Abstract
Adenine nucleotide translocase (Ant) facilitates the exchange of adenosine triphosphate across the mitochondrial inner membrane and plays a critical role for bioenergetics in eukaryotes. Mice have 3 Ant paralogs, Ant1 (Slc25a4), Ant2 (Slc25a5), and Ant4 (Slc25a31), which are expressed in a tissue-dependent manner. We previously identified that Ant4 was expressed exclusively in testicular germ cells in adult mice and essential for spermatogenesis and subsequently male fertility. Further investigation into the process of spermatogenesis revealed that Ant4 was particularly highly expressed during meiotic prophase I and indispensable for normal progression of leptotene spermatocytes to the stages thereafter. In contrast, the expression and roles of Ant4 in female germ cells have not previously been elucidated. Here, we demonstrate that the Ant4 gene is expressed during embryonic ovarian development during which meiotic prophase I occurs. We confirmed embryonic ovary-specific Ant4 expression using a bacterial artificial chromosome transgene. In contrast to male, however, Ant4 null female mice were fertile although the litter size was slightly decreased. They showed apparently normal ovarian development which was morphologically indistinguishable from the control animals. These data indicate that Ant4 is a meiosis-specific gene expressed during both male and female gametogenesis however indispensable only during spermatogenesis and not oogenesis. The differential effects of Ant4 depletion within the processes of male and female gametogenesis may be explained by meiosis-specific inactivation of the X-linked Ant2 gene in male, a somatic paralog of the Ant4 gene.
Keywords: oogenesis, mitochondria, adenine nucleotide translocase, meiosis
Introduction
Adenine nucleotide translocase (Ant), also called adenosine diphosphate (ADP)/adenosine triphosphate (ATP) carrier, belongs to the mitochondrial solute carrier Slc25 family that supports a variety of transport activities across the mitochondrial inner membrane.1 Ant particularly mediates the exchange of ADP and ATP across the mitochondrial membrane, thus playing an essential role in energy metabolism within eukaryotic cells.2 Under conditions of aerobic respiration, ATP produced within the mitochondria is exported to the cytosol through Ant to support cellular activities. In exchange, ADP is imported and utilized as a substrate for the conversion of ADP to ATP by the ATP synthase. All known eukaryotes possess multiple paralogs encoding this critical protein for bioenergetics, and mammals have 4 Ant paralogs, Ant1 (Slc25a4), Ant2 (Slc25a5), Ant3 (Slc25a6), and Ant4 (Slc25a31), except rodents that have Ant1, 2, 4 but not Ant3.3 Interestingly, some of these Ant paralogs are expressed in a tissue-specific manner.4–7 Ant1 is particularly highly expressed in cardiac and skeletal muscles, whereas Ant4 is exclusively expressed in germ cells, as discussed more in detail subsequently. In contrast, Ant2 and Ant3 are ubiquitously expressed, although it has been shown that human ANT2 expression is rather inducible by proliferative stimuli while ANT3 is constitutively expressed.
About a decade ago, we originally identified the Ant4 gene through a screening of genes that were hypermethylated during differentiation of murine pluripotent embryonic stem cells.8 In adult tissues, the Ant4 gene is only expressed in testis but completely repressed in other organs with high CpG methylation marks within its promoter region.8,9 Upon closer examination of the testis, Ant4 expression was indeed expressed in germ cells but not in Sertoli cells or other interstitial tissues.10 Further, through isolation of stage-specific spermatogenic cells by differential sedimentation velocity at unit gravity,11 we demonstrated that Ant4 messenger RNA (mRNA) expression began to increase from the preleptotene stage forward, with continued increase through the leptotene and zygotene phases, peaking in pachytene cells and then decreasing thereafter.10,12 This stage-specific expression pattern of the Ant4 gene indicates that Ant4 is highly expressed during meiotic prophase I. Along with this expression profile, Ant4 null mice demonstrated maturation arrest of meiotic spermatocytes and resultant male infertility.10,12
The expression and role of the Ant4 gene in female germ cell development, however, have not been previously fully investigated. Unlike male meiosis, female meiosis occurs at different stages of development. Primary oocytes enter meiotic prophase I at approximately 13.5 dpc.13 The primary oocytes undergo leptotene, zygotene, and pachytene stages and arrest at the diplotene stage.14 Then, the primary oocytes remain in a dormant state until they reach puberty. After birth, during a menstrual cycle, selected oocytes reenter and complete meiosis I. After ovulation, the oocytes arrest in metaphase II of meiosis II. Once fertilization occurs, the second meiotic division is completed. In order to further elucidate the role and regulations of the Ant4 gene in gametogenesis, here we investigated expression of Ant4 in murine embryonic ovaries and characterized the Ant4 null female mice.
