Summary
Objective
We conducted a survey of nursing home physicians to learn about (1) the laboratory value thresholds that clinical event monitors should use to generate alerts about potential adverse drug events (ADEs); (2) the specific information to be included in the alerts; and (3) the communication modality that should be used for communicating them.
Methods
Nursing home physician attendees of the 2010 Conference of AMDA: The Society for Post-Acute and Long-Term Care Medicine.
Results
A total of 800 surveys were distributed; 565 completed surveys were returned and seven surveys were excluded due to inability to verify that the respondents were physicians (a 70% net valid response rate). Alerting threshold preferences were identified for eight laboratory tests. For example, the majority of respondents selected thresholds of ≥ 5.5 mEq/L for hyperkalemia (63%) and ≤ 3.5 without symptoms for hypokalemia (54%). The majority of surveyed physicians thought alerts should include the complete active medication list, current vital signs, previous value of the triggering lab, medication change in the past 30 days, and medication allergies. Most surveyed physicians felt the best way to communicate an ADE alert was by direct phone/voice communication (64%), followed by email to a mobile device (59%).
Conclusions
This survey of nursing home physicians suggests that the majority prefer alerting thresholds that would generally lead to fewer alerts than if widely accepted standardized laboratory ranges were used. It also suggests a subset of information items to include in alerts, and the physicians’ preferred communication modalities. This information might improve the acceptance of clinical event monitoring systems to detect ADEs in the nursing home setting.
Keywords: Nursing homes, therapeutic drug monitoring, clinical decision support systems, adverse drug event
1. Introduction
Drug-related injury is a common and costly problem among patients in the nursing home (NH) setting. These adverse drug events (ADEs) [1] are associated with an estimated 93,000 deaths annually and as much as $4 billion of excess healthcare expenditures [2–4]. As many as half of ADEs are the result of preventable errors occurring in the medication use process (e.g., prescribing, order communication, dispensing, administration, and monitoring) [4]. Data about preventable ADEs in NHs suggest that most (70–80%) are associated with monitoring errors which include inadequate laboratory evaluation of drug therapies or delayed/failed responses to signs or symptoms of drug toxicity [5, 6].
Medication monitoring by clinicians in the NH setting is difficult because NH patients are typically older, have greater medical comorbidity, are prescribed more medications, and suffer from greater functional and cognitive impairment than their community-dwelling counterparts [7, 8]. An additional challenge is that the NH healthcare workforce is generally understaffed and staff turnover rates are high [9–12]. Interventions that leverage informatics show promise for overcoming these obstacles. Of particular interest are clinical event monitors - systems that can detect ADEs by processing signals generated from laboratory test results and pharmacy orders [13–15]. In hospital and ambulatory care settings, these systems have been shown to prevent the development, progression, or mitigate the seriousness of ADEs by promoting the early detection of adverse events and an appropriate response [16–22]. Evidence from other care settings suggests that clinical event monitors might be particularly effective in the NH setting because that they can identify ADEs missed by clinicians more quickly and inexpensively than methods such as voluntary reporting (i.e., incident reports), direct observation of medication passes, and retrospective chart review [15, 23–27].
Although most NH facilities have yet to fully implement health information technology (HIT),[28] the majority generate laboratory, pharmacy, and Minimum Data Set [29] data in electronic format that can be used to build a clinical event monitoring system. However, careful research is necessary to ensure that the new systems have a positive impact on quality of care and do not result in operational inefficiencies such as alert burden and fatigue that have previously been identified as barriers to NH HIT interventions [30–32]. To this end, our investigative team determined a list of consensus-based triggers and tested their positive predictive values using a prototype clinical event monitoring system [33–35].
To translate the prototype system into production, we needed additional information to develop alerts that would be perceived specifically by NH physicians as clinically relevant, actionable, and communicated in a way that was consistent with their clinical workflow. The objective of this study was to survey NH physicians to better understand
the laboratory value thresholds that should be used to generate alerts about potential ADEs;
the specific information to be included in the alerts; and
physician’s preference for communicating the alerts.
