Abstract
Fertilization is an indispensable step for formation of a zygote in sexual reproduction, leading to species survival. When mating occurs, sperm is transported to the female reproductive tracts via the seminal plasma (SP). SP is derived from male accessory sex glands and it plays pivotal roles for fertilization in animals. However, molecular mechanisms of SP or a fluid derived from male accessory sex glands for successful fertilization remain unclear. Here, we report that in male quail the cloacal gland (CG) produces prostaglandin F2α (PGF2α) that contributes to successful fertilization. PGF2α, as well as the secretion of CG (CGS), induced vaginal contractions and caused the opening of the entrance of the sperm storage tubules, the structures responsible for the long-term sperm storage and fertilization. The removal of CGS from the male before mating reduced the fertility, but the supplementation of CGS or PGF2α rescued the subfertility. We further showed that male CG contains glucose that is utilized as energy source for the intrinsic sperm mobility after transportation to female vagina. This mechanism, in concert with the excitatory effects of PGF2α enables successful fertilization in the domestic bird.
Fertilization is of paramount importance to species survival and its success depends on ejaculated sperm traversing the female reproductive tract to reach the oocytes where fertilization occurs. During mating, sperm are transported to the female reproductive tract within a fluid medium, generally referred to seminal plasma (SP) secreted from rete testis, epididymis and accessory sex glands of male genital tract1. The studies on the SP in many species highlight pivotal roles of SP in successful fertilization, including augmentation of sperm motility, modification of female receptivity and behavior, decreasing immune responses against allogenic spermatozoa and enhancing sperm transport within the oviduct2,3,4. Most of the semen of murine rodent coagulates to form a copulatory plug, which reduces the fertilization success of rival males and prevents sperm loss by a backward flow1. SP contains signaling substances such as sex steroids, prostaglandins and glycoproteins including cytokines and growth factors2,4. These molecules bind to their respective receptors on the target cells of the female reproductive tract to modulate functions related to fertilization. For example, immune-regulatory molecules such as transforming growth factor β (TGFβ) and prostaglandin E, found in the SP, had been suggested to modulate female immune response for male antigen-specific tolerance2. Indeed, an earlier study demonstrated that mated female mice fail to reject skin grafts of paternal origin5. The direct effects of SP components on the sperm had also been suggested. A family of heparin-binding proteins in bovine SP, that are referred to as bovine seminal plasma proteins (BSPs), coat the sperm surface and enable sperm to bind to the oviductal epithelium to prolong sperm motile life span in the oviduct6. Prostaglandin F2α (PGF2α), the acidic soluble substance, which is produced in the seminal vesicle of male mammals is detected in the SP of various animals, although its concentration varies among species7. Studies in boar suggest that this hormone is important for sperm transport to the site of fertilization in the oviduct probably due to the stimulation of uterine contractility8,9. Indeed, it is reported that exogenous PGF2α added to boar semen improved conception and farrowing rates after artificial insemination10. In addition to the effects of PGF2α, it is reported in pigs that SP estrogens can trigger local uterine endometrium PGF2α release and thus increase uterine activity11. Moreover, PGF2α also thought to be involved in excessive sperm elimination from the uterus since polymorphonuclear neutrophils are activated by sperm to release PGF2α via the cyclooxygenase pathway to cause contraction of smooth muscle to remove accumulated fluid including sperm in the lumen12. It is considered that PGF2α might improve reproductive efficiency by enhancing sperm transport within female reproductive tract to the fertilization site in mammals as PGF2α dose not increase any sperm motility parameter13. Although PGF2α in the SP is generally recognized as an agent that contracts smooth muscles of female reproductive tract, molecular mechanisms of how the myometrial contraction by the action of PGF2α improves sperm transport remain unclear.
In avian species, unique reproductive strategies, such as polyspermic fertilization and sperm storage in the oviduct, are employed for successful fertilization. Thus, the characterization of avian reproductive strategies will provide deeper understanding of the reproductive system of vertebrates. The male reproductive system in birds is quite different in morphology from that in mammals, as it lacks the prostate and seminal vesicles14. Mature spermatozoa travel down the vas deferens, which is expanded into a sac-like ending near the cloaca (Fig. 1a). At copulation, male cloaca is everted and applied to the opening of the oviduct, i.e. vagina (Fig. 1a, b). Male cloacal gland (CG), a unique structure present in the dorsal wall of the cloaca, is noticeable as the exocrine gland in galliform species15. Mature male quail produce foam as an accessory sexual fluid in the CG (Supplementary Movie 1). This foam, which is obviously different from a lymph-like fluid produced by roosters, drakes and male turkeys, but rather close to the secretion by mammalian accessory sex glands16, is ejected with sperm in the opening of the oviduct, i.e. vagina, during copulatory behavior17. Thus, the CG of mature birds has been thought to produce essential substance(s) implicated in fertilization. However, the chemical features of CG products that play a role for successful fertilization have yet to be characterized.
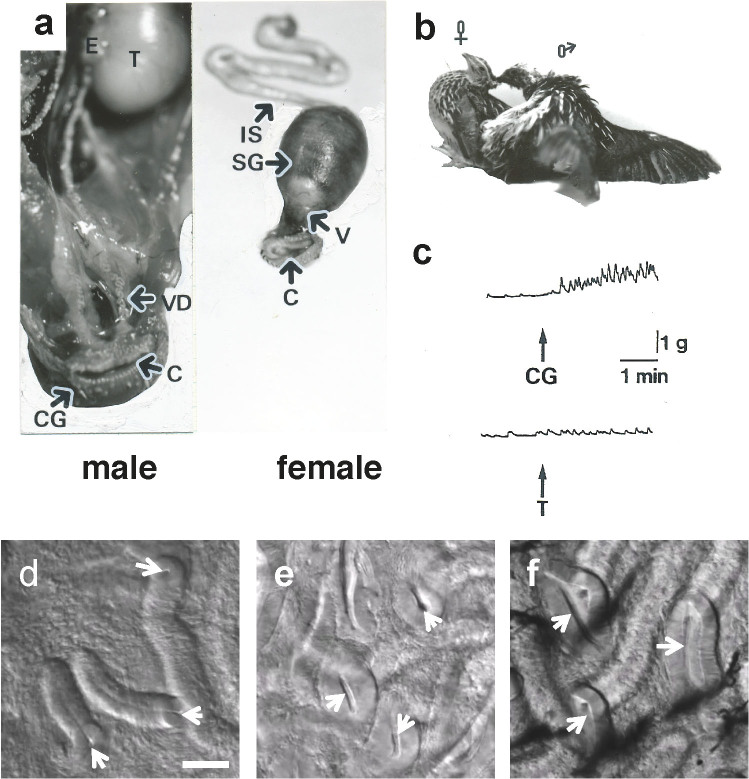
Figure 1. The reproductive system in the male and female Japanese quail.
(a), Male, T. testis; E. epididymis; VD. vas deferens; C. cloaca