Materials and Methods
RNA Isolation
The RNAqueous Kit (Ambion, Austin, Texas) was used for total RNA extraction from fetal ovarian tissues of Ant4 +/− and Ant4 −/− mice at different time points (13.5, 14.5, 15.5, 16.5, 17.5, and 18.5 dpc) according to the manufacturer’s instructions. Contamination of genomic DNA in extracted RNA was excluded by the TURBO DNA-free Kit (Ambion). Complementary DNA (cDNA) was synthesized from the total RNA by the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) under the following thermocycler conditions: 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 seconds.
Quantitative Reverse Transcriptase Polymerase chain Reaction
Taqman Gene Expression Master Mix for the Ant4, Ant2, and β-actin expressions and Power SYBR Green PCR Master Mix for the Sycp3 expression (Applied Biosystems, Foster City, California) were used for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) under the following conditions: 95°C for 10 minutes and 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Template equivalent to 2.5 ng of total RNA was used for the amplification, and each reaction was performed in triplicate. Transcript level of each sample was normalized to β-actin and relatively quantified by the 2−▵Ct method. The primer sets used are as follows: An4: Taqman probe (Mm00617754_m1, Applied Biosystems), Ant2: Taqman probe (Mm00846873_g, Applied Biosystems), Sycp3: 5′-GCAGCAGTGGGAACTGGATA and 5′-TTCATTCTCTGGCTCTGAACAA, and β-actin: Taqman probe (4352341E, Applied Biosystems).
Mice Husbandry
Mice were bred and maintained on a 12-hour light–dark cycle and given ad libitum access to food and water. All in vivo experimental procedures carried out in the study were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Generation of Bacterial Artificial Chromosome Transgenic Mice
Transgenic constructs and targeting of bacterial artificial chromosome DNA
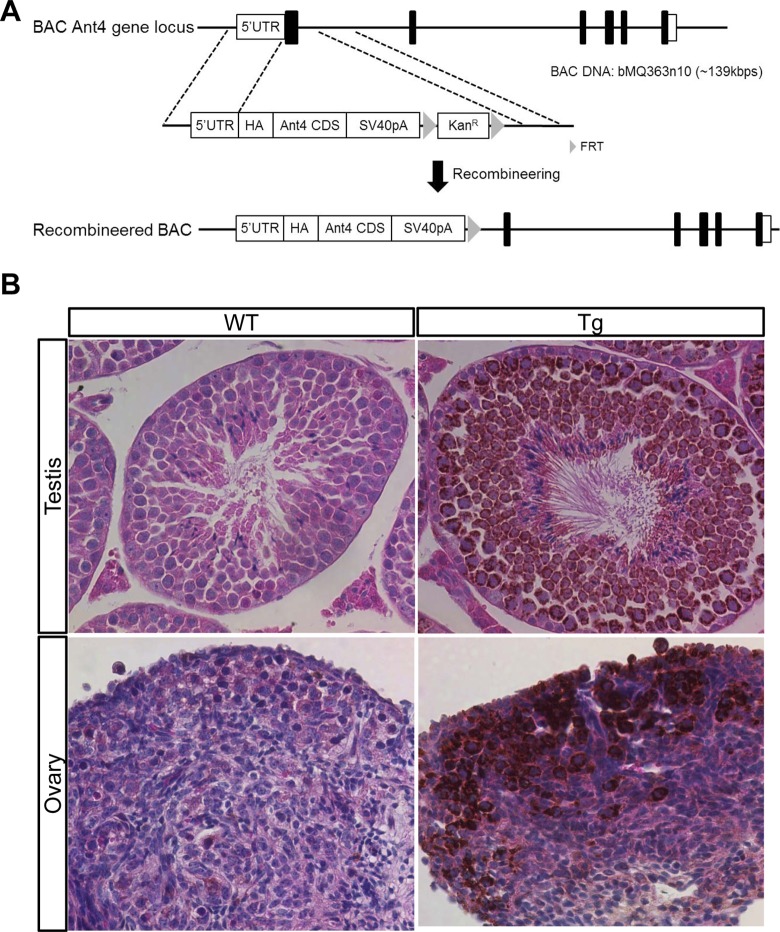
A bacterial artificial chromosome (BAC) clone including the Ant4 locus (bMQ363n10 [˜139 kbps]; Source Bioscience, Nottingham) was genetically manipulated for the generation of BAC HA-Ant4 transgenic mice; bMQ363n10 (˜139 kbp) has 58 kbp upstream and 64 kbp downstream of Ant4. First, a transgenic construct for recombineering was generated in pCR2.1-TOPO (Invitrogen, Carlsbad, California) vector for the genetic modification. The 5′ homologous arm which is 1 kbp upstream from the ATG initiation site of the Ant4 gene and the Ant4 cDNA sequence were fused by PCR and introduced into the pCR2.1-TOPO vector. SV40 polyadenylation (pA) signal and kanamycin resistance gene cassette flanked by FLP recombinase target (FRT) sequences were then inserted downstream of the Ant4 cDNA sequence. Finally, the 3′ homologous arm, which is 1 kbp downstream of exon 1 of the Ant4 gene, was introduced into downstream of the kanamycin resistance gene cassette (Figure 1A). To recombine the transgenic construct with BAC DNAs, Escherichia coli strain EL250 which can produce recombination proteins by heat shock at 42°C and flippase under 0.1% l-arabinose was used. The recombineering was performed as described by the Frederick National Laboratory for cancer research (http://ncifrederick.cancer.gov/research/brb/recombineeringInformation.aspx). Briefly, the BAC DNA was transformed into EL250 by electroporation and selected on chloramphenicol (12.5 µg/mL)-containing plates. After overnight culture at 32°C in 5 mL of low salt-containing Lysogeny broth medium, the selected BAC DNA containing EL250 was incubated for 15 minutes at 42°C for activation of