Abstract
The synthetic retinoid fenretinide (N-[4-hydroxyphenyl] retinamide or 4HPR) has been shown to not only inhibit cell growth but also to induce apoptosis in a variety of malignant cell lines. It is being tested presently for its potential as a chemopreventive agent against several cancers. A related retinoid, 13-cis-retinoic acid (cRA), has been shown to have activity against gliomas in vitro as well as in a recent clinical study. The present study aimed at assessing the activity of fenretinide against glioma cells in vitro and comparing it with that of cRA at pharmacologically relevant doses. We hypothesized that the ability of fenretinide to induce apoptosis would make it more potent against gliomas than cRA. Four glioma cell lines (D54, U251, U87MG, and EFC-2) were treated with fenretinide (1–100 µM) and showed dose- and time-dependent induction of cell death. At pharmacologically relevant doses, fenretinide was more active against glioma cells than cRA because of its ability to induce apoptosis. Flow cytometric studies using D54 cells demonstrated no significant changes in the cell cycle distribution compared with untreated control, but a sub-G1 fraction consistent with apoptosis was detected. Terminal deoxynucleotidyl transferase-mediated nick end labeling assay indicated that the apoptotic fraction was cell cycle nonspecific. Fenretinide treatment resulted in cleavage of poly ADP-ribose polymerase, indicating an activation of the caspase 3. Immunofluorescence studies using the nuclear stain 4′,6-diamidine-2′-phenylindole dihydrochloride showed nuclear condensation and an apoptotic morphology. Hence, this study demonstrates that, at clinically relevant doses, fenretinide is a potent inducer of apoptosis in gliomas acting via the caspase pathway. We also show that at clinically achievable doses, fenretinide has more activity against gliomas than comparable doses of cRA. The favorable side effect profile seen in previous clinical studies and the in vitro activity against gliomas demonstrated in this study suggest that fenretinide could be a promising therapeutic agent against gliomas.
INTRODUCTION
Malignant gliomas are a significant cause of mortality in the adult cancer population causing ~7% of all cancer-related deaths. Chemotherapeutic approaches presently in use have failed to make an impact on gliomas because of significant toxicity, problems with drug delivery, and the high degree of chemoresistance demonstrated by these tumors (1). Newer agents that target cell characteristics such as differentiation, angiogenesis, invasion, DNA repair, and apoptosis and that have better side-effect profiles are presently being investigated for their efficacy against this malignancy (2). Retinoids are one such class of drugs that have demonstrated an ability to inhibit growth and induce differentiation in a variety of malignancies (3). However, many natural and synthetic retinoids have limited therapeutic utility because of side effects (4, 5).
Fenretinide2 is a synthetic retinoid that is associated with minimal side effects in humans and does not accumulate in the liver (6, 7). It has been shown to have biological activity against several malignancies in vitro, including leukemia (8), neuroblastoma (9), and cancers of the breast (10), prostate (11), ovary (12), uterine cervix (13), and head and neck (14). The efficacy of fenretinide in chemoprevention has also been demonstrated in animal models of mammary (15) and prostatic tumors (16). Fenretinide has been evaluated in humans in several clinical trials and appears to have a role in chemoprevention of malignancies in humans (3, 17, 18). Clinically achievable plasma concentrations ranging from 1 to 3 µM have been well tolerated in patients treated with fenretinide in chemoprevention trials (18). More studies are being conducted presently to clarify the role of fenretinide in controlling and preventing malignancy (19–22).
A recent clinical trial demonstrated the efficacy of high-dose cRA in delaying time to progression and increasing survival in patients with malignant gliomas (23). This result was possibly related to the diverse effects that retinoids exhibit against malignant cells, including induction of differentiation and inhibition of angiogenesis (24). On the basis of previous reports that fenretinide induces both differentiation and apoptosis in comparison to cRA, we hypothesized that fenretinide may demonstrate activity against gliomas. In this study, we compare the activity of fenretinide to that of cRA in vitro using concentrations that are clinically achievable. We demonstrate that fenretinide is superior in its in vitro activity against glioma cell lines compared with cRA. We also show that this effect is predominantly based on its ability to induce apoptosis by caspase activation. Our results suggest a strong potential for fenretinide as a novel therapeutic agent against malignant gliomas.
MATERIALS AND METHODS
Cell Lines
The cytotoxicity assays were performed using four human glioma cell lines: U87MG, U251, D54, and EFC-2. U87MG was commercially obtained (American Type Culture Collection, Rockville, MD). The D54 cell line was a generous gift of Dr. Darrell Bigner (Duke University, Durham, IL). The EFC-2 cell line, developed at the University of Texas M. D. Anderson Cancer Center, was derived from a glioblastoma. All cell lines were established from glioblastomas. Cells were maintained in DMEM-F12 (1:1 v/v) medium (Sigma Chemical Co., St. Louis, MO) supplemented with 5% FCS at 37°C in a humidified atmosphere containing 5% CO2.
