Our understanding of how feedbacks between plants and soil microbial communities may contribute to plant invasions and exotic dominance is limited by our understanding of how feedbacks may shift in the light of other ecological processes. In a greenhouse experiment, we found that the strength of plant–soil feedbacks shifted for both a native and exotic as soil microbial communities changed along a gradient of soil nitrogen (N) availability. Moreover, competition from an exotic grass minimized the beneficial feedback between the native grass and its soil microbial community when the soil community was from a high N environment.
Keywords: Avena fatua, California grasslands, competition, exotic species, native species, nitrogen enrichment, plant–soil feedbacks, Stipa pulchra.
Abstract
Feedbacks between plants and soil biota are increasingly identified as key determinants of species abundance patterns within plant communities. However, our understanding of how plant–soil feedbacks (PSFs) may contribute to invasions is limited by our understanding of how feedbacks may shift in the light of other ecological processes. Here we assess how the strength of PSFs may shift as soil microbial communities change along a gradient of soil nitrogen (N) availability and how these dynamics may be further altered by the presence of a competitor. We conducted a greenhouse experiment where we grew native Stipa pulchra and exotic Avena fatua, alone and in competition, in soils inoculated with conspecific and heterospecific soil microbial communities conditioned in low, ambient and high N environments. Stipa pulchra decreased in heterospecific soil and in the presence of a competitor, while the performance of the exotic A. fatua shifted with soil microbial communities from altered N environments. Moreover, competition and soil microbial communities from the high N environment eliminated the positive PSFs of Stipa. Our results highlight the importance of examining how individual PSFs may interact in a broader community context and contribute to the establishment, spread and dominance of invaders.
Introduction
Increasingly, feedbacks between plants and soil biota are being identified as key determinants of the abundance and composition of plant communities (Wardle et al. 2004; van der Putten et al. 2013). Negative feedbacks, where plant species are less productive in their ‘home’ soil biota, are thought to be important in the maintenance of plant diversity (Reynolds et al. 2003; Vogelsang et al. 2006) and promote species coexistence at small scales. Positive feedbacks, where species are more productive in ‘home’ soil biota, can contribute to species dominance and patch dynamics on a landscape scale (Chase and Leibold 2003; Shurin 2007). Introduced species seem to be exceptions to the rule, as soil biota is often found to have little impact on invasion success (Callaway et al. 2004; Inderjit and van der Putten 2010; Suding et al. 2013). However within the introduced range, the positive effects of ‘home’ soil biota may contribute to exotic dominance (Grman and Suding 2010). Translating demonstrated plant–soil feedbacks (PSFs) to abundance patterns has had varied results (Klironomos 2002; Yelenik and Levine 2011), as these effects are often considered in isolation from other ecological processes. Environmental factors can affect the dependency of plants on soil biota (Johnson et al. 2003) and the composition of the soil communities (Zeglin et al. 2007). However, the relative strength of these feedbacks may be small compared with interactions such as plant competition (Shannon et al. 2012). Addressing this context dependency of PSFs is key to our understanding of the role of PSFs in plant invasions and exotic dominance.
Soil nitrogen (N) enrichment, via fertilization, atmospheric deposition or other anthropogenic inputs, can alter soil microbial communities (Bissett et al. 2013) and facilitate plant invasions (Vitousek et al. 1997; Brooks 2003). Despite this evidence, our understanding of how feedbacks may shift in light of these changes to impact plant performance and subsequent invasion dynamics is limited. Under elevated soil N, microbial composition can shift towards a more bacterial dominated community (Bardgett et al. 1999; Allison 2002; Bradley et al. 2006; Zeglin et al. 2007) and can experience a loss of arbuscular mycorrhizal fungal (AMF) species within soil microbial communities (Egerton-Warburton et al. 2007; Liu et al. 2012). However, the net effect of these soil microbial community shifts on PSFs and invasions is unclear. In addition to these changes in soil microbial communities, host plant identity, which plays a significant role in dictating soil microbial community composition and feedback strength (Bardgett and Cook 1998; Hausmann and Hawkes 2009), can also shift in tandem with resources. For example, native and exotic species loss has been observed with increasing resource availability across multiple grassland systems, but resident natives had a greater likelihood of loss than exotics (Suding et al. 2005). Synergistic interactions between shifts in soil microbial communities due to altered resources and shifts in exotic abundance may result in enhanced PSFs that benefit the exotic vs. the native, contributing to invasion; yet these interactive effects are seldom studied.
Plant–soil feedbacks are often assessed at the individual plant level in isolation of other ecological processes such as plant–plant interactions, although they can jointly operate in regulating community diversity and abundance (Hodge and Fitter 2013). Plants can actively secrete compounds within their rhizosphere to promote the acquisition of resources (Hartmann et al. 2009), but the presence of the competitor can cause resources to be more limiting and potentially alter the magnitude of PSFs, either intensifying the PSF (Van der Putten and Peters 1997) or eliminating them (Casper and Castelli 2007). Scaling up individual plant responses to soil communities to the community level requires an understanding of how competitive hierarchies may interact with existing PSFs; however, only a handful of studies have investigated both (Van der Putten and Peters 1997; Casper and Castelli 2007; Hol et al. 2013) and rarely in the context of invasion (Yelenik and Levine 2011; Shannon et al. 2012).