homologous recombination proteins. After immediate cooling on wet ice, the EL250 strains were washed with ice-cold water, then the transgenic construct was transformed into the corresponding BAC DNA-containing EL250 strains by electroporation, and screened on kanamycin (50 µg/mL)-containing plates. After 2 to 3 days of culture, several clones were picked up to screen transformants for recombineered BAC DNAs and their genotypes were checked by PCR (wildtype Ant4: 5′-GCTGTGCACTGATTGAGCAT, 5′-TGTCAACGTCACCTCCTCTG and Tg Ant4:5′-GCTGTGCACTGATTGAGCAT, 5′-TGCCACGCCAATAACTTAAA and thermocycling condition: 95°C for 5 minutes and 35 cycles at 95°C for 30 seconds and 55°C for 30 seconds and 72°C for 45 seconds and 72°C for 10 minutes), and the positive clones by PCR were confirmed by Southern blot analysis with a Digoxigenin (DIG) DIG-labeled DNA probe. To confirm the integrity of the recombineered BAC DNAs, restriction patterns of the BACs were compared to that of the corresponding original BACs using EcoRI. To remove the kanamycin resistance gene cassette, clones were first cultured overnight at 32°C in 5 mL of medium. Next, they were incubated for 1 hour in 0.1% l-arabinose-containing medium to induce expression of flippase. Afterward, they were spread on chloramphenicol-containing plates. After 2 to 3 days, several clones were isolated and checked by PCR and Southern blot analyses to confirm cassette deletion. The selected clones were cryopreserved at −80°C until further processes.
Figure 1.
Adenine nucleotide translocase 4 (Ant4) promoter is active in fetal ovaries. A, Illustration of the strategy to generate bacterial artificial chromosome (BAC) human influenza hemagglutinin (HA)-Ant4 transgene. B, HA-tagged Ant4 was detected in mature testis (10-week old, top) and fetal ovary (17.5 dpc, bottom). Each section was counterstained with hematoxylin and eosin. Adult testis and fetal ovary from wild-type mice were used as a negative controls.
Purification of the targeted BACs
The targeted BACs were purified by cesium chloride (CsCl) density gradient centrifugation for pronuclear injection. Cultures of 1.5 L were collected and lysed to extract the targeted BAC DNAs. The extracted BAC DNAs were precipitated by ethanol and rehydrated in 8 mL of Tris-EDTA (TE) buffer. To carry out CsCl density gradient centrifugation, CsCl was dissolved in the BAC DNAs–TE mixture in order to make a final concentration of 1.55 g/mL, and ethidium bromide (10 mg/mL) was added to each tube to visualize the BAC DNAs. Three consecutive ultracentrifugations, then, were performed with an NVT-90 fixed angle rotor at 25°C: (1) 55 000 rpm overnight, (2) 78 000 rpm for 4 hours, and (3) 55 000 rpm overnight. After each ultracentrifugation, red bands (DNA) were extracted using an 18-gauge needle and were transferred to a new ultracentrifuge tube. The tube was filled with premade 1.55 g/mL TE-RNAse solution. After the third ultracentrifugation, the red bands including the BAC DNA (˜1.5 mL) were collected, and ethidium bromide was removed from the DNA by phase extraction with saltwater-saturated butanol. Finally, the CsCl was removed from the BAC DNA solution by 2 consecutive dialyses (Molecular weight cut-off [MWCO] 12 000-14 000, Spectra/Por, Spectrum Labs, Rancho Dominguez, California) in injection buffer (10 mmol/L tris pH 7.5, 0.1 mmol/L EDTA, 100 mmol/L NaCl). After measuring the concentration of the purified BAC DNAs by a spectrophotometer (Synergy HT Multi-Mode Microplate Reader; BioTek, Winooski, Vermont), the DNAs were stored at −80°C until pronuclear injection.
