Abstract
It was revealed previously that B10.BR(Ydel) females sired by males with the Y-chromosome long arm deletion differ from genetically identical B10.BR females sired by males with the intact Y chromosome. This is interpreted as a result of different epigenetic information which females of both groups inherit from their fathers. In the following study, we show that cumulus–oocyte complexes ovulated by B10.BR(Ydel) females synthesize increased amounts of progesterone, which is important sperm stimulator. Because their extracellular matrix is excessively firm, the increased progesterone secretion belongs presumably to factors that compensate this feature enabling unchanged fertilization ratios. Described compensatory mechanism can act only on sperm of high quality, presenting proper receptors. Indeed, low proportion of sperm of Ydel males that poorly fertilize B10.BR(Ydel) oocytes demonstrates positive staining of membrane progesterone receptors. This proportion is significantly higher for sperm of control males that fertilize B10.BR(Ydel) and B10.BR oocytes with the same efficiency.
Keywords: cumulus cells, progesterone, spermatozoa, steroidogenesis, Y chromosome
Introduction
In the mouse, the male-specific region of the Y-chromosome long arm (MSYq) is composed of highly repetitive DNA sequences that play a crucial role in sperm development (spermiogenesis). Four multicopy gene families: Ssty1/2, Sly, Asty, and Orly have been identified in this region.1–3 Most information about their function derives from analysis of mutant male mice, which are charged with various deletions in the long arm of the Y chromosome.2 Such analysis enrich the knowledge about contribution of the Y chromosome to the processes associated with male fertility, also in people. Deletions in the long arm of the Y chromosome affect many men with sperm dysfunctions and fertility disorders.4,5
B10.BR and B10.BR-Ydel congenic strains of mice belong to very useful experimental models for studies on MSYq genes. Animals of both strains have the same genotypes, but while B10.BR males have an intact Y chromosome, B10.BR-Ydel males carry the Y chromosome with a large deletion covering approximately two-third of the long arm.6 Despite the loss of the great part of MSYq sequences, B10.BR-Ydel males are fertile, but they produce spermatozoa with definitely lower quality than control B10.BR males.6–11 Additionally, in the offspring of mutant males, a characteristic sex ratio skew in favor of females is observed, which does not occur in the offspring of control males (published data,1,12 breeding records of the authors’ laboratory). Experiments on XYRIIIqdel male mice (with a very similar to B10.BR-Ydel deletion removing 2/3 of the MSYq) proved that this phenomenon is caused not by a reduced frequency of Y-bearing sperm in ejaculate but by their poorer fertilizing ability in comparison with X-bearing counterparts.13 Genetically identical females of the both congenic strains vary in some reproductive features as well,14–16 which can be explained only by transgenerational influence of parental Y chromosomes. Epigenetic modifications of genetic material in X-bearing spermatozoa seem to be the possible mechanism of such influence. We hypothesize that genes of the long arm of the Y chromosome are engaged in the establishment of epigenetic marks in male germ cells, and the partial loss of Yq sequences in mutant males affects the regulation of this complicated process. Altered epigenetic information may be inherited by female progeny modulating some of its qualities.15,16
The involvement of the Y chromosome into sperm epigenetics has been already demonstrated in many studies. The comparison of sex-specific methylation patterns in germ cells of sex-reversed and control mouse embryos revealed a clear methylating activity dependent on the presence of the Y chromosome and autonomous from the effect of gonadal environment. This activity is not associated with Sry expression, because it was observed in cells with the Sry-deleted Y chromosome.17 In spermatids of mice with partial and entire deletions of the male-specific Yq content, increased transcription from both sex chromosomes and various histone code abnormalities were discovered, which imply that MSYq genes are engaged in postmeiotic sex chromosome repression accompanied by chromatin remodeling.18 Construction of transgenic shSLY mice allowed to prove that multicopy Sly (Sycp3-like Y-linked) gene plays the predominant role in these processes,19 although contribution of other genes cannot be excluded. Comet assays, which showed the dramatic increase in DNA damage in sperm of mice with a severe Yq deletion, confirmed the significance of MSYq genes for the correct chromatin remodeling and thus for maintaining DNA integrity during spermiogenesis.20
As mentioned earlier, our previous experiments have revealed some phenotypic differences between female mice sired by B10.BR-Ydel and B10.BR males. We named the first group of females B10.BR(Ydel) to distinguish them from genetically identical females of the control B10.BR strain. Extracellular matrix of cumuli oophori surrounding ovulated oocytes of B10.BR(Ydel) females is more abundant with proteins than matrix of cumuli oophori surrounding oocytes of B10.BR females.16 It is also more resistant to enzymatic treatment as well as to sperm activity in vitro.14,15 These features decrease fertilization efficiency of B10.BR(Ydel) oocytes, but only in the case of mating with B10.BR-Ydel males, producing poor-quality sperm.15 Additionally, females sired by males with the deleted Y chromosome exhibit increased prostaglandin E2-EP2 receptor signaling in their periovulatory processes.16 Apart from many other important functions, this signaling stimulates progesterone synthesis in cumulus granulosa cells.21,22 In the present study, we compared progesterone production in cumulus–oocyte complexes (COCs) ovulated by B10.BR and B10.BR(Ydel) females. We assessed expression levels of Cyp11a1 gene (encoding cholesterol side-chain cleavage cytochrome P450, first enzyme on steroid biosynthetic pathway converting cholesterol to pregnenolone) as well as Hsd3b1 gene (encoding 3β-hydroxysteroid dehydrogenase, enzyme transforming pregnenolone to progesterone). Additionally, we measured messenger RNA (mRNA) level of Cyp19a1 gene encoding P450 aromatase responsible for conversion of testosterone into 17β-estradiol.23,24 This is proved that although COCs synthesize predominantly progesterone after ovulation, small amounts of estradiol are also secreted at that time.25–28 Next, we measured progesterone accumulation in the medium where B10.BR and B10.BR(Ydel) COCs were incubated.