Here, we propose that (i) soil microbial communities from differing resource environments and host plants and (ii) the interaction between plant competition and microbial community can influence the magnitude and direction of PSFs. We focus our study on California grasslands, which have experienced a large-scale shift from native perennial grasses mixed with annual forbs to exotic annual grasses over the last century (Jackson 1985), as well as an increase in atmospheric N deposition (Fenn et al. 2003). In this system, annual exotic grasses can shift the composition of soil microbial communities (Hawkes et al. 2005, 2006) and can alter the community of AMF colonizing roots of native grasses (Hausmann and Hawkes 2009, 2010), reducing the growth of native species (Vogelsang and Bever 2009).
We conducted a greenhouse experiment where we grew a native, Stipa pulchra, and exotic, Avena fatua (hereafter, Stipa and Avena, respectively), in soils inoculated with conspecific (‘home’) and heterospecific (‘away’) soil communities. To examine the interactive effects of resource environment and plant species identity on microbial communities, soil inocula were collected from a field experiment where Avena and Stipa plots had been treated with either carbon or N addition to alter soil resource availability. To examine the interaction between competitive interactions and microbial function on plant species performance, we grew plants individually or with a neighbour. We hypothesized that if positive PSFs contributed to invasion, then Avena would grow better in its ‘home’ soil than ‘away’ soil communities (note: we refer to ‘home’ soil as soils conditioned by the exotic in the introduced range vs. in its native range). Conversely, if Stipa were to grow better in its ‘home’ soil compared with ‘away’, positive PSFs would prevent invasion. Moreover, we hypothesized that soil communities from different soil resource environments would contribute to invasion if Avena were to grow better with soil communities from high N sites. Lastly, we hypothesized that plant–plant interactions would contribute to invasion if the presence of a competitor weakened the benefit that Stipa has when grown in its ‘home’ soil communities.
Methods
Study species and soil
We focused on two grass species common to southern California grasslands: the native perennial, S. pulchra, and the exotic annual, A. fatua (nomenclature follows Baldwin et al. 2012). Soils for the experiment were collected from Loma Ridge in Irvine, CA within the Irvine Ranch Land Reserve (N: 33.7501, W: −117.71787)—a grassland largely dominated by a mixture of exotic annual grasses and native perennial grasses (Larios et al. 2013). Background soil was collected from this site and upon collection the soil was air dried, sieved through a 2-mm sieve to remove rocks and debris and steam sterilized at 120 °C. This soil was then mixed 1 : 1 with sterile coarse sand and used as the sterile background soil to fill 164 mL cone-tainers for the greenhouse experiment described below.
To test how soil communities from varying N environments affected the strength of PSFs on plant performance, we collected soil inocula in March 2010 from a field experiment where native and exotic plants had been grown separately under low, ambient and high soil N (L. Larios and K. N. Suding, unpubl. data). Within the experiment, N was increased at a rate of 6 g N m−2 year−1, which we applied in the form of slow-release calcium nitrate (Florikan®, Sarasota, FL), and was decreased using table sugar at a rate of 421 g C m−2 year−1. In similar sites, this level of carbon addition decreased N by ∼30 % (Cleland et al. 2013). Soil amendments were applied three times over each growing season, beginning in the 2009 growing season (i.e. 2009 growing season is defined as October 2008 to June 2009) until the end of the 2011 growing season. In total, the experiment consisted of 30 plots (5 replicate blocks × 2 neighbourhood types × 3 soil N). Within each of the five experimental blocks, we collected soils from both the native and exotic plots. Within the native plots, soils were collected directly under a Stipa individual and for the exotics, under a stand of Avena, ensuring that roots were collected with each soil sample. This soil was kept cool (∼4–6 °C) and shipped to the University of California, Berkeley. Within 3 weeks of collection, the soils from each block were bulked to form the soil inocula used in the experiment. Spatial variation can contribute to high variability in microbial communities within a site (Pereira e Silva et al. 2012). Our goal was not to assess this spatial variability by testing the effects of the field soil resource additions on soil microbial communities per se, but to ask how soil communities from different resource environments impact plant growth and feedbacks. Therefore, we composited the soils from each block to form the soil inocula used in our soil treatments to ensure that we inoculated with the entire microbial taxa found across a resource environment. We additionally included a sterile soil treatment with no inoculum. Therefore, we had a total of seven soil-community treatments: Stipa-conditioned, (i) low N, (ii) ambient N, (iii) high N; Avena-conditioned, (iv) low N, (v) ambient N, (vi) high N and (vii) sterile control. The inoculum was added to the cone-tainers at a ratio of 30 : 1, sterile background soil (described above) to inoculum (Bever 1994).