Pronuclear injection
The BAC DNAs were injected into 200 to 300 embryos obtained from B6D2F1/J (C57BL/6JXDBA2J) at the Mouse Model Core in the Department of Animal Care Services at the University of Florida. The BAC injected embryos were implanted to recipient mice. After 6 weeks, tail snips from new born mice were collected and genotyped by PCR as described in the section of “Transgenic constructs and targeting of BAC DNA” to select transgenic founders.
Histology and immunohistochemistry
Ovaries and testes were collected at various time points, washed in phosphate-buffered saline (PBS), and fixed overnight in 4% paraformaldehyde in PBS. The next day, the fixed organs were washed with PBS, dehydrated in 70% ethanol, infiltrated, and embedded in paraffin. The paraffin-embedded tissues were cut into 5-µm slices, and the sections were placed on glass slides and dried until further staining. For immunohistochemistry, HA-tagged Ant4 protein expression was visualized using an anti-HA antibody (1:400, rabbit, Cell Signaling Technology, Danvers, Massachusetts) and VECTASTAIN Elite ABC kit (Vector Labs, Burlingame, California) according to manufacturer’s instructions. Briefly, the paraffin sections were deparaffinized in xylene and hydrated through an ethanol series and water. After heat-induced epitope retrieval (Dako, Carpinteria, California), preblocking was carried out by treatment with Peroxo Block (Invitrogen) for 45 seconds, goat serum for 20 minutes, and with the Avidin/Biotin Blocking Kit (Vector Labs) sequentially, according to manufacturer’s instructions. After overnight incubation with the anti-HA antibody, the sections were incubated for 30 minutes with a biotinylated secondary antibody then for 30 minutes with ABC reagent. Finally, HA-tagged Ant4 was visualized by the perxosidase-diaminobenzidine (Vector Labs) reaction. After rinsing with water and dehydration, the sections were mounted and observed under the microscope (Olympus IX70 Center Valley, Pennsylvania). All staining steps were performed at room temperature except for the incubation with the primary antibody (at 4°C).
Results
Ant4 is Expressed During Embryonic Ovarian Development
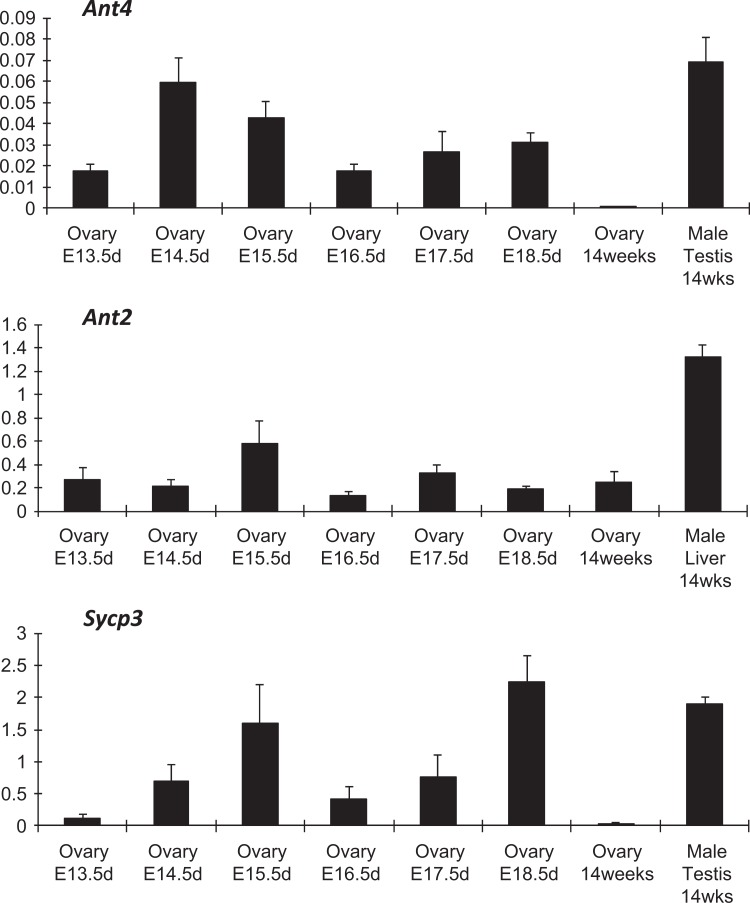
To investigate whether Ant4 is expressed during fetal ovarian development, murine ovaries were collected at different embryonic days (13.5, 14.5, 15.5, 16.5, 17.5, and 18.5 dpc). Transcript levels of each gene were detected by qRT-PCR (Figure 2). The Ant4 transcripts were found in fetal ovaries over the selected time points but not in the adult ovary (14 weeks old). Adult testis (14 weeks old) was utilized as positive control for Ant4 mRNA expression. As a control, mRNA expression of a well-known meiotic gene, Sycp3, was investigated.15 Likewise, expression of Sycp3 was detected during embryonic ovary development but not in adult ovary samples. These data indicate that Ant4 is specifically expressed during meiosis in both female and male reproductive organs. Since the homogametic sex chromosome (XX) in female mice can escape meiotic sex chromosome inactivation,16 we expected that the X-linked Ant2 gene would be expressed in fetal ovary. Indeed, Ant2 expression was found in the fetal and adult ovaries regardless of meiotic stages (Figure 2). Adult liver was used as a positive control for Ant2 expression.