2. Methods
2.1 Participants
Eligible participants included all 1,061 NH physician attendees of the 2010 Conference of AMDA: The Society for Post-Acute and Long-Term Care Medicine, held in Long Beach, California. Physicians were selected as the target audience based on their medical-legal responsibility for all medication prescribing and responding to ADEs in the NH setting.
2.2 Survey Development
We developed a multi-component paper survey iteratively with input from our health professional co-investigators. The final survey was tested prior to distribution by co-investigators and an additional eight geriatricians within the Division of Geriatric Medicine at the University of Pittsburgh who provide care in the NH setting. It is provided as supplemental data.
2.2.1 Laboratory Value Thresholds
In the laboratory value thresholds component of the survey, physicians were asked to respond to eight questions. The 8 lab studies were selected based on our prior research where they met any of the following criteria:
reached the highest degree of consensus agreement that if present that it is likely caused by an ADE [33];
had excellent positive predictive validity for the detection of ADEs [35]; or
if the laboratory study was abnormal, a clinical intervention could be carried out to mitigate it (i.e., they were actionable).
For each question, the physicians surveyed were to keep in mind that their responses were to be based on the laboratory and pharmacy data associated with a hypothetical 85-year-old NH resident who had been taking all medications for at least three months at the current dose (i.e., achieved steady state). The resident’s baseline labs were normal, and there were no previous ADEs, and/or drug-drug interactions. The respondents were asked to elect the single best abnormal laboratory value threshold at which the clinical event monitoring system should generate an alert about a potential ADE.
The laboratory/medication combinations included chemistry tests (drug-induced hyperkalemia, hypokalemia, hyponatremia, hypoglycemia, hypertransaminasemia, and acute kidney injury as indicated by high serum creatinine) as well as hematology tests (thrombocytopenia and elevated International Normalized Ratio (INR)). Each of the laboratory value response options and thresholds were derived from a variety of review articles, consensus statements and professional society white papers [36–46]. If there was disagreement across the aforementioned references, we used the Common Terminology Criteria for Adverse Events (CTCAE) developed and maintained by the National Cancer Institute [45]. These resources provided laboratory reference ranges that lie between the upper and lower limits constituting 95% of all values determined on a defined population of healthy individuals from all clinical settings.
2.2.2 Information to be Included in the Alerts
The goal of this part of the survey was to identify the information items that should be provided to physicians so that they would require no additional information or communication with the NH prior to responding to an ADE alert. Nineteen questions listed a patient- or facility-specific information item such as: admission/readmission date, active medication regimen, vital signs, allergy information, laboratory data preceding the alert, and history of same/similar ADE. For each question, the physician was presented with five response options ranging from “Strongly Disagree” to “Strongly Agree.”
2.2.3 Preference for Communication Modality for Alerts
In this part of the survey, physicians were asked to respond to six questions indicating their degree of agreement that a particular communication modality was the best way to communicate an ADE alert in real-time without disrupting the physician’s work-flow. The communication modalities included: alphanumeric pager, direct phone/voice communication, email accessible from a personal computer, email accessible from a smartphone, electronic medical record system, and fax machine. For each of these questions, the physician was presented with five response options ranging from “Strongly Disagree” to “Strongly Agree.” There were no open-ended response options provided throughout the survey.
2.3 Survey Distribution
Following University of Pittsburgh institutional review board approval, the survey was distributed at the 2010 AMDA conference to a total of 800 physicians. Respondents who returned a completed survey by the end of the conference were given a complimentary AMDA-endorsed Clinical Practice Guideline worth approximately $15.
2.4 Data analysis
Completed surveys were manually transferred using dual data entry from paper to a Microsoft® ACCESS database for analysis. We used frequencies and percentages to summarize survey responses and bar charts for graphical summarization. SAS® version 9.2 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
3. Results
3.1 Survey Participants
A total of 800 surveys were distributed; 565 completed surveys were returned and seven surveys were excluded due to inability to verify that the respondents were physicians (a 70% net valid response rate). The majority (89%) of surveyed physicians provided medical direction in at least one NH. As ▶Table 1 shows, most were male (69%) and doctors of allopathic medicine (90%), the majority completed an internal medicine residency program (56%), approximately one-third (34%) completed a fellowship in geriatric medicine, and almost half had been practicing clinical medicine for >20 years (46%). Most (57%) reported spending at least 25% of their clinical time providing care in the NH. Seven respondents indicated that they did not provide care in any NH. These observations were retained because, as medical directors, these individuals would still be responsible for setting institutional policies and procedures.
