Abstract
Purpose
Astaxanthin is a type of carotenoid known to have strong antioxidant effects. The purpose of this study was to investigate whether astaxanthin confers a neuroprotective effect against glutamate stress, oxidative stress, and hypoxia-induced apoptotic or necrotic cell death in primary cultures of rat retinal ganglion cells (RGCs).
Methods
Purified rat RGCs were exposed to three kinds of stressors induced by 25 μM glutamate for 72 h, B27 medium without an antioxidant for 4 h, and a reduced oxygen level of 5% for 12 h. Each assay was repeated 12 times, with or without 1 nM, 10 nM, and 100 nM astaxanthin. The number of live RGCs was then counted using a cell viability assay. RGC viability in each condition was evaluated and compared with controls. In addition, we measured apoptosis and DNA damage.
Results
We found that under glutamate stress, RGC viability was reduced to 58%. Cultures with 1 nM, 10 nM, and 100 nM astaxanthin showed an increase in RGC viability of 63%, 74%, and 84%, respectively. Under oxidative stress, RGC viability was reduced to 40%, and astaxanthin administration resulted in increased viability of 43%, 50%, and 67%, respectively. Under hypoxia, RGC viability was reduced to 66%, and astaxanthin administration resulted in a significant increase in viability to 67%, 77%, and 93%, respectively. These results indicate that 100 nM astaxanthin leads to a statistically significant increase in RGC viability under the three kinds of stressors tested, compared to controls (Dunnett’s test, p<0.05). The apoptotic activity of RGCs under glutamate stress increased to 32%, but was reduced to 15% with 100 nM astaxanthin administration. Glutamate stress led to a 58% increase in DNA damage, which was reduced to 43% when cultured with 100 nM astaxanthin. Thus, 100 nM astaxanthin showed a statistically significant reduction in apoptosis and DNA damage in RGCs (Wilcoxon rank-sum test, p<0.05).
Conclusions
Our results suggest that astaxanthin has a neuroprotective effect against RGC death induced by glutamate stress, oxidative stress, and hypoxia, which induce apoptotic and necrotic cell death.
Introduction
Glaucoma is a chronic neurodegenerative disease mainly characterized by higher intraocular pressure, which can lead to apoptosis of retinal ganglion cells (RGCs) and the progressive loss of optic nerve axons resulting in structural and functional deficits in patients with glaucoma, and is a leading cause of blindness all over the world [1]. Glaucomatous optic neuropathy (GON) can occur in the optic nerve head and has been associated with multiple pathogenic mechanisms following lamina cribrosa deformation by elevated intraocular pressure [2]. For example, blood flow disturbances at the optic nerve head may be involved in the pathogenesis of axon loss and RGC apoptosis by hypoxia and the production of reactive oxygen species (ROS) [3]. Additionally, reactive glial cells in the optic nerve head may release excitatory amino acids such as glutamate [4] or nitric oxide [5].
Astaxanthin (AST), a type of carotenoid found in many marine organisms such as salmon, trout, red sea-bream, shrimp, lobster, and fish eggs, can decrease the formation of reactive oxygen species (ROS) induced by biologic molecules [6]. AST can induce various pharmacological effects, including antioxidative activity [7-11], antitumor effects [12,13], anti-inflammatory actions [14], antidiabetic [15] and hepatoprotective effects [16], and immunomodulatory activity [17,18], suggesting that AST may have considerable potential for beneficial applications in human health and nutrition.
In a previous study, AST displayed a neuroprotective effect against oxidative stress in an in vivo experiment using mice [19]. However, no data have yet been reported in RGC primary cultures using other stressors related to GON, such as glutamate toxicity or hypoxia. In this study, we investigate the influence of AST against glutamate- and hypoxia-induced apoptosis, as well as oxidative stress-induced necrosis, in primary rat RGC cultures.
Methods
Materials
All animal studies were performed in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Resolution on the Use of Animals in Research. Poly-L-lysine, BSA, L-glutamine, human recombinant brain-derived neurotrophic factor (BDNF), and rat recombinant ciliary neurotrophic factor (CNTF) were obtained from Sigma (St. Louis, MO). The papain dissociation system was obtained from Worthington Biochemical (Lakewood, NJ); mouse anti-rat SIRP (CD172a) monoclonal antibodies (MAB 1407P) and mouse anti-rat Thy1.1 monoclonal antibodies (MAB 1406) were obtained from Chemi-Con International (Temecula, CA). The live/dead viability cytotoxicity kit (L-3224) was obtained from Molecular Probes (Eugene, OR). The B27 supplement minus antioxidants (AO-) was obtained from Gibco (Grand Island, NY). Unless otherwise stated, the B27 supplement contains antioxidants. AST tartrate was obtained from Fuji Chemical Industry Co., Ltd. (Toyama, Japan).
