Abstract
We examined the relationships of ante-mortem vascular risk factors to post-mortem cerebrovascular and AD pathologies. Eighty-four AD patients underwent assessment of vascular risk (blood pressure, cholesterol, smoking, cardiovascular disease, diabetes, atrial fibrillation, transient ischemic attack [TIA] or stroke) and later underwent brain autopsy. Given our aim to examine mild cerebrovascular changes (CVC), individuals were excluded if autopsy revealed large stroke. The most common forms of CVC were circle of Willis atherosclerosis followed by arteriosclerosis, lacunes, and microinfarcts. Excluding history of TIA/clinical stroke, individual vascular risk factors were not associated with CVC. However, the presence of multiple vascular risk factors was associated with CVC. Further, presence of CVC was associated with lower Braak and Braak stage. Findings highlight the importance of aggregate risk in the vascular contribution to dementia. Interventions designed to maintain cerebrovascular health may represent important opportunities for preventing or delaying dementia, even when AD is the dominant pathology.
Keywords: Alzheimer’s disease, vascular risk, cerebrovascular disease
1. INTRODUCTION
Vascular risk factors are common and increase risk for Alzheimer’s disease (AD) (1, 2). Most studies have focused on individual vascular risk factors, although multiple vascular risk factors often co-exist (3) and have been shown to incrementally increase risk for AD (1, 2). Studies commonly examine individual risk factors while adjusting for additional risk factors, but this approach may result in over-adjustment and underestimation of effects (1, 4).
Accumulating evidence suggests that vascular risk factors increase risk for AD via cerebrovascular disease (CVD) (5). Although the majority of evidence suggests that vascular risk factors do not increase plaques and tangles per se (5), some studies demonstrate positive correlations between CVD and AD pathology (6–8). Notably, most studies examining the association between vascular risk and AD have characterized participants as AD based on clinical data alone, without autopsy-based data to confirm clinical diagnosis and allow for assessment of multiple forms of neuropathology (e.g., neurofibrillary tangles, CVD, cerebral amyloid angiopathy [CAA]). Given that individuals with clinically diagnosed ‘probable’ AD commonly exhibit mixed pathologies (9, 10), previous findings may be explained in part by misclassification of participants with mixed or vascular pathologies as pure AD (1).
To improve our understanding of mechanisms linking vascular risk burden and AD, we assessed whether ante-mortem assessment of aggregate vascular risk factors is related to cerebrovascular changes (CVC), CAA, and AD pathology in individuals with autopsy-confirmed AD. We were particularly interested in whether vascular risk factors were associated with occult CVC (cerebral arteriosclerosis, circle of Willis atherosclerosis, lacunes, microinfarcts) at autopsy in patients with ante-mortem clinical diagnoses of AD. We hypothesized that greater vascular risk burden would be associated with the presence of CVC and that individual vascular risk factors would show attenuated associations with neuropathology. A secondary aim was to examine the association between CVC and AD pathology in autopsy-confirmed AD. Given evidence that CVC and AD pathology have additive effects on risk for AD, we expected that CVC would be associated with less severe AD pathology at a given level of dementia severity. The current study adds to the existing literature by including neuropathologic confirmation of AD diagnosis, examining both AD and CVC as underlying neuropathological substrates, focusing on AD patients with subclinical or mild CVC, and including a comprehensive vascular risk assessment and scoring system.
2. METHODS
2.1 Participants and clinical evaluation
Autopsy-based neuropathological data from 602 participants of various ages at autopsy (range=36–104 years) and with various neuropathological diagnoses (e.g., normal, Alzheimer’s disease, Pick’s disease) recruited through the University of California San Diego (UCSD) Alzheimer’s Disease Research Center (ADRC) were initially reviewed. From the subset of individuals meeting criteria for probable or definite AD at autopsy based on semi-quantitative estimates of neuritic plaque density as recommended by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (11) and Braak score as recommended by the National Institute on Aging-Reagan criteria (12) (n=277), we selected all individuals with “pure” AD on neuropathology (i.e., individuals who did not show evidence of CVC, medial temporal lobe sclerosis, Lewy body pathology, or Pick’s disease at autopsy; n=34). We included all 34 individuals in this group given its relatively low prevalence (9). Participants with both AD and CVC (i.e., individuals who showed lacunes, cortical microinfarcts, cerebral arteriosclerosis, and/or circle of Willis atherosclerosis) were then randomly sampled (n=124). Individuals were excluded if autopsy revealed any significant pathological process other than AD or CVC. Also, given our aim to examine subclinical or mild CVC, individuals were excluded if autopsy revealed large (macroscopic) stroke. Twenty-seven individuals were excluded for significant Lewy body pathology, 8 for large infarcts (>10 mm in diameter), 7 for medial temporal lobe sclerosis, 6 for macroscopic cerebral hemorrhage, and none for Pick’s disease. Clinical data were reviewed and those who underwent vascular risk assessment and met National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association criteria (13) for probable or possible AD at the time of the vascular risk assessment were included. Sixteen individuals were excluded for missing vascular risk data. Groups of AD patients with and without CVC (AD+CVC and AD-CVC, respectively) were matched on mean age. These inclusion and exclusion criteria resulted in a final sample of 84 participants (AD-CVC: n=34; AD+CVC: n=50).
On the day of vascular risk assessment, demographic and clinical data were recorded and the Mattis Dementia Rating Scale (DRS) was administered to estimate dementia severity. Apolipoprotein E (APOE) genotyping was performed. Data were collected in accordance with UCSD institutional review board-approved procedures.
2.2 Vascular risk assessment
Participants underwent clinical interview and physical examination to determine the presence or absence of vascular risk factors. Brachial artery blood pressure measures were obtained while the participant was seated. A blood draw was performed and plasma glucose and serum cholesterol levels were obtained.
The presence or absence of the following risk factors was determined from clinical interview, physical exam, and laboratory studies: cardiovascular disease (coronary artery disease, cardiac failure, or intermittent claudication); diabetes (self-reported diabetes, use of anti-diabetic therapy, or casual blood glucose ≥200 mg/dL); high total cholesterol (≥240 mg/dL); hypertension (untreated systolic blood pressure ≥140 mm Hg, untreated diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications (14, 15)); atrial fibrillation; and current smoking (16).