Table 1.
Physician and Practice Characteristics of Survey Respondents
| Characteristic | No. | % |
|---|---|---|
| Gender | ||
| Male | 384 | 69% |
| Female | 172 | 31% |
| No Response | 2 | <1% |
| Medical School Training | ||
| Allopathic physicians (MD) | 504 | 90% |
| Osteopathic physicians (DO) | 44 | 8% |
| Unspecified | 10 | 2% |
| Residency Training* | ||
| Internal medicine | 310 | 56% |
| Family medicine | 214 | 38% |
| Currently a resident/fellow | 21 | 4% |
| Fellowship Training* | ||
| Geriatrics fellowship | 192 | 34% |
| Number of Years Practicing Medicine | ||
| 1 to 10 years | 122 | 22% |
| 11 to 20 years | 140 | 25% |
| >20 years | 258 | 46% |
| Still in training | 36 | 6% |
| No Response | 2 | <1% |
| Percentage of clinical time in the NH | ||
| None-10% | 64 | 12% |
| 10–25% | 175 | 31% |
| 26–50% | 139 | 25% |
| 51–75% | 72 | 13% |
| >75% | 108 | 19% |
| Number of NHs where clinical care is provided | ||
| 1 | 166 | 30% |
| 2–5 | 296 | 53% |
| >5 | 88 | 16% |
| No response | 8 | 1% |
NH = nursing home
*Residency and Fellowship training responses add up to more or less than 100% due to multiple responses and omitted responses, respectively. Others may not add up to 100% due to roundin
3.2 Laboratory Value Thresholds
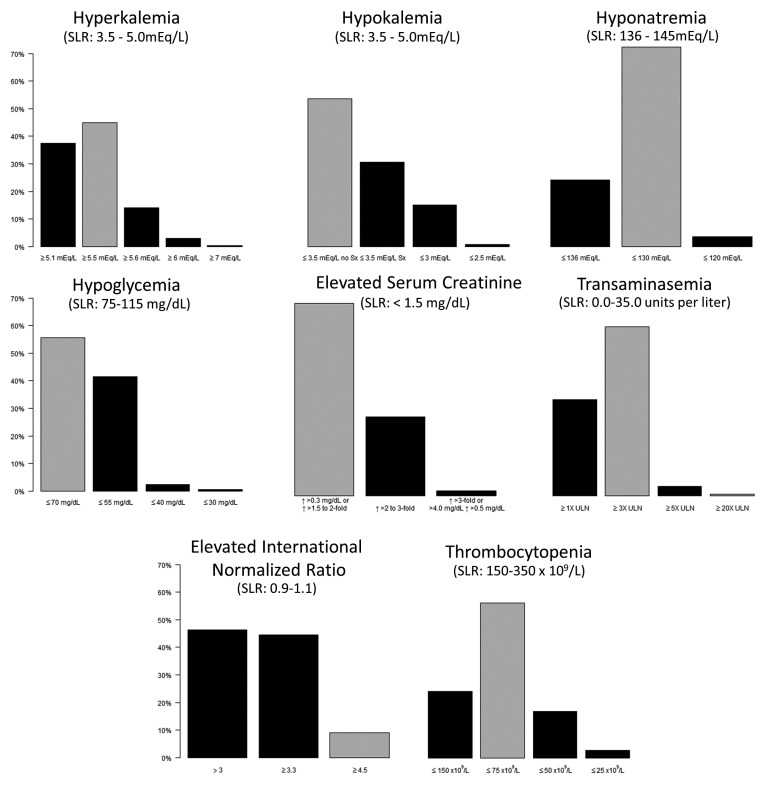
▶Figure 1 shows the percentage of NH physicians surveyed who prefer to be notified about a potential ADE by a clinical event monitoring system at a given laboratory threshold for the eight laboratory tests. For the chemistry lab tests, the majority of respondents selected thresholds of ≥5.5 mEq/L for hyperkalemia (a combined 63%) and ≤3.5 without symptoms for hypokalemia (54%). Seventy percent of respondents selected ≤130 mEq/L as the threshold for hyponatremia. For hypoglycemia, the two most commonly selected thresholds were ≤55 mg/dL and ≤70 mg/dL by 40% and 55% of respondents, respectively. The threshold selected for serum creatinine by the majority of respondents (70%) was an increase of >0.3 mg/dL or a 1.5 to 2-fold increase in serum creatinine. For transaminasemia, 61% of physicians selected a threshold of ≥3 times the upper limit of normal. For the hematology tests, the majority of respondents selected thresholds >3.3 for INR (a combined percentage of 54%) and ≤75 x 109/L as the platelet count threshold for thrombocytopenia (56%).