Experimental design
To assess the interaction between soil communities from different resource environments and plant host on plant–soil interactions in the absence of competitive interactions, we planted three individual seeds of each species by themselves into cone-tainers with the soil inoculated with either conspecific or heterospecific soil communities from low N, ambient and high N sites. To examine the effect of competitive interactions on plant–soil interactions, we also planted species mixtures (consisting of one Stipa and one Avena) with the seven soil-community treatments described above. After initial germination we removed individuals from all cone-tainers so that each cone had a single individual for the no-competition Stipa and Avena treatments and one individual of each species for the competitive mixtures. We transplanted seedlings into the cones if no seeds germinated. The transplanted seedlings were planted at the same time as the other seeds so that they were comparable in size upon transplant. Thus we had a total of 420 cone-tainers (7 soil-community inocula × 3 species plantings × 10 blocks × 2 replicates within each block). The multiple replicates within a single block were averaged so that only block means were used in subsequent analyses.
The plants were grown at the Oxford Tract Greenhouse at the University of California, Berkeley, and were watered regularly with distilled water, without supplemental lighting or fertilizer. The blocks were rotated every week to minimize any differential effects of lighting and temperature within the greenhouse. Additionally, the cone-tainers were spaced such that there were never two cone-tainers adjacent to each other, to minimize any potential cross-contamination of soil inocula with watering. All above- and below-ground biomass was harvested 10 weeks after initial planting. Transplanted individuals were harvested 10 weeks after transplanting. The biomass was sorted to species for the competition treatment, and all biomass was dried for 48 h at 60 °C.
Statistical analysis
To evaluate how plant growth varied across the experiment, we analysed total biomass (sum of above- and below-ground biomass) with a three-way ANOVA, specifying block as a random factor, using the Proc Mixed module (SAS Institute, v 9.1).
We calculated the effect of the soil inoculum pairwise between the sterile soil treatment and the other soil inocula within each block with a natural log-response ratio, ‘ln(Bi/Bc)’, where B was the total biomass of the plant in either an inoculated soil treatment (‘i’) or sterile soil (‘c’). We assessed the directionality of the response ratio using t-tests, where a value >0 indicated a significant positive response and a value <0 indicated a significant negative response. To assess whether the effect of simply adding soil inocula changed with culturing species or soil resource site, we ran a mixed effects model using the Proc Mixed module separately for each species with the inoculum response ratio as the response variable, soil-community sources (plant species, soil resource site) as two fixed factors and block as a random effect.
To assess whether soil communities from varying soil resources affect plant performance, we calculated for each species a natural log-response ratio (i.e. ln(BalteredN/BambN)), separately for the conspecific and heterospecific soil communities. We then analysed this soil resource response ratio in a mixed model with soil-community sources (i.e. species and soil resource environment) as fixed effects and block as a random effect. We assessed directionality where a positive value would indicate that the individual grew better in the altered soil communities, while a negative value would indicate that it grew worse using t-tests as described above. A significant effect of soil resource environment for Avena would indicate that the changes in soil communities due to resource environment do alter performance, supporting our second hypothesis. A significant effect of the species soil inocula would indicate whether the effect of the soil communities from varied resourced environments varied between conspecific and heterospecific soil inocula.
Plant–soil feedback strength was calculated as ‘ln(Bhome/Baway)’, where Bhome is the total biomass of an individual when grown in their conspecific soil communities and Baway is the total biomass when grown in heterospecific soil communities. Plant–soil feedback strength was calculated within each soil resource soil microbial community and competition treatment (i.e. Avena feedback for no-competition and low N would be the comparison of Avena biomass when grown alone, between conspecific (home) and heterospecific (away) cultured soils at low N sites). For blocks where individuals of a specific treatment died, we averaged biomass across the other blocks for that species as a substitute. We did this five times for Stipa when grown alone. For the competition treatments, we replaced the biomass of both the species nine times. However, we dropped any blocks that had lost replicates for three or more soil inocula treatments, resulting in a loss of one block for the no-competition treatment and three for the competition treatments.
To assess how PSF responses changed with competition or across soil communities from different soil N environments, we ran a mixed effects model with PSF as the response variable and soil N inocula sources, target species identity and competition as fixed factors. Block was included as a random factor and any significant interactions were evaluated with post-hoc Tukey pairwise difference tests. A significant culturing species–target species interaction would indicate that PSFs could facilitate invasion, if Avena experienced no feedbacks when grown in ‘away’ soil communities, but would indicate invasion resistance if Stipa experienced positive feedbacks when grown in ‘home’ soil communities. A significant competition–species interaction would indicate that PSFs changed in the presence of a competitor, where a negative shift in feedbacks for Stipa when grown in competition would support our third hypothesis.
