Abstract
Objectives
There are both direct and indirect evidence suggesting the abnormalities of GSK-3β and β-catenin, two important components of the Wnt signaling pathway in the pathophysiology of bipolar illness and possibly schizophrenia (SZ). In order to further clarify the role of Wnt-signaling pathway in the pathophysiology of bipolar disorder (BP) and SZ, we studied GSK-3β and β-catenin in the postmortem brain of subjects with these disorders.
Methods
We determined the protein expression of GSK-3β, pGSK-3β-ser-9, and β-catenin using the Western blot technique, and mRNA using qPCR method, in the dorsolateral prefrontal cortex (DLPFC), cingulate gyrus (CG), and temporal cortex (TEMP) obtained from 19 subjects with BP, 20 subjects with SZ, and 20 normal control (NC) subjects.
Results
We found that the protein expression of GSK-3β, pGSK-3β-ser-9, and β-catenin was significantly decreased in the DLPFC and TEMP, but not in CG, of subjects with BP compared with NC subjects. The mRNA expression of GSK-3β and β-catenin was significantly decreased in the DLPFC and TEMP, but not in the CG, of subjects with BP compared with NC subjects. There were no significant differences in the protein or mRNA expression of GSK-3β, pGSK-3β-ser-9, or β-catenin between subjects with SZ and NC subjects in any of the brain areas studied.
Conclusions
These studies show region-specific abnormalities of both protein and mRNA expression of GSK-3β and β-catenin in postmortem brain of subjects with BP but not in subjects with SZ. Thus, abnormalities of Wnt signaling pathway may be associated with the pathophysiology of bipolar illness.
Keywords: β-catenin, bipolar illness, cingulate gyrus, GSK-3β, pGSK-3β-ser-9, postmortem brain, prefrontal cortex, schizophrenia, temporal cortex
Several lines of evidence suggest that disturbances in GSK-3β and β-catenin, two important components of the Wnt signaling pathway, may be involved in the pathophysiology of bipolar illness and schizophrenia (SZ). The Wnt signaling pathway is activated by the interaction of a Wnt protein with a frizzled receptor, which leads to the phosphorylation of disheveled (DVL) and the inactivation of GSK-3β by DVL. The inactivation of GSK-3β results in the cytoplasmic accumulation of β-catenin and its subsequent translocation to the nucleus where it binds to TCF/LEF (T-cell factor and Lymphoid Enhancer Factor), thus forming a transcription factor complex that activates the transcription of several genes (1-4).
The main evidence suggesting a disturbance in Wnt signaling in bipolar disorder (BP) is derived from the observation that mood stabilizing drugs, such as lithium, used in the treatment of BP affect the Wnt signaling pathway, presumably by inhibiting GSK-3β activity and thus increasing β-catenin levels. It was first shown by Klein and Menton (5) that lithium causes inhibition of GSK-3β activity. Subsequently, it was shown that lithium inhibits GSK-3 both in intact cells and in the mammalian brain in vivo. Jope and Roh (6) and De Sarno et al. (7) found that chronic in vivo treatment with lithium increased the phosphorylation of Ser-9 in GSK-3β in the brain of mice. It was later shown by other investigators (8) that valproic acid treatment, an anticonvulsant and a mood-stabilizing drug, also directly inhibited GSK-3β activity. However, not all groups agree that valproate is a GSK-3β inhibitor (9). It was shown by Gould et al. (10) that lithium treatment inhibited GSK-3β in brain of rats. The observation that both mood-stabilizing drugs, lithium and valproic acid, cause inhibition of GSK-3β suggests that abnormalities of the Wnt signaling pathway in general, and GSK-3β in particular, may be associated with the pathophysiology of BP.
The involvement of GSK-3β in BP has also been supported by the observation that monozygotic twins discordant for BP have differential expression of genes in Wnt signaling (11). It has been observed that a single nucleotide polymorphism in the GSK-3β promoter gene influences the onset of illness in patients with BP (12).
GSK-3β has been studied in the platelets, lymphocytes, and postmortem brain of subjects with BP and SZ. Pandey et al. (13) found decreased GSK-3β protein expression in platelets of drug-free patients with BP. Nadri et al. (14) found no difference in GSK-3α and GSK-3β mRNA levels, GSK-3β protein levels, or total GSK-3 activity between patients with SZ and normal control (NC) subjects. Some studies used postmortem brain samples from subjects with SZ and BP. Lesort et al. (15) determined Wnt signaling in the postmortem brains of subjects with BP and observed that there are no significant differences in the protein expression levels of GSK-3β or β-catenin in subjects with BP compared with matched NC subjects. Similar results were reported by Kozlvosky et al. (16) who also did not find differences in the levels of GSK-3β between subjects with BP and NC subjects. Since antipsychotics also increase β-catenin levels in the rat brain and inhibit GSK-3β activity (17), GSK-3β and β-catenin have been studied in the postmortem brain of patients with SZ. Cotter et al. (18) and Lesort et al. (15) found decreased β-catenin in the postmortem brains of the patients with SZ. Emamian et al. (19) found decreased GSK-3β phosphorylation (i.e., increased activity) in the brains of subjects with SZ and a non-significant decrease in total GSK-3β. Beasley et al. (20) found significantly decreased GSK-3β protein levels in subjects with SZ. Kozlovsky et al. (16, 21) found lower GSK-3β levels in the frontal cortex of patients with SZ compared with NC subjects. However, they did not find significant differences in GSK-3β between subjects with BP and NC subjects (21). Studies of GSK-3β in the postmortem brain of subjects with SZ by Nadri et al. (22) were inconclusive.
The suggestion that abnormalities of GSK-3β and/or β-catenin may be involved in the pathophysiology of bipolar illness and SZ is, thus, generally indirect, since the studies of GSK-3β in the postmortem brain were unclear. One of the reasons that the studies in the postmortem brain were inconclusive or unclear was because they studied only the protein expression of GSK-3β in the brain samples. However, determination of GSK-3β protein expression does not indicate or does not provide any index of its activity. GSK-3β is a multi-functional serine/threonine kinase, and its activity is regulated negatively by serine 9 (pGSK-3β-ser-9) and positively by the tyrosine 216 (pGSK-3β-tyr-216) phosphorylation. GSK-3β phosphorylation at Ser-9 appears to be an index of GSK-3β activity (23), and it is therefore important to determine both the protein expression of GSK-3β and pGSK-3β-ser-9, as well as its mRNA in order to obtain a comprehensive evaluation of the role of GSK-3β in the postmortem brain of subjects with BP and SZ.