Fig. 1.
The percentage of nursing home physicians surveyed who prefer to be notified about a potential adverse drug event by an active medication monitoring system at a given laboratory threshold. Standardized laboratory ranges (SLRs) are shown for those laboratory test if they were reported in in a widely cited reference [47]. The gray bars show the threshold chosen for use in a clinical event monitoring system being tested in five nursing homes (see Discussion). ULN – upper limit of normal.
3.3 Information to be Included in the Alerts
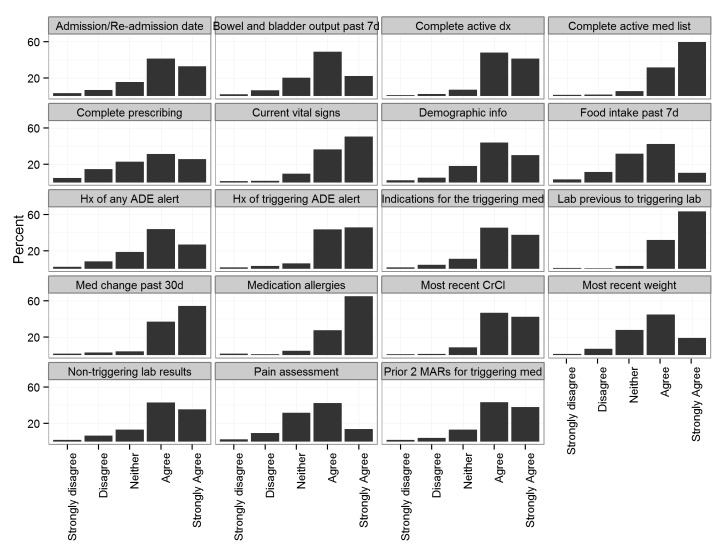
▶Figure 2 shows the distribution of physician preferences on information items to be included with a drug/lab ADE alert. While general agreement (i.e., more than 50% of physicians agreed or strongly agreed) was indicated for the inclusion of all surveyed items, strong agreement by the majority of surveyed physicians was found for showing the complete active medication list, current vital signs, previous value of the triggering lab to the value that triggered the alert, medication change in the past 30 days, and medication allergies. At least one-fifth of physicians were ambivalent about the inclusion of complete prescribing information, last 7 days food intake, and a pain assessment. The survey included an open-ended question soliciting “other information that should be included in the alert.” Several themes emerged from the respondents including:
Fig. 2.
Physician preferences on information items to be included with a drug/lab ADE alert. Abbreviations: d – days, dx – diagnosis, med – medication, info – information, Hx – history, lab – laboratory result, CrCl – creatinine clearance, MAR – medication administration record.
clarification of the patient’s advanced directives or code status;
listing who the ordering clinician was;
stating the reason or indication as to why the lab test was ordered; and
the signs or symptoms associated with the laboratory abnormality (e.g., fall, bleeding, mental status changes).
3.4 Preference for Communication Modality for Alerts
The communication modality that most of the surveyed physicians agreed or strongly agreed would be the best way to communicate an ADE alert was direct phone/voice communication (64%), followed by email to a mobile device (59%), an electronic medical record system (53%), fax machine (52%), alphanumeric pager (52%), and email accessible from a personal computer (43%).