Drug Treatment
cRA (Sigma Chemical Co.) and fenretinide (Cilag A.G., Schaffhausen, Switzerland) were prepared as stock solutions in DMSO (with the final concentration of DMSO being <0.1% during treatment). Freshly prepared dilutions were used for each experiment. Drug addition was done in yellow light because of the photosensitivity of the drugs. Dose response and time course experiments were initially carried out in three cell lines (U87MG, U251, and EFC-2) using a range of concentrations from 0.1 to 100 µM for each drug. A second set of experiments with clinically relevant drug concentrations, i.e., 1, 3, and 5 µM, were performed by continuously exposing D54 cells to the respective drug. The effect on cell proliferation was determined by harvesting the cells at the time points mentioned above and counting them in a hemocytometer.
Cell Morphology
The morphology of cells treated with fenretinide (3 µM) was studied using the immunofluorescent nuclear stain DAPI. D54 cells were plated in two-chamber slides (Nalge Nunc International, Naperville, IL) and treated with fenretinide 24 h after plating. The treated cells and untreated control cells were harvested on days 3 and 6, fixed with 1% paraformaldehyde for 15 min, washed with PBS and stained with DAPI for 5 min. Cell morphology was visualized by fluorescent microscopy using a 340-nm excitation filter.
Flow Cytometry
D54 cells were plated at a density of 105 cells/dish in 100-mm dishes. Fenretinide (3 µM) was added 24 h after plating the cells. The cells were harvested at 24, 72, and 96 h and fixed in 70% ethanol. The cells were subsequently treated with propidium iodide (50 mg/ml) and RNase (20 mg/ ml) and subjected to flow cytometry using a EPICS II flow cytometer (Coulter Corp., Hialeah, FL) equipped with an aircooled argon ion laser and emitting at a wavelength of 488 nm at 15 MW. A minimum of 10,000 events was analyzed per sample using the Coulter cytological program. Cells were sorted according to their DNA content into various phases of the cell cycle with the sub-G1 cell population representing the apoptotic fraction.
TUNEL Assay
A TUNEL assay was performed using the APO-DIRECT kit according to the protocol recommended by the manufacturer (Phoenix Flow Systems, Inc., San Diego, CA). Briefly, D54 cells at a density of 105 cells were harvested after treatment with fenretinide as described in flow cytometric studies. The cells were fixed using freshly prepared 1% paraformaldehyde in PBS and incubated in a staining solution containing TdT enzyme, fluorescein-dUTP, and TdT reaction buffer for 1 h at 37°C. Following this, the cells were rinsed and resuspended in a propidium iodide/RNase A solution prior to flow cytometric analysis.
Western Blot Analysis
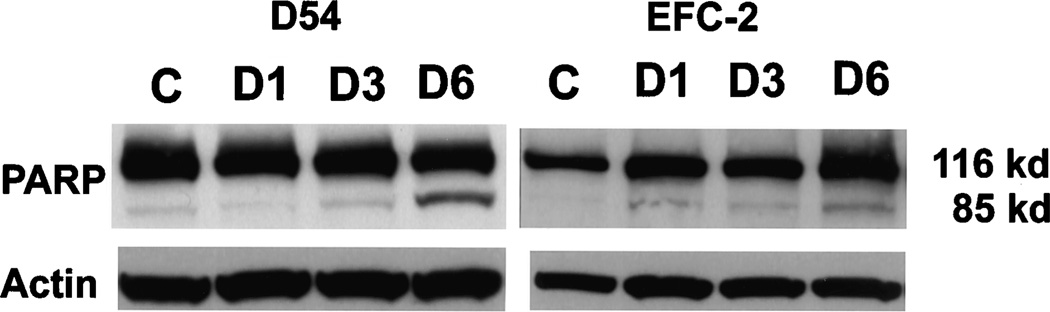
Cleavage of PARP and levels of expression of cpp32 were studied by Western blot analysis at the indicated times after exposure to drug (Fig. 4). Briefly, equal amounts of protein from fenretinide-treated and untreated D54 cells were subjected to SDS-PAGE and then transferred to a nitrocellulose membrane (Schleicher and Schuell Inc., Keene, NH). The membrane was blocked using Blotto-Tween (5% nonfat dry milk, 0.05% Tween, 0.9% NaCl, and 50 mM Tris, pH 7.5). The following antibodies were used to detect the respective protein: anti-human cpp32 rabbit polyclonal IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-human PARP mouse monoclonal IgG (PharMingen, San Diego, CA) used in a 1:2000 dilution. Horseradish peroxidase-conjugated donkey anti-rabbit and goat anti-mouse antibodies (Amersham Corp., Arlington Heights, IL) were used as secondary antibodies. Actin levels were determined as a loading control using a mouse monoclonal antihuman β-actin antibody (Amersham Corp.).