To further clarify the role of the Wnt signaling pathway in general, and GSK-3β and β-catenin in particular, we determined the protein expression of GSK-3β, pGSK-3β-ser-9, and β-catenin (in both cytosol and nuclear fractions) in the dorsolateral prefrontal cortex (DLPFC), cingulate gyrus (CG), and temporal cortex (TEMP) from subjects with BP and SZ and in NC subjects.
Materials and methods
Acquisition of human postmortem brain samples and clinical assessment
The frozen postmortem brain samples from DLPFC [Brodmann area 9 (BA9)], CG (BA24), and TEMP (BA38) were obtained from the Harvard Brain Tissue Resource Center (HBTRC) at McLean Hospital (Belmont, MA, USA) known as the McLean 66 Cohort, and consisting of subjects with BP and SZ and NC subjects. Diagnoses for the subjects in the collection were established by two psychiatrists at the HBTRC via retrospective review of all available medical records and extensive questionnaires about social and medical history completed by family members of the donors. The criteria of Feighner et al. (24) for the diagnosis of SZ and DSM-III-R10 for the diagnosis of schizoaffective disorder and BP were applied. Individuals with a documented history of substance dependence or neurological illnesses were excluded from the collection. The BP and SZ group also included subjects who died by suicide. This study was approved by the Institutional Review Boards of McLean Hospital and the University of Illinois at Chicago (Chicago, IL, USA).
The demographics associated with these subjects are listed in Table 1. The sample included 19 subjects with BP, 20 subjects with SZ, and 20 NC subjects. Mean age, postmortem interval (PMI), and pH of the frozen brain samples did not differ significantly between the three groups: age (years) (NC: 60.35 ± 16.94; BP: 62.68 ± 17.91; SZ: 56.25 ± 17.90); PMI (hours) (NC: 21.19 ± 6.18; BP: 20.64 ± 9.70; SZ: 20.76 ± 5.35); and brain pH (NC: 6.37 ± 0.26; BP: 6.45 ± 0.23; SZ: 6.43 ± 0.25). The subjects with BD and SZ had been exposed to various psychotropic medications (Table 1).
Table 1.
Summary of demographic and clinical data available on the subjects and tissue samples used in the present study
| Group and subject # |
Age (years) |
Race | Gender | PMI (hours) |
Brain pH |
Cause of death | Psychiatric diagnosis |
Medication at the time of death |
|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||
| 1 | 49 | W | M | 24.60 | 6.76 | Myocardial Infarction | NC | |
| 2 | 53 | NA | F | 24.00 | 5.80 | Cancer | NC | |
| 3 | 74 | W | F | 12.50 | 6.33 | NA | NC | |
| 4 | 54 | W | M | 24.20 | 6.53 | Congestive heart failure | NC | |
| 5 | 70 | W | F | 22.50 | 6.26 | Cancer | NC | |
| 6 | 67 | W | M | 22.33 | 6.42 | Heart attack/disease | NC | |
| 7 | 37 | W | M | 18.75 | 6.68 | Accidental | NC | |
| 8 | 73 | NA | M | 20.53 | 6.05 | NA | NC | |
| 9 | 35 | NA | M | 25.67 | 6.33 | NA | NC | |
| 10 | 89 | W | M | 7.42 | 6.39 | Cancer | NC | |
| 11 | 79 | W | M | 20.92 | 6.28 | Cancer | NC | |
| 12 | 78 | W | F | 23.91 | 6.67 | Cancer | NC | |
| 13 | 38 | W | M | 28.83 | 6.53 | Myocardial Infarction | NC | |
| 14 | 65 | W | F | 24.25 | 6.40 | NA | NC | |
| 15 | 66 | W | F | 7.42 | 6.03 | Cancer | NC | |
| 16 | 50 | NA | M | 24.13 | 6.01 | NA | NC | |
| 17 | 84 | W | M | 28.58 | 6.42 | Pneumonia | NC | |
| 18 | 40 | W | M | 16.60 | 6.24 | Myocardial infarction | NC | |
| 19 | 66 | W | M | 18.70 | 6.76 | Heart attack/disease | NC | |
| 20 | 40 | W | M | 28.00 | 6.50 | Ski accident | NC | |
|
| ||||||||
| Mean | 60.35 | 21.19 | 6.37 | |||||
| SD | 16.94 | 6.18 | 0.26 | |||||
| Bipolar disorder | ||||||||
| 1 | 80 | NA | F | 11.60 | 6.38 | NA | BP | NA |
| 2 | 74 | W | M | 7.18 | 6.70 | Pneumonia | BP | Gabapentin, zolpidem, olanzapine, lorazepam |
| 3 | 73 | W | F | 20.83 | 6.30 | Sepsis | BP | Carbamazepine, risperidone, diltiazem |
| 4 | 74 | W | M | 14.25 | 6.27 | Pneumonia | BP | Lithium,olanzapine, divalproex, zolpidem, lorazepam |
| 5 | 73 | W | F | 17.00 | 6.40 | Renal failure | BP | Divalproex, risperidone, sertraline, donepezil |
| 6 | 40 | W | M | 30.75 | 6.03 | Suicide (hanging) | BP | Risperidone, gabapentin, nefazodone, topiramate, ziprasidone |
| 7 | 38 | W | M | 22.00 | 6.24 | Suicide (CO poisoning) | BP | Divalproex, paroxetine, clonazepam, olanzapine, metoclopramide |
| 8 | 83 | NA | M | 17.50 | 6.60 | Cardiopulmonary arrest | BP | Divalproex, paroxetine |
| 9 | 72 | W | M | 27.66 | 6.24 | Sepsis | BP | Lithium, trazadone, |
| 10 | 82 | W | M | 5.02 | 6.37 | Cardiopulmonary arrest | BP | NA |
| 11 | 78 | W | M | 30.20 | 6.30 | Cardio-respiratory arrest | BP | Thorazine, clonazepam, divalproex, lithium, levodopa-carbidopa |