4. Discussion
To the best of our knowledge, this is the first survey of laboratory thresholds for ADE alerting among generally highly experienced NH physicians. A strength of this study is that it provides guidance on the optimal thresholds, alert content, and communication preference for a care setting that is relatively understudied in spite of the large number of patients at risk for adverse events.
An important finding is that, for all but one of the laboratory tests (hypokalemia), the majority of NH physicians prefer alerting thresholds that would generally lead to fewer alerts than if widely accepted standardized laboratory ranges (SLRs) were used [47]. The clinicians’ preferred thresholds reflect intuitive estimates that might reduce false positive results and hence alert fatigue. However, further work is needed to establish the actual sensitivity, specificity, and positive predictive value of these alert thresholds when used in clinical practice. We are currently testing a system that uses the modal-selected thresholds chosen here (▶Figure 1) for all of the laboratory tests except for INR. Although the majority of respondents wanted the INR threshold for notification to be >3, our research group decided to use a higher cut-off ≥4.5. This higher level was selected primarily because recent evidence suggests that levels ≥4.5 are associated with increased risk of serious bleeding events and the American College of CHEST physician consensus guidelines provide specific recommendations using an INR cut-point of ≥4.5 as actionable [48].
Another important finding is that NH physicians strongly agree that five information items should be included in any laboratory ADE alert – complete active medication list, current vital signs, previous value of the triggering lab to value that triggered the alert, medication change in the past 30 days, and medication allergies. While there was strong agreement on other information items, these five reflect standard information needs that NH physicians have when reviewing an ADE alert that future clinical event monitors designed for the NH setting should address.
It is noteworthy that, of the six communication modalities, direct phone/voice communication and email to a mobile device were indicated by a greater proportion of NH physicians as the best ways to communicate an ADE alert. This finding suggests that alert communication by mobile device might be broadly accepted by NH physicians. The finding also is concordant with recent evidence that providing clinical event monitor alerts within the context of an electronic charting or order entry system is less likely to improve process or outcome measures [49]. Future work should explore if communication modality preference is influenced by age, gender, or other factors.
We previously reported that the proportion of physicians who use mobile devices to assist with prescribing is 42% [50], which is a rate lower than reported in other clinical environments [51]. However, it is likely that the rate of mobile device use with prescribing has increased since the survey was conducted. Further research is needed to better characterize the facilitators and barriers to adoption of technology in the NH and its precise impact on NH ADEs. The design of the alerts, workflow changes, and impact on ADEs will be reported in a separate publication.
A potential limitation of this study is that it was a descriptive cross-sectional survey of physicians sampled by convenience at a national conference. A high proportion of the NH physicians attending the AMDA Annual Symposium participated. However, they might not be representative of NH physicians in general. Other types of clinicians that prescribe (e.g., nurse practitioners or physician assistants), make prescribing recommendations (e.g., consultant pharmacists), or administer medications (e.g., nurses and medication technicians) were not included in this survey.
Another potential limitation is that, with the exception of acute kidney injury, the survey did not explore lab value changes that would be of concern to NH physicians. Future work should seek to fill in this knowledge gap and identify methods for tailoring laboratory alert triggering thresholds to specific clinical contexts. For example, acute kidney injury alerts for patients on hemodialysis might be more acceptable if they fired less frequently to account for expected weekly variations. A decision rule refinement approach similar to that used by Boussadi et al. to customize rules for medication dosage based on renal function thresholds might be applicable [52].
An alternate approach to probing physician’s desired information items would have been to ask participants to rank the items rather than consider them independently. While this approach might have led to a more concise list of preferred information items, we thought it would be difficult for participants to rank nineteen information items.
5. Conclusions
This survey of nursing home physicians suggests that the majority prefer alerting thresholds that would generally lead to fewer alerts than if widely accepted standardized laboratory ranges were used. It also suggests a subset of information items to include in alerts, and the physicians’ preferred communication modalities. This information might improve the acceptance of clinical event monitoring systems to detect ADEs in the nursing home setting.