Results
Stipa pulchra response
Soil inocula and competitive environment both affected Stipa growth. Stipa total biomass was affected by soil microbial inoculum from Avena and from different soil N environments (culturing species × soil N interaction: F2,76 = 8.22, P < 0.001; [see Supporting Information]). Competition decreased Stipa biomass by almost 90 % (0.327 vs. 0.036 g, F1,76 = 595.9, P < 0.0001). Additionally, the competitive environment influenced the effect of soil inoculum on Stipa (competition × culturing species interaction: F1,76 = 9.72, P < 0.01; Fig. 1, square symbols). Comparisons of growth in sterilized soil indicate that Avena-cultured soil communities decreased Stipa growth while conspecific-cultured soils had a combination of neutral and negative effects compared with sterilized conditions (culturing species: F1,40 = 14.18, P < 0.0001; soil N: F2,40 = 0.90, P = 0.41; Fig. 2A).
Figure 1.
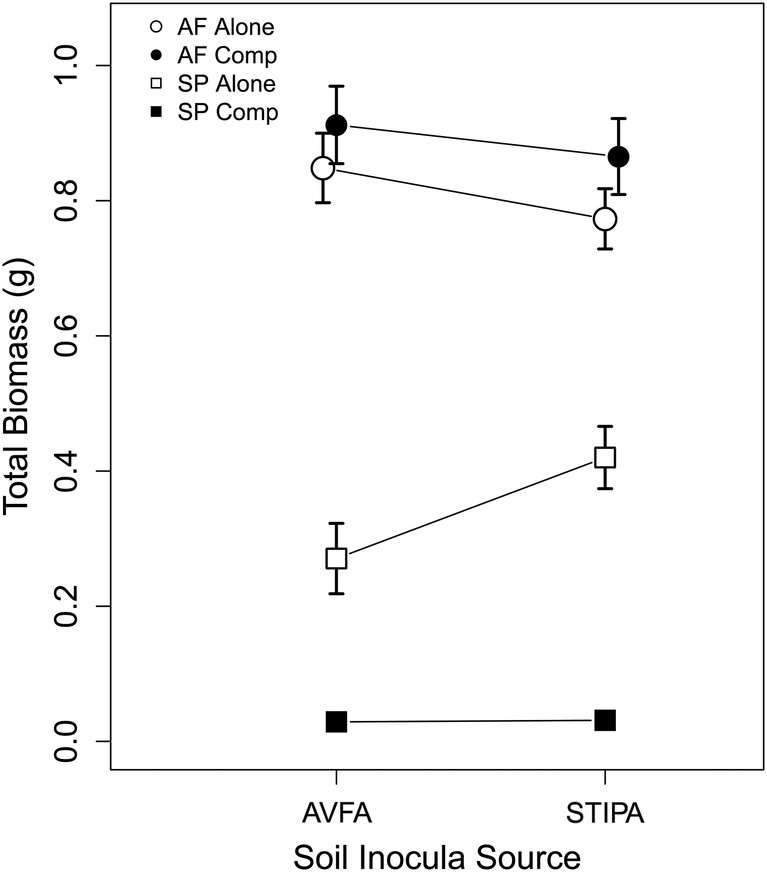
Total (above- and below-ground) biomass for S. pulchra (squares) and A. fatua (circles) when grown alone (open symbols) or with a competitor (filled symbols) with soil inocula cultured under ambient resources by conspecifics and heterospecifics. Competition decreased Stipa biomass, regardless of which soil community Stipa was grown. Avena grew similarly in both conspecific (Stipa) and heterospecific (Avena) soils regardless of the presence of a competitor. Mean ± 1 SE. Error bars for Stipa with competitors are hidden by symbols.
Figure 2.
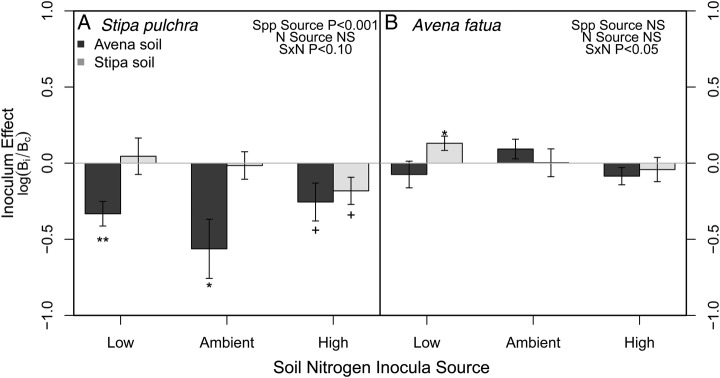
Effect of inoculating soil on plant performance for S. pulchra (A) and A. fatua (B). Stipa experienced negative effects (i.e. grew worse in the inoculated soil treatments compared with sterile) when grown in heterospecific (Avena) soil inoculum. Soil inocula affected Avena growth only when grown with inoculum from the heterospecific (Stipa) grown under low N environments. Mean ± 1 SE. Significantly different from zero: +P < 0.07, *P < 0.05, **P < 0.01.