In the female genital tract, progesterone secreted by cumulus cells is sperm chemoattractant.29–31 It also stimulates capacitation, hyperactivated motility, and acrosomal reaction of spermatozoa.32–34 All these effects of progesterone support successful fertilization. Progesterone interacts with specific sperm membrane receptors, activating nongenomic second messenger-mediated pathways.32,33,35 Biochemical signals derived from cumulus cells can be recognized only by competent spermatozoa, presenting proper receptors on their surfaces. For that reason at the end of our work, we assessed availability of progesterone receptors on spermatozoa produced by males with the normal (B10.BR) and with the deleted (B10.BR-Ydel) Y chromosomes.
Materials and Methods
Animals and Hormonal Treatment
Adult, 10- to 12-week-old female and male mice from the 2 congenic B10.BR/SgSn and B10.BR-Ydel strains were used in the experiments. Males from the mutant B10.BR-Ydel strain are characterized by a partial deletion in the long arm of the Y chromosome. Genetically, identical males from the control B10.BR strain have the intact Y chromosome. To avoid genetic divergence between the 2 congenic strains, in each generation B10.BR-Ydel males are backcrossed to B10.BR females. Females sired by males with the normal and with the deleted Y chromosomes have the same genotype. To distinguish these 2 experimental groups of females, we named them as B10.BR and B10.BR(Ydel), respectively.
Mice were maintained under a 12-hour light–dark cycle and fed on a commercial laboratory diet. To stimulate superovulation, female mice were injected intraperitoneally with 7 IU of pregnant mare serum gonadotrophin (Inter Vet, Holland) and 48 hours later with 7 IU of human chorionic gonadotrophin (hCG; Inter Vet). The experiments were performed in accordance with Polish legal requirements, under the license of the First Local Ethics Commission for Animal Experiments at the Jagiellonian University.
RNA Isolation From Cumulus Cells and Real-Time Reverse Transcriptase-Polymerase Chain Reaction
The B10.BR and B10.BR(Ydel) female mice were killed by cervical dislocation, 14 hours after hCG injection. The COCs collected from both oviducts of each female were dispersed with hyaluronidase (Sigma-Aldrich, Germany) dissolved in phosphate-buffered saline (PBS; 50 IU/mL). The oocytes were removed using thin capillaries. The remaining cumulus cells were pelleted by gentle centrifugation (300g, 10 minutes, 20°C) and immediately subjected to RNA isolation with the Nucleo Spin II kit (Macherey-Nagel, Germany). Integrity of the obtained RNA was confirmed by gel electrophoresis. RNA purity and concentration were assessed with Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham). Total RNA of 0.5 µg of each sample was reverse transcribed into complementary DNA (cDNA) using the High-Capacity cDNA Reverse Transcription Kit with random hexamers (Applied Biosystems, Foster City, California).Real-time polymerase chain reactions (PCRs) were run in a final volume of 15 μL on StepOne-Plus Real-Time PCR System (Applied Biosystems) using 96-well optical plates (Applied Biosystems). The reaction mixtures included 2 µL of cDNA sample (diluted 1:2), RNase-free water (Sigma-Aldrich), TaqMan Gene Expression Master Mix (Applied Biosystems), and TaqMan Gene Expression Assay (Applied Biosystems; Cyp11a1: Mm00490735_m1, Hsd3b1: Mm01261921_mH, Cyp19a1: Mm00484049_m1, Actb: Mm00607939_s1). Polymerase chain reactions were incubated at 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. β-Actin (Actb) was used as the endogenous reference gene. All reactions were run in triplicate, and average threshold cycle (Ct) was calculated. For each sample, the Ct value of the endogenous reference gene was subtracted from the Ct values of the target genes to obtain ΔCt values and to normalize the PCRs for the amount of cDNA added to the subsequent reactions. For graphical presentation and statistical analysis, relative mRNA level indexes of Cyp11a1, Hsd3b1, and Cyp19a1 genes were generated with 2−ΔCt formulae.36
Culture of COCs
The B10.BR and B10.BR(Ydel) females were killed at 14 hours post-hCG injection. Cumulus–oocyte complexes isolated from both oviducts of each female were counted and transferred into humidified cell culture dish (Corning Inc, New York) containing 1 mL of Eagle minimum essential medium with Earle salts, l-glutamine, and sodium bicarbonate (Sigma-Aldrich) supplemented with 5% heat-inactivated fetal bovine serum (Sigma-Aldrich).After 4.5 hours incubation (37°C, 5% CO2), culture medium was separated from the COCs by gentle centrifugation (300g, 10 minutes, 20°C) and frozen at −20°C until further investigation. Groups counting less than 20 COCs were excluded from the experiment.