Acknowledgments
We would like to thank the American Medical Directors Association for collaborating with us to allow the distribution of the survey at their annual symposium, Cleat Szczepaniak for survey distribution, Chad Kimmel for data entry, and Joseph T. Hanlon for his helpful comments on the manuscript. This study was supported by the Agency for Healthcare Research and Quality (R01HS018721, K12HS019461), a NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (KL2 RR024154–01), National Institute of Aging grants (R01AG027017; P30AG024827; K07AG033174, K01AG044433), and a VA Health Services Research grant (IIR-06–062). The content is solely the responsibility of the authors and does not represent the official views of the Agency for Healthcare Research and Quality or any of the other funding sources.
Footnotes
Clinical Relevance Statement:
Adverse drug events are particularly common among older nursing home residents. Clinical event monitoring systems that automate the detection and management of these events in this particularly vulnerable population might be particularly effective. This manuscript reports on an approach to involve physicians who practice in the nursing home in the development of a clinical event monitoring system. We anticipate that this approach should lead to increased acceptance, use and satisfaction associated with adverse drug event alerts generated by a laboratory-value based clinical event monitoring system.
Conflict of interest
The authors declare that they have no conflicts of interest in the research
Human Subjects Protections
Ethics approval was provided by the University of Pittsburgh Institutional Review Board.
References
- 1.Institute of Medicine. To Err Is Human: Building a Safer Health System. Kohn L, Corrigan J, Donaldson M, Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 2.Bootman JL, Harrison DL, Cox E.The health care cost of drug-related morbidity and mortality in nursing facilities. Arch Intern Med 1997; 157(18):2089–2096. [PubMed] [Google Scholar]
- 3.Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, Lee M, White K, LaPrino J, Erramuspe-Mainard J, DeFlorio M, Gavendo L, Baril JL, Reed G, Bates DW.Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc 2008; 56(12):2225–2233. [DOI] [PubMed] [Google Scholar]
- 4.Handler SM, Wright RM, Ruby CM, Hanlon JT.Epidemiology of medication-related adverse events in nursing homes. Am J Geriatr Pharmacother 2006; 4(3):264–272. [DOI] [PubMed] [Google Scholar]
- 5.Gurwitz JH, Field TS, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Edmondson AC, Bates DW.Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000; 109(2):87–94. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz JH, Field TS, Judge J, Rochon P, Harrold LR, Cadoret C, Lee M, White K, LaPrino J, Erramuspe-Mainard J, DeFlorio M, Gavendo L, Auger J, Bates DW.The incidence of adverse drug events in two large academic long-term care facilities. Am J Med 2005; 118(3):251–258. [DOI] [PubMed] [Google Scholar]
- 7.Ouslander JG, Bonner A.SNFs: Opening the Black Box. Web M&M: Morbidity and Mortality Rounds on the Web. 2013. Available from: http://www.webmm.ahrq.gov/case.aspx?caseID=313. Last Accessed 8/27/2014. [Google Scholar]
- 8.Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde R.Long-Term Care Services inthe United States: 2013 Overview. Hyattsville, MD: National Center for Health Statistics, 2013. [PubMed] [Google Scholar]
- 9.Castle NG, Engberg J, Men A.Nursing home staff turnover: impact on nursing home compare quality measures. Gerontologist 2007; 47(5):650–661. [DOI] [PubMed] [Google Scholar]
- 10.Decker F, Gruhn P, Matthews-Martin L, Dollard K, Dollard Tucker A, Bizette L.Results of the 2002 AHCA Survey of Nursing Staff Vacancy and Turnover in Nursing Homes. American Health Care Association, 2003. February 12, 2003. [Google Scholar]
- 11.Donoghue C.Nursing Home Staff Turnover and Retention An Analysis of National Level Data. J Appl Gerontol 2010; 29(1):89–106. [Google Scholar]