2.3 Aggregate vascular risk
Aggregate vascular risk was computed using a modified algorithm developed to predict lifetime risk for cardiovascular disease (17, 18). We modified this algorithm to be more relevant to CVC. Specifically, we included history of stroke/TIA, cardiovascular disease, and atrial fibrillation as additional major risk factors given that they have each been shown to increase stroke risk (16). We classified participants into five mutually exclusive categories depending on whether they had (1) no risk factors above threshold levels, (2) ≥1 risk factor at mildly elevated levels, (3) ≥1 moderately-to-severely elevated risk factor(s), (4) 1 major risk factor only, or (5) ≥2 major risk factors. See Table 2 for specific criteria for each of the five aggregate risk categories.
Table 2.
Comparison of Alzheimer’s disease groups with cerebrovascular changes (AD+CVC) and without cerebrovascular changes (AD−CVC) on vascular risk factors and neuropathologic measures
| AD+CVC, n = 50 mean (sd) |
AD−CVC, n = 34 mean (sd) |
t or χ2 value |
p- value |
|||
|---|---|---|---|---|---|---|
| Individual Vascular Risk Factors, N (%) | ||||||
| Cardiovascular Disease | 23 (46.00%) | 9 (26.47%) | 3.27 | .07 | ||
| Diabetes | 7 (14.00%) | 1 (2.94%) | 2.87 | .09 | ||
| Hypertension | 33 (66.00%) | 18 (53.00%) | 1.48 | .23 | ||
| High Total Cholesterol | 19 (38.00%) | 9 (26.47%) | 1.21 | .27 | ||
| Atrial Fibrillation | 6 (12.00%) | 1 (2.94%) | 2.17 | .14 | ||
| Current smoker | 3 (6.00%) | 3 (8.82%) | .24 | .62 | ||
| TIA/Stroke | 7 (14.00%) | 0 (0.0%) | 5.19 | .02 | ||
| Total cholesterol (mg/dL) | 223.08 | (46.86) | 224.15 | (35.33) | .11 | .91 |
| Triglycerides (mg/dL) | 166.23 | (99.94) | 151.32 | (64.45) | .82 | .42 |
| Systolic blood pressure (mm Hg) | 140.21 | (20.19) | 138.18 | (20.50) | .45 | .65 |
| Diastolic blood pressure (mm Hg) | 79.26 | (10.88) | 81.44 | (9.30) | .96 | .34 |
| Taking antihypertensive medication | 17 (34.00%) | 8 (23.53%) | 1.06 | .30 | ||
| Casual glucose (mg/dL) | 98.82 | (34.38) | 94.00 | (19.72) | .74 | .46 |
| BMI | 24.13 | (3.71) | 24.01 | (4.17) | .14 | .89 |
| Aggregate Vascular Risk Profile* | 5.54 | .14 | ||||
| No elevated risk factors | 0 (0%) | 0 (0%) | ||||
| ≥1 Mildly elevated risk factor | 2 (4.00%) | 1 (2.94%) | ||||
| ≥1 Moderately-to-severely elevated risk factor | 9 (18.00%) | 10 (29.41%) | ||||
| 1 Major risk factor | 15 (30.00%) | 15 (44.12%) | ||||
| ≥2 Major risk factors | 24 (48.00%) | 8 (23.53%) | ||||
| Aggregate vascular risk (low/high)† | 26/24 | 26/8 | 5.14 | .02 | ||
| Vascular Risk Factor Sum Score‡ | ||||||
| One or more risk factor(s) | 44 (88.00%) | 26 (76.47%) | 1.94 | .16 | ||
| Two or more risk factors | 30 (60.00%) | 11 (32.35%) | 6.19 | .01 | ||
| Three or more risk factors | 16 (32.00%) | 4 (11.76%) | 4.57 | .03 | ||
| Neuropathology | ||||||
| Braak and Braak stage (low/high) | 20/30 | 5/29 | 6.19 | .01 | ||
| CAA (absent/present) § | 11/39 | 4/29 | 1.31 | .25 | ||
Participants were classified into five mutually exclusive categories depending on whether they had (1) no risk factors above threshold levels (total cholesterol<180 mg/dL; untreated systolic blood pressure<120 mm Hg; untreated diastolic blood pressure<80 mm Hg; no history of stroke/TIA, diabetes, cardiovascular disease, or atrial fibrillation; and nonsmoker), (2) ≥1 risk factor at mildly elevated levels (total cholesterol 180–199 mg/dL; untreated systolic blood pressure 120–139 mm Hg or untreated diastolic blood pressure 80–89 mm Hg; no history of stroke/TIA, diabetes, cardiovascular disease, or atrial fibrillation; and nonsmoker), (3) ≥1 moderately-to-severely elevated risk factor(s) (total cholesterol 200–239 mg/dL; untreated systolic blood pressure 140–159 mm Hg or untreated diastolic blood pressure 90–99 mm Hg; no history of stroke/TIA, diabetes, cardiovascular disease, or atrial fibrillation; and nonsmoker), (4) 1 major risk factor only (total cholesterol ≥240 mg/dL; treated hypertension, untreated systolic blood pressure ≥160 mm Hg, or untreated diastolic blood pressure ≥100 mm Hg; history of stroke/TIA, diabetes, cardiovascular disease, or atrial fibrillation; or current smoker), or (5) ≥2 major risk factors.
Aggregate vascular risk burden classified as Low (≥1 mildly elevated risk factor, ≥1 moderately-to-severely elevated risk factor(s), or 1 major risk factor only) or High (≥2 major risk factors)
Vascular risk sum score is based on the presence or absence of the following seven risk factors: high total cholesterol, hypertension, history of TIA/stroke, diabetes, cardiovascular disease, atrial fibrillation, and current smoking
cerebral amyloid angiopathy was not assessed for one participant
Abbreviations: TIA = transient ischemic attack; BMI = body mass index; CAA = cerebral amyloid angiopathy
We calculated a second vascular risk composite score based on the summation of individual vascular risk factors (1). First, each of the following risk factors was assigned a value of 0 if absent and 1 if present based on the criteria described above: cardiovascular disease, diabetes, high total cholesterol, hypertension, current smoking, atrial fibrillation, and history of TIA/stroke. We summed these seven vascular risk factors to create a composite score for each participant. Unlike the aggregate risk score described above, this sum score does not differentially weigh individual risk factors based on severity.