| 12 | 42 | W | F | 15.80 | 6.26 | Medication overdose | BP | Divalproex, lithium, perphenazine, zolpidem |
| 13 | 29 | W | F | 10.70 | 6.70 | Suicide (hanging) | BP | Valproic acid, lithium, clonozepam, phenelzine, olanzapine, propranolol |
| 14 | 64 | W | F | 11.00 | 6.69 | Emphysema | BP | Divalproex, carbamazepine, trifluoperazine, Doxepin, trihexyphenidil, clonazepam |
| 15 | 38 | W | M | 41.50 | 6.52 | Suicide (gunshot wound) | BP | NA (? Not taking meds at time of death) |
| 16 | 51 | W | M | 31.00 | 7.02 | NA | BP | NA |
| 17 | 76 | W | F | 22.80 | 6.60 | Heart attack/disease | BP | Lithium, lorazepam |
| 18 | 50 | W | M | 30.50 | 6.35 | Heart attack/disease | BP | Lithium |
| 19 | 74 | W | M | 24.80 | 6.53 | Pneumonia | BP | Divalproex, quetiapine |
|
| ||||||||
| Mean | 62.68 | 20.64 | 6.45 | |||||
| SD | 17.91 | 9.70 | 0.23 | |||||
| Schizophrenia | ||||||||
| 1 | 55 | NA | F | 18.00 | 6.48 | NA | SA | NA |
| 2 | 66 | W | M | 22.10 | 6.43 | Pneumonia | SZ | Haloperidol |
| 3 | 61 | W | M | 19.90 | 6.68 | Renal failure | SZ | Haloperidol, lorazepm |
| 4 | 73 | W | F | 24.00 | 6.08 | Cancer | SZ | Risperidone,fluoxetine, clorazepate, fentanyl |
| 5 | 63 | W | M | 22.35 | 6.55 | Cardiac arrest | SZ | Clozapine, haloperidol, trazadone, lorazepam |
| 6 | 44 | W | M | 19.00 | 6.05 | Pneumonia | SZ | Clozapine |
| 7 | 35 | W | M | 28.00 | 6.25 | Heart attack/disease | SZ | None |
| 8 | 42 | W | M | 18.10 | 6.26 | Suicide (CO poisoning) | SZ | Trazadone |
| 9 | 78 | W | F | 13.40 | 6.81 | Sick sinus syndrome | SA | NA |
| 10 | 46 | W | M | 18.50 | 6.31 | Cancer (sepsis) | SZ | Olanzapine,divalproex |
| 11 | 26 | W | M | 16.00 | 6.75 | Suicide (hanging) | SZ | Prolixin decanoate |
| 12 | 42 | W | M | 27.10 | 6.64 | Cancer | SZ | None |
| 13 | 47 | W | M | 19.25 | 6.57 | Cancer | SZ | Clonazepam, hydroxyzine |
| 14 | 83 | W | F | 23.25 | 5.91 | GI bleed | SZ | Haloperidol decanoate |
| 15 | 84 | W | F | 25.75 | 6.14 | Congestive heart failure | SA | Risperidone, divalproex, temazepam |
| 16 | 31 | W | M | 15.00 | 6.46 | NA | SZ | Risperidone, olanzapine, bupropion |
| 17 | 72 | W | F | 21.75 | 6.65 | Cancer | SZ | Risperidone, paroxetine, clonidine |
| 18 | 48 | W | F | 33.78 | 6.63 | Heart attack/disease | SA | Risperidone, divalproex |
| 19 | 80 | W | M | 10.97 | 6.44 | Heart attack/disease | SZ | Thioridazine, mirtazapine |
| 20 | 49 | W | M | 19.08 | 6.60 | Suicide (hanging) | SZ | Haloperidol decanoate, lorazepam |
|
| ||||||||
| Mean | 56.25 | 20.76 | 6.43 | |||||
| SD | 17.90 | 5.35 | 0.25 | |||||
PMI = postmortem interval; W = white; NA = not available; M = male; F = female; CO = carbon monoxide; NC = normal control; BP = bipolar disorder; SA = schizoaffective; SZ = schizophrenia; SD = standard deviation.
Determination of mRNA levels
RNA isolation
Total RNA was extracted from 100 mg of tissue using the TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions and treated with DNAse 1 (Invitrogen, USA). The RNA yield was determined by absorbance at 260 nm using NanoDrop®ND-1000 (NanoDrop Technologies, Montchanin, DE, USA). RNA quality was assessed using Agilent Bioanalyzer 2100 (Agilent), and only samples with 28S/18S ratios >1.2 and RIN 7.0 were included.
mRNA determination
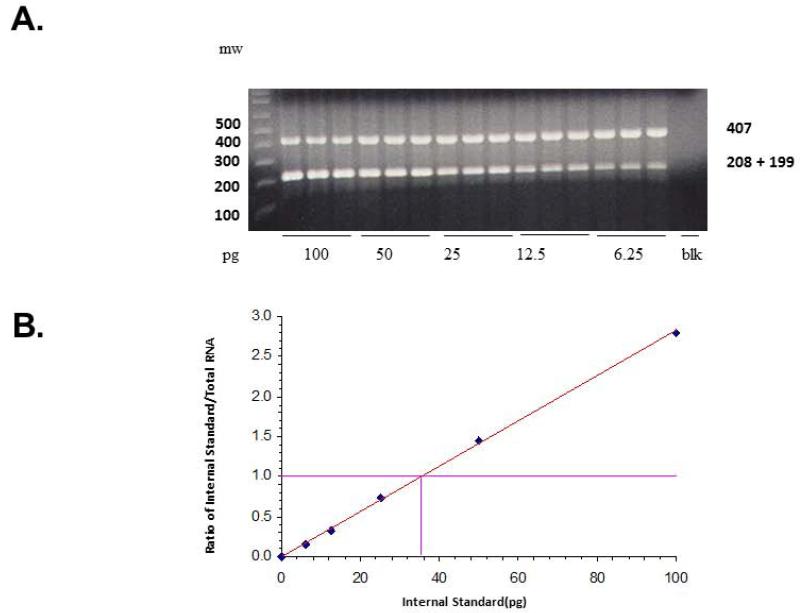
A constant amount (0.5 μg) of total RNA was reverse transcribed using 50 ng random hexamers, 2mM dNTP mix, 10 units ribonuclease inhibitor, and 200 units MMLV-reverse transcriptase enzyme in a final reaction volume of 20 μl. GSK-3β mRNA expression levels were determined using competitive RT-PCR method with internal standard as described earlier (25). Briefly, primer pairs were designed to allow amplification from 235–641 for GSK-3β (GenBank accession no. NM002093). The internal standard has the restriction site XhoI, engineered so that after digest the standard will give two bands of size 199 and 208 bp. cDNA aliquots are amplified using Hot Tub DNA polymerase and trace amounts of 32P-dCTP. For accurate determination of the target concentration PCR products were digested with XhoI and CPM was determined by excising target and internal standard (IS) bands from an ethidium bromide stained gel and running them through a scintilation counter. Standard curve is plotted, using EXCEL, for each sample as the counts incorporated into the IS divided by the counts incorporated into the target mRNA versus the known amount of IS added (Fig. 1) The amount of target molecule was calculated using the formula, y = mx + b, where x represents the concentration of the target when y = 1 (a molar ratio of 1:1). Results are expressed as attomoles target gene/μg of total RNA.