Figure 2.
Adenine nucleotide translocase 4 (Ant4) is expressed in fetal ovaries. The levels of Ant4, Ant2, and Sycp3 messenger RNA (mRNA) expression were examined in fetal and adult ovaries by qualitative reverse transcriptase polymerase chain reaction (qRT-PCR). Each mRNA amounts were normalized based upon the expression of the actin mRNA. Testis and liver tissues were used as positive controls for Ant4 and Sycp3 expressions and Ant2 expression, respectively. Error bars indicate standard deviations of triplicate samples.
Ant4 Promoter is Active During Fetal Ovarian Development
The BAC transgenic approach was used to examine the Ant4 promoter activity in fetal ovaries. The chosen BAC DNA including the Ant4 gene locus was modified via genetic recombineering techniques (Figure 1A). Briefly, the construct including the 5′-homologous arm-HA-Ant4CDS-SV40 pA-FRT-KanR-FRT-3′-homologus arm was inserted downstream of the Ant4 promoter by homologous recombination. After the selection by kanamycin, the Kan R cassette was removed in the selected clones by flippase. Through pronuclear injection with the modified BAC HA-Ant4 construct, 1 male and 2 females were identified as transgenic founders with a 20% success rate (3 of 15). The female descendants from 2 female founders were used for immunohistochemical analysis using antibodies against HA because heterogeneous transgene expression was observed in the male founder line. As seen with endogenous Ant4, which is strongly expressed in meiotic spermatocytes but not in somatic tissues of testis,10 the transgenic Ant4 expression was detected strongly in spermatocytes (Figure 1B). Closer investigation revealed that the HA-tagged Ant4 expression was also observed in round and elongated spermatids as well as in the midpiece of spermatozoa, similar to the endogenous Ant4 (Supplemental Figure S1).10 In addition, somatic cells such as Sertoli cells did not express the transgene Ant4 either (Supplemental Figure S1). Taken together, the expression pattern of the BAC transgenic Ant4 was comparable to that of the endogenous Ant4 gene. It should also be noted here that the transgene expression was not detected in any somatic organs investigated including brain, heart, lung, liver, spleen, and kidney (data not shown). We then assessed the Ant4 promoter activity in fetal ovaries (17.5 dpc) by immunostaining with anti-HA antibodies. Transgene expression was noted in the fetal ovary, indicating that the Ant4 promoter is indeed active during meiosis in female as well.
Ant4 Null Ovaries are Morphologically and Histologically Normal
In order to scrutinize the effects of Ant4 depletion in oogenesis, we investigated the size and histology of Ant4 +/− and Ant4 −/− ovaries. Adult ovaries were extracted from Ant4 +/− and Ant4 −/− female animals at various ages (7-13 months old). Five pairs of Ant4 +/− ovaries were compared to 4 pairs of Ant4 −/− ovaries for size and histology. As shown in Figure 3A, average sizes of ovaries from Ant4 null and control animals were similar (Figure 3A). Indeed, ovary weight was not significantly different between the 2 groups (mean value: 4.21 mg for Ant4 +/− and 4.31 mg for Ant4 −/−, P = .84; Figure 3B). To further examine oogenesis, hematoxylin and eosin staining was carried out with ovaries from 12-month-old Ant4 +/− and Ant4 −/− animals. Morphology of developing follicles and surrounding cells was apparently normal in both Ant4 +/− and Ant4 −/− ovaries (arrows; Figure 4).