Radioimmunoassay
Steroid concentrations in culture media were estimated by specific radioimmunoassays as previously described.37 Progesterone levels were determined using (1,2,6,7-3H)progesterone (specific activity 96 Ci/mmol; GE Healthcare, Amersham International, United Kingdom) as a tracer and an antibody induced in sheep against 11α-hydroxyprogesterone succinyl–bovine serum albumin (BSA; a gift from Professor B. Cook, University of Glasgow, Scotland). Cross-reactions were 1.8% with pregnenolone, 1.5% with corticosterone, 0.8% with 17α-hydroxyprogesterone, and 0.1% with testosterone. Binding of other steroids was below 0.01%. The lower level of sensitivity of the assays was 20 pg. Coefficients of variation within and between assays were below 5.0% and 9.8%, respectively.
Estradiol concentrations were measured using (2,4,6,7-3H)estradiol (specific activity 88 Ci/mmol; GE Healthcare, Amersham International) as a tracer and rabbit antibody against estradiol-17β-6-carboxymethyloxime–BSA (a gift from Prof R. Rembiesa, Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland). Cross-reactions were 1% with 16-keto-estradiol-17β, 0.8% with estrone, 0.8% with estriol, 0.1% with testosterone, and less than 0.1% with other steroids. The lower level of sensitivity was 5 pg. Coefficients of variation within and between assays were below 4% and 7.5%, respectively. All samples were assayed in duplicate. The results obtained for subsequent incubations were recalculated per single COC.
Detection of Surface Progesterone Receptors in Spermatozoa
Sperm preparation was performed using human tubal fluid (HTF) medium38 containing 101.6 mmol/L NaCl, 4.69 mmol/L KCl, 0.37 mmol/L KH2PO4, 2.04 mmol/L CaCl2, 0.2 mmol/L MgSO4, 25 mmol/L NaHCO3, 2.78 mmol/L glucose, 0.33 mmol/L Na pyruvate, 21.4 mmol/L Na lactate, penicillin 100 U/mL, streptomycin sulfate 50 µg/mL, and phenol red 10 µg/mL (all chemicals obtained from Sigma-Aldrich). Sperm were collected from both cauda epididymides of B10.BR and B10.BR-Ydel males and preincubated for 0.5 hours (37°C, 5% CO2) in 1 mL of HTF medium supplemented with 4 mg of BSA (Sigma-Aldrich; cat. no A-6003).
After centrifugation (300g, 10 minutes, 25°C) and removal of BSA-containing supernatant, the sperm pellet was resuspended in 2 mL of HTF medium and loaded onto a discontinuous gradient of 45% and 90% Percoll (Pharmacia, Sweden) in HTF medium. The gradient was centrifuged (560g, 20 minutes, 25°C), allowing motile sperm to sediment as a pellet at the bottom of conical tube. The top 2 layers (containing debris, nonsperm cells, and immotile sperm) were removed together with Percoll solutions. The bottom motile sperm pellet was washed in HTF (300g, 10 minutes, 25°C) and then capacitated in 1 mL of HTF with BSA (1 hour, 37°C, 5% CO2). After next centrifugation (300g, 10 minutes, 25°C) and supernatant removal, spermatozoa were suspended in PBS without calcium and magnesium (Biomed-Lublin, Poland) at a concentration of 1 × 106 cells/mL.
Progesterone 3-(o-carboxymethyl)oxime–BSA-fluorescein isothiocyanate conjugate (P-BSA-FITC; Sigma-Aldrich) was dissolved in PBS at a concentration of 200 µg/mL and treated with dextran-coated charcoal (Sigma-Aldrich) to remove nonconjugated progesterone.39 Sperm suspension of 25 µL was added to 75 µL of P-BSA-FITC solution, mixed gently, and incubated overnight at 4°C in the dark. As a negative control, another sperm sample was incubated at the same conditions in BSA–FITC solution (Sigma-Aldrich). After incubation, the cells were washed with PBS, centrifuged (300g, 10 minutes, 4°C), and resuspended in PBS. Sperm suspension of 10 µL was smeared onto a glass slide, stained for 5 minutes with 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich; diluted 1:2000 in PBS), mounted with Citifluor AF1 medium (Citifluor, United Kingdom), covered with a coverslip, and sealed with nail polish. The preparations were examined with Nikon Eclipse TS100 microscope (Nikon Instruments Inc, Japan) equipped with epifluorescence attachment. For each preparation, 200 successive spermatozoa were classified as positive or negative for P-BSA-FITC staining.
Statistical Analyses
Statistical analyses of gene expression levels were performed on 2−ΔCt values. The results obtained in percentages were normalized using angular transformation (arcsin). Differences between groups were evaluated with the Student t test (for equal variances). Significance was assumed at P < .05.
Results
Expression of all examined genes: Cyp11a1, Hsd3b1, and Cyp19a1 was detected in cumulus cells surrounding ovulated oocytes of B10.BR and B10.BR(Ydel) females (Figure 1). Cyp11a1 gene is characterized with the highest expression, persisting on the same level in cells of the both groups of females (P = .5). Relatively high expression is also typical for Hsd3b1 gene which is, however, significantly more active in B10.BR(Ydel) cumulus cells in comparison with control B10.BR cumulus cells (P = .03). The level of Cyp19a1 mRNA is very low in postovulatory cumuli oophori and almost 2-fold higher in the cells surrounding oocytes of B10.BR(Ydel) females than in the cells surrounding gametes of B10.BR females (P = .004).