Purified rat retinal ganglion cell culture
RGC cultures were obtained from retinas dissected from enucleated eyes of 5–6 day-old Wistar rats (Saitama Jikken Doubutsu, Saitama, Japan), euthanized using CO2 inhalation, followed by a two-step immunopanning procedure, as follows [20]. Retinal tissue was incubated at 37 °C for 30 min in a solution containing 15 U/ml papain and 70 U/ml collagenase in Hank’s balanced salt solution containing 0.2 mg/ml BSA and 0.2 mg/ml DL-cysteine. To yield a single cell suspension, the tissue was then triturated sequentially through a narrow-bore Pasteur pipette in a solution containing 2 mg/ml ovomucoid, 0.004% DNase, and 1 mg/ml BSA. After centrifugation at 120 ×g for 5 min, cells were washed once in an ovomucoid-BSA solution containing 10 mg/ml of each reagent. Cells were then centrifuged again at 120 ×g for 5 min and resuspended in 0.1% BSA in PBS (155 mM NaCl, 2.97 mM Na2HPO4-7H2O, 1.06 mM KH2PO4, pH 7.4; Gibco). Antibodies were then removed, and the cell suspension was incubated in an anti-macrophage antibody-coated flask for 30 min and an anti-Thy1.1 antibody-coated flask for 1 h at room temperature. Cells adhering to the flask (RGCs) were resuspended in serum-free neurobasal medium (Gibco) supplemented with 2% B27 supplement (Gibco), BDNF (40 ng/ml), CNTF (40 ng/ml), and forskolin (10 μM) and seeded at 2×105 cells/well onto 13 mm coverslips placed in 24-well plates. The coverslips had been autoclaved and then coated with 0.05 mg/ml poly-L-lysine (Sigma) overnight, rinsed twice with Hank’s buffered saline solution (HBSS), and then coated for 2 h with 1 μg/ml of laminin (Gibco). RGCs were cultured for 72 h under normoxic conditions (20% O2, 5% CO2, 75% N2 at 37 °C) before each experiment in serum-free B27 complete medium containing neurobasal medium with 1 mM L-glutamine (Sigma), B27 supplement, 40 ng/ml BDNF, 40 ng/ml rat CNTF, and 10 μM forskolin. After incubating for 72 h, RGCs were exposed to neurobasal medium plus each concentration of astaxanthin for 1 h, and then, RGCs were exposed to the following stress.
Glutamate neurotoxicity
Control coverslips were moved to freshly prepared neurobasal medium containing B27 supplement and placed in normoxic conditions without glutamate. Experimental coverslips for glutamate neurotoxicity were transferred to freshly prepared neurobasal medium containing B27 supplement and glutamate (25 μM), which were incubated for a further 72 h.
Oxidative stress
Control coverslips were moved to freshly prepared B27 supplemented neurobasal medium containing potent antioxidants (reduced glutathione, vitamin E, vitamin E acetate, catalase, and superoxide dismutase), and coverslips for oxidative treatment were transferred to neurobasal medium containing B27 without these five antioxidants (AO-), a condition inductive for oxidative stress [21,22]. RGCs in both conditions were then incubated for 4 h.
Hypoxic stress
Control coverslips were moved to freshly prepared neurobasal medium with B27 supplement and placed in normoxic conditions. Hypoxic stress was induced by placing the cultures in a controlled atmosphere of 5% O2, 5% CO2, and 90% N2 at 37 °C for 12 h.
Application of astaxanthin
All experiments were performed using three concentrations (1 nM, 10 nM, and 100 nM) of AST. AST was added separately for a final concentration in each test culture.
RGC survival rate assay
After incubation, the surviving RGCs were processed for viability by labeling with calcein-AM (2 mM), a component of the Live/Dead Viability/Cytotoxicity kit. Live RGCs were defined as positive for calcein-stained cell bodies with neurites extending at least three cell diameters from the cellular body. RGCs were counted manually in a total of eight fields at a standardized location at 10× magnification. Live RGCs in each well were expressed as a percentage of live cells relative to the control cultures without glutamate, oxidative agents, or hypoxia. The average cell survival percentage of 12 experiments for each condition was expressed in text and figures as the mean ± standard deviation (SD). The cells were counted in a masked manner.
RGC apoptotic activity assay
After incubation, we used the Vybrant Apoptosis Assay Kit to quantify apoptotic RGCs that underwent glutamate stress. RGCs were incubated under glutamate stress followed by Alexa Fluor 488-conjugated annexin V binding combined with propidium iodide labeling. Apoptotic RGCs were stained as annexin V [+]/propidium iodide [-], and necrotic cells were stained as annexin V [+]/propidium iodide [+]. Undamaged RGCs remained negative for both stains [23,24]. At the end of the double-staining procedure, Hoechst 33,342 was added to the culture medium to a final concentration of 8 mM. Cells were counted in at least ten random fields of each well at 200× magnification using a fluorescence microscope (BZ-9000, KEYENCE, Tokyo, Japan). The percentages of apoptotic RGCs and necrotic RGCs were quantified by determining the ratio of (annexin V [+]/propidium iodide [-]) cells and (annexin V [+]/propidium iodide [+]) cells to Hoechst 33,342 [+] RGCs, respectively. The cells were counted in a masked manner.