- 12.Handler S, Castle N, Studenski S, Perera S, Fridsma D, Nace D, Hanlon J.Patient safety culture assessment in the nursing home. Qual Saf Health Care 2006; 15(6):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Classen DC, Pestotnik SL, Evans RS, Burke JP.Computerized surveillance of adverse drug events in hospital patients. JAMA 1991; 266(20):2847–2851. [PubMed] [Google Scholar]
- 14.Jha AK, Kuperman GJ, Teich JM, Leape L, Shea B, Rittenberg E, Burdick E, Seger DL, Vander Vliet M, Bates DW.Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 1998; 5(3):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honigman B, Lee J, Rothschild J, Light P, Pulling RM, Yu T, Bates DW.Using computerized data to identify adverse drug events in outpatients. J Am Med Inform Assoc 2001; 8(3):254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality. Reducing and Preventing Adverse Drug Events To Decrease Hospital Costs. Rockville, MD: 2001AHRQ Publication Number 01–0020. [Google Scholar]
- 17.Evans RS, Pestotnik SL, Classen DC, Horn SD, Bass SB, Burke JP.Preventing adverse drug events in hospitalized patients. Ann Pharmacother 1994; 28(4):523–527. [DOI] [PubMed] [Google Scholar]
- 18.Hartis CE, Gum MO, Lederer JW., Jr.Use of specific indicators to detect warfarin-related adverse events. Am J Health Syst Pharm 2005; 62(16):1683–1688. [DOI] [PubMed] [Google Scholar]
- 19.Jha AK, Laguette J, Seger A, Bates DW.Can Surveillance Systems Identify and Avert Adverse Drug Events? A Prospective Evaluation of a Commercial Application. J Am Med Inform Assoc 2008; 15(5):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson JF, Williams DH, Seger A, Gandhi TK, Bates DW.Drug-lab triggers have the potential to prevent adverse drug events in outpatients. J Am Med Inform Assoc 2002; 9(6): S39–S40. [Google Scholar]
- 21.Raschke RA, Gollihare B, Wunderlich TA, Guidry JR, Leibowitz AI, Peirce JC, Lemelson L, Heisler MA, Susong C.A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA 1998; 280(15):1317–1320. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JB, Stapinski CD, Huber C, Ghandi TK, Churchill WW.Computer-based system for preventing adverse drug events. Am J Health Syst Pharm 2004; 61(15):1599–1603. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW.Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care 2004; 13(4):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy M, Azaz-Livshits T, Sadan B, Shalit M, Geisslinger G, Brune K.Computerized surveillance of adverse drug reactions in hospital: implementation. Eur J Clin Pharmacol 1999; 54(11):887–892. [DOI] [PubMed] [Google Scholar]
- 25.Murff HJ, Patel VL, Hripcsak G, Bates DW.Detecting adverse events for patient safety research: a review of current methodologies. J Biomed Inform 2003; 36(1–2): 131–143. [DOI] [PubMed] [Google Scholar]
- 26.Tegeder I, Levy M, Muth-Selbach U, Oelkers R, Neumann F, Dormann H, Azaz-Livshits T, Criegee-Rieck M, Schneider HT, Hahn E, Brune K, Geisslinger G.Retrospective analysis of the frequency and recognition of adverse drug reactions by means of automatically recorded laboratory signals. Br J Clin Pharmacol 1999; 47(5):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handler SM, Altman RL, Perera S, Hanlon JT, Studenski SA, Bost JE, Saul MI, Fridsma DB.A systematic review of the performance characteristics of clinical event monitor signals used to detect adverse drug events in the hospital setting. J Am Med Inform Assoc 2007; 14(4):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abramson EL, McGinnis S, Moore J, Kaushal R, investigators H. A statewide assessment of electronic health record adoption and health information exchange among nursing homes. Health Serv Res 2014; 49(1 Pt 2): 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin CM.Getting ready for MDS 3.0: patient evaluation takes a new turn. Consult Phar 2010; 25(7):404–406, 11–15. [DOI] [PubMed] [Google Scholar]
- 30.Judge J, Field TS, DeFlorio M, Laprino J, Auger J, Rochon P, Bates DW, Gurwitz JH.Prescribers’ responses to alerts during medication ordering in the long term care setting. J Am Med Inform Assoc 2006; 13(4):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochon PA, Field TS, Bates DW, Lee M, Gavendo L, Erramuspe-Mainard J, Judge J, Gurwitz JH.Computerized physician order entry with clinical decision support in the long-term care setting: insights from the baycrest centre for geriatric care. J Am Geriatr Soc 2005; 53(10):1780–1789. [DOI] [PubMed] [Google Scholar]