2.4 Brain autopsy procedures
Brain autopsies were performed at UCSD using established ADRC protocols (19) described elsewhere (20). Briefly, brains were staged for degree of neurofibrillary tangle pathology by one neuropathologist (L.A.H.) using a modification (21) of Braak and Braak’s staging scheme (22). Semi-quantitative estimates of neuritic plaque density were calculated using methods recommended by CERAD (11). Brains were assessed for four forms of CVC: 1) lacunes (small [<10 mm in diameter] artery infarcts and/or hemorrhages), 2) cortical microinfarcts, 3) cerebral arteriosclerosis (small parenchymal arteriolar disease), and 4) atherosclerosis in the circle of Willis (20, 23). Severity of CAA was assessed semi-quantitatively on thioflavin-S stained preparations (24). See Appendix (part A) for a more detailed description of the brain autopsy procedures.
For each AD+CVC participant, a semi-quantitative measure of CVC severity was calculated using a modification of a published method (20). Briefly, four forms of CVC (cerebral arteriosclerosis, circle of Willis atherosclerosis, lacunes, and cortical microinfarcts) were each scored 0 if absent or 1 if present. The points were then summed to create a measure of CVC severity ranging from 0 to a maximum of 4 possible points.
2.5 Participant groupings
For analysis of neuropathologic variables, participants were classified in three ways depending on the presence and/or severity of different forms of pathology. First, participants were classified into AD+CVC (n=50) or AD-CVC (n=34) based on the presence or absence of CVC at autopsy. Second, participants were categorized as having CAA (AD+CAA, n=68) or no CAA (AD-CAA, n=15). Third, participants were grouped into less severe AD (Braak scores less than VI, n=25) or more severe AD (Braak score of VI, n=59).
2.6 Statistical analyses
Independent samples t-tests for continuous variables and χ2 tests for categorical variables were performed. All statistical tests were two-tailed with a significance cutoff of p < .05.
3. RESULTS
3.1 Participant groupings
Of the 84 participants, 50 (59.5%) displayed CVC at autopsy whereas 34 (40.5%) did not. See Table 3 for frequencies of the individual forms of CVC. Among those with CVC, the mean semi-quantitative score for CVC severity was 1.34 (standard deviation=.63; range=1–3). Across the entire sample, CAA was present in 81.0% of participant brains. Brains of all participants demonstrated AD tangle pathology at Braak and Braak stage IV or higher (IV: 4.8%; V: 25.0, %; VI: 70.2%). Brains of participants demonstrated neuritic plaque density rated as sparse (3.6%), moderate (16.7%), or frequent (73.8%).
Table 3.
Prevalence of the individual forms of cerebrovascular changes among Alzheimer’s disease patients with cerebrovascular changes
| Lacunes* | 18% |
| Cortical microinfarcts | 8% |
| Cerebral arteriosclerosis † | 18% |
| Atherosclerosis in the circle of Willis | 90% |
Small (<10 mm in diameter) artery infarcts and/or hemorrhages
Small parenchymal arteriolar disease
3.2 Demographic and clinical data
The AD+CVC and AD-CVC groups did not significantly differ in terms of mean age (at vascular risk assessment and DRS administration), mean years of education, sex distribution, APOE genotype, or cognitive functioning (Table 1). Compared to the AD-CVC group, the AD+CVC group was significantly older at estimated age of dementia onset and age at death, however, there were no significant differences between the two groups in terms of duration of dementia or dementia severity (based on DRS) or in clinical diagnosis (probable versus possible AD). Further, there were no significant differences between the AD+CVC and AD-CVC groups on DRS performance when additional analyses were conducted including age as a covariate (See Appendix [part B] for detailed results).
Table 1.
Comparison of Alzheimer’s disease groups with cerebrovascular changes (AD+CVC) and without cerebrovascular changes (AD−CVC) on demographic and clinical characteristics
| AD+CVC, n = 50 mean (sd) |
AD−CVC, n = 34 mean (sd) |
t or χ2 value |
p- value |
|||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age at Vascular Risk Assessment, yrs | 73.18 | (7.97) | 70.38 | (6.33) | 1.71 | .09 |
| Education, yrs | 13.00 | (3.21) | 13.53 | (2.67) | .79 | .43 |
| Women/men | 26/24 | 16/18 | .20 | .66 | ||
| APOE Genotype (ε4+/ε4−) | 34/16 | 22/9 | .08 | .78 | ||
| Diagnosis at Vascular Risk Assessment | 40/10 | 28/6 | .07 | .79 | ||
| (Probable AD/Possible AD) | ||||||
| Age at Dementia Onset | 69.24 | (8.28) | 64.85 | (6.35) | 2.59 | .01 |
| Age at Death | 79.10 | (8.07) | 75.41 | (6.01) | 2.27 | .03 |
| Duration of Dementia | 9.86 | (4.14) | 10.36 | (2.98) | .60 | .55 |
| Time Between Vascular Risk Assessment and Death, yrs | 6.11 | (3.35) | 5.21 | (2.48) | 1.50 | .14 |
| Cognitive Functioning (raw scores) | ||||||
| DRS Total Score | 104.42 | (25.24) | 98.56 | (25.59) | 1.04 | .30 |
| DRS Attention | 32.47 | (5.51) | 30.91 | (7.72) | 1.07 | .29 |
| DRS Initiation/Perseveration | 24.98 | (9.10) | 21.65 | (9.22) | 1.63 | .11 |
| DRS Construction | 4.45 | (1.67) | 4.15 | (1.69) | .81 | .42 |
| DRS Conceptualization | 31.00 | (6.64) | 28.74 | (8.11) | 1.39 | .17 |
| DRS Memory | 12.76 | (5.20) | 11.50 | (5.55) | 1.05 | .30 |
Abbreviations: APOE = apolipoprotein E; DRS = Dementia Rating Scale
3.3 Individual vascular risk factors and CVC
At least one vascular risk factor was present in 70 participants across the entire sample (83.3%; Table 2). Seven of the AD+CVC participants and none of the AD-CVC participants had a self-reported history of possible stroke or TIA (p=.02). Although there were no other significant group differences in terms of frequencies of vascular risk factors (p-values > .05), there were trends toward the AD+CVC group having higher rates of cardiovascular disease and diabetes (p-values of .07 and .09, respectively).