Oxidative DNA damage in RGC assay
To detect 8-hydroxy-20-deoxyguanosine (8-OHdG) protein in RGCs, immunostaining was performed as previously reported [19,25], using an anti-8-OHdG monoclonal antibody (clone N45.1; JaICA, Shizuoka, Japan).
8-OHdG staining: At the end of incubation, RGCs attached to coverslips placed in the bottoms of 24 well plates were fixed in 4% paraformaldehyde and treated with RNase (100 g/ml) in Tris buffer (10 mM Tris–HCl, pH 7.5, 1 mM EDTA, and 0.4 M NaCl) at 37 °C for 1 h. After rinsing with PBS, cells were treated with proteinase K (10 g/ml) at room temperature for 7 min. After rinsing with PBS, DNA was denatured by treatment with 4 N HCl for 7 min at room temperature. The pH was adjusted with 50 mM Tris–HCl, pH 7.5, for 5 min at room temperature [26]. After rinsing with 0.2% Triton X-100 in PBS, cells were stained with a mouse monoclonal anti-8-OHdG antibody (1:50). Alexa Fluor 488-conjugated goat anti-mouse IgG (1:200) was used as the secondary antibody. Slides were mounted with Vectashield H-1000 (Vector laboratories, Inc. (Burlingame, CA). The percentages of DNA oxidative damage were quantified by determining the ratio of 8-OHdG (+)/DAPI(+) cells to DAPI(+) RGCs, respectively. The cells were counted in a masked manner.
DAPI staining
Cells immunostained for 8-OHdG were rinsed and then counterstained with DAPI (2 g/ml) for 5 min. After rinsing with PBS, the slides were mounted with Vectashield. Fluorescence images were captured using a fluorescence microscope (BZ-9000, KEYENCE, Tokyo, Japan) and merged with the 8-OHdG-stained images.
Statistical analysis
Dunnett’s test was used to compare the survival ratio of cells treated with AST that underwent the three stresses to the control cultures without AST. The Wilcoxon rank-sum test was used to compare the ratio of apoptotic and necrotic cells (%) and 8-OHdG positive cells (%) under glutamate-induced stress treated with AST to the controls without AST. A p value of <0.05 or 0.01 was considered significant.
Results
Culture condition of RGCs
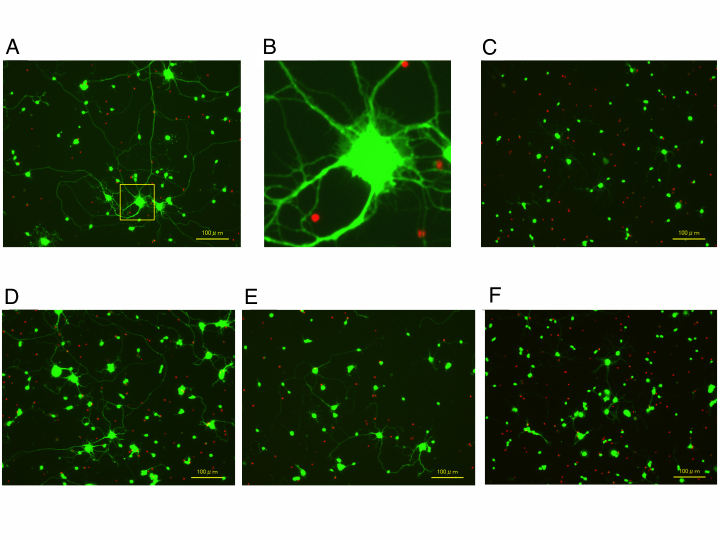
Figure 1 shows representative culture images of RGCs in each condition. Compared with the non-stress control cultures (Figure 1A,B), exposure to glutamate for 72 h induced apparent shrinkage of cell bodies and dendrites (Figure 1C). The 100 nM astaxanthin concentration decreased the shrinkage of cell bodies and dendrites (Figure 1D), but there were no differences between the 10 and 1 nM astaxanthin-treated cells (Figure 1E,F).
Figure 1.
Fluorescent images of rat purified RGC cultures exposed to glutamate with or without astaxanthin. Live cells were stained green with calcein-AM and dead cells were red by ethidium homodimer-1. A: Non-treated control. B: Magnified figure of A. C: 25 μM glutamate only. D: 25 μM glutamate + 100 nM astaxanthin. E: 25 μM glutamate + 10 nM astaxanthin. F: 25 μM glutamate + 1 nM astaxanthin.