When grown alone, Stipa grew better with conspecific-cultured soil communities compared with heterospecific (better in home vs. away soils), resulting in positive feedbacks when Stipa was grown alone (Fig. 3A, dark grey bars). These positive feedbacks diminished when Stipa was grown with Avena (Spp × Comp, F1,76 = 7.45, P < 0.01; Fig. 3A, light grey bars) and with high N soil communities (soil N × Spp, F2,76 = 6.24, P < 0.01, low and ambient N vs. high N Tukey HSD P < 0.01 and P < 0.05, respectively), resulting in the development of a strong negative feedback when in competition with Avena and in high N soil communities (Fig. 3).
Figure 3.
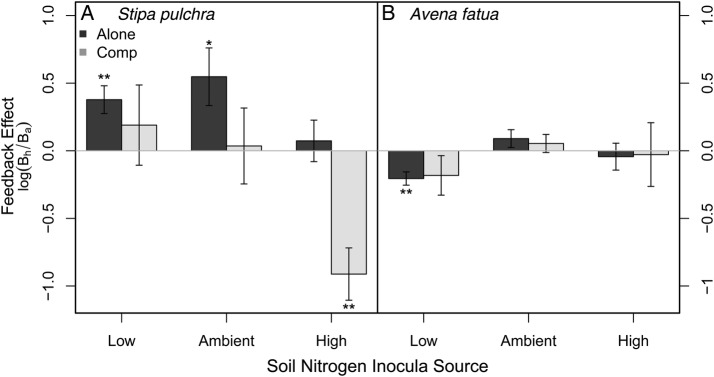
Plant–soil feedbacks for S. pulchra (A) and A. fatua (B) grown alone or with a competitor, across soils cultured by conspecifics or heterospecifics under varying resources. Stipa experienced positive feedbacks (i.e. grew better with its home soil communities) when grown alone in low N and ambient N soil communities, but these feedbacks became negative when grown in high N soil communities. Avena grew worse in its conspecific soil compared with heterospecific low N soil communities, resulting in a negative feedback. Means ± 1 SE. Significantly different from zero: *P < 0.05, **P < 0.01.
Soil microbial communities from different N environments did not alter Stipa growth; however, Stipa grew better with soil communities cultured by the heterospecific, Avena (culturing species: F1,24 = 4.25, P = 0.05; soil N: F1,24 = 0.23, P = 0.63; Spp × soil N: F1,24 = 0.95, P = 0.34; Fig. 4).
Figure 4.
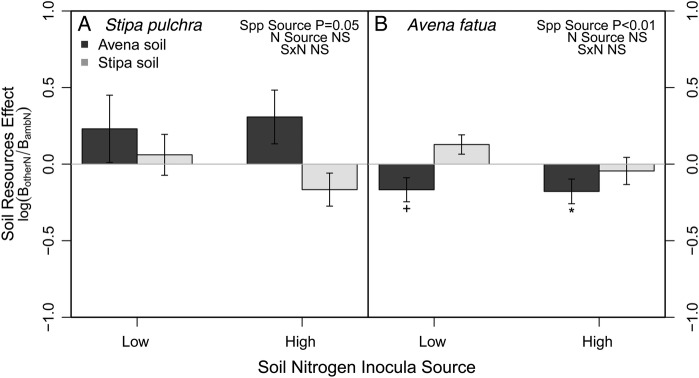
Effect of changes in soil community due to changes in soil nitrogen (N) resources on S. pulchra (A) and A. fatua (B) growth. Stipa grew better in soil communities from ambient N availability compared with low or high N availability when these soils were also cultured by heterospecifics, Avena. Conversely, Avena grew better in soil communities from ambient N availability when these soils were cultured by conspecifics. Means ± 1 SE. Significantly different from zero: +P < 0.07, *P < 0.05.
Avena fatua response
Avena exhibited little response to different soil communities (Fig. 3). The only exception to this pattern was a negative feedback at low N, where it grew worse in ‘home’ low N soil communities (soil N × Spp, low vs. ambient N: Tukey HSD, P < 0.05). Interactions with Stipa did not alter Avena growth (F1,76 = 0.01, P = 0.91; Fig. 1, circles) nor change PSFs (Fig. 3). Additionally, Avena growth was greater in ‘away’, low N soil communities than under sterile soil conditions (Fig. 2B, culturing species × soil N: F2,40 = 3.36, P < 0.05).
The soil resource environment did not alter the soil community in a way that altered Avena biomass. Much like Stipa's response, Avena grew better in soils conditioned by heterospecifics compared with conspecifics (culturing species: F1,24 = 10.22, P < 0.01; soil N: F1,24 = 1.87, P = 0.18; Spp × soil N: F1,24 = 1.45, P = 0.23).
Discussion
Plant–soil feedbacks involve two effects: soil-community effects on plant growth and plant species effects on soil communities (Bever 1994). As such, these feedbacks have most often been studied by isolating these two factors (Kulmatiski et al. 2008). However, many other factors can affect the composition of microbial communities (Waldrop et al. 2006; Bissett et al. 2013), as well as the growth of plant species (Chase and Leibold 2003), leading us to expect that PSFs may be dependent on the broader environmental context (Kardol et al. 2013). Indeed, we find that two of these additional factors (soil resource environment effects on soil microbial communities and competitive effects on plant growth) strongly impact the strength and even direction of PSFs.