Figure 1.
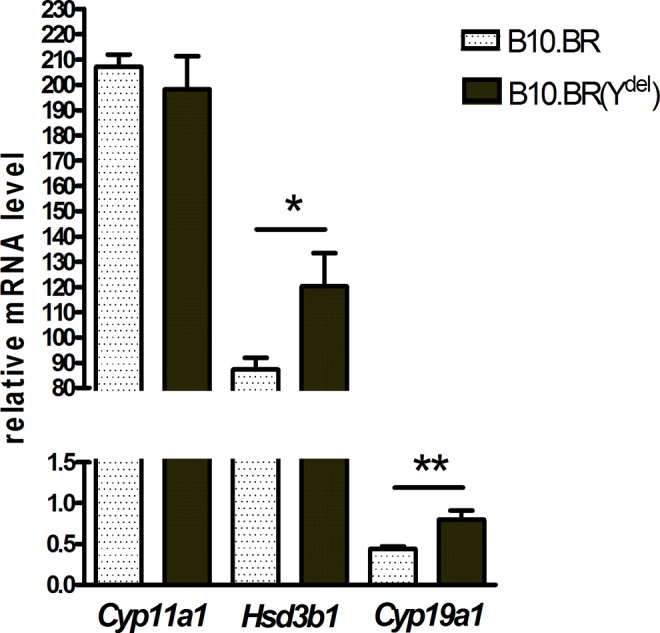
Messenger RNA levels of Cyp11a1, Hsd3b1, and Cyp19a1 genes in relation to the endogenous reference gene Actb in cumulus cells surrounding ovulated oocytes of B10.BR and B10.BR(Ydel) females (2−ΔCt values × 103 ± standard error of the mean [SEM]), *P = .03 and **P = .004; n = 6.
As illustrated in Figure 2, COCs ovulated by B10.BR(Ydel) females secrete more progesterone during incubation in vitro than COCs ovulated by females from the control B10.BR strain. The difference is statistically significant (P = .002). Because of marginal aromatase activity in postovulatory cumulus cells (Figure 1), the amount of estradiol released to the medium by cultured COCs appeared to be below the detection level.
Figure 2.
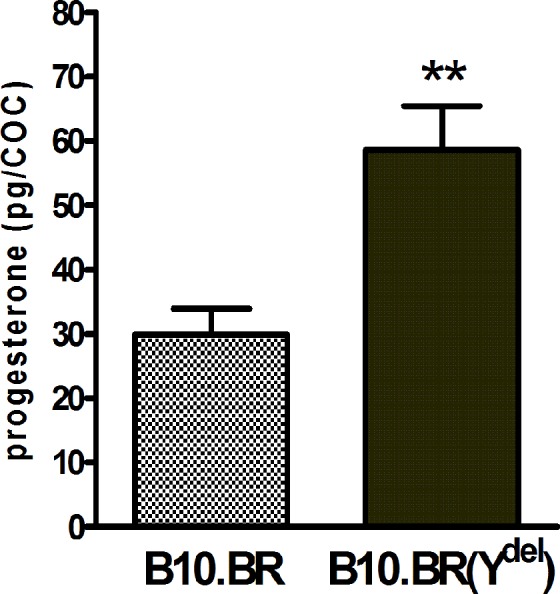
Amount of progesterone (pg) secreted by ovulated B10.BR and B10.BR(Ydel) COCs during 4.5 hours incubation in vitro (mean ± standard error of the mean [SEM]), **P = .002; for both groups of females n = 10 independent incubations of minimum 20 COCs. COC indicates cumulus–oocyte complex.
Despite the selection of exclusively viable spermatozoa and their subsequent incubation under capacitating conditions, in all experiments only a fraction of male gametes presented typical staining of their acrosomal region with P-BSA-FITC complex (Figure 3). This fraction was significantly less numerous for spermatozoa of B10.BR-Ydel males than for spermatozoa produced by B10.BR males (P < .001): 40.27% of positively stained B10.BR-Ydel spermatozoa versus 59.80% of positively stained B10.BR spermatozoa (Figure 4).
Figure 3.
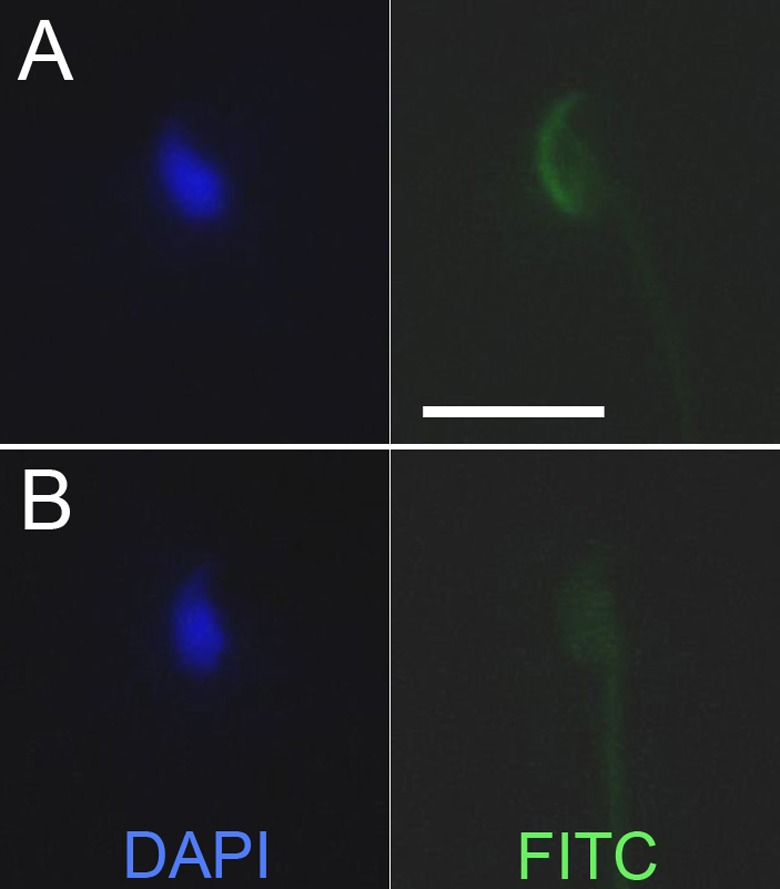
Exemplary spermatozoa positive (A) and negative (B) for staining with progesterone 3-(o-carboxymethyl)oxime: bovine serum albumin-fluorescein isothiocyanate conjugate (P-BSA-FITC) complex. Scale bar = 10 µm.