Effect of AST on glutamate-induced RGC death
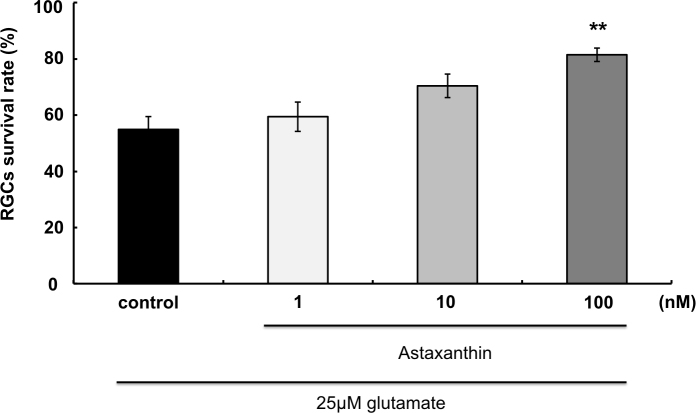
After 72 h of incubation, in the presence of glutamate alone, 57.5±13.0% of the RGCs survived compared to controls without glutamate (Figure 2). In the presence of 1 nM, 10 nM, and 100 nM AST, RGC survival was 63.1±12.6%, 73.7±8.5%, and 83.5±4.3%, respectively (n=12). The 100 nM AST concentration significantly increased the RGC survival rate compared to glutamate alone (p<0.01).
Figure 2.
Neuroprotective effect of astaxanthin on glutamate-induced RGC death. Asterisks indicate significant increase in the retinal ganglion cell (RGC) survival rate compared to glutamate 25 μM (black column, as control). ** indicate p<0.01, Dunnett’s test; n=12. Error bar indicates standard deviation (SD).
Effect of AST on oxidative stress-induced RGC death
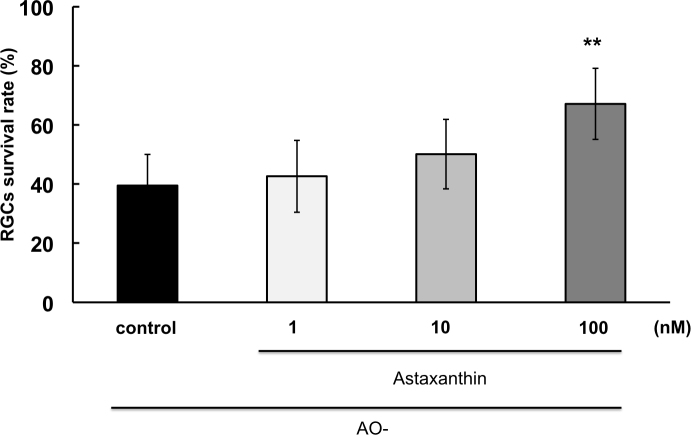
After 4 h of oxidative stress, in the presence of oxidative stress (AO-) alone, 39.5±10.5% of the RGCs survived compared to controls with AO added (Figure 3). In the presence of 1 nM, 10 nM, and 100 nM AST, RGC survival was 42.6±12.2%, 50.1±11.7%, and 67.0±12.0%, respectively (n=12). The 100 nM AST concentration significantly increased the RGC survival rate compared to cultures without AO added (p<0.01).
Figure 3.
Neuroprotective effect of astaxanthin on oxidative stress-induced RGC death. Asterisks indicate a significant increase in the retinal ganglion cell (RGC) survival rate compared to the antioxidants (AO-) only (control). ** indicate p<0.01, Dunnett’s test; n=12. Error bar indicates standard deviation (SD).
Effect of AST on hypoxia-induced RGC death
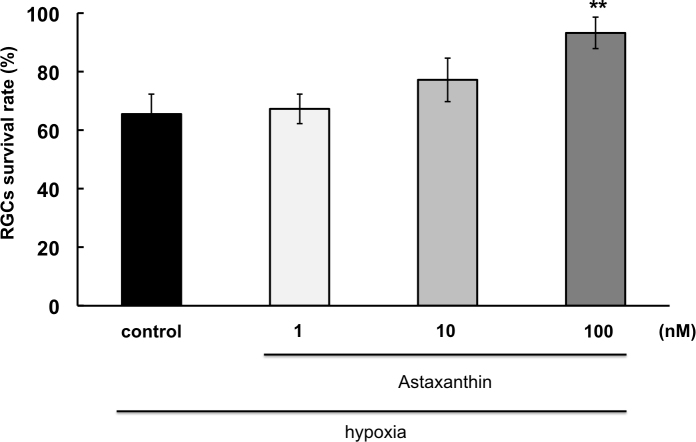
After 12 h of hypoxia, 65.5±6.8% of the RGCs survived compared to the controls cultured under normoxic conditions (Figure 4). In the presence of 1 nM, 10 nM, and 100 nM AST, RGC survival was 67.1±5.0%, 77.2±7.5% and 93.2±5.3%, respectively (n=12). The 100 nM AST concentration significantly increased the RGC survival rate compared to hypoxia alone (p<0.01).