Fig. 1.
(A) Representative agarose gel electrophoresis showing a competitive reverse transcriptase-polymerase chain reaction (RT-PCR) for GSK-3β messenger RNA (mRNA) in dorsolateral prefrontal cortex (DLPFC) of one normal control (NC) subject. Decreasing concentrations of internal standard (IS) for GSK-3β (100–6.25 pg) were added to a constant amount (0.5 μg) of total RNA. The mixtures were reverse transcribed and polymerase chain reaction amplified in the presence of trace amounts of 32P-dCTP (cytidine triphosphate); aliquots were digested by XhoI and electrophoresed on 1.5% agarose gel. The higher molecular size band corresponds to the amplification product arising from the target RNA, whereas the lower band arises from the IS RNA for GSK-3β after it is digested with XhoI. (B) Standard curve is plotted for each sample as the counts incorporated into the IS divided by the counts incorporated into the target mRNA versus the known amount of IS added. The amount of target molecule was calculated using the formula, y = mx + b, where x represents the concentration of the target when y = 1 (a molar ratio of 1:1). Results are expressed as attomoles target gene/μg of total RNA.
β-catenin mRNA was quantified with pre-developed TaqMan gene expression assays (Applied Biosystems) for relative quantitation. qPCR was performed with the MX3005p sequence detection system (Agilent) and TaqMan Universal PCR Master Mix, with UNG (Applied Biosystems) according to the manufacturer’s instructions. The TaqMan assay IDs were as follows: ACTB, Hs99999903; PPIA, Hs99999904_m1; and CTNNB1, Hs00355049_m1. The stability and optimal number of housekeeping genes was determined using geNORM version 3.4 (PrimerDesign Ltd, UK) according to the manufacturer’s instructions (26). This comparison identified β-actin and cyclophilin A (PPIA) as the most stable housekeeping genes. PCR efficiency after 5-log dilution series was 92–105%. Both β-actin and PPIA had similar amplification efficiencies as the target genes and were run in parallel. For each primer/probe set, the PCR reaction was carried out using 10 μl of cDNA diluted 1:10 fold and included a non-RT and no template control. All measurements were performed in triplicates. The amount of target gene mRNA normalized to β-actin and PPIA and relative to the control reference samples is expressed as 2−(ΔΔCt).
Immunolabeling of GSK-3β, pGSK-3β-ser-9, and β-catenin
Preparation of cytosol and nuclear fractions for Western blot determination
The preparation of cytosol and nuclear fraction followed the protocol from Piece Biotechnology Inc. (Rockford, IL, USA). Briefly, tissue was homogenized in ice-cold cytoplasmic extraction regent (CER-I) containing 0.5 mg/ml benzamidine, 2ug/ml aprotinin, 2 μg/ml leupeptin, and 0.75 mM phenylmethysulfonyl fluoride (PMSF). The homogenate was added to cytoplasmic extraction reagent-II (CER-II) and then centrifuged at 16.000 g for 5 min. The supernatant was the cytosol. The resulting pellet was suspended in ice-cold nuclear extraction (NER) containing 0.5 mg/ml benzamide, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 mM PMSF and incubated for 40 min on ice with frequent agitation. The nuclear extracts were separated by centrifugation at 16,000 g for 10 min. The protein content of the nuclear fraction was determined by the method of Lowry et al. (27). The cytosol was used to determine the GSK-3β protein, pGSK-3β-ser-9, and β-catenin. The nuclear fraction was used to determine the protein expression of β-catenin in the DLPFC, CG, and TEMP.
Quantitation of GSK-3β and β-catenin by Western blot
Immunolabeling of individual GSK-3β and pGSK-3β-ser-9 in cytosol fraction, and β-catenin in cytosol, and nuclear fractions was determined by Western blot. Equal volumes of cytosol and nuclear fractions (20 μl containing 20 μg protein) were resolved onto 7.5% (w/v) SDS-polyacrylamide gel and blotted on enhanced chemiluminescence (ECL) membrane (Amersham, Arlington Heights, IL, USA). Membranes were incubated with polyclonal antibodies for GSK-3β, pGSK-3β-ser-9, and β-catenin overnight at 4°C. The dilution for each antibody was as follows: GSK-3β (1:3000); pGSK-3β-ser-9 (1:1000); and β-catenin (1:1000). The three antibodies were purchased from the Cell Signaling Technology, Inc. (Danvers, MA, USA). They were then exposed to ECL autoradiography film. The same membranes were stripped and reprobed with β-actin (Sigma Chemical Co., St. Louis, MO, USA), which was used as a housekeeping protein. The optical densities (OD) of the bands were quantified using the Loats Image Analysis System (Loats Associates, Inc., Westminster, MD, USA), and the OD of each band was corrected by the OD of the corresponding β-actin band.
Statistical analysis and effect of confounding variables
We analyzed the data using SAS 9.2 statistical software package. We used ANCOVA (Proc GLM) to jointly compare NC subjects and subjects with BP and SZ adjusting the effects of age, gender, postmortem interval (PMI), and pH of brain. For comparisons among the subjects with BP and SZ we used t-test with Bonferroni correction to adjust the type I error rates. We also performed a post-hoc t-test for group comparison separately.
Results
Effect of age, gender, and brain pH on protein and gene expression of GSK-3β, pGSK-3β-ser-9, and β-catenin
The protein expression of GSK-3β, pGSK-3β-ser-9, and β-catenin, and gene expression of GSK-3β and β-catenin were determined in the DLPFC, CG, and TEMP obtained from subjects with BP and SZ and NC subjects. There was no significant correlation of either GSK-3β or β-catenin with age. Also, when we compared male and female subjects, we did not find any significant difference in any of the outcome measures in any group. Our results from regression analyses showed that age has a significant effect (in positive direction) on pGSK-3β-ser-9 only in the TEMP. Brain pH has a significant effect (in positive direction) on protein and mRNA expression of β-catenin only in the TEMP.