Figure 4.
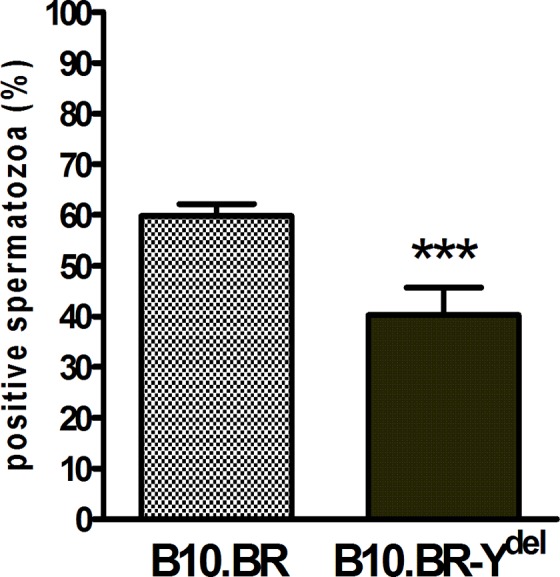
Percent of B10.BR and B10.BR-Ydel spermatozoa positive for staining with progesterone 3-(o-carboxymethyl)oxime: bovine serum albumin-fluorescein isothiocyanate conjugate (P-BSA-FITC) complex (mean ± standard error of the mean [SEM]), ***P < .001; n = 5.
Discussion
Previous experiments carried out in our laboratory have revealed many subtle but noticeable differences in reproductive qualities of females from the congenic B10.BR and B10.BR-Ydel strains of mice.14–16 These differences between genetically identical females were explained by indirect influence of paternal Y chromosomes that participate in the establishment of heritable epigenetic marks in X-bearing sperm. Cumulus cells surrounding oocytes of B10.BR(Ydel) females just prior and after ovulation present more prostaglandin EP2 receptors and produce more prostaglandin E2 than analogous cells of B10.BR females.16 Prostaglandin signaling plays very important role in female reproduction, promoting ovulation40,41 and keeping ovulated COCs in competence for fertilization.42 Additionally, prostaglandin E2, acting via specific membrane receptors on cumulus cells, stimulates their steroidogenic activity.21,22 The present study shows that ovulated COCs of both B10.BR and B10.BR(Ydel) females characterize with typical for their stage expression of genes encoding key steroidogenic enzymes.43,44 High expression of Cyp11a1 and Hsd3b1 genes and very low expression of Cyp19a1 gene confirm that postovulatory COCs are steroidogenically active and synthesize predominantly progesterone.25–28 Simultaneously, COCs of B10.BR(Ydel) females exhibit higher activity of Hsd3b1 gene (Figure 1) and release more progesterone during in vitro incubation (Figure 2) in comparison with COCs of control B10.BR females. Above-mentioned regularities imply that, according to our expectations, increased prostaglandin signaling in cumulus cells surrounding ovulated oocytes of B10.BR(Ydel) females is associated with increased synthesis of progesterone in these cells. Intensive progesterone production by COCs of B10.BR(Ydel) females can influence positively their fertilization efficiency. In oviducts, progesterone secreted by cumulus cells is a well-characterized sperm chemoattractant that not only guide male gametes to oocytes but additionally stimulates their capacitation, hyperactivated motility, rise of intracellular Ca2+ concentration, and acrosomal reaction.29–35,45 Expression level of Cyp19a1 gene (which is likely to correspond with intensity of testosterone aromatization to 17β-estradiol) is also significantly higher in COCs of B10.BR(Ydel) females than in COCs of B10.BR females (Figure 1). This characteristic of COCs of females sired by males with the Y chromosome deletion has presumably smaller physiological relevance. Cumulus cells surrounding ovulated oocytes secrete only slight amounts of estradiol27,28 (in the present study below the detection level). Besides, effect of estradiol on sperm function is ambiguous, and various studies concerning this problem gave conflicting results.33,46–50
Classically, steroids bind to intracellular receptors that induce various transcriptional events. In the case of transcriptionally inactive spermatozoa, they interact with specific cell surface receptors initiating rapid second messenger-mediated responses. Progesterone secreted by cumuli oophori is the predominant steroid that acts on spermatozoa in the female genital tract and considerably facilitates fertilization.32,33,35 Staining of mouse, stallion, and human spermatozoa with cell-impermeable fluorescein-tagged progesterone-BSA complex (P-FITC-BSA) revealed that progesterone receptors are masked by coating factors in freshly ejaculated sperm and become available after incubation under capacitating conditions, but only in a limited proportion of male gametes.39,51,52 Similarly, in the present