Figure 4.
Neuroprotective effect of astaxanthin on hypoxia-induced RGC death. Asterisks indicate a significant increase in the retinal ganglion cell (RGC) survival rate compared to hypoxia only (control). p<0.01, Dunnett’s test; n=12. Error bar indicates standard deviation (SD).
Effect of AST on glutamate-induced apoptotic and necrotic RGC death
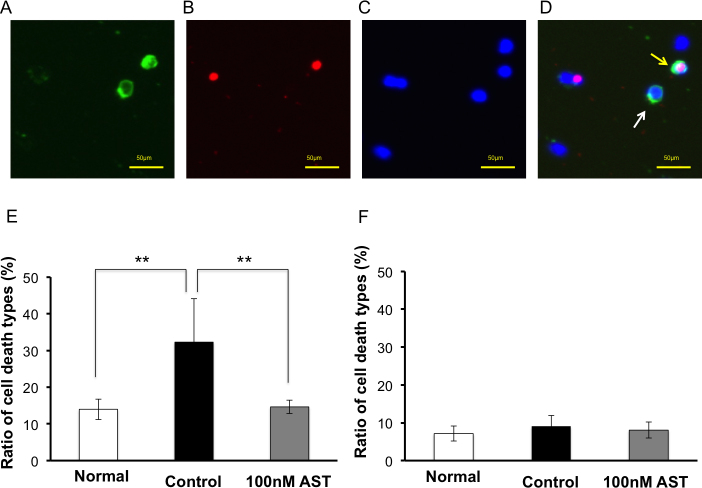
Figure 5A–D show representative fluorescence microscopy of Hoechst 33,342 and annexin V/propidium iodide staining at 72 h under glutamate-induced RGC death. Green staining is annexin V-positive cells (Figure 5A). Red staining is propidium iodide-positive staining cells (Figure 5B). Blue staining is Hoechst 33,342-positive staining cells (Figure 5C). Figure 5D is a merged image; annexin V(+)/propidium iodide (-) apoptotic cells (white arrow) and annexin V(+)/propidium iodide(+) necrotic cells (yellow arrow) are detectable. Compared to normal controls, the application of glutamate significantly increased apoptosis (32.3±11.8% versus 14.0±2.8%, p<0.01, Wilcoxon rank-sum test) but not necrosis (9.1±2.8% versus 7.1±2.0%, p=0.3095; Figure 5E–F). The percentage of apoptotic and necrotic RGCs in the 100 nM AST group was 14.6±1.8% and 8.1±2.1%, respectively (n=5). AST significantly reduced apoptotic RGC death compared to glutamate only (p<0.01, Wilcoxon rank-sum test) but did not reduce necrosis (p=0.5476).
Figure 5.
Detection of apoptotic and necrotic RGCs in glutamate and astaxanthin. Representative fluorescence microscopy of Hoechst 33,342 and annexin V/propidium iodide staining at 72 h under glutamate-induced retinal ganglion cell (RGC) death. A: Green staining is annexin V-positive cells. B: Red staining is propidium iodide-positive staining cells. C: Blue staining is Hoechst 33,342-positive staining cells. D: Merged image; annexin V(+)/propidium iodide (-) apoptotic cells (white arrow) and annexin V(+)/propidium iodide(+) necrotic cells (yellow arrow) are detectable. E: Ratio of apoptotic cells. Glutamate 25 µM increased the percentage of apoptotic cells. Astaxanthin (AST) 100 nM significantly decreased apoptotic cells induced by glutamate. F: Ratio of necrotic cells. No significant difference was observed in all groups. Each value represents mean ± standard deviation (SD; n=5). Asterisks indicate p<0.01 (Wilcoxon rank-sum test).
Effect of AST on glutamate-induced DNA oxidative damage
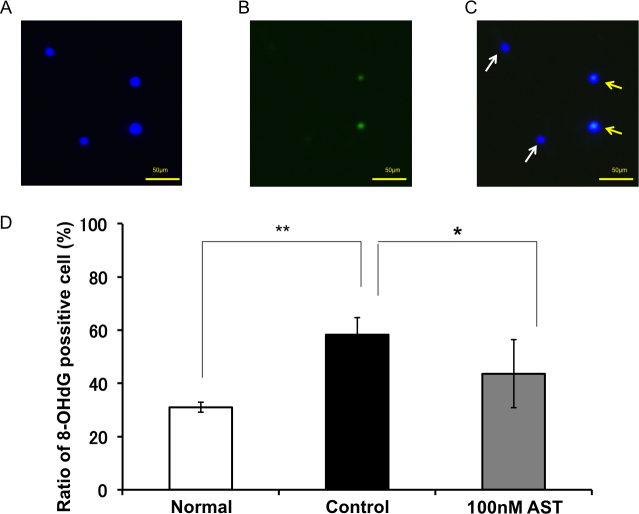
Figure 6A-C show representative fluorescence microscopy of 4',6-diamidino-2-phenylindole (DAPI) and 8-OHdG staining at 72 h under glutamate-induced RGC death. Blue staining is DAPI-positive cells (Figure 6A), and green staining is 8-OHdG-positive staining cells (Figure 6B). Figure 6C is a merged image; DAPI(+)/8-OHdG(-) living cells (white arrow) and DAPI(+)/8-OHdG(+) DNA oxidative damage cells (yellow arrow) are detectable. Application of glutamate significantly increased the DNA oxidative damage marker 8-OHdG, compared to the normal controls (58.3±6.4% versus 31.0±1.9%, p=0.0022, Wilcoxon rank-sum test, n=6; Figure 6D). The 100 nM AST concentration significantly reduced DNA oxidative damage compared to glutamate only (42.7±10.3%, p=0.0152, Wilcoxon rank-sum test, n=6).