Immunolabeling of GSK-3β and pGSK-3β-ser-9 in the cytosol fraction of the DLPFC, CG, and TEMP of subjects with BP and SZ and NC subjects
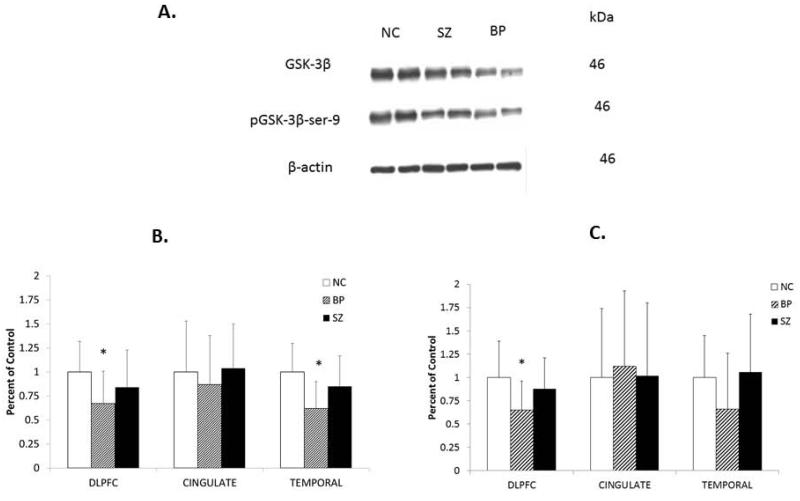
Representative Western blots showing the immunolabeling of GSK-3β and pGSK-3β-ser-9 in the cytosol fractions of the DLPFC obtained from two NC subjects, two subjects with BP, and two subjects with SZ are shown in Figure 2A. GSK-3β and pGSK-3β-ser-9 migrated to 46 kDa. As can be seen, GSK-3β appears to be decreased in the subjects with BP compared with NC subjects, with little or no change in the subjects with SZ. Similar decreases in the protein expression of GSK-3β and pGSK-3β-ser-9 were observed in the TEMP, but not in the CG of subjects with BP (results not shown).
Fig. 2.
Representative Western blots (A) showing the immunolabeling of GSK-3β and pGSK-3β-ser-9 in the cytosol fraction of dorsolateral prefrontal cortex (DLPFC) of two normal control (NC) subjects, two subjects with schizophrenia (SZ), and two subjects with bipolar disorder (BP). kDa indicates kilo Daltons. Mean protein expression levels of GSK-3β (B) and pGSK-3β-ser-9 (C) in the DLPFC cytosol fractions of NC subjects (n = 20), subjects with BP (n = 19), and subjects with SZ (n = 20) in the in the cingulate gyrus (CG) cytosol fractions of NC (n = 12), BP (n = 12), and SZ (n = 12), and in the temporal cortex (TEMP) cytosol fraction of NC (n = 16), BP (n = 16), and SZ (n = 15). The results are expressed as optical density (O.D.). Values are mean ± standard deviation. *p < 0.05.
To examine whether there were any group differences in the GSK-3β or pGSK-3β-ser-9 protein levels between the three groups, we performed ANCOVA, which showed differences between the three groups. We then performed the post-hoc analysis using post-hoc t-test and group t-test with Bonferroni correction for group comparisons. We found that there was a significant decrease in the protein expression of GSK-3β (p = 0.003) and pGSK-3beta-ser-9 (p = 0.003) in the DLPFC of subjects with BP compared with NC subjects without any significant changes in the protein expression of GSK-3β (p = 0.15) or pGSK-3β-ser-9 (p = 0.30) in the DLPFC of subjects with SZ compared with NC subjects, as shown in Figures 2B and 2C.
Since abnormalities of CG have been implicated in bipolar illness and SZ, we then determined the protein expression levels of GSK-3β and pGSK-3β-ser-9 in the CG of these subjects. However, we did not find any significant differences in the expression of either GSK-3β or pGSK-3β-ser-9 in the subjects with BP or SZ compared with NC subjects (Figs. 2B and 2C), suggesting that there were no differences between the groups.
Since we did not observe changes in the CG but observed changes in the DLPFC, we examined if there was a region-specific change. We determined the protein expression of GSK-3β and pGSK-3β-ser-9 in the TEMP of these subjects. ANCOVA analysis showed significant differences between groups (p = 0.0005). When we compared the groups, we found that the mean protein expression of GSK-3β (p = 0.001), but not pGSK-3β-ser-9 (p = 0.087), was significantly decreased in the TEMP of subjects with BP compared with NC subjects. There were no significant differences in the protein expression of either GSK-3β or the pGSK-3β-ser-9 in the TEMP of subjects with SZ compared with NC subjects.
Immunolabeling of β-catenin in the cytosol and nuclear fraction of the DLPFC, CG, and TEMP of subjects with BP and SZ and NC subjects
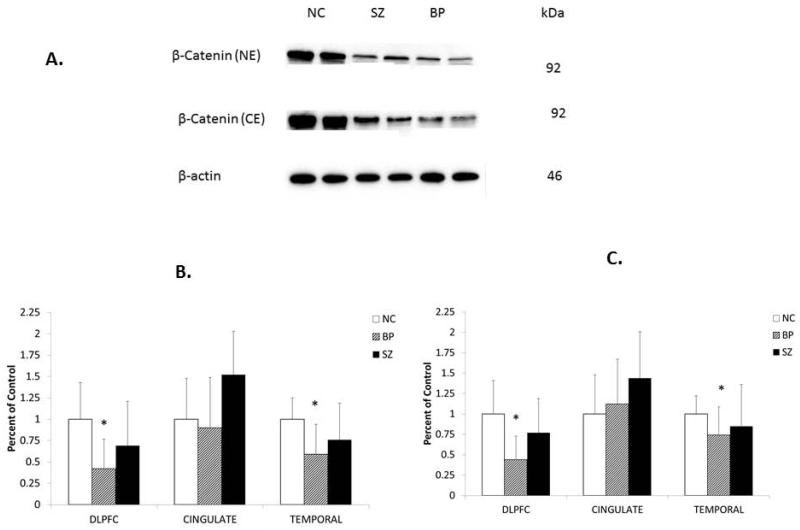
Immunolabeling of β-catenin was determined in the cytosol and nuclear fractions of the DLPFC, CG, and TEMP obtained from subjects with BP and SZ and NC subjects. Representative Western blots of β-catenin immunolabeling (cytosol and nuclear fractions) in the DLPFC obtained from two NC subjects, two subjects with BP, and two subjects with SZ are shown in Figure 3A. β-catenin migrated to 92 kDa. As can be seen, β-catenin in both cytosol and nuclear fractions appears to be decreased in subjects with BP, but not in subjects with SZ, compared with NC subjects.