Plant species effects on microbial communities can strongly regulate species establishment and performance (Bever et al. 2010) and the presence or lack of these effects may have strong implications for plant invasions (Inderjit and van der Putten 2010). Here, we observed that the native Stipa responded to culturing plant identity, where it grew less in soils conditioned by Avena, suggesting that Avena is able to culture a distinct soil community that negatively affects the native Stipa. On the other hand, we found that Avena was not responsive to culturing plant species identity as it grew similarly in soil conditioned by either conspecifics (Avena-conditioned) or heterospecifics (Stipa-conditioned) compared with sterile soil. While recent reviews have suggested that sterilized and unsterilized comparisons can be biased towards detecting negative responses to soil inocula (Kulmatiski et al. 2008; Brinkman et al. 2010), the strong response of Stipa to soil conditioned by Avena suggests that Avena may foster soil pathogens at a high enough density to affect Stipa growth. Interestingly, we observed an interaction between culturing plant host and soil N environment for both species, but the directionality varied for the native and exotic. Stipa grew worse in home soils compared with sterile when the soils came from the high N environment, and Avena grew better in heterospecific soils that were cultured at low N compared with sterile soil.
Our results support the idea that resource-induced changes to soil communities can impact PSFs, but the response may be species specific (Manning et al. 2008). Across the resource environments, we observed that neither Stipa nor Avena responded to changes in soil communities conditioned by Stipa. However both species responded to shifts in the Avena-conditioned soil communities, regardless of whether the conditioning was in low or high N environments, where Stipa's performance improved, while Avena's worsened (Fig. 4, dark grey bars). These results support previous findings that Stipa is able to foster a more diverse assemblage of soil biota compared with exotic annual grasses (Hausmann and Hawkes 2009), and thus, resource-induced shifts in soil communities may not have a large impact on plant growth. The positive response of Stipa to Avena-conditioned soil communities in different resource environments has interesting applications for management efforts aimed at native recovery. Soil N reduction activities are traditionally used to alter competitive interactions in favour of the natives (Blumenthal et al. 2003), and our results suggest that these soil N reductions may also minimize some of the negative effects on native species' growth that result from the soil conditioning of an exotic species like Avena. The small amount of inocula that we used may have resulted in lower densities of harmful pathogens and beneficial symbionts and contributed to the positive/neutral feedbacks that we observed for Stipa and Avena, respectively (Brinkman et al. 2010). However by assessing both the inocula effects and feedback effects, our results suggest that Stipa's positive feedback is likely a result of Avena culturing a microbial community that negatively impacts Stipa. Additional experiments that explore the spatial variability in the soil community and partition the members of the community to assess the groups driving this pattern are needed to further our understanding of how consistent this response will be across a landscape.
Integrating PSFs into other ecological processes such as competition is key to scaling the impact of PSFs observed at the individual plant level up to the community level (Hodge and Fitter 2013; Kardol et al. 2013). Competition had no impact on Avena growth, either independently or through a PSF interaction. Independently we observed: (i) when grown alone, Stipa grew better in its home soil compared with Avena-conditioned soil and (ii) Stipa had a strong negative response to competition by Avena. However, when we assessed the potential interactive effects of competition and feedbacks, we observed that Stipa's positive feedback was eliminated under competition. While this result is consistent with the competitive hierarchy previously observed between Avena and Stipa seedlings (Dyer and Rice 1997, 1999), this study does not allow us to decipher whether this result is also due to the strong control that Avena species may have on the soil community (Hausmann and Hawkes 2009). The strong effect of Avena on Stipa performance suggests that restoration efforts should continue to focus on ways to reduce the abundance of exotics in order to promote native species recovery.
Our approach also allowed us to examine how feedbacks may change in the presence of a competitor and soil communities conditioned in different soil N environments. We observed that soils from high N environments eliminated Stipa's positive feedback and interacted strongly with competition such that Stipa grew worse in its ‘home’ soil compared with ‘away’ soils. Similarly to the individual effects of soil communities from different resource environments, we observed that Avena grew worse in its ‘home’ soil compared with ‘away’ soils. Our results highlight the importance of future studies to explore how PSFs may interact with ongoing environmental change such as atmospheric N deposition to influence the resilience of existing native communities to invasion.
Conclusions
In conclusion, we found that both plant host and soil resource environment effects on soil communities may alter plant growth and that these impacts can shift in the presence of a competitor. Although the relationships of plant host and soil microbial communities are often assessed in isolation, our ability to understand how they may contribute to observed abundance patterns require us to investigate them in light of other key ecological processes. This more integrated assessment is key to our improved understanding of how plant–soil interactions may contribute to invader establishment, spread and dominance.
Sources of Funding
This work was supported by the NSF Graduate Research Fellowship Program (DEB 1106400 to L.L.) and NSF (DEB 09-19569 to K.N.S.).