3.4 Aggregate vascular risk and CVC
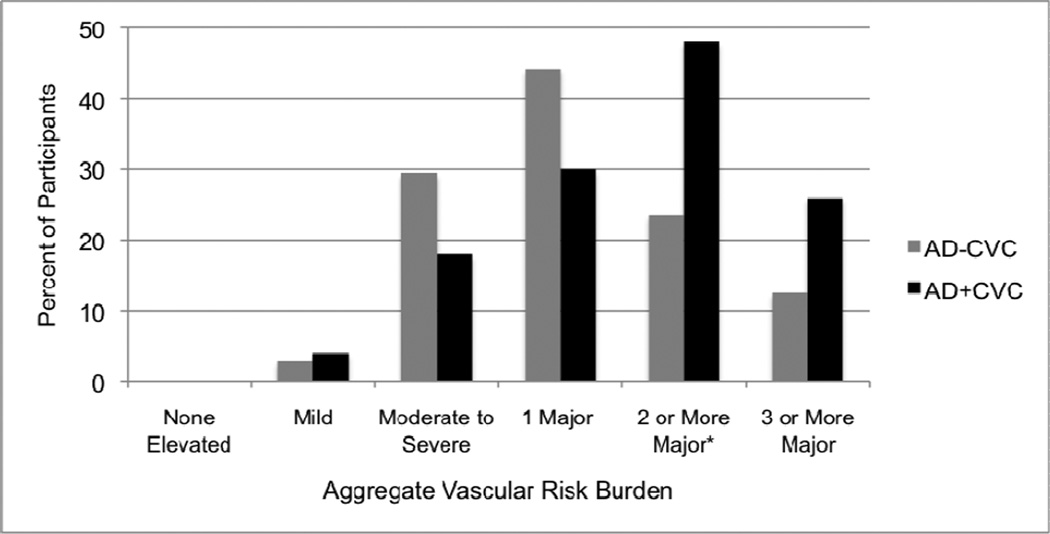
AD patients with the most severe aggregate risk (≥2 major risk factors) were more likely to have CVC compared to those with less severe aggregate risk (χ2=5.14, p=.02, ϕ=.25; Table 2 and Figure 1). Among those participants with the most severe aggregate risk, 16 had 2 major risk factors (11 AD+CVC, 5 AD-CVC), 9 had 3 major risk factors (6 AD+CVC, 3 AD-CVC), 6 had 4 major risk factors (6 AD+CVC, 0 AD-CVC), and 1 had 5 major risk factors (1 AD+CVC, 0 AD-CVC). When different forms of CVC were analyzed separately, AD patients with the most severe aggregate vascular risk were significantly more likely to have arteriosclerosis/circle of Willis atherosclerosis (χ2=3.90, p=.048, ϕ=.22) and nearly significantly more likely to have lacunes and/or infarcts (χ2=3.50, p=.061, ϕ=.20). There were no significant differences between the AD+CAA and AD-CAA groups (χ2=1.09, p=.30, ϕ=.12), between lower and higher Braak stage groups (χ2=2.92, p=.09, ϕ=.19), or between neuritic plaque density groups (χ2=5.24, p=.07, ϕ=.26) in terms of aggregate vascular risk.
Figure 1. Aggregate vascular risk burden for Alzheimer’s disease groups with cerebrovascular changes (AD+CVC) and without cerebrovascular changes (AD−CVC).
Bar graph showing the percent of participants in the AD-CVC and AD+CVC groups classified into each of six categories: (1) no elevated risk factor levels (0% of participants fit this category), (2) ≥1 mildly elevated risk factor, (3) ≥1 moderately-to-severely elevated risk factor(s), (4) 1 major risk factor only, (5) ≥2 major risk factors, and (6) ≥3 major risk factors.
* χ2 = 5.14 (p = .02) for comparison between AD-CVC and AD+CVC
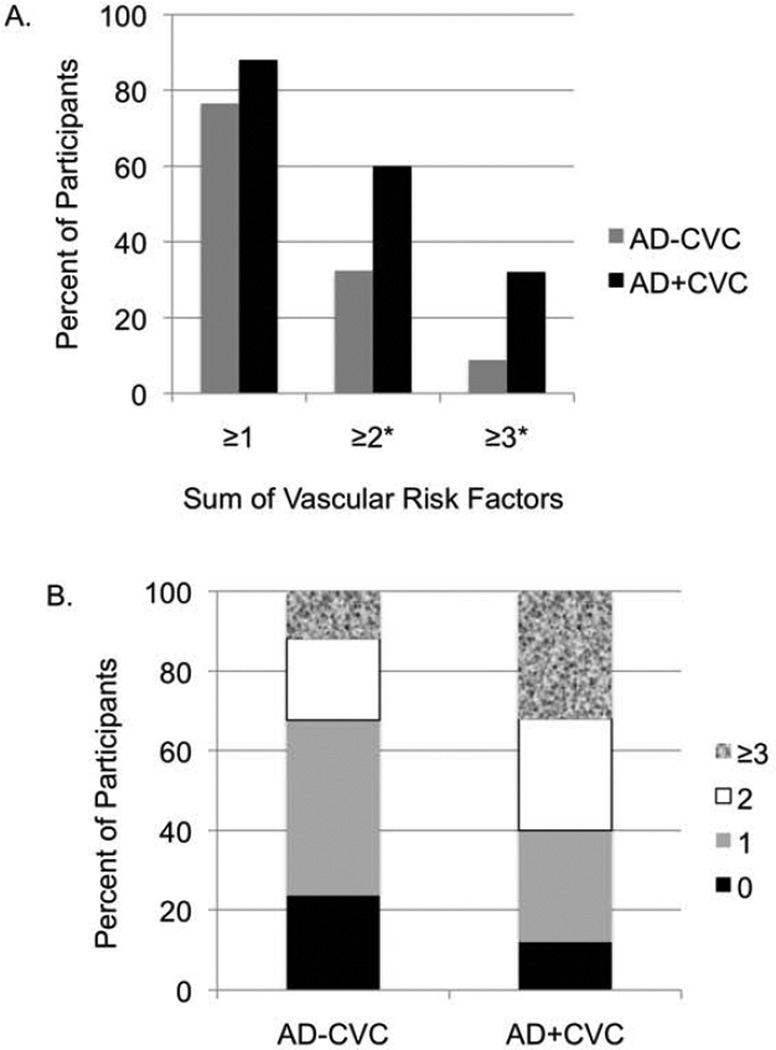
When the sum of vascular risk factors based on the presence or absence of seven risk factors was assessed, the AD+CVC group showed significantly greater vascular risk burden relative to the AD-CVC group (Table 2 and Figure 2). Although having one or more vascular risk factor did not distinguish the AD+CVC and AD-CVC groups (χ2=1.94, p=.16, ϕ=.15), significantly more individuals in the AD+CVC group had two or more vascular risk factors (χ2=6.19, p=.01, ϕ=.27) and three or more vascular risk factors (χ2=4.57, p=.03, ϕ=.23). Given that few individuals had four or more vascular risk factors (n=7), we did not compare groups in terms of aggregate risk beyond three or more risk factors. These analyses did not involve independent groups (e.g., the “two or more risk factors'” category includes participants from the “one or more risk factor(s)” category). There were no significant differences between the AD+CAA and AD-CAA groups, between the lower and higher Braak and Braak stage groups, or between neuritic plaque density groups in terms of the sum of vascular risk factors (p-values >.05).