Figure 3.
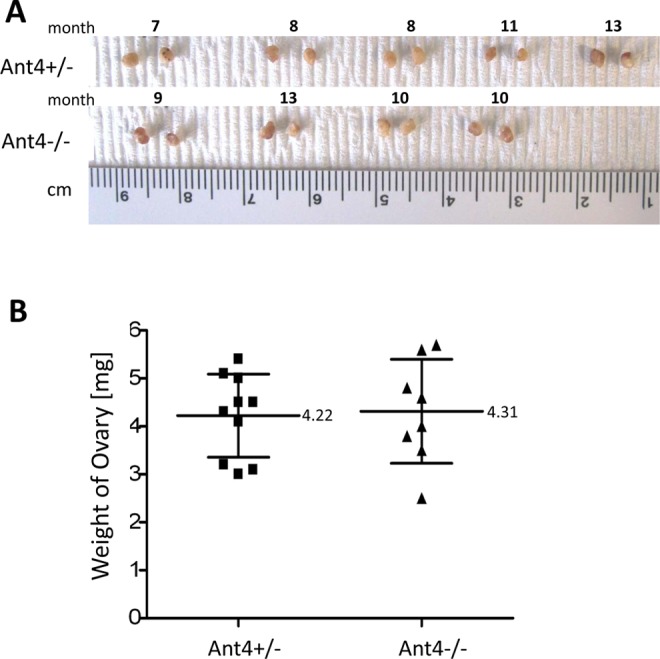
Size and weight of adenine nucleotide translocase 4 (Ant4) −/− ovary. A, Gross size and morphology of ovaries from adult Ant4+/− and Ant4−/− female mice (7-13 months old). B, Weight of ovaries. Error bars indicate standard deviations (P = .84).
Figure 4.
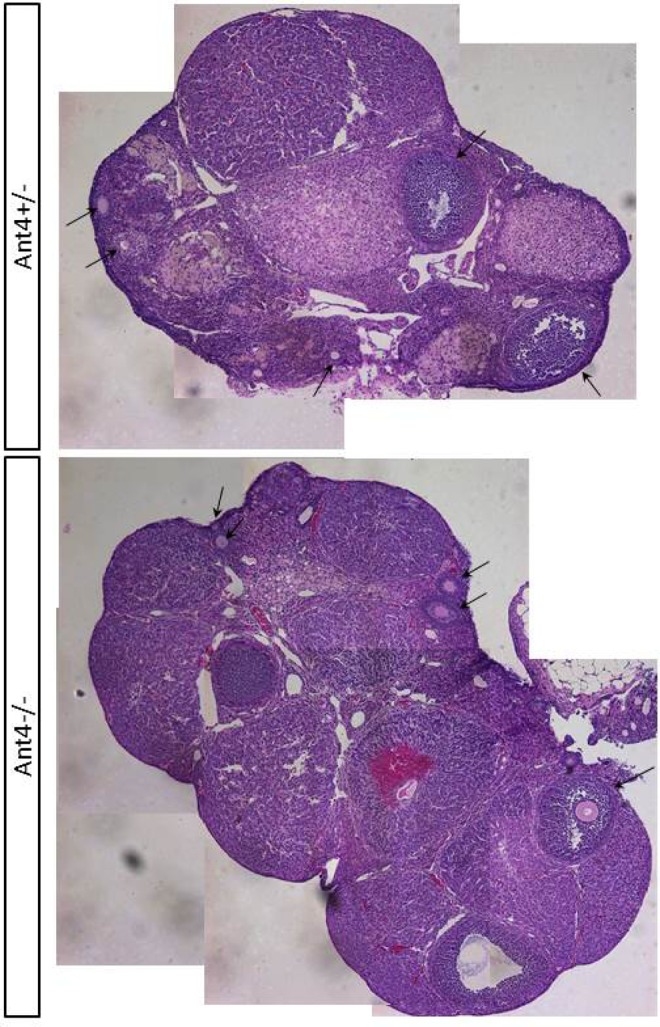
Adenine nucleotide translocase 4 (Ant4)−/− ovary is histologically normal. Representative histological images of adult Ant4+/− and Ant4−/− ovaries stained with hematoxylin and eosin (X40). Arrows indicate developing follicles.