Fig. 4.
PARP cleavage in fenretinide-treated D54 cells. Western blot analysis of D54 cell lysates demonstrated cleavage of PARP, indicating activation of caspases. The effect was minimal at day 1 and increased to a maximum by day 6.
RESULTS
Comparison of Effects of cRA and Fenretinide on Glioma Cell Growth
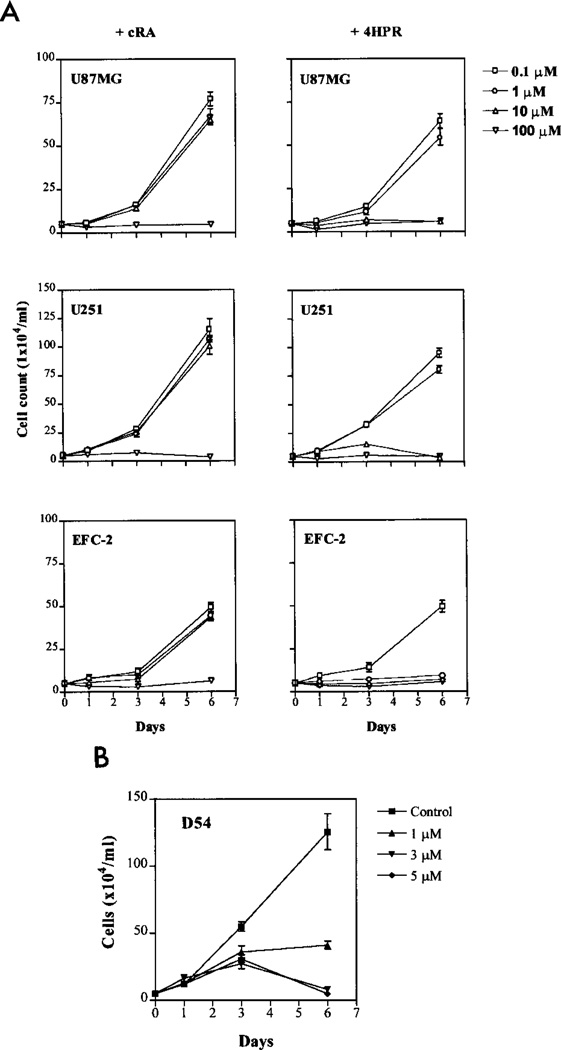
We compared the effect of exposing four different glioma cell lines to cRA and to fenretinide in time course and dose-response experiments (Fig. 1A). Dose-dependent growth inhibition was seen with both agents in all cell lines. However, fenretinide treatment resulted in pronounced growth inhibition at 10 µM concentration, whereas this degree of inhibition was seen only at 100 µM concentration with cRA, suggesting one log-order higher potency for fenretinide compared with cRA against glioma cells. All of the cell lines tested were seen to be equally susceptible to the effects of the two agents.
Fig. 1.
A dose-response curves for cRA and fenretinide. The cells were plated in six-well dishes at a density of 5 × 105 cells/well, treated with either cRA or fenretinide at concentrations of 0.1–100 µM, and harvested on days 1, 3, and 6. Cell survival was determined by the trypan blue exclusion method. B, dose-response curves for D54 cells at pharmacologically relevant concentrations of fenretinide by the trypan blue exclusion method. Bars, SE.
To determine whether the effect of fenretinide occurred at clinically relevant concentrations, D54 glioma cells were treated with the agent at concentrations of 1, 3, and 5 µM. Dose-dependent growth inhibition was again seen (Fig. 1B). The inhibitory effect at both the 3 and 5 µM concentrations was similar to that achieved by the 10 µM concentration for fenretinide and 100 µM concentration for cRA. On the basis of this result, fenretinide was added at a concentration of 3 µM in subsequent experiments.