Fig. 3.
Representative Western blots (A) showing the immunolabeling of β-catenin in the cytosol and nuclear fractions of dorsolateral prefrontal cortex (DLPFC) of two normal control (NC) subjects, two subjects with schizophrenia (SZ), and two subjects with bipolar disorder (BP). kDa indicates kilo Daltons. Mean protein expression levels of β-catenin in the cytosol fraction (B) and nuclear fraction (C) in the DLPFC of NC subjects (n = 20), subjects with BP (n = 19), and subjects with SZ (n = 20), in the in the cingulate gyrus (CG) of NC (n = 15), BP (n = 14), and SZ (n = 13), and in the temporal cortex (TEMP) of NC (n = 16), BP (n = 16), and SZ (n = 15). The results are expressed as optical density (O.D.). Values are mean ± SD. *p < 0.05.
ANCOVA analysis showed significant differences in the protein expression of β-catenin in both cytosol (p = 0.0008) and nuclear (p < 0.0001) fractions in the DLPFC and TEMP, but not in the CG. When we compared the protein expression of β-catenin between the groups, the protein expression of β-catenin was significantly decreased in both the cytosol and nuclear fractions of DLPFC and TEMP, but not in CG of BP with BP compared with NC (Fig. 3B and C). There was no significant difference in the protein expression of β-catenin in either cytosol or nuclear fraction of any of the three brain areas (DLPFC, CG, TEMP) between SZ and NC subjects, as shown in Fig. 3B and C.
mRNA expression levels of GSK-3β and β-catenin in the DLPFC, CG, and TEMP of subjects with BP and SZ and NC subjects
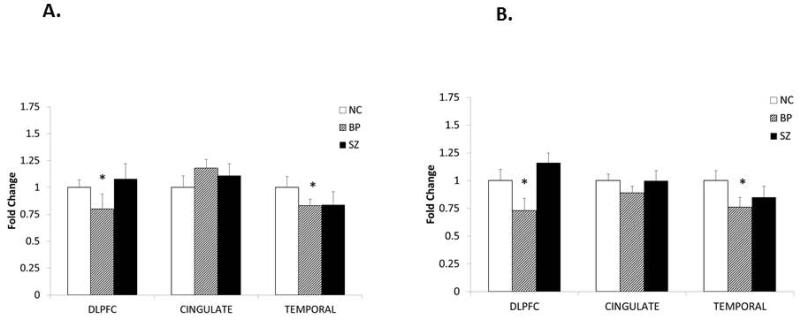
We determined the mRNA expression of GSK-3β in the DLPFC, CG, and TEMP of BP, SZ, and NC subjects to examine if the gene expression of GSK-3β is altered in the subjects with BP or SZ. The results of the mRNA expression of GSK-3β are shown in Figure 4A. When we compared the subjects with BP and SZ with NC subjects, we found that the mRNA expression of GSK-3β was significantly decreased in the DLPFC (p = 0.025) and TEMP (p = 0.042), but not in the CG (p = 0.109), of subjects with BP compared with NC subjects. On the other hand, there were no significant changes in GSK-3β mRNA expression in any of these three areas of subjects with SZ compared with NC subjects. These results suggest that, similar to the protein expression, the mRNA expression of GSK-3β is decreased only in the subjects with BP in the DLPFC and TEMP, but not in the CG.
Fig. 4.
(A) The mean mRNA expression levels of GSK-3β in the dorsolateral prefrontal cortex (DLPFC) of normal control (NC) subjects [1.00 ± standard error (SEM) 0.07, n = 20], subjects with bipolar disorder (BP) (0.80 ± SEM 0.14, n = 19), and subjects with schizophrenia (SZ) (1.08 ± SEM 0.14, n = 19); in the cingulate gyrus (CG) of NC (1.00 ± SEM 0.11, n = 15), BP (1.18 ± SEM 0.08, n = 12), and SZ (1.11 ± SEM 0.11, n = 15); and in the temporal cortex (TEMP) of NC (1.00 ± SEM 0.10, n = 15), BP (0.83 ± SEM 0.06, n = 12), and SZ (0.84 ± SEM 0.12, n = 13). (B) The mean mRNA expression levels of β-catenin in the DLPFC of NC (1.00 ± SEM 0.10, n = 20), BP (0.73 ± SEM 0.11, n = 19), and SZ (1.16 ± SEM 0.09, n = 20); in the CG of NC (1.00 ± SEM 0.06, n = 15), BP (0.89 ± SEM 0.06, n = 12), and SZ (1.00 ± SEM 0.09, n = 13); and in the TEMP of NC (1.00 ± 0.09, n = 15), BP (0.76 ± SEM 0.09, n = 12), and SZ (0.85 ± 0.10, n = 13). The data are shown as fold change in mRNA levels. Values are fold change ± S.E.M. *p < 0.05.
With Bonferroni correction we did not find significant differences in the mRNA expression of GSK-3β between subjects with BP and NC subjects in any of the three brain areas (i.e., DLPFC, CG, and TEMP). The reason may be that the post-hoc t-test without Bonferroni correction was not strong (BP versus NC group in DLPFC, p = 0.025; BP versus NC in TEMP, p = 0.042).
We then compared mRNA expression of β-catenin in the DLPFC, CG, and TEMP of BP, SZ, and NC subjects, as shown in Figure 4B. We found that the mRNA expression of β-catenin was significantly decreased in the DLPFC (p = 0.006) and TEMP (p = 0.003), but not in CG (p = 0.074) of subjects with BP compared with NC subjects. In contrast, the mRNA expression of β-catenin was not significantly different in the subjects with SZ in any of the three brain areas compared with NC subjects.
Effect of suicide on GSK-3β and β-catenin expression
Since both GSK-3β and β-catenin have been shown to be altered in the postmortem brain of suicide victims (28), we examined if GSK-3β and β-catenin changes observed in subjects with BP are related to suicide. There were three subjects who died by suicide in the BP group and four subjects in the SZ group. When we compared the means of suicide subjects and non-suicide subjects in the BP and SZ groups, we did not find any significant differences in the protein or mRNA expression of GSK-3β or β-catenin between those subjects who died by suicide and those subjects who died of natural causes, indicating that there was no significant effect of suicide either on GSK-3β or β-catenin.