Figure 6.
Suppressive effect of astaxanthin on glutamate-induced DNA oxidative damage. Representative fluorescence microscopy of 4',6-diamidino-2-phenylindole (DAPI) and 8-hydroxy-20-deoxyguanosine (8-OHdG) staining at 72 h under glutamate-induced retinal ganglion cell (RGC) death. A: Blue staining is DAPI-positive cells. B: Green staining is 8-OHdG-positive staining cells. C: Merged image of A and B. DAPI(+)/8-OHdG(-) living cells (white arrow) and DAPI(+)/8-OHdG(+) DNA oxidative damage cells (yellow arrow) are detectable. Each value represents mean ± standard deviation (SD; n=6). *, ** indicate p<0.05 or 0.01 (Wilcoxon rank-sum test).
Discussion
Previous studies have indicated AST has various pharmacological effect against oxidative stress. However, no data have yet been reported in an in vitro experiment related to GON. Thus, we investigated the use of an in vitro model of purified rat RGC cultures with properties similar to an in vivo [27] to examine whether AST was neuroprotective for RGC death induced by three stressors: glutamate-induced neurotoxicity, hypoxia, and oxidative stress. In previous studies, we examined the cell death types using three different stresses on primary cultured RGCs in vitro. We found that 100 nM AST significantly increased RGC viability exposed to the three stressors, all of which have been implicated in the pathogenesis of GON. Glutamate and hypoxia mainly induced apoptosis, and oxidative stress mainly induced necrosis [28,29]. Thus, our result (Figure 2, Figure 3, Figure 4) suggests AST may have a protective effect on apoptosis and necrosis. Based on the results of these three experiments, we are interested that AST suppressed apoptotic cell death because it is obvious that an antioxidant such as AST has a protective effect against oxidative stress–induced necrosis. Since hypoxia induces various signals leading to cell death, it may be vague to reveal the mechanism of cell death. However, the signal by stimulation with glutamate is narrow than that with hypoxia. Thus, we focused on glutamate-induced cell death and performed the experiments shown in Figure 5 and Figure 6.
In the present study, AST displayed a neuroprotective effect against glutamate-induced RGC apoptosis (Figure 2 and Figure 5). Glutamate is present in the retina at appropriate concentrations to maintain signal transduction by activating several types of glutamate receptors, including N-methyl D-aspartate (NMDA) receptors, that enhance intracellular levels of ROS and intracellular calcium concentrations [Ca2+]i [30,31]. A high level of glutamate has been shown to result in apoptosis of RGCs in in vivo injections into the vitreous and in in vitro treatments using cultured retinal neuronal cells [32-35]. Recently, it was revealed that AST reduced the ROS production and cytotoxicity of SH-SY5Y human neuroblastoma cells [36]. In addition, 2 μM AST has been shown to partially decrease ROS production without a decrease in [Ca2+]i during oxidative stress induced by a fatty acid mixture in human lymphocytes [37]. Thus, AST may have a neuroprotective effect against glutamate toxicity through the reduction of ROS, and not by lowering [Ca2+]i. One mechanism of ROS-induced cell death is DNA damage [38]. Thus, in our study, we confirmed that AST reduced the generation of the DNA damage marker, 8-OHdG, which is induced by glutamate stress (Figure 6). It has been reported that some carotenoids prevent DNA damage caused by various stressors [38], and oral administration of AST reduced DNA damage induced by NMDA receptor stimulation [39] or cyclophosphamide [38]. Reduction of ROS by AST may protect against apoptosis resulting from DNA damage. Future studies should be undertaken to elucidate the mechanisms by which AST leads to DNA repair.
We previously reported that in rat RGC cultures, hypoxia-induced apoptotic cell death and Bax-mediated mitochondrial apoptotic pathways were involved [28]. In the rat brain, AST may reduce the translocation of cytochrome c leading to apoptotic activity [40]. During hypoxia, some carotenoids can activate antiapoptotic pathways through the maintenance of mitochondrial potential and the prevention of cytochrome c release from mitochondria [41]. Thus, the neuroprotective effect of AST, a type of carotenoid, against hypoxia-induced RGC death may be attributed to the maintenance of normal mitochondrial function.