Changes in Cell Morphology
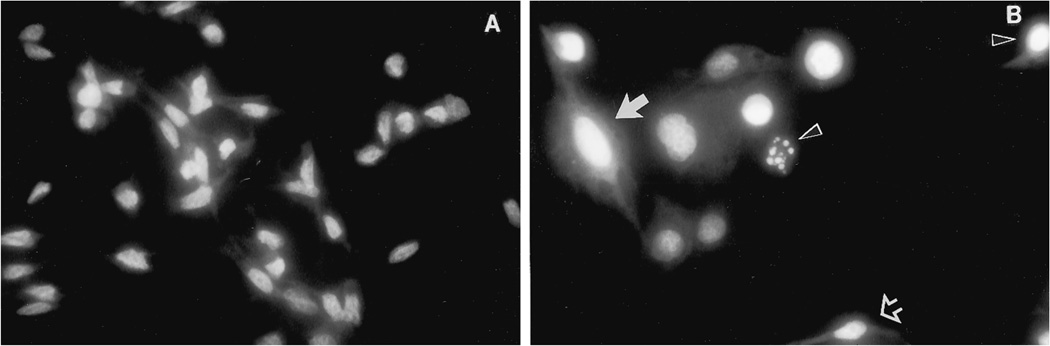
D54 cells treated with 3 µM fenretinide demonstrated morphological changes within 72 h of drug addition. Cell detachment was seen by light microscopy by day 3, with individual cells assuming a rounded appearance and often demonstrating membrane blebbing. With DAPI staining, three types of morphological appearance were evident: small cells with chromatin condensation, shrinkage of the cytoplasm, and the formation of apoptotic bodies, typical of apoptosis; large flattened cells without nuclear condensation suggestive of senescent cells; and cells that were similar to control cells that appeared unaffected by drug exposure (Fig. 2).
Fig. 2.
Morphological appearance of D54 cells with and without treatment with 3 µM fenretinide. The cells were stained with DAPI, and their morphology was visualized by fluorescence microscopy. A, untreated control cells. B, fenretinide-treated cells: three types of cell morphology were seen; small cells with chromatin condensation, shrinkage of the cytoplasm, and the formation of apoptotic bodies, typical of apoptosis (black arrowhead); large flattened cells without nuclear condensation suggestive of senescence (solid white arrow); and small cells similar to control cells that appeared unchanged after drug exposure (open white arrow).
Cell Cycle Kinetics and DNA Fragmentation
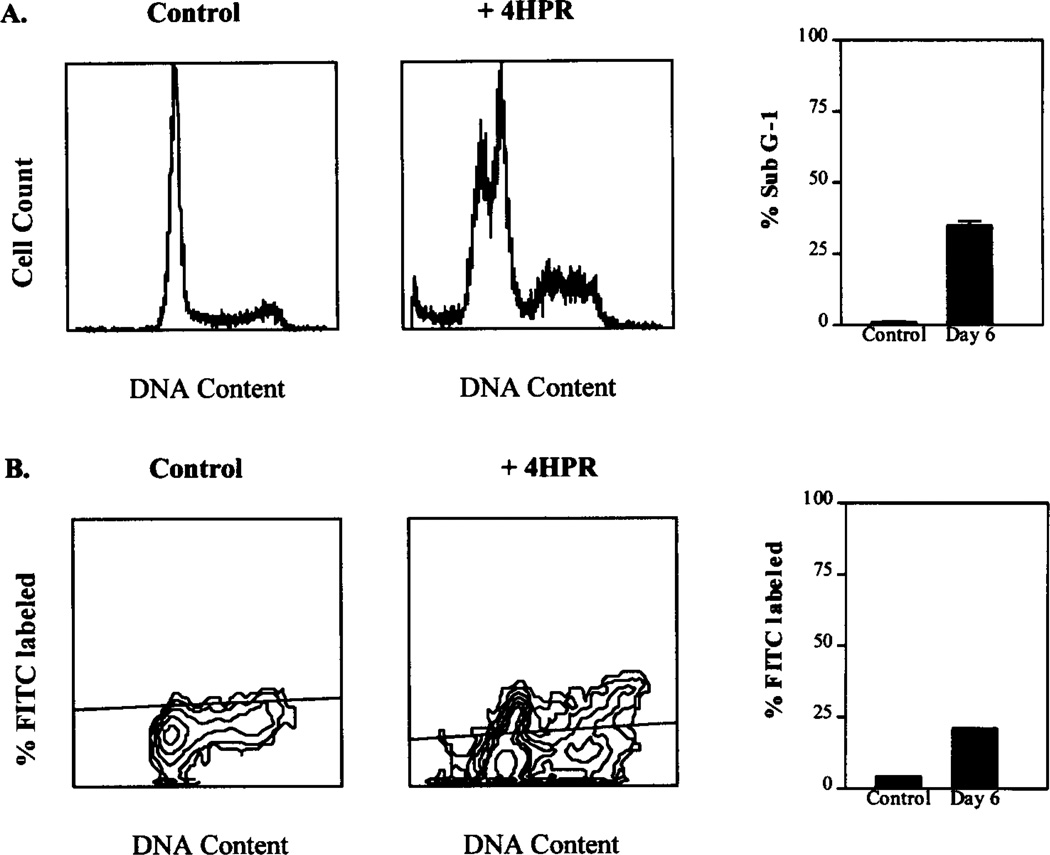
To determine whether the growth inhibition seen in proliferation assays was a result of the cytostatic or the cytotoxic effects of fenretinide, we studied the cell cycle distribution and occurrence of DNA fragmentation in D54 cells treated with 3 µM fenretinide. Flow cytometric analysis demonstrated the appearance of a sub-G1 fraction indicative of apoptosis on day 6 of fenretinide treatment without other changes in the cell cycle distribution (Fig. 3A). The TUNEL assay demonstrated the occurrence of DNA fragmentation by day 6 of fenretinide treatment (Fig. 3B).