Contributions by the Authors
Both L.L. and K.N.S. designed the experiment and edited the manuscript. L.L. conducted the data collection and statistical analyses and wrote the first draft of the manuscript.
Conflicts of Interest Statement
None declared.
Supporting Information
The following Supporting Information is available in the online version of this article –
Figure S1. The average individual total biomass for Stipa pulchra (A) and Avena fatua (B) as above- and below-ground biomass across soil inocula and competition treatments.
Acknowledgements
We thank L. August-Schmidt, J. Butler, A. Carlson, H. Gao and J. Martinez for help in the greenhouse and H. Bueno, E. Stone for help in the lab. We also thank K. Baer, J. Maron, M. Spasojevic, L. Waller and two anonymous reviewers for their helpful comments on this manuscript. Lastly, we thank the Irvine Ranch Conservancy for access to our research sites.
Literature Cited
- Allison VJ. Nutrients, arbuscular mycorrhizas and competition interact to influence seed production and germination success in Achillea millefolium. Functional Ecology. 2002;16:742–749. [Google Scholar]
- Baldwin BG, Goldman DH, Keil DJ, Patterson R, Rosatti TJ, Wilken DH, editors. The Jepson manual: vascular plants of California. 2nd edn. Berkeley, CA: University of California Press; 2012. [Google Scholar]
- Bardgett RD, Cook R. Functional aspects of soil animal diversity in agricultural grasslands. Applied Soil Ecology. 1998;10:263–276. [Google Scholar]
- Bardgett RD, Mawdsely JL, Edwards S, Hobbs PJ, Rodwell JS, Davies WJ. Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Functional Ecology. 1999;13:650–660. [Google Scholar]
- Bever JD. Feedback between plants and their soil communities in an old field community. Ecology. 1994;75:1965–1977. [Google Scholar]
- Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M. Rooting theories of plant community ecology in microbial interactions. Trends in Ecology and Evolution. 2010;25:468–478. doi: 10.1016/j.tree.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett A, Brown MV, Siciliano SD, Thrall PH. Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecology Letters. 2013;16:128–139. doi: 10.1111/ele.12109. [DOI] [PubMed] [Google Scholar]
- Blumenthal DM, Jordan NR, Russelle MP. Soil carbon addition controls weeds and facilitates prairie restoration. Ecological Applications. 2003;13:605–615. [Google Scholar]
- Bradley K, Drijber RA, Knops J. Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi. Soil Biology and Biochemistry. 2006;38:1583–1595. [Google Scholar]
- Brinkman EP, Van der Putten WH, Bakker EJ, Verhoeven KJF. Plant–soil feedback: experimental approaches, statistical analyses and ecological interpretations. Journal of Ecology. 2010;98:1063–1073. [Google Scholar]
- Brooks ML. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave desert. Journal of Applied Ecology. 2003;40:344–353. [Google Scholar]
- Callaway RM, Thelen GC, Barth S, Ramsey PW, Gannon JE. Soil fungi alter interactions between the invader Centaurea maculosa and North American natives. Ecology. 2004;85:1062–1071. [Google Scholar]
- Casper BB, Castelli JP. Evaluating plant–soil feedback together with competition in a serpentine grassland. Ecology Letters. 2007;10:394–400. doi: 10.1111/j.1461-0248.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago, IL: University of Chicago Press; 2003. [Google Scholar]
- Cleland EE, Larios L, Suding KN. Strengthening invasion filters to re-assemble native plant communities: soil resources and phenological overlap. Restoration Ecology. 2013;21:390–398. [Google Scholar]
- Dyer AR, Rice KJ. Intraspecific and diffuse competition: the response of Nassella pulchra in a California grassland. Ecological Applications. 1997;7:484–492. [Google Scholar]
- Dyer AR, Rice KJ. Effects of competition on resource availability and growth of a California bunchgrass. Ecology. 1999;80:2697–2710. [Google Scholar]
- Egerton-Warburton LM, Johnson NC, Allen EB. Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecological Monographs. 2007;77:527–544. [Google Scholar]
- Fenn ME, Haeuber R, Tonnesen GS, Baron JS, Grossman-Clarke S, Hope D, Jaffe DA, Copeland S, Geiser L, Rueth HM, Sickman JO. Nitrogen emissions, deposition, and monitoring in the western United States. Bioscience. 2003;53:391–403. [Google Scholar]
- Grman E, Suding KN. Within-year soil legacies contribute to strong priority effects of exotics on native California grassland communities. Restoration Ecology. 2010;18:664–670. [Google Scholar]
- Hartmann A, Schmid M, van Tuinen D, Berg G. Plant-driven selection of microbes. Plant and Soil. 2009;321:235–257. [Google Scholar]
- Hausmann NT, Hawkes CV. Plant neighborhood control of arbuscular mycorrhizal community composition. New Phytologist. 2009;183:1188–1200. doi: 10.1111/j.1469-8137.2009.02882.x. [DOI] [PubMed] [Google Scholar]