study, we obtained positive staining of progesterone receptors just for a fraction of sperm derived from B10.BR and B10.BR-Ydel males. The sperm cells that were negative for P-FITC-BSA staining lack progesterone receptors or alternatively receptors in their membranes remained covered because of inefficient passing through the capacitating process.39,52 Availability of progesterone receptors is very important for sperm function. Experiments in vitro with human spermatozoa showed very strong correlation between positive staining of progesterone receptors and ability to undergo acrosome reaction. Gametes of men with various fertility disorders characterize with definitely lower frequency of P-FITC-BSA binding than gametes of fertile men. Therefore, visualization of progesterone receptors at sperm surfaces has been proposed as a simple and reliable method for the evaluation of their functional competence.51,53 The present study demonstrates that also in the mouse, proportion of spermatozoa positively stained with P-FITC-BSA complex is a good indicator of their general quality. This proportion appeared to be significantly lower for sperm of males with the Y-chromosome deletion than for sperm of control males (Figure 4). Gametes of B10.BR-Ydel males are characterized with frequent morphological and ultrastructural irregularities,6–8 delay in epididymal maturation,8 decreased effectiveness of movement,8,10,11 and lower fertilization efficiency in relation to sperm of B10.BR males.8,9 Now, the reduced availability of surface progesterone receptors has been added to the list.
Extracellular matrix of cumuli oophori surrounding ovulated oocytes of B10.BR(Ydel) females is more resistant and abundant with proteins than matrix of cumuli oophori surrounding oocytes of B10.BR females.15,16 Simultaneously, COCs of B10.BR(Ydel) females synthesize and secrete higher amounts of molecules supporting fertilization, such as prostaglandin and progesterone. Because biochemical signaling sent by cumulus cells is recognized only by competent spermatozoa (presenting proper receptors on their surfaces), the described features of COCs of B10.BR(Ydel) females can act together as a selective barrier for male gametes. Indeed, sperm of B10.BR males, probably answering molecular signals derived from cumulus cells, fertilize oocytes of B10.BR(Ydel) females with the same efficiency as oocytes of B10.BR females. For spermatozoa produced by B10.BR-Ydel males, the firm extracellular matrix of B10.BR(Ydel) COCs is, however, a serious obstacle that considerably reduces fertilization ratio.15 We postulate that low availability of progesterone receptors on sperm of males with the Y-chromosome deletion contributes to the described regularity. B10.BR-Ydel spermatozoa may be insufficiently stimulated and hence less effective in disintegration of resistant cumulus layer that surround oocytes of B10.BR(Ydel) females.
It was shown that deficiency of MSYq-encoded multicopy genes in various mouse models (carrying spontaneous deletions or specific shSLY transgene) causes postmeiotic predominance of multicopy X-linked genes acting antagonistically to MSYq genes during sperm differentiation.54 This disruption of balance between X- and Y-linked genes leads to functional advantage of X-bearing sperm13 and consequently to sex ratio skew in favor of females in the offspring.1,12 The present study implies that this imbalance in the activity of X- and Y-linked genes in mutant male mice can lead additionally to the strengthening of selective barriers for spermatozoa in their female progeny.
Footnotes
Authors’ Note: The work reported was done at Institute of Zoology, Jagiellonian University, Krakow, Poland.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by funding from the Jagiellonian University within the SET project cofinanced by the European Union and by Polish National Science Centre (grant number N N303 816840).