Fig. 3.
A, effects of fenretinide (3 µM) on cell cycle kinetics of D54 cells. Control and fenretinide-treated D54 cells were harvested on days 1, 3, and 6 and studied by flow cytometric analysis. Fenretinide-treated cells showed no changes in cell cycle distribution compared with control cells. A sub-G1 population indicating cells with fragmented DNA (<2N) was seen in fenretinide-treated cells by day 3 and increased to a maximum by day 6. Bars, SE. B, D54 cells treated with 3 µM fenretinide were harvested on days 3 and 6 with untreated control cells and studied by TUNEL assay using the APODIRECT kit. FITC-labeled cells representing the apoptotic subpopulation undergoing DNA fragmentation were quantified. Apoptosis was seen maximally by day 6 and occurred in a cell cycle-independent manner as shown.
Induction of Caspases by Fenretinide
We determined the effect of fenretinide treatment on PARP, a downstream target of cpp32, to define whether fenretinide-induced apoptosis was mediated through caspase activation. Western blot analysis of D54 and EFC-2 cell lysates obtained on days 1, 3, and 6 after exposure to 3 µM fenretinide showed evidence for PARP cleavage with detectable levels of the cleavage product being evident by day 1 and increasing incrementally by day 6 (Fig. 4).
DISCUSSION
The development of most retinoids as anticancer agents was significantly hindered by the adverse effects associated with their use, including dermatological and hepatic toxicity. Fenretinide, however, has been reported to be tolerated well by humans at concentrations of 1–3 µM that are clinically achievable (25–27). In contrast to the other retinoids, this agent was seen to accumulate not in the liver but instead in tissues with a high adipose content such as the breast (22, 28). Preclinical studies have suggested a lower incidence of adverse effects with fenretinide compared with other retinoids. Hence, it is increasingly entering the clinical trial phase as a chemopreventive agent (6, 17, 20, 21, 29).
The relevance of fenretinide as therapy for gliomas lies in the efficacy of this agent in preventing tumor recurrence and development of new tumors in animal models of carcinogenesis (7, 15, 16). The results of preclinical studies indicate that the retinoid-mediated inhibition of cancer resulted from blocking the progression of early lesions. In gliomas, morbidity and mortality is usually caused by progression of residual tumor after initial surgery and radiotherapy (30, 31). Agents that can potentially prevent progression of tumor would hence be expected to have a significant impact on the prognosis. Synthetic retinoids such as fenretinide appear to have the potential for preventing glioma progression and inducing differentiation similar to their effects in other human malignancies in preclinical studies and early clinical trials. Furthermore, fenretinide appears to inhibit cell proliferation by inducing apoptosis independent of cell cycle status and hence could better target the heterogeneous cell populations in a malignant glioma compared with conventional cytotoxic agents. Additionally, a recent clinical study in our institution had demonstrated that cRA administered at a high dose was effective in eliciting tumor response and prolonging both time to progression and survival in patients with anaplastic astrocytomas and glioblastomas independent of age (23). We hypothesized that the fenretinide would be superior to cRA in inhibiting glioma cell growth and would induce apoptosis in gliomas. We tested the effect of fenretinide at a clinically achievable serum concentration of 3 µM, although higher concentrations are potentially attainable in lipophilic tissues.
The results of the present study indicate that fenretinide has activity against glioma cells in vitro. We show that fenretinide induces apoptosis in glioma cells by activating the caspase cascade as demonstrated by cleavage of PARP, a downstream target of cpp32. The effects of cRA are known to be predominantly mediated through its interaction with the retinoic acid receptor and retinoid X receptor families of nuclear retinoid receptors. This interaction results in the transcriptional activation of several genes that promote cell differentiation and inhibit cell growth. The mediators of the cellular effects of fenretinide, however, are poorly understood. Previous studies have shown activation of caspases after fenretinide treatment. This effect has been shown to occur independently of nuclear retinoid receptor binding and appears to occur via a distinct apoptotic pathway activated either directly by fenretinide or by one of its metabolites (32, 33).