Discussion
In this study we examined the role of the Wnt signaling pathway in bipolar illness and SZ by determining the protein and mRNA expression levels of GSK-3β and β-catenin, the major components and regulators of this pathway, in the DLPFC, CG, and TEMP of subjects with BP and SZ and NC subjects. We found that the protein and mRNA expression of GSK-3β was significantly decreased in the DLPFC and TEMP of subjects with BP but not in the CG. In contrast, neither protein nor mRNA expression of GSK-3β were significantly altered in any of these three brain areas obtained from subjects with SZ, suggesting a region-specific GSK-3β decrease in bipolar illness.
As stated earlier, β-catenin levels are regulated by GSK-3β. Increased activity of GSK-3β decreases, and decreased GSK-3β activity increases, β-catenin levels. Our observation of decreased protein and mRNA expression of GSK-3β in subjects with BP would suggest decreased GSK-3β activity leading to increased β-catenin levels in the DLPFC and TEMP of subjects with BP compared with NC subjects. Contrary to this expectation, we found that β-catenin levels are also decreased in the DLPFC and TEMP of subjects with BP compared with NC subjects, suggesting that the GSK-3β activity may, in fact, be increased in subjects with BP compared with NC subjects.
There are several mechanisms that regulate β-catenin levels, one being the GSK-3β activity. Lower GSK-3β levels in subjects with BP do not necessarily indicate low GSK-3β activity. Since there is no direct method of determining GSK-3β activity, some investigators tried to assess it indirectly (23). One approach was to use the GSK-3β phosphorylation at Ser-9 as an index of GSK-3β activity (23). Thus, we also measured pGSK-3β-ser-9 levels in the three brain areas obtained from subjects with BP and SZ and NC subjects. Similar to GSK-3β protein and mRNA expression levels, we observed that pGSK-3β-ser-9 levels were significantly decreased in the DLPFC and TEMP of subjects with BP compared with NC subjects, suggesting increased GSK-3β activity. We observed decreased β-catenin (both protein and mRNA expression) levels in the DLPFC and TEMP of subjects with BP, suggesting that decreased β-catenin in these brain areas may be related, at least in part, to increased GSK-3β activity.
There are several lines of evidence that suggest the involvement of GSK-3β and probably the Wnt pathway in the pathophysiology of BP. The initial evidence was provided by the observation of Klein and Melton (5) and other investigators (5, 10, 29) indicating that lithium causes an inhibition of GSK-3β activity both in vitro and in vivo. Some genetic studies show that polymorphisms of the GSK-3β gene confer vulnerability to mood disorders, as well as BP (12, 30, 31). Animal studies also provide supporting evidence. For example, it was observed that GSK-3β over-expression in transgenic mice results in hyperactivity (32). Valproate and antipsychotic drugs increase GSK-3β phosphorylation at Ser-9, thus providing evidence of direct inhibition of GSK-3β activity by mood stabilizers and antipsychotics (7, 33, 34).
The present study clearly indicates decreased levels of GSK-3β, pGSK-3beta-ser-9, and β-catenin in specific areas (i.e., in the DLPFC and TEMP) of the postmortem brain of patients with BP. However, these changes appear to be specific to subjects with BP, since no changes in GSK-3β or β-catenin were observed in subjects with SZ in any of the brain areas we studied. Another interesting finding of our study is that β-catenin and GSK-3β changes in subjects with BP are specific to certain brain areas, as we did not find any changes in the Wnt signaling in the CG. The significance of this observation is still not clear.
Because of the importance of β-catenin in regulating many neuronal function and because its levels are regulated by GSK-3β, we also studied the protein and mRNA expression of β-catenin in the DLPFC, CG, and TEMP from subjects with BP and SZ. β-catenin is located in the cell membrane and in the cytoplasm. The cytoplasmic fraction is involved in the signaling of Wnt, while the membrane-associated fraction is involved in providing the structural support in cell adhesions. The unphosphorylated β-catenin then translocates into the nucleus where it binds to the transcription factors of TCF/LEF family to promote the transcription of Wnt-related genes. Since we observed decreased protein expression levels of β-catenin in cytosol fraction, we examined whether this decrease is related to changes in its translocation to the nucleus. We therefore determined β-catenin levels in the nuclear fraction and found that it was also significantly decreased in the DLPFC and TEMP of subjects with BP compared with NC subjects, thus suggesting an overall decrease of β-catenin, in both cytosol and nuclear fractions.
Phosphorylation of GSK-3β by the Wnt signaling pathway is one of the mechanisms regulating its activity. However, the phosphorylation of GSK-3β at Ser-9 positions by several other kinases, such as protein kinase A (PKA), Akt, and protein kinase C (PKC), can also regulate GSK-3β activity (35, 36). As stated before, the phosphorylation of GSK-3β at the serine-9 position inhibits GSK-3β activity and causes an increase in β-catenin levels, while phosphorylation at Tyr-216 increases GSK-3β activity and decreases β-catenin levels. This suggests that the phosphorylation of GSK-3β and regulation of β-catenin levels are regulated by several other kinases.
The main limitation of this study is that most of the subjects with BP and SZ were taking psychoactive medication. It is therefore not entirely clear if the changes observed in GSK-3β and β-catenin expression were related to the presence of psychoactive medication. However, both mood stabilizers and antipsychotics have been shown to decrease GSK-3β and β-catenin expression in the rat brain. In this study we observed changes in GSK-3β and β-catenin expression only in the DLPFC and TEMP, but not in the CG, of subjects with BP. If our findings were the result of drug treatments, one would expect them to have similar effects in all three brain areas of subjects with BP. Similarly, one would also expect to see the drug effect in the subjects with SZ, as antipsychotics also produce changes in GSK-3β and β-catenin expression. It is therefore possible, but unlikely, that the region-specific changes in GSK-3β and β-catenin expression in subjects with BP are related to prior exposure to mood stabilizing drugs. Another possibility is that drugs do not necessarily distribute homogeneously among the brain regions and this may be one of the reasons for the absence of any significant effect in in the CG of patients with BP. Nonetheless, the results need to be interpreted with caution.