References
- 1. Conway SJ, Mahadevaiah SK, Darling SM, Capel B, Rattigan AM, Burgoyne PS. Y353/B: a candidate multiple-copy spermiogenesis gene on the mouse Y chromosome. Mamm Genome. 1994;5 (4):203–210. [DOI] [PubMed] [Google Scholar]
- 2. Touré A, Clemente EJ, Ellis P, et al. Identification of novel Y chromosome encoded transcripts by testis transcriptome analysis of mice with deletions of the Y chromosome long arm. Genome Biol. 2005;6 (12):R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellis PJI, Ferguson L, Clemente EJ, Affara NA. Bidirectional transcription of a novel chimeric gene mapping to mouse chromosome Yq. BMC Evol Biol. 2007;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts KP. Y chromosome deletions and male infertility: state of the art and clinical implications. J Androl. 1998;19 (3):255–259. [PubMed] [Google Scholar]
- 5. Koh E, Sin HS, Fukushima M, Namiki M. Azoospermia factor and male infertility. Reprod Med Biol. 2010;9(3):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Styrna J, Klag J, Moriwaki K. Influence of partial deletion of the Y chromosome on mouse sperm phenotype. J Reprod Fertil. 1991;92 (1):187–195. [DOI] [PubMed] [Google Scholar]
- 7. Styrna J, Imai HT, Moriwaki K. An increased level of sperm abnormalities in mice with a partial deletion of the Y chromosome. Genet Res. 1991;57 (2):195–199. [DOI] [PubMed] [Google Scholar]
- 8. Styrna J, Bilińska B, Krzanowska H. The effect of a partial Y chromosome deletion in B10.BR-Ydel mice on testis morphology, sperm quality and efficiency of fertilization. Reprod Fertil Dev. 2002;14 (1-2):101–108. [DOI] [PubMed] [Google Scholar]
- 9. Xian M, Azuma S, Naito K, Kunieda T, Moriwaki K, Toyoda Y. Effect of a partial deletion of Y chromosome on in vitro fertilizing ability of mouse spermatozoa. Biol Reprod. 1992;47 (4):549–553. [DOI] [PubMed] [Google Scholar]
- 10. Grzmil P, Gołas A, Müller C, Styrna J. The influence of the deletion on the long arm of the Y chromosome on sperm motility in mice. Theriogenology. 2007;67 (4):760–766. [DOI] [PubMed] [Google Scholar]
- 11. Kotarska K, Lenartowicz M. Sperm migration and selection in the reproductive tract of female mice is mostly affected by male genotype. Folia Biol. 2011;59 (1-2):71–75. [DOI] [PubMed] [Google Scholar]
- 12. Moriwaki K, Suh DS, Styrna J. Genetic factors affecting sperm morphology in the mouse. Mouse News Lett. 1988;82:138. [Google Scholar]
- 13. Ward MA, Burgoyne PS. The effects of deletions of the mouse Y chromosome long arm on sperm function—intracytoplasmic sperm injection (ICSI)-based analysis. Biol Reprod. 2006;74 (4):652–658. [DOI] [PubMed] [Google Scholar]
- 14. Styrna J. Partial deletion of the Y chromosome removes the effect of paternal genome imprinting on periovum sensitivity to hyaluronidase in mice. Genet Res Camb. 1995;65 (3):229–231. [DOI] [PubMed] [Google Scholar]
- 15. Kotarska K, Styrna J. Can the partial deletion in the Y chromosome of male mice affect the reproductive efficiency of their daughters? Syst Biol Reprod Med. 2012;58 (2):81–87. [DOI] [PubMed] [Google Scholar]
- 16. Kotarska K, Lenartowicz M, Przybyło M, Gołas A, Styrna J. Increased prostaglandin E2-EP2 signalling in cumulus cells of female mice sired by males with the Y-chromosome long-arm deletion. Reprod Fertil Dev. 2013;25 (6):900–906. [DOI] [PubMed] [Google Scholar]
- 17. Durcova-Hills G, Hajkova P, Sullivan S, Barton S, Surani MA, McLaren A. Influence of sex chromosome constitution on the genomic imprinting of germ cells. Proc Natl Acad Sci USA. 2006;103 (30):11184–11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reynard LN, Turner JMA. Increased sex chromosome expression and epigenetic abnormalities in spermatids from male mice with Y chromosome deletions. J Cell Sci. 2009;122 (pt 22):4239–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cocquet J, Ellis PJI, Yamauchi Y, et al. The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biol. 2009;7(11):e1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamauchi Y, Riel JM, Stoytcheva Z, Burgoyne PS, Ward MA. Deficiency in mouse Y chromosome long arm gene complement is associated with sperm DNA damage. Genome Biol. 2010;11 (6):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci USA. 2000;97 (18):10288–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nuttinck F, Marquant-Le Guienne B, Clément L, Reinaud P, Charpigny G, Grimard B. Expression of genes involved in prostaglandin E2 and progesterone production in bovine cumulus–oocyte complexes during in vitro maturation and fertilization. Reproduction. 2008;135 (5):593–603. [DOI] [PubMed] [Google Scholar]
- 23. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25 (6):947–970. [DOI] [PubMed] [Google Scholar]
- 24. LaVoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med. 2009;234 (8):880–907. [DOI] [PubMed] [Google Scholar]
- 25. Sherizly I, Kraicer PF. Progesterone secretion by the post-ovulatory rat cumulus oophorus. Mol Reprod Dev. 1980;3 (2):115–119. [Google Scholar]
- 26. Schuetz AW, Dubin NH. Progesterone and prostaglandin secretion by ovulated rat cumulus cell–oocyte complexes. Endocrinology. 1981;108 (2):457–463. [DOI] [PubMed] [Google Scholar]
- 27. Vanderhyden BC, Macdonald EA. Mouse oocytes regulate granulosa cell steroidogenesis throughout follicular development. Biol Reprod. 1998;59 (6):1296–1301. [DOI] [PubMed] [Google Scholar]