Fenretinide did not induce changes in the cell cycle kinetics of glioma cells in this study. Marth et al. (10) reported a G1 phase accumulation of breast cancer cells after 4–7 days of fenretinide or retinoic acid exposure at a concentration of 1 µM. Several investigators also have reported a G1 phase accumulation in other cell lines with both fenretinide and retinoic acid (32, 34, 35). In these studies, it was suggested that the growth-inhibitory effect of fenretinide was a result of both the alteration of cell cycle kinetics as well as induction of apoptosis. However, in glioma cells, the absence of a G1 phase cell cycle arrest suggests that this effect may be tissue specific. Hence, the greater potency of growth inhibition seen with fenretinide in our study appeared to be the result of induction of apoptosis compared with cRA, which predominantly causes cellular differentiation (36).
Fenretinide is a highly lipophilic agent that would be expected to easily traverse the blood-brain barrier and achieve significant concentrations in the brain. It can be p.o. administered and is well tolerated in humans, based on recent trials in other cancers. It appears to be more potent in vitro than natural retinoids (8, 37), some of which (all-trans retinoic acid and cRA) appear to have efficacy against malignant gliomas (23, 38–40). These characteristics, combined with the results of our study, suggest that fenretinide could potentially be a valuable agent in the treatment of malignant gliomas both as a single agent as well as in combination with cytotoxic therapies presently in use.
ACKNOWLEDGMENTS
We thank Vickie J. Williams for editorial assistance.
Footnotes
The abbreviations used are: fenretinide, N-[4-hydroxyphenyl] retinamide or 4HPR; cRA, 13-cis-retinoic acid; DAPI, 4′,6-diamidine-2′-phenylindole dihydrochloride; TUNEL, terminal deoxynucleotidyl transferase-mediated nick end labeling; PARP, poly(ADP-ribose) polymerase.
REFERENCES
- 1.Brock CS, Bower M. Current perspectives in gliomas. Med. Oncol. 1997;14:103–120. doi: 10.1007/BF02990955. [DOI] [PubMed] [Google Scholar]
- 2.Yung WK. New approaches to molecular therapy of brain tumors. Curr. Opin. Neurol. 1994;7:501–505. doi: 10.1097/00019052-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Man T. Solid tumours–chemoprevention with retinoids. Leukemia (Baltimore) 1994;8:1785–1790. [PubMed] [Google Scholar]
- 4.David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol. Adverse Drug Exp. 1988;3:273–288. doi: 10.1007/BF03259940. [DOI] [PubMed] [Google Scholar]
- 5.van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, van de Kerkhof PC, Yap SH. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin) J. Hepatol. 1990;11:185–188. doi: 10.1016/0168-8278(90)90111-4. [DOI] [PubMed] [Google Scholar]
- 6.Costa A, De Palo G, Decensi A, Formelli F, Chiesa F, Nava M, Camerini T, Marubini E, Veronesi U. Retinoids in cancer chemoprevention. Clinical trials with the synthetic analogue fenretinide. Ann. NY Acad. Sci. 1995;768:148–162. doi: 10.1111/j.1749-6632.1995.tb12118.x. [DOI] [PubMed] [Google Scholar]
- 7.Moon RC, Thompson HJ, Becci PJ, Grubbs CJ, Gander RJ, Newton DL, Smith JM, Phillips SL, Henderson WR, Mullen LT, Brown CC, Sporn MB. N-(4-Hydroxyphenyl) retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39:1339–1346. [PubMed] [Google Scholar]
- 8.Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F, Formelli F, Menard S, Costa A, Veronesi U. N-(4-Hydroxyphenyl) retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res. 1993;6036;53 [PubMed] [Google Scholar]
- 9.Ponzoni M, Bocca P, Chiesa V, Decensi A, Pistoia V, Raffaghello L, Rozzo C, Montaldo PG. Differential effects of N-(4-hydroxyphenyl)retinamide and retinoic acid on neuroblastoma cells: apoptosis versus differentiation. Cancer Res. 1995;55:853–861. [PubMed] [Google Scholar]