Figure 2. Sum of vascular risk factors for Alzheimer’s disease groups with cerebrovascular changes (AD+CVC) and without cerebrovascular changes (AD−CVC).
A. Bar graph showing the percent of participants in the AD-CVC and AD+CVC groups classified based on the presence of: (1) one or more vascular risk factors, (2) two or more vascular risk factors, and (3) three or more vascular risk factors. Note that these analyses did not involve independent groups (e.g., the “one or more risk factor(s)” category includes participants from the “two or more risk factors” category.)
*p < .05 for comparison between AD-CVC and AD+CVC
B. Stacked bar chart showing the percent of participants in the AD-CVC and AD+CVC groups with zero, one, two, or three or more vascular risk factors.
Although we excluded potential participants for evidence of large (macroscopic) stroke on autopsy, to ensure that findings were not determined by a self-reported history of TIA or possible stroke, we computed the aggregate vascular risk composite withholding history of TIA/stroke as a risk factor. We conducted further χ2 analyses to determine whether severity of aggregate risk was related to CVC and results remained identical to those described above. In addition, we conducted analyses excluding those participants with self-reported history of possible stroke and results remained qualitatively and statistically very similar.
3.5 Relationships among cerebrovascular pathology, Alzheimer’s disease pathology, and cerebral amyloid angiopathy
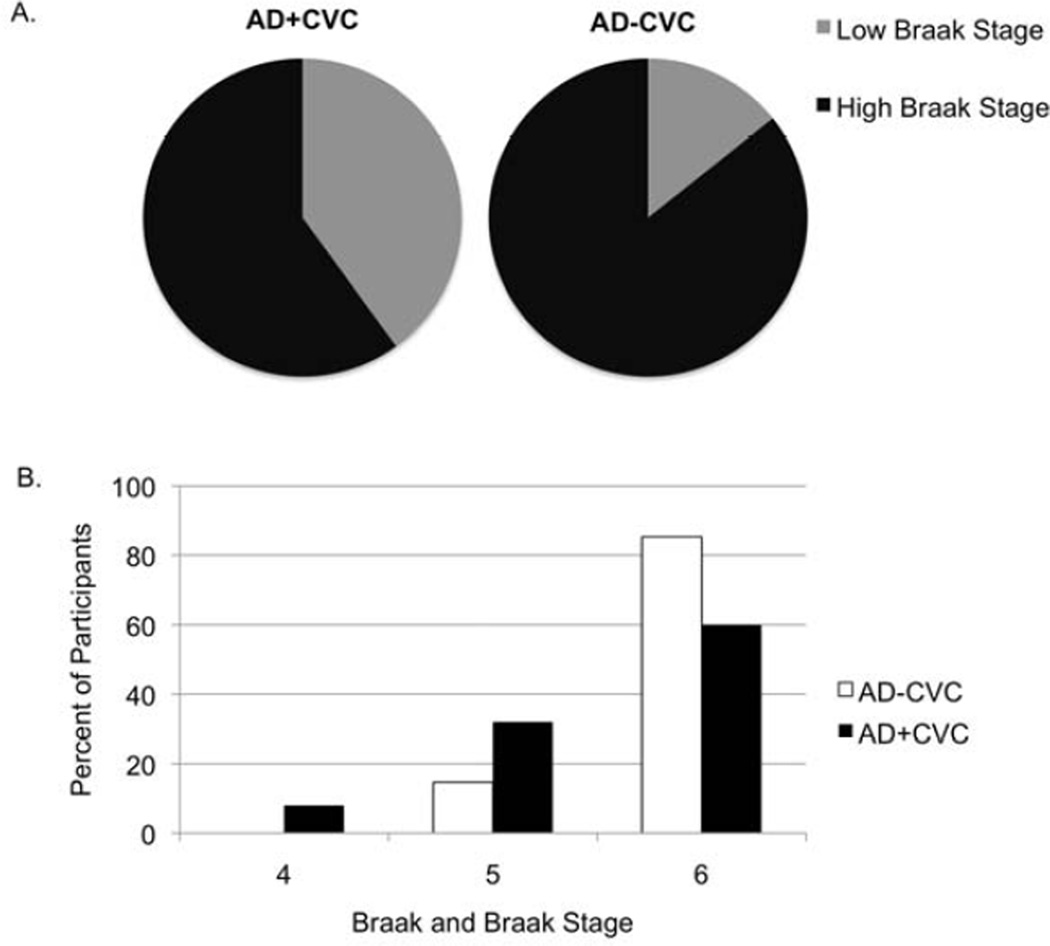
The presence of CVC among AD patients was associated with a significantly lower Braak Stage (χ2=6.19, p=.01; Figure 3). When excluding those with lacunar infarcts or cortical microinfarcts (n = 11), there was a marginally significant relationship between lower Braak stage and the presence of CVC (χ2=3.39, p=.06, ϕ=.22). This suggests that infarcts play an important role in this relationship. However, this could also reflect a power issue given that, after these 11 participants are removed and the sample size of AD+CVC participants is reduced from 50 to 39, the effect size does not substantially differ (ϕ=.27 versus ϕ=.22). There was no relationship between the presence of CVC and CAA (χ2=1.31, p=.25) or neuritic plaque density (χ2=4.05, p=.26).