- 32.Kramer A, Richard AA, Epstein A, Winn D, May K.Understanding the costs and benefits of health information technology in nursing homes and home health agencies: case study findings. University of Colorado, Denver, 2009. [Google Scholar]
- 33.Handler SM, Hanlon JT, Perera S, Roumani YF, Nace DA, Fridsma DB, Saul MI, Castle NG, Studenski SA.Consensus list of signals to detect potential adverse drug reactions in nursing homes. J Am Geriatr Soc 2008; 56(5):808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handler SM, Hanlon JT.Detecting Adverse Drug Events Using a Nursing Home Specific Trigger Tool. Ann Longterm Care 2010; 18(5):17–22. [PMC free article] [PubMed] [Google Scholar]
- 35.Handler SM, Hanlon JT, Perera S, Saul MI, Fridsma DB, Visweswaran S, Studenski SA, Roumani YF, Castle NG, Nace DA, Becich MJ.Assessing the performance characteristics of signals used by a clinical event monitor to detect adverse drug reactions in the nursing home. AMIA Annu Symp Proc 2008: 278–282. [PMC free article] [PubMed] [Google Scholar]
- 36.Gennari FJ.Hypokalemia. N Engl J Med 1998; 339(7):451–458. [DOI] [PubMed] [Google Scholar]
- 37.Green RM, Flamm S.AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002; 123(4):1367–1384. [DOI] [PubMed] [Google Scholar]
- 38.Cryer PE, Davis SN, Shamoon H.Hypoglycemia in diabetes. Diabetes Care. 2003June;26(6):1902–12. [DOI] [PubMed] [Google Scholar]
- 39.Palmer BF.Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351(6):585–592. [DOI] [PubMed] [Google Scholar]
- 40.Holt MP, Ju C.Mechanisms of drug-induced liver injury. AAPS Journal 2006; 8(1): E48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurwitz JH, Field TS, Radford MJ, Harrold LR, Becker R, Reed G, DeBellis K, Moldoff J, Verzier N.The safety of warfarin therapy in the nursing home setting. Am J Med 2007; 120(6):539–544. [DOI] [PubMed] [Google Scholar]
- 42.Levin A, Warnock DG, Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco CAcute Kidney Injury Network Working G. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis 2007; 50(1):1–4. [DOI] [PubMed] [Google Scholar]
- 43.Wallach JB.Interpretation of diagnostic tests. 8th ed. Philadelphia: Wolters Kluwer Health/Lippincott Wiliams & Wilkins; 2007. [Google Scholar]
- 44.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G, American College of Chest P. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(6 Suppl): 160S-98S. [DOI] [PubMed] [Google Scholar]
- 45.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 National Institutes of Health, 2009NIH publication; # 09–7473 [Google Scholar]
- 46.Rose AJ, Ozonoff A, Berlowitz DR, Henault LE, Hylek EM.Warfarin dose management affects INR control. J Thromb Haemost 2009; 7(1):94–101. [DOI] [PubMed] [Google Scholar]
- 47.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values.[Erratum appears in N Engl J Med 2004; 351(23): 2461. N Engl J Med. 2004; 351(15):1548–1563. [DOI] [PubMed] [Google Scholar]
- 48.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH.Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl): e152S-e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roshanov PS, Fernandes N, Wilczynski JM, Hemens BJ, You JJ, Handler SM, Nieuwlaat R, Souza NM, Beyene J, Van Spall HG, Garg AX, Haynes RB.Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013; 346: f657. [DOI] [PubMed] [Google Scholar]
- 50.Garritty C, El Emam K.Who’s using PDAs? Estimates of PDA use by health care providers: a systematic review of surveys. J Med Internet Res 2006; 8(2): e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Handler SM, Boyce RD, Ligons FM, Perera S, Nace DA, Hochheiser H.Use and perceived benefits of mobile devices by physicians in preventing adverse drug events in the nursing home. J Am Med Dir Assoc 2013; 14(12):906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boussadi A, Caruba T, Zapletal E, Sabatier B, Durieux P, Degoulet P.A clinical data warehouse-based process for refining medication orders alerts. J Am Med Inform Assoc 2012; 19(5):782–775. [DOI] [PMC free article] [PubMed] [Google Scholar]