Female Ant4 Null Mice are Fertile but Produce a Smaller Number of Progeny Than Control Mice
In order to evaluate the effects of Ant4 deletion in female fertility, the litter size of Ant4 +/− (n = 22) and Ant4 −/− (n = 17) female mice was compared. Regardless of genotypes, all female mice produced healthy pups without any appreciable abnormalities, indicating that Ant4 is dispensable during female gametogenesis in contrast to male. However, when the number of pups per litter from the 2 groups was analyzed in detail, a small but significant difference was detected. On average, the litter size of Ant4 +/− female was 5.7 while that of Ant4 −/− was 4.5 (P = .01; Figure 5).
Figure 5.
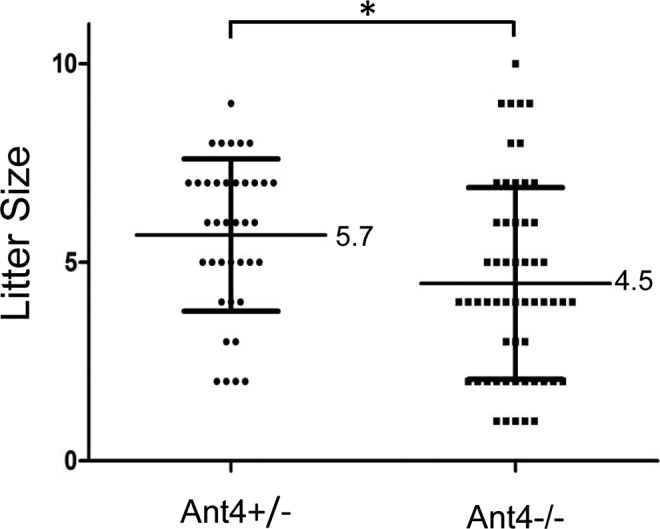
Adenine nucleotide translocase 4 (Ant4)+/− and Ant4 −/− female mice. 22 Ant4 +/− and 17 Ant4 −/− females were mated with Ant4+/− males, and the litter size was counted. Error bars indicate standard deviations. The asterisk indicates P < .05 in t test (P = .01).
Discussion
The present study demonstrates that Ant4 is not a male germ cell-specific gene but rather expressed during meiosis within both male and female germ cells. The highest expression of the Ant4 gene was noted during prophase I of testicular germ cells.10 In female mice, the Ant4 gene was expressed in embryonic ovaries in which prophase 1 proceeds along with the control meiosis-specific gene Sycp3. Indeed, we previously demonstrated that repression of the Ant4 gene during somatic cell specification is, at least in part, regulated by the E2F6 transcriptional repressor, and this mechanism is apparently conserved in many other meiosis-specific genes, including Stag3 and Smc1β,17,18 further supporting the finding here. The meiosis-specific expression appears to be governed by flanking regions of the Ant4 gene, as the BAC transgene showed identical expression profiles to the endogenous Ant4 gene. This transgene system should be valuable for further determining regulatory regions rendering the Ant4 gene expression pattern to be exclusively limited to meiosis.
Despite expression of the Ant4 gene in both male and female gametogenesis, the outcomes of genetic silencing via knockout were contrasting between male and female mice. Although we observed a partial decrease in litter sizes, female fertility was largely conserved in Ant4 null mice demonstrating normal ovary and oocyte development based on histological analyses. In contrast, male Ant4 null mice were infertile demonstrating premature arrest of spermatogenesis at the leptotene stage.12 Since meiosis is a complex and energy-demanding process, the removal of mitochondrial ATP transport activity is expected to result in dire effects in both sexes. Although the discrepancy could be potentially delineated by a differential energy metabolism during male and female gametogenesis, there has been no particular supporting evidence suggesting that male meiosis would require more oxidative phosphorylation.
Of interest, a somatic paralog of Ant4, Ant2 gene, is located on the X chromosome in mammals.10 It is known that many of the X-linked genes are repressed during murine male meiosis by unpaired meiotic sex chromosome inactivation (MSCI) during male meiosis.16,19 Indeed Ant2 gene expression was shown to be repressed during murine male meiosis10 but not in embryonic ovaries as demonstrated herein. This may explain why the Ant4 null phenotype appears only in male animals. The Ant4 gene is conserved throughout eutherian and metatherian mammals, in which the Ant2 gene is localized to the X chromosome. This supported the idea that Ant4 is conserved in mammals to “compensate” for the loss of Ant2 expression due to MSCI. In our earlier phylogenetic analysis using the available Ensemble database at the time,10 Ant4 orthologs were not found in other vertebrates, which was consistent with the “compensation” hypothesis.