Figure 3. Braak and Braak stage for Alzheimer’s disease groups with cerebrovascular changes (AD+CVC) and without cerebrovascular changes (AD−CVC).
A. Proportion of participants in the AD+CVC and AD-CVC groups with lower versus higher severity of neurofibrillary tangle pathology as determined by Braak and Braak stage. Braak and Braak stages were divided into Low (stages IV and V) and High (stage VI). χ2 = 6.19 (p = .01) for comparison between AD-CVC and AD+CVC groups on low versus high Braak and Braak stages.
B. Proportion of participants in the AD+CVC and AD-CVC groups as a function of Braak and Braak stage (IV, V, or VI). χ2 = 6.99 (p = .03) for comparison between AD-CVC and AD+CVC groups on Braak and Braak stages (IV, V, or VI).
4. DISCUSSION
Results indicate that AD patients with multiple vascular risk factors are more likely to show CVC at autopsy. These observed relationships are consistent with our previously published report demonstrating that vascular risk factors are associated with CVD in AD patients (20), as well as neuroimaging studies showing that increased vascular risk burden is associated with MRI markers of CVD (e.g., white matter hyperintensities) in mild cognitive impairment (25) and AD (26). Of the 84 participants in the present sample, 50 (59.5%) displayed CVC at autopsy whereas 34 (40.5%) did not. Although this finding demonstrates a high comorbidity of CVC among AD patients in our sample, this ratio of AD+CVC to AD-CVC is not necessarily representative of the true prevalence of CVC given that we deliberately selected all available cases of “pure” AD, which represented a minority of autopsy-confirmed AD cases in the broader sample. Nonetheless, our findings are consistent with a growing body of evidence indicating that CVC is a common co-morbidity and may be integrally involved in the development of AD (5, 9, 10). However, it should be noted that a methodological difference between these previous studies (9, 10) and the current study is that the former restricted their definitions of CVD to macroscopic cerebral infarcts whereas we excluded those with large infarcts and focused on mild forms of CVC (i.e., cerebral arteriosclerosis, circle of Willis atherosclerosis, lacunes, microinfarcts). In the present sample, which involved a relatively limited range of vascular risk burden, traditional vascular risk factors including cardiovascular disease, diabetes, hypertension, high cholesterol, atrial fibrillation, and current smoking were not associated with the presence of CVC when examined individually. In contrast, the presence of multiple vascular risk factors and aggregate vascular burden were associated with CVC. These findings highlight the potential importance of considering aggregate risk when examining the vascular contribution to cognitive impairment.
An area of continuing debate involves whether vascular risk factors directly increase AD pathology or whether the effect is mediated through CVD (5). Regarding the latter possibility, some evidence suggests that vascular dysfunction may reduce clearance of β-amyloid (Aβ) or indirectly increase deposition of Aβ (27). In the present study, aggregate vascular risk factors were associated with CVC but not severity of AD or presence of CAA. This finding is in contrast to some previous autopsy studies from AD/neurodegenerative disease brain banks reporting associations between increased atherosclerosis and greater plaque and tangle densities (8, 28, 29). However, it has been argued that such findings may reflect selection bias given that they are not confirmed when samples are drawn from non-dementia clinic/center findings (5). Further, such findings are not consistent with a growing body of evidence suggesting that vascular risk factors do not increase plaques and tangles per se (5).
We found that the presence of CVC was associated with lower Braak stage, yet there were no differences in severity of cognitive impairment (based on the DRS) between the AD+CVC and AD-CVC groups. The fact that the AD+CVC group showed the same degree of overall cognitive impairment as the AD-CVC group, despite having a significantly lower burden of neurofibrillary tangle pathology, suggests that vascular pathology has an additive effect on cognitive impairment, even in patients with autopsy-confirmed AD and relatively mild CVC. However, given the cross-sectional nature of the neuropathologic exam, causality of this association cannot be inferred. In addition, there was no difference between the groups in terms of neuritic plaque density. Although this lack of association is in line with previous studies demonstrating that CVD is not related to amyloid deposition (30), a recent neuroimaging study showed a relationship between vascular risk and amyloid deposition (31). However, the latter finding was reported in a sample of mainly cognitively normal and mildly impaired older adults and involved in vivo neuroimaging rather than autopsy findings from confirmed AD cases. Further, compared to tau, amyloid has been proposed to be an earlier marker during the course of AD and may be particularly sensitive prior to the onset of cognitive impairment (32). Taken together, the present results corroborate previous findings demonstrating that AD patients with CVD have lower Braak stages than AD patients without CVD (33, 34).
In the present study, the AD+CVC group was significantly older than the AD-CVC group at dementia onset and at death. However, there was no difference between the two groups in terms of duration of dementia or severity of cognitive impairment. We found that the presence of CVC was associated with less AD pathology (i.e., lower Braak stage) among autopsy-confirmed AD patients. It is possible that less AD pathology is required to clinically unmask dementia in AD patients who also have some degree of cognitive impairment related to coexisting CVC (5, 20). Taken together with evidence that the prevalence of CVC increases with advancing age (35, 36), AD patients with a later age at dementia onset may be more likely to have both vascular and AD pathology contributing to their dementia. Future community-based, longitudinal studies may help elucidate potential differences in the course of AD with underlying CVC versus “pure” AD.