- 10.Marth C, Bock G, Daxenbichler G. Effect of 4-hydroxyphenylretinamide and retinoic acid on proliferation and cell cycle of cultured human breast cancer cells. J. Natl. Cancer Inst. 1985;75:871–875. doi: 10.1093/jnci/75.5.871. [DOI] [PubMed] [Google Scholar]
- 11.Roberson KM, Penland SN, Padilla GM, Selvan RS, Kim CS, Fine RL, Robertson CN. Fenretinide: induction of apoptosis and endogenous transforming growth factor β in PC-3 prostate cancer cells. Cell Growth Differ. 1997;8:101–111. [PubMed] [Google Scholar]
- 12.Supino R, Crosti M, Clerici M, Warlters A, Cleris L, Zunino F, Formelli F. Induction of apoptosis by fenretinide (4HPR) in human ovarian carcinoma cells and its association with retinoic acid receptor expression. Int. J. Cancer. 1996;65:491–497. doi: 10.1002/(SICI)1097-0215(19960208)65:4<491::AID-IJC17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Oridate N, Lotan D, Mitchell MF, Hong WK, Lotan R. Inhibition of proliferation and induction of apoptosis in cervical carcinoma cells by retinoids: implications for chemoprevention. J. Cell. Biochem. Suppl. 1995;23:80–86. doi: 10.1002/jcb.240590911. [DOI] [PubMed] [Google Scholar]
- 14.Scher RL, Saito W, Dodge RK, Richtsmeier WJ, Fine RL. Fenretinide-induced apoptosis of human head and neck squamous carcinoma cell lines. Otolaryngol. Head Neck Surg. 1998;118:464–471. doi: 10.1177/019459989811800406. [DOI] [PubMed] [Google Scholar]
- 15.Dowlatshahi K, Mehta RG, Thomas CF, Dinger NM, Moon RC. Therapeutic effect of N-(4-hydroxyphenyl)retinamide on N-methyl-N-nitrosourea-induced rat mammary cancer. Cancer Lett. 1989;47:187–192. doi: 10.1016/0304-3835(89)90089-x. [DOI] [PubMed] [Google Scholar]
- 16.Pienta KJ, Nguyen NM, Lehr JE. Treatment of prostate cancer in the rat with the synthetic retinoid fenretinide. Cancer Res. 1993;53:224–226. [PubMed] [Google Scholar]
- 17.Veronesi U, De Palo G, Costa A, Formelli F, Decensi A. Chemoprevention of breast cancer with fenretinide. IARC. Sci Publ. 1996;136:87–94. [PubMed] [Google Scholar]
- 18.Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G, Moglia D, De Palo G, Costa A, Veronesi U. Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J. Clin. Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 19.Kelloff GJ, Crowell JA, Boone CW, Steele VE, Lubet RA, Greenwald P, Alberts DS, Covey JM, Doody LA, Knapp GG. Clinical development plan: N-(4-hydroxyphenyl)retinamide. J. Cell. Biochem. Suppl. 1994;20:176–196. [PubMed] [Google Scholar]
- 20.Modiano MR, Dalton WS, Lippman SM, Joffe L, Booth AR, Meyskens FLJ. Phase II study of fenretinide (N-[4-hydroxyphenyl]retinamide) in advanced breast cancer and melanoma. Invest. New Drugs. 1990;8:317–319. doi: 10.1007/BF00171846. [DOI] [PubMed] [Google Scholar]
- 21.Pienta KJ, Esper PS, Zwas F, Krzeminski R, Flaherty LE. Phase II chemoprevention trial of oral fenretinide in patients at risk for adenocarcinoma of the prostate. Am. J. Clin. Oncol. 1997;20:36–39. doi: 10.1097/00000421-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 22.De Palo G, Camerini T, Marubini E, Costa A, Formelli F, Del Vecchio M, Mariani L, Miceli R, Mascotti G, Magni A, Campa T, Di Mauro MG, Attili A, Maltoni C, Del Turco MR, Decensi A, D’Aiuto G, Veronesi U. Chemoprevention trial of contralateral breast cancer with fenretinide. Rationale, design, methodology, organization, data management, statistics and accrual. Tumori. 1997;83:884–894. doi: 10.1177/030089169708300603. [DOI] [PubMed] [Google Scholar]
- 23.Yung WK, Kyritsis AP, Gleason MJ, Levin VA. Treatment of malignant gliomas with high dose 13-cis retinoic acid. Clin. Cancer Res. 1996;2:1931–1935. [PubMed] [Google Scholar]
- 24.Majewski S, Szmurlo A, Marczak M, Jablonska S, Bollag W. Inhibition of tumor cell-induced angiogenesis by retinoids, 1,25-dihydroxyvitamin D3 and their combination. Cancer Lett. 1993;75:35–39. doi: 10.1016/0304-3835(93)90204-m. [DOI] [PubMed] [Google Scholar]
- 25.De Palo G, Formelli F. Risks and benefits of retinoids in the chemoprevention of cancer. Drug Saf. 1995;13:245–256. doi: 10.2165/00002018-199513040-00004. [DOI] [PubMed] [Google Scholar]