It should be noted, however, that an updated database showed that reptiles have an Ant4 ortholog.20 In the case of the anole lizard, we demonstrated that Ant4 was also expressed in reptile testis but not in somatic organs.20 In contrast to mammals, anole Ant2 was not encoded on the sex chromosome; alternatively, some reptiles do not have sex chromosomes at all. These implied the conservation of Ant4 may not be simply driven by the sex chromosomal localization of the Ant2 gene and its subsequent inactivation during male meiosis. It is still possible that additional as of yet unidentified functional properties of Ant4, which are critical for male germ cells, may drive Ant4 gene conservation in limited vertebrates.20 Further studies are required for a more comprehensive understanding of this uniquely conserved mitochondrial protein in germ cells.
Acknowledgments
We thank Dr Katherine Santostefano (University of Florida) for critical reading of the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NIH grant U01 HD060474 to N.T.
Supplemental Material: The online supplemental figure is available at http://rs.sagepub.com/supplemental.
References
- 1. Palmieri F, Pierri CL, De Grassi A, Nunes-Nesi A, Fernie AR. Evolution, structure and function of mitochondrial carriers: a review with new insights. Plant J. 2011;66 (1):161–181. [DOI] [PubMed] [Google Scholar]
- 2. Klingenberg M. The ADP and ATP transport in mitochondria and its carrier. Biochim Biophys Acta. 2008;1778 (10):1978–2021. [DOI] [PubMed] [Google Scholar]
- 3. Traba J, Satrustegui J, del Arco A. Adenine nucleotide transporters in organelles: novel genes and functions. Cell Mol Life Sci. 2011;68 (7):1183–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579 (3):633–637. [DOI] [PubMed] [Google Scholar]
- 5. Doerner A, Pauschinger M, Badorff A, et al. Tissue-specific transcription pattern of the adenine nucleotide translocase isoforms in humans. FEBS Lett. 1997;414 (2):258–262. [DOI] [PubMed] [Google Scholar]
- 6. Levy SE, Chen YS, Graham BH, Wallace DC. Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene. 2000;254 (1-2):57–66. [DOI] [PubMed] [Google Scholar]
- 7. Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem. 1992;267 (21):14592–14597. [PubMed] [Google Scholar]
- 8. Rodic N, Oka M, Hamazaki T, et al. DNA methylation is required for silencing of ant4, an adenine nucleotide translocase selectively expressed in mouse embryonic stem cells and germ cells. Stem Cells. 2005;23 (9):1314–1323. [DOI] [PubMed] [Google Scholar]
- 9. Brower JV, Lim CH, Han C, Hankowski KE, Hamazaki T, Terada N. Differential CpG island methylation of murine adenine nucleotide translocase genes. Biochim Biophys Acta. 2009;1789 (3):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brower JV, Rodic N, Seki T, et al. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem. 2007;282 (40):29658–29666. [DOI] [PubMed] [Google Scholar]
- 11. Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74 (1):68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brower JV, Lim CH, Jorgensen M, Oh SP, Terada N. Adenine nucleotide translocase 4 deficiency leads to early meiotic arrest of murine male germ cells. Reproduction. 2009;138 (3):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44 (12):622–632. [DOI] [PubMed] [Google Scholar]
- 14. Hartshorne GM, Lyrakou S, Hamoda H, Oloto E, Ghafari F. Oogenesis and cell death in human prenatal ovaries: what are the criteria for oocyte selection? Mol Hum Reprod. 2009;15 (12):805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kouznetsova A, Novak I, Jessberger R, Hoog C. SYCP2 and SYCP3 are required for cohesin core integrity at diplotene but not for centromere cohesion at the first meiotic division. J Cell Sci. 2005;118 (pt 10):2271–2278. [DOI] [PubMed] [Google Scholar]
- 16. Turner JM, Mahadevaiah SK, Fernandez-Capetillo O, et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet. 2005;37 (1):41–47. [DOI] [PubMed] [Google Scholar]
- 17. Kehoe SM, Oka M, Hankowski KE, et al. A conserved E2F6-binding element in murine meiosis-specific gene promoters. Biol Reprod. 2008;79 (5):921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leseva M, Santostefano KE, Rosenbluth AL, Hamazaki T, Terada N. E2f6-mediated repression of the meiotic Stag3 and Smc1β genes during early embryonic development requires Ezh2 and not the de novo methyltransferase Dnmt3b. Epigenetics. 2013;8 (8):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10 (3):207–216. [DOI] [PubMed] [Google Scholar]
- 20. Lim CH, Hamazaki T, Braun EL, Wade J, Terada N. Evolutionary genomics implies a specific function of Ant4 in mammalian and anole lizard male germ cells. PLoS One. 2011;6(8):e23122. [DOI] [PMC free article] [PubMed] [Google Scholar]