The prevalence of vascular risk factors and conditions is rising and, for many individuals, these risk factors are undiagnosed or undertreated (37). In the present study we were particularly interested in whether we could predict occult vascular disease in patients with a primary diagnosis of AD. This approach differs from many previous studies that have included participants with mixed or vascular dementias. We found that aggregate vascular risk was significantly associated with asymptomatic or mild forms of CVC. These findings suggest that aggregate vascular risk burden may be a useful predictor of occult CVC. Taken together with recent research suggesting that vascular pathology but not amyloid pathology is related to poorer cognition among older adults (30), these findings raise the possibility that aggregate vascular risk measures may be useful predictors of cognitive decline.
Although atherosclerosis may result in infarcts, most of the AD+CVC participants in the current sample evidenced atherosclerotic changes in the absence of ischemic brain damage. The mechanisms through which atherosclerotic cerebrovascular pathology may directly lead to cognitive impairment in the absence of infarcts are not well established. Proposed mechanisms include chronic cerebral hypoperfusion; cerebral embolism originating from ruptured or thrombotic plaques; increased parenchymal oxidative stress; or blood pressure dysregulation contributing to reduced integrity of the blood-brain barrier (38). In addition, evidence from a clinical-pathological-imaging study demonstrated significant associations between atherosclerosis/arteriosclerosis and cortical gray matter atrophy in an autopsy sample (39). Such findings suggest that vascular disease leads to cognitive decline not only through its effects on white matter and subcortical structures but also by contributing to cortical atrophy. When both AD and vascular pathologies are present, the two processes may converge to affect cortical structures involved in cognition (39).
The present study is not without limitations. First, data related to the age of onset of vascular risk factors were unavailable. The relationship between some vascular risk factors and dementia may be age-dependent (40) and midlife exposure may be most closely linked to late life cognitive impairment (41). We cannot be sure how findings may have differed if we examined exposure earlier in life or adequacy of treatment of vascular risk factors. Second, as is the case in all studies based on neuropathologic data, we cannot determine the pathology at the time of ante-mortem assessment and examine how the relationship between vascular risk factors and neuropathology may differ over time. Third, the sample was selected for likely neurodegenerative rather than vascular dementia. Findings may have differed in a sample including individuals with more severe CVC. Also, we did not assess white matter integrity, which may have provided additional information regarding ischemic vascular pathology. Finally, as is common in neuropathology studies (9, 20, 42), we assessed only one hemisphere and, therefore, may have underestimated existing pathology.
Despite these limitations, the present study has several strengths. We collected extensive neuropathologic data from a well-characterized sample, and the ADRC has maintained an autopsy rate of 90% among subjects followed clinically. Pathologic diagnoses were made blind to clinical diagnoses. Given autopsy confirmation of diagnoses, we did not misclassify individuals with mixed or vascular dementia as AD and, therefore, are not positively biasing the relationships between the measured vascular risk factors and CVC. Further, unlike most previous studies, which have focused on individual vascular risk factors, we assessed several vascular risk factors, objectively measured multiple risk factors (blood pressure, glucose, cholesterol), and computed an aggregate burden score.
Growing evidence for vascular contributions to AD has potential implications for models of AD neuropathology, diagnostic criteria, and treatment (43). Given that many vascular risk factors are modifiable, together with the current lack of effective therapies to treat neurodegenerative cognitive impairment, interventions designed to target vascular risk factors in order to maintain cerebrovascular health may represent important opportunities for preventing or delaying the onset of AD (38). Future studies assessing ante-mortem vascular risk burden at time intervals from midlife to late life, incorporating ante-mortem MRI measures with neuropathologic data, and including participants with a wide range of vascular risk burden may further elucidate associations between vascular risk and AD and inform approaches for earlier identification and treatment for those at risk for dementia.
Systematic review: We reviewed the literature on the influence of vascular risk factors on cerebrovascular disease (CVD) and Alzheimer’s pathology in patients with Alzheimer’s disease (AD). We included neuropathological and neuroimaging studies.
Interpretation: The present findings highlight the potential relative importance of aggregate vascular risk burden compared to individual risk factors in terms of the vascular contribution to AD. Findings suggest that even relatively low vascular risk burden is associated with cerebrovascular changes (CVC). Taken together, results suggest that aggregate vascular risk measures may be a useful predictor of occult CVC and of AD risk.
Future directions: The role of vascular risk burden as a predictor of AD should be evaluated with longitudinal studies that assess vascular risk from midlife to late life, incorporate ante-mortem MRI measures with neuropathologic data, and include participants with a wide range of vascular risk burden.
ACKNOWLEDGMENTS
The current study was supported by the National Institute of Health (R01 AG012674, K24 AG026431, P50 AG05131, and T32 MH19934-17). The authors thank the staff, volunteers, and participants of the UCSD ADRC for their important contributions.
APPENDIX
A. Description of Brain Autopsy Procedures
Brain autopsies were performed at University of California San Diego using established ADRC protocols (19). The left hemisphere was fixed in formalin for 5–7 days. After fixation, the hemibrain was examined grossly and cut into 1-cm thick coronal slabs. Tissue blocks were taken from the midfrontal cortex, inferior parietal cortex, superior temporal gyrus, inferior temporal gyrus, anterior cingulate gyrus, posterior cingulate gyrus, anterior hippocampus, posterior hippocampus, amygdala, basal ganglia including substantia innominata and adjacent insular cortex, mesencephalon, rostral pons, and cerebellar vermis as well as all gross lesions. Tissue blocks were embedded in paraffin, cut into 10-µm thick sections, and stained with hematoxylin and eosin and thioflavin-S (44). For a subset of cases, selected tissue blocks were also stained with antibodies against ubiquitin or α-synuclein and a phosphorylated form of tau in order to rule out other forms of neurodegeneration. Diffuse plaques, neuritic plaques, and neurofibrillary tangles were counted in midfrontal, rostral superior temporal, inferior parietal, entorhinal, and hippocampal regions. Brains were then staged for the degree of neurofibrillary tangle pathology by one neuropathologist (L.A.H.) using a modification (21) of Braak and Braak’s staging scheme (22). Participant brains were assessed for four forms of CVC: 1) lacunes (small [<10 mm in diameter] artery infarcts and/or hemorrhages), 2) cortical microinfarcts, 3) cerebral arteriosclerosis (small parenchymal arteriolar disease), and 4) atherosclerosis in the circle of Willis. Using a method previously described (24), the severity of CAA was assessed semiquantitatively on thioflavin-S stained preparations of the midfrontal cortex, superior temporal gyrus, inferior parietal cortex, and posterior hippocampus by the study neuropathologist (L.A.H.) using a four-point scale ranging from 0 to 3 (absent, mild, moderate, severe). Criteria for classification of non-AD pathologies used by the National Alzheimer’s Disease Coordinating Center were applied (45–51).