- Hausmann NT, Hawkes CV. Order of plant host establishment alters the composition of arbuscular mycorrhizal communities. Ecology. 2010;91:2333–2343. doi: 10.1890/09-0924.1. [DOI] [PubMed] [Google Scholar]
- Hawkes CV, Wren IF, Herman DJ, Firestone MK. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecology Letters. 2005;8:976–985. doi: 10.1111/j.1461-0248.2005.00802.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CV, Belnap J, D'Antonio C, Firestone MK. Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant and Soil. 2006;281:369–380. [Google Scholar]
- Hodge A, Fitter AH. Microbial mediation of plant competition and community structure. Functional Ecology. 2013;27:865–875. [Google Scholar]
- Hol WHG, de Boer W, ten Hooven F, van der Putten WH. Competition increases sensitivity of wheat (Triticum aestivum) to biotic plant–soil feedback. PLoS ONE. 2013;8:6. doi: 10.1371/journal.pone.0066085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderjit, van der Putten WH. Impacts of soil microbial communities on exotic plant invasions. Trends in Ecology and Evolution. 2010;25:512–519. doi: 10.1016/j.tree.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Jackson LE. Ecological origins of California's Mediterranean grasses. Journal of Biogeography. 1985;12:349–361. [Google Scholar]
- Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology. 2003;84:1895–1908. [Google Scholar]
- Kardol P, De Deyn GB, Laliberte E, Mariotte P, Hawkes CV. Biotic plant–soil feedbacks across temporal scales. Journal of Ecology. 2013;101:309–315. [Google Scholar]
- Klironomos JN. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- Kulmatiski A, Beard KH, Stevens JR, Cobbold SM. Plant–soil feedbacks: a meta-analytical review. Ecology Letters. 2008;11:980–992. doi: 10.1111/j.1461-0248.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- Larios L, Aicher RJ, Suding KN. Effect of propagule pressure on recovery of a California grassland after an extreme disturbance. Journal of Vegetation Science. 2013;24:1043–1052. [Google Scholar]
- Liu YJ, Shi GX, Mao L, Cheng G, Jiang SJ, Ma XJ, An LZ, Du GZ, Johnson NC, Feng HY. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytologist. 2012;194:523–535. doi: 10.1111/j.1469-8137.2012.04050.x. [DOI] [PubMed] [Google Scholar]
- Manning P, Morrison SA, Bonkowski M, Bardgett RD. Nitrogen enrichment modifies plant community structure via changes to plant–soil feedback. Oecologia. 2008;157:661–673. doi: 10.1007/s00442-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Pereira e Silva MC, Dias ACF, van Elsas JD, Salles JF. Spatial and temporal variation of archaeal, bacterial and fungal communities in agricultural soils. PLoS ONE. 2012;7:e51554. doi: 10.1371/journal.pone.0051554. doi:10.1371/journal.pone.0051554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds HL, Packer A, Bever JD, Clay K. Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology. 2003;84:2281–2291. [Google Scholar]
- Shannon S, Flory SL, Reynolds H. Competitive context alters plant–soil feedback in an experimental woodland community. Oecologia. 2012;169:235–243. doi: 10.1007/s00442-011-2195-6. [DOI] [PubMed] [Google Scholar]
- Shurin JB. How is diversity related to species turnover through time? Oikos. 2007;116:957–965. [Google Scholar]
- Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proceedings of the National Academy of Sciences of the USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suding KN, Harpole WS, Fukami T, Kulmatiski A, MacDougall AS, Stein C, van der Putten WH. Consequences of plant–soil feedbacks in invasion. Journal of Ecology. 2013;101:298–308. [Google Scholar]
- Van der Putten WH, Peters BAM. How soil-borne pathogens may affect plant competition. Ecology. 1997;78:1785–1795. [Google Scholar]
- van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, Van de Voorde TFJ, Wardle DA. Plant–soil feedbacks: the past, the present and future challenges. Journal of Ecology. 2013;101:265–276. [Google Scholar]
- Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications. 1997;7:737–750. [Google Scholar]
- Vogelsang KM, Bever JD. Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology. 2009;90:399–407. doi: 10.1890/07-2144.1. [DOI] [PubMed] [Google Scholar]
- Vogelsang KM, Reynolds HL, Bever JD. Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytologist. 2006;172:554–562. doi: 10.1111/j.1469-8137.2006.01854.x. [DOI] [PubMed] [Google Scholar]
- Waldrop MP, Zak DR, Blackwood CB, Curtis CD, Tilman D. Resource availability controls fungal diversity across a plant diversity gradient. Ecology Letters. 2006;9:1127–1135. doi: 10.1111/j.1461-0248.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- Yelenik SG, Levine JM. The role of plant–soil feedbacks in driving native-species recovery. Ecology. 2011;92:66–74. doi: 10.1890/10-0465.1. [DOI] [PubMed] [Google Scholar]
- Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia. 2007;154:349–359. doi: 10.1007/s00442-007-0836-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