- 26.Paulson JD, Oldham JW, Preston RF, Newman D. Lack of genotoxicity of the cancer chemopreventive agent N-(4-hydroxyphenyl) retinamide. Fundam. Appl. Toxicol. 1985;5:144–150. doi: 10.1016/0272-0590(85)90058-2. [DOI] [PubMed] [Google Scholar]
- 27.Costa A, Malone W, Perloff M, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M, Andreoli C, Del Vecchio M. Tolerability of the synthetic retinoid fenretinide (HPR) Eur. J. Cancer Clin. Oncol. 1989;25:805–808. doi: 10.1016/0277-5379(89)90124-7. [DOI] [PubMed] [Google Scholar]
- 28.Hultin TA, Filla MS, McCormick DL. Distribution and metabolism of the retinoid, N-(4-methoxyphenyl)-all- trans-retinamide, the major metabolite of N-(4-hydroxyphenyl)-all-trans- retinamide, in female mice. Drug Metab. Dispos. 1990;18:175–179. [PubMed] [Google Scholar]
- 29.Decensi A, Bruno S, Costantini M, Torrisi R, Curotto A, Gatteschi B, Nicolo G, Polizzi A, Perloff M, Malone WF. Phase IIa study of fenretinide in superficial bladder cancer, using DNA flow cytometry as an intermediate end point. J. Natl. Cancer Inst. 1994;86:138–140. doi: 10.1093/jnci/86.2.138. [DOI] [PubMed] [Google Scholar]
- 30.Durmaz R, Erken S, Arslantas A, Atasoy MA, Bal C, Tel E. Management of glioblastoma multiforme: with special reference to recurrence. Clin. Neurol. Neurosurg. 1997;99:117–123. doi: 10.1016/s0303-8467(97)00014-0. [DOI] [PubMed] [Google Scholar]
- 31.Yung WK. Chemotherapy for malignant brain tumors. Curr. Opin. Oncol. 1990;2:673–678. [PubMed] [Google Scholar]
- 32.DiPietrantonio AM, Hsieh TC, Olson SC, Wu JM. Regulation of G1/S transition and induction of apoptosis in HL-60 leukemia cells by fenretinide (4HPR) Int. J Cancer. 1998;78:53–61. doi: 10.1002/(sici)1097-0215(19980925)78:1<53::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Piedrafita FJ, Pfahl M. Retinoid-induced apoptosis and Sp1 cleavage occur independently of transcription and require caspase activation. Mol. Cell. Biol. 1997;17:6348–6358. doi: 10.1128/mcb.17.11.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igawa M, Tanabe T, Chodak GW, Rukstalis DB. N-(4-Hydroxyphenyl) retinamide induces cell cycle specific growth inhibition in PC3 cells. Prostate. 1994;24:299–305. doi: 10.1002/pros.2990240605. [DOI] [PubMed] [Google Scholar]
- 35.Lotan R. Retinoids and apoptosis: implications for cancer chemoprevention and therapy. J. Natl. Cancer Inst. 1995;87:1655–1657. doi: 10.1093/jnci/87.22.1655. [DOI] [PubMed] [Google Scholar]
- 36.Dipietrantonio A, Hsieh TC, Wu JM. Differential effects of retinoic acid (RA) and N-(4-hydroxyphenyl) retinamide (4-HPR) on cell growth, induction of differentiation, and changes in p34cdc2, Bcl-2, and actin expression in the human promyelocytic HL-60 leukemic cells. Biochem. Biophys. Res. Commun. 1996;224:837–842. doi: 10.1006/bbrc.1996.1109. [DOI] [PubMed] [Google Scholar]
- 37.Zou CP, Kurie JM, Lotan D, Zou CC, Hong WK, Lotan R. Higher potency of N-(4-hydroxyphenyl)retinamide than all-trans-retinoic acid in induction of apoptosis in non-small cell lung cancer cell lines. Clin. Cancer Res. 1998;4:1345–1355. [PubMed] [Google Scholar]
- 38.Defer GL, Adle-Biassette H, Ricolfi F, Martin L, Authier FJ, Chomienne C, Degos L, Degos JD. All-trans retinoic acid in relapsing malignant gliomas: clinical and radiological stabilization associated with the appearance of intratumoral calcifications. J. Neurooncol. 1997;34:169–177. doi: 10.1023/a:1005701507111. [DOI] [PubMed] [Google Scholar]
- 39.Kaba SE, Langford LA, Yung WK, Kyritsis AP. Resolution of recurrent malignant ganglioglioma after treatment with cis-retinoic acid. J. Neurooncol. 1996;30:55–60. doi: 10.1007/BF00177443. [DOI] [PubMed] [Google Scholar]
- 40.Phuphanich S, Scott C, Fischbach AJ, Langer C, Yung WK. All-trans-retinoic acid: a phase II Radiation Therapy Oncology Group study (RTOG 91-13) in patients with recurrent malignant astrocytoma. J. Neurooncol. 1997;34:193–200. doi: 10.1023/a:1005765915288. [DOI] [PubMed] [Google Scholar]