1538-7836.2011.04483.x. [DOI] [PubMed] [Google Scholar]
- 28.Gladding P, Webster M, Zeng I, Farrell H, Stewart J, Ruygrok P, et al. The pharmacogenetics and pharmacodynamics of clopidogrel response: an analysis from the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc Interv. 2008;1:620–627. doi: 10.1016/j.jcin.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Frere C, Cuisset T, Morange PE, Quilici J, Camoin-Jau L, Saut N, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol. 2008;101:1088–1093. doi: 10.1016/j.amjcard.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 30.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 31.Frere C, Cuisset T, Gaborit B, Alessi MC, Hulot JS. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost. 2009;7:1409–1411. doi: 10.1111/j.1538-7836.2009.03500.x. [DOI] [PubMed] [Google Scholar]
- 32.Muhlestein JB, Horne BD, Bair TL, Li Q, Madsen TE, Pearson RR, et al. Usefulness of in-hospital prescription of statin agents after angiographic diagnosis of coronary artery disease in improving continued compliance and reduced mortality. Am J Cardiol. 2001;87:257–261. doi: 10.1016/s0002-9149(00)01354-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.