B. Statistical Results from Regression Models Including Age as a Covariate
There were no significant differences between the AD+CVC and AD-CVC groups on DRS performance when analyses were conducted including age as a covariate. Results from the analyses including age as a covariate were as follows. When entered as the only predictor on step 1, age did not account for a significant amount of overall variance in DRS performance (DRS Total Score: F = .05, p = .82, R2 = .001, β = .03; DRS Attention subscale: F = 2.71, p = .10, R2 = .03, β = .18; DRS Initiation/Perseveration subscale: F = .03, p = .86, R2 = <.001, β = .02; DRS Construction subscale: F = 2.37, p = .13, R2 = .03, β = .17; DRS Conceptualization subscale: F = .006, p = .94, R2 = <.01, β = .008; DRS Memory subscale: F = 3.59, p = .06, R2 = .04, β = −.21). When entered on step 2, participant group (AD+CVC versus AD-CVC) did not account for a significant amount of overall variance in DRS performance (DRS Total Score: F = .53, p = .59, R2 = .01; DRS Attention subscale: F = 1.65, p = .20, R2 = .04; DRS Initiation/Perseveration subscale: F = 1.32, p = .27, R2 = .03; DRS Construction subscale: F = 1.31, p = .28, R2 = .03; DRS Conceptualization subscale: F = .98, p = .38, R2 = .02; DRS Memory subscale: F = 2.92, p = .06, R2 = .07). Further, participant group (AD+CVC versus AD-CVC) was not associated with cognitive performance (DRS Total Score: β = .11, p = 32; DRS Attention subscale: β = .09, p = .45; DRS Initiation/Perseveration subscale: β = .18, p = 11; DRS Construction subscale: β = .06, p = .61; DRS Conceptualization subscale: β = .16, p = .17; DRS Memory subscale: β = .16, p = .14.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose related to the manuscript.
REFERENCES
- 1.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 3.Genest J, Jr, Cohn JS. Clustering of cardiovascular risk factors: targeting high-risk individuals. Am J Cardiol. 1995;76(2):8A–20A. doi: 10.1016/s0002-9149(05)80010-4. [DOI] [PubMed] [Google Scholar]
- 4.Szklo M, Nieto F. Epidemiology: beyond the basics. Gaithersburg, Maryland: Aspen Publishers; 2000. [Google Scholar]
- 5.Chui HC, Zheng L, Reed BR, Vinters HV, Mack WJ. Vascular risk factors and Alzheimer's disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimers Res Ther. 2012;4(1):1. doi: 10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113(1):13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 7.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 8.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(Pt 12):3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 11.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 12.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, et al. APOE Genotype Modifies the Relationship between Midlife Vascular Risk Factors and Later Cognitive Decline. J Stroke Cerebrovasc Dis. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Leip EP, Larson MG, D'Agostino RB, Beiser A, Wilson PW, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 19.Hansen LA, Samuel W. Criteria for Alzheimer's disease and the nosology of dementia with Lewy bodies. Neurology. 1997;48(1):126–132. doi: 10.1212/wnl.48.1.126. [DOI] [PubMed] [Google Scholar]
- 20.Nation DA, Delano-Wood L, Bangen KJ, Wierenga CE, Jak AJ, Hansen LA, et al. Antemortem pulse pressure elevation predicts cerebrovascular disease in autopsyconfirmed Alzheimer's disease. J Alzheimers Dis. 2012;30(3):595–603. doi: 10.3233/JAD-2012-111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuel W, Galasko D, Masliah E, Hansen LA. Neocortical lewy body counts correlate with dementia in the Lewy body variant of Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55(1):44–52. doi: 10.1097/00005072-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 23.Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology. 1995;45(3 Pt 1):461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- 24.Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, et al. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer's disease and Lewy body variant. Neurology. 1996;47(1):190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- 25.Lo RY, Jagust WJ. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79(13):1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AY, Jeong SH, Choi BH, Sohn EH, Chui H. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Arch Gerontol Geriatr. 2006;42(2):157–166. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathology. 2005;15(1):78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beeri MS, Rapp M, Silverman JM, Schmeidler J, Grossman HT, Fallon JT, et al. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology. 2006;66(9):1399–1404. doi: 10.1212/01.wnl.0000210447.19748.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology. 2005;64(3):494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 30.Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, et al. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol. 2013;70(4):488–495. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012;33(9):1979–1987. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354(9182):919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 34.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136(Pt 9):2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 37.DeCarli C. Clinically asymptomatic vascular brain injury: a potent cause of cognitive impairment among older individuals. J Alzheimers Dis. 2013;33(Suppl 1):S417–S426. doi: 10.3233/JAD-2012-129004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63(1):72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 41.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer's disease: evidence from epidemiological studies. J Alzheimers Dis. 2012;32(3):531–540. doi: 10.3233/JAD-2012-120802. [DOI] [PubMed] [Google Scholar]
- 42.Au R, Seshadri S, Knox K, Beiser A, Himali JJ, Cabral HJ, et al. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9(6):673–686. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, et al. Regional White Matter Hyperintensity Volume, Not Hippocampal Atrophy, Predicts Incident Alzheimer Disease in the Community. Arch Neurol. 2012:1–7. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58(11):1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 46.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 48.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117(1):15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 50.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 51.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDSAIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]