- 28. Ebeling S, Töpfer D, Meinecke B. Steroidogenesis and the influence of MAPK activity during in vitro maturation of porcine cumulus oocyte complexes. Reprod Dom Anim. 2011;46 (3):513–519. [DOI] [PubMed] [Google Scholar]
- 29. Guidobaldi HA, Teves ME, Uñates DR, Anastasía A, Giojalas LC. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS ONE. 2008;3 (8):e3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Reprod. 2008;23 (10):2339–2345. [DOI] [PubMed] [Google Scholar]
- 31. Teves ME, Guidobaldi HA, Unates DR, et al. Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS ONE. 2009;4 (12):e8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calogero AE, Burrello N, Barone N, Palermo I, Grasso U, D’Agata R. Effects of progesterone on sperm function: mechanisms of action. Hum Reprod. 2000;15 (suppl 1):28–45. [DOI] [PubMed] [Google Scholar]
- 33. Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol Cell Endocrinol. 2009;308 (1-2):39–46. [DOI] [PubMed] [Google Scholar]
- 34. Thomas P, Tubbs C, Garry VF. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): identification of mPRα on human sperm and its association with sperm motility. Steroids 2009;74 (7):614–621. [DOI] [PubMed] [Google Scholar]
- 35. Harper CV, Publicover SJ. Reassessing the role of progesterone in fertilization—compartmentalized calcium signalling in human spermatozoa? Hum Reprod. 2005;20 (10):2675–2680. [DOI] [PubMed] [Google Scholar]
- 36. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25 (4):402–408. [DOI] [PubMed] [Google Scholar]
- 37. Szołtys M, Galas J, Jabłonka A, Tabarowski Z. Some morphological and hormonal aspects of ovulation and superovulation in the rat. J Endocrinol. 1994;141 (1):91–100. [DOI] [PubMed] [Google Scholar]
- 38. Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9 (6):557–582. [DOI] [PubMed] [Google Scholar]
- 39. Cheng FP, Gadella BM, Voorhout WF, Fazeli A, Bevers MM, Colenbrander B. Progesterone-induced acrosome reaction in stallion spermatozoa is mediated by a plasma membrane progesterone receptor. Biol Reprod. 1998;59 (4):733–742. [DOI] [PubMed] [Google Scholar]
- 40. Davis BJ, Lennard DE, Lee CA, et al. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology. 1999;140 (6):2685–2695. [DOI] [PubMed] [Google Scholar]
- 41. Duffy DM, McGinnis LK, VandeVoort CA, Christenson LK. Mammalian oocytes are targets for prostaglandin E2 (PGE2) action. Reprod Biol Endocrin. 2010;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tamba S, Yodoi R, Segi-Nishida E, Ichikawa A, Narumiya S, Sugimoto Y. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc Natl Acad Sci USA. 2008;105 (38):14539–14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McRae RS, Johnston HM, Mihm M, O’Shaughnessy PJ. Changes in mouse granulosa cell gene expression during early luteinization. Endocrinology. 2005;146 (1):309–317. [DOI] [PubMed] [Google Scholar]
- 44. Su YQ, Nyegaard M, Overgaard MT, Qiao J, Giudice LC. Participation of mitogen-activated protein kinase in luteinizing hormone-induced differential regulation of steroidogenesis and steroidogenic gene expression in mural and cumulus granulosa cells of mouse preovulatory follicles. Biol Reprod. 2006;75 (6):859–867. [DOI] [PubMed] [Google Scholar]
- 45. Kobori H, Miyazaki S, Kuwabara Y. Characterization of intracellular Ca2+ increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol Reprod. 2000;63 (1):113–120. [DOI] [PubMed] [Google Scholar]
- 46. Luconi M, Muratori M, Forti G, Baldi E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. J Clin Endocrinol Metab. 1999;84:1670–1678. [DOI] [PubMed] [Google Scholar]
- 47. Adeoya-Osiguwa SA, Markoulaki S, Pocock V, Milligan SR, Fraser LR. 17beta-estradiol and environmental estrogens significantly affect mammalian sperm function. Hum Reprod. 2003;18:100–107. [DOI] [PubMed] [Google Scholar]
- 48. Francavilla F, Romano R, Pandolfi C, et al. Evaluation of the effect of 17alphaOH-progesterone and 17beta-oestradiol on human sperm ability to fuse with oocytes: comparison and possible interference with the effect of progesterone. Int J Androl. 2003;26 (6):342–347. [DOI] [PubMed] [Google Scholar]
- 49. Tsakmakidis IA, Lymberopoulos AG, Alexopoulos C, Boscos CM, Kyriakis SC. In vitro effect of zearalenone and alpha-zearalenol on boar sperm characteristics and acrosome reaction. Reprod Domest Anim. 2006;41(5):394–401. [DOI] [PubMed] [Google Scholar]
- 50. Vigil P, Toro A, Godoy A. Physiological action of oestradiol on the acrosome reaction in human spermatozoa. Andrologia. 2008;40 (3):146–151. [DOI] [PubMed] [Google Scholar]
- 51. Gadkar S, Shah CA, Sachdeva G, Samant U, Puri CP. Progesterone receptor as an indicator of sperm function. Biol Reprod. 2002;67 (4):1327–1336. [DOI] [PubMed] [Google Scholar]
- 52. Pietrobon EO, Monclus M, Alberdi AJ, Fornes MW. Progesterone receptor availability in mouse spermatozoa during epididymal transit and capacitation: ligand blot detection of progesterone-binding protein. J Androl. 2003;24 (4):612–620. [DOI] [PubMed] [Google Scholar]
- 53. Jacob A, Hurley I, Mandel FS, Hershlag A, Cooper GW, Benoff S. Human sperm non-nuclear progesterone receptor expression is a novel marker for fertilization outcome. Mol Hum Reprod. 1998;4 (6):533–542. [DOI] [PubMed] [Google Scholar]
- 54. Cocquet J, Ellis PJI, Mahadevaiah SK, Affara NA, Vaiman D, Burgoyne PS. A genetic basis for a postmeiotic X versus Y chromosome intragenomic conflict in the mouse. PLoS Genet. 2012;8(9):e1002900. [DOI] [PMC free article] [PubMed] [Google Scholar]