In summary, our study shows the following: there is a region-specific alteration in GSK-3β and pGSK-3β-ser-9 protein levels in certain brain areas of subjects with BP, and the decrease in GSK-3β is specific to subjects with BP, as it is not observed in the subjects with SZ. In addition, there is a comparable decrease in the protein and mRNA expression levels of β-catenin in the DLPFC and TEMP of subjects with BP, but not in the CG of either subjects with BP or in any of the three brain areas of subjects with SZ. These observations suggest: (i) there is a disturbance in the Wnt signaling pathway in bipolar illness; (ii) GSK-3β and β-catenin abnormalities may be involved in the pathophysiology of bipolar illness; and (iii) that mood stabilizing drugs, such as lithium, may create their beneficial effect by acting on the GSK-3β and β-catenin in the Wnt signaling pathway.
Acknowledgements
This study was supported by a grant RO1 MH077254 to GNP from the National Institute of Mental Health, Rockville, MD, USA. This agency had no role in study design, acquisition and interpretation of data or writing of the report.
We thank Dr. Runa Bhaumik for her help with the statistical analyses of the data, and Ms. Miljana Petkovic for organizing the brain tissue samples and for helping with the preparation and the submission of the manuscript. We thank the Harvard Brain Tissue Resource Center, Boston, MA, USA for providing the postmortem brain samples under PHS grant number R24 MH068855.
Footnotes
Disclosures
The authors of this paper do not have any financial interest or potential conflict of interest related directly or indirectly to this work.
References
- 1.Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329:209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 4.Peifer M. Regulating cell proliferation: as easy as APC. Science. 1996;272:974–975. doi: 10.1126/science.272.5264.974. [DOI] [PubMed] [Google Scholar]
- 5.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryves WJ, Dalton EC, Harwood AJ, Williams RSB. GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid. Bipolar Disord. 2005;7:260–265. doi: 10.1111/j.1399-5618.2005.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- 11.Matigian N, Windus L, Smith H, et al. Expression profiling in monozygotic twins discordant for bipolar disorder reveals dysregulation of the WNT signalling pathway. Mol Psychiatry. 2007;12:815–825. doi: 10.1038/sj.mp.4001998. [DOI] [PubMed] [Google Scholar]
- 12.Benedetti F, Bernasconi A, Lorenzi C, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Pandey GN, Ren X, Rizavi HS, Dwivedi Y. Glycogen synthase kinase-3beta in the platelets of patients with mood disorders: effect of treatment. J Psychiatr Res. 2010;44:143–148. doi: 10.1016/j.jpsychires.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Nadri C, Kozlovsky N, Agam G, Bersudsky Y. GSK-3 parameters in lymphocytes of schizophrenic patients. Psychiatry Res. 2002;112:51–57. doi: 10.1016/s0165-1781(02)00191-9. [DOI] [PubMed] [Google Scholar]
- 15.Lesort M, Greendorfer A, Stockmeier C, Johnson GV, Jope RS. Glycogen synthase kinase-3beta, beta-catenin, and tau in postmortem bipolar brain. J Neural Transm. 1999;106:1217–1222. doi: 10.1007/s007020050235. [DOI] [PubMed] [Google Scholar]
- 16.Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry. 2000;157:831–833. doi: 10.1176/appi.ajp.157.5.831. [DOI] [PubMed] [Google Scholar]
- 17.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Cotter D, Kerwin R, al-Sarraji S, et al. Abnormalities of Wnt signalling in schizophrenia--evidence for neurodevelopmental abnormality. Neuroreport. 1998;9:1379–1383. doi: 10.1097/00001756-199805110-00024. [DOI] [PubMed] [Google Scholar]
- 19.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 20.Beasley C, Cotter D, Khan N, et al. Glycogen synthase kinase-3beta immunoreactivity is reduced in the prefrontal cortex in schizophrenia. Neurosci Lett. 2001;302:117–120. doi: 10.1016/s0304-3940(01)01688-3. [DOI] [PubMed] [Google Scholar]
- 21.Kozlovsky N, Belmaker RH, Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr Res. 2001;52:101–105. doi: 10.1016/s0920-9964(00)00174-2. [DOI] [PubMed] [Google Scholar]
- 22.Nadri C, Dean B, Scarr E, Agam G. GSK-3 parameters in postmortem frontal cortex and hippocampus of schizophrenic patients. Schizophr Res. 2004;71:377–382. doi: 10.1016/j.schres.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Forlenza OV, Torres CA, Talib LL, et al. Increased platelet GSK3B activity in patients with mild cognitive impairment and Alzheimer’s disease. J Psychiatr Res. 2011;45:220–224. doi: 10.1016/j.jpsychires.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr., Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 25.Pandey GN, Dwivedi Y, Rizavi HS, et al. GSK-3beta gene expression in human postmortem brain: regional distribution, effects of age and suicide. Neurochem Res. 2009;34:274–285. doi: 10.1007/s11064-008-9770-1. [DOI] [PubMed] [Google Scholar]
- 26.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Ren X, Rizavi HS, Khan MA, Dwivedi Y, Pandey GN. Altered Wnt signalling in the teenage suicide brain: focus on glycogen synthase kinase-3beta and beta-catenin. Int J Neuropsychopharmacol. 2013;16:945–955. doi: 10.1017/S1461145712001010. [DOI] [PubMed] [Google Scholar]
- 29.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 30.Lachman HM, Pedrosa E, Petruolo OA, et al. Increase in GSK3beta gene copy number variation in bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:259–265. doi: 10.1002/ajmg.b.30498. [DOI] [PubMed] [Google Scholar]
- 31.Zandi PP, Belmonte PL, Willour VL, et al. Association study of Wnt signaling pathway genes in bipolar disorder. Arch Gen Psychiatry. 2008;65:785–793. doi: 10.1001/archpsyc.65.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prickaerts J, Moechars D, Cryns K, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlovsky N, Amar S, Belmaker RH, Agam G. Psychotropic drugs affect Ser9-phosphorylated GSK-3 beta protein levels in rodent frontal cortex. Int J Neuropsychopharmacol. 2006;9:337–342. doi: 10.1017/S1461145705006097. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 35.Bhat RV, Budd SL. GSK3beta signalling: casting a wide net in Alzheimer’s disease. Neurosignals. 2002;11:251–261. doi: 10.1159/000067423. [DOI] [PubMed] [Google Scholar]
- 36.Chen RH, Ding WV, McCormick F. Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem. 2000;275:17894–17899. doi: 10.1074/jbc.M905336199. [DOI] [PubMed] [Google Scholar]