We have previously shown that, in contrast to glutamate- or hypoxia-induced neurotoxicity, oxidative stress induced by the depletion of antioxidative agents from the culture medium mainly induced necrosis by activating the calpain/cathepsin pathway [42]. AST was neuroprotective not only against RGC apoptosis but also against necrosis. Unlike apoptotic programmed cell death, necrotic cell death occurs when cells are exposed to extreme stress. For example, exposure to NMDA over a short duration or low concentrations generated low levels of peroxynitrate and induced apoptosis, while exposure to high concentrations of NMDA or peroxynitrate induced necrotic cell damage [43]. Our results suggest that AST has a strong neuroprotective effect against necrosis induced by severe stress.
AST is a natural product found in ingestible seafood and can be extracted for use as an oral supplement. A previous report in humans showed that the plasma concentration of AST was 62.4 nM after oral AST supplementation of 3 mg for 12 weeks [44]. This suggests that it is possible to ingest an AST supplement that will lead to an effective plasma concentration close to what we used in our in vitro study. Therefore, we expect that AST supplementation may be protective in neurodegenerative diseases such as glaucoma. Future animal model studies and clinical trials will be required to further elucidate the neuroprotective mechanisms and optimal dosage for AST supplementation.
In conclusion, AST is a compound that has potent antioxidant activity that can inhibit apoptotic and necrotic RGC cell death caused by glutamate, hypoxia, and oxidative stress. We suggest that AST could be used as a potent supplement to protect RGCs against toxic factors related to glaucoma and other neurodegenerative diseases.
Acknowledgments
This experiment was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (JSPS KAKENHI grant number 22390321 to M.A.).
References
- 1.Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma - Is there a future role? Exp Eye Res. 2010;91:554–66. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Tezel G, Wax MB. Glaucoma. Chem Immunol Allergy. 2007;92:221–7. doi: 10.1159/000099273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30:275–80. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- 4.Dreyer EB. A proposed role for excitotoxicity in glaucoma. J Glaucoma. 1998;7:62–7. [PubMed] [Google Scholar]
- 5.Neufeld AH. Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S129–35. doi: 10.1016/s0039-6257(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Shibata T, Hisaka S, Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009;1254:18–27. doi: 10.1016/j.brainres.2008.11.076. [DOI] [PubMed] [Google Scholar]
- 7.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR. 1990;22:27–38. [PubMed] [Google Scholar]
- 8.O'Connor I, O'Brien N. Modulation of UVA light-induced oxidative stress by beta-carotene, lutein and astaxanthin in cultured fibroblasts. J Dermatol Sci. 1998;16:226–30. doi: 10.1016/s0923-1811(97)00058-3. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto T, Hosoda K, Hirano R, Kurata H, Matsumoto A, Miki W, Kamiyama M, Itakura H, Yamamoto S, Kondo K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J Atheroscler Thromb. 2000;7:216–22. doi: 10.5551/jat1994.7.216. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol. 2000;54:550–5. doi: 10.1007/s002530000416. [DOI] [PubMed] [Google Scholar]
- 11.Aoi W, Naito Y, Sakuma K, Kuchide M, Tokuda H, Maoka T, Toyokuni S, Oka S, Yasuhara M, Yoshikawa T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid Redox Signal. 2003;5:139–44. doi: 10.1089/152308603321223630. [DOI] [PubMed] [Google Scholar]
- 12.Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19(3A):1849–53. [PubMed] [Google Scholar]
- 13.Jyonouchi H, Sun S, Iijima K, Gross MD. Antitumor activity of astaxanthin and its mode of action. Nutr Cancer. 2000;36:59–65. doi: 10.1207/S15327914NC3601_9. [DOI] [PubMed] [Google Scholar]
- 14.Ohgami K, Shiratori K, Kotake S, Nishida T, Mizuki N, Yazawa K, Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44:2694–701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama K, Naito Y, Hasegawa G, Nakamura N, Takahashi J, Yoshikawa T. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002;7:290–3. doi: 10.1179/135100002125000811. [DOI] [PubMed] [Google Scholar]
- 16.Kang JO, Kim SJ, Kim H. Effect of astaxanthin on the hepatotoxicity, lipid peroxidation and antioxidative enzymes in the liver of CCl4-treated rats. Methods Find Exp Clin Pharmacol. 2001;23:79–84. doi: 10.1358/mf.2001.23.2.627931. [DOI] [PubMed] [Google Scholar]
- 17.Okai Y, Higashi-Okai K. Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. Int J Immunopharmacol. 1996;18:753–8. doi: 10.1016/s0192-0561(97)85558-0. [DOI] [PubMed] [Google Scholar]
- 18.Chew BP, Wong MW, Park JS, Wong TS. Dietary beta-carotene and astaxanthin but not canthaxanthin stimulate splenocyte function in mice. Anticancer Res. 1999;19(6B):5223–7. [PubMed] [Google Scholar]
- 19.Nakajima Y, Inokuchi Y, Shimazawa M, Otsubo K, Ishibashi T, Hara H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J Pharm Pharmacol. 2008;60:1365–74. doi: 10.1211/jpp/60.10.0013. [DOI] [PubMed] [Google Scholar]
- 20.Uchida S, Suzuki Y, Araie M, Kashiwagi K, Otori Y, Sakuragawa N. Factors secreted by human amniotic epithelial cells promote the survival of rat retinal ganglion cells. Neurosci Lett. 2003;341:1–4. doi: 10.1016/s0304-3940(02)01454-4. [DOI] [PubMed] [Google Scholar]
- 21.Perry SW, Norman JP, Litzburg A, Gelbard HA. Antioxidants are required during the early critical period, but not later, for neuronal survival. J Neurosci Res. 2004;78:485–92. doi: 10.1002/jnr.20272. [DOI] [PubMed] [Google Scholar]
- 22.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 23.Schutte B, Nuydens R, Geerts H, Ramaekers F. Annexin V binding assay as a tool to measure apoptosis in differentiated neuronal cells. J Neurosci Methods. 1998;86:63–9. doi: 10.1016/s0165-0270(98)00147-2. [DOI] [PubMed] [Google Scholar]
- 24.Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–59. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inokuchi Y, Imai S, Nakajima Y, Shimazawa M, Aihara M, Araie M, Hara H. Edaravone, a free radical scavenger, protects against retinal damage in vitro and in vivo. J Pharmacol Exp Ther. 2009;329:687–98. doi: 10.1124/jpet.108.148676. [DOI] [PubMed] [Google Scholar]
- 26.Yarborough A, Zhang YJ, Hsu TM, Santella RM. Immunoperoxidase detection of 8-hydroxydeoxyguanosine in aflatoxin B1-treated rat liver and human oral mucosal cells. Cancer Res. 1996;56:683–8. [PubMed] [Google Scholar]
- 27.Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen YN, Yamada H, Mao W, Matsuyama S, Aihara M, Araie M. Hypoxia-induced retinal ganglion cell death and the neuroprotective effects of beta-adrenergic antagonists. Brain Res. 2007;1148:28–37. doi: 10.1016/j.brainres.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Yamagishi R, Aihara M, Araie M. Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp Eye Res. 2011;93:265–70. doi: 10.1016/j.exer.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–73. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26:544–57. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Aoun P, Simpkins JW, Agarwal N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2003;44:2999–3004. doi: 10.1167/iovs.02-1060. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2374–83. doi: 10.1167/iovs.03-1239. [DOI] [PubMed] [Google Scholar]
- 34.Asomugha CO, Linn DM, Linn CL. ACh receptors link two signaling pathways to neuroprotection against glutamate-induced excitotoxicity in isolated RGCs. J Neurochem. 2010;112:214–26. doi: 10.1111/j.1471-4159.2009.06447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, Cordeiro MF. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–33. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DH, Kim CS, Lee YJ.Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem Toxicol 201149271–80.Epub 2010 Nov 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campoio TR, Oliveira FA, Otton R.Oxidative stress in human lymphocytes treated with fatty acid mixture: role of carotenoid astaxanthin. Toxicol In Vitro 2011251448–56.Epub 2011 Apr 27 [DOI] [PubMed] [Google Scholar]
- 38.Azqueta A, Collins AR.Carotenoids and DNA damage. Mutat Res 20127334–13.Epub 2012 Mar 21 [DOI] [PubMed] [Google Scholar]
- 39.Tripathi DN, Jena GB.Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res 201069669–80.Epub 2009 Dec 28 [DOI] [PubMed] [Google Scholar]
- 40.Shen H, Kuo CC, Chou J, Delvolve A, Jackson SN, Post J, Woods AS, Hoffer BJ, Wang Y, Harvey BK.Astaxanthin reduces ischemic brain injury in adult rats. FASEB J 2009231958–68.Epub 2009 Feb 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chucair AJ, Rotstein NP, Sangiovanni JP, During A, Chew EY, Politi LE. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:5168–77. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]
- 42.Yu ZK, Chen YN, Aihara M, Mao W, Uchida S, Araie M. Effects of beta-adrenergic receptor antagonists on oxidative stress in purified rat retinal ganglion cells. Mol Vis. 2007;13:833–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–6. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazawa T, Nakagawa K, Kimura F, Satoh A. Plasma carotenoid concentrations before and after supplementation with astaxanthin in middle-aged and senior subjects. Biosci Biotechnol Biochem. 2011;75:1856–8. doi: 10.1271/bbb.110368. [DOI] [PubMed] [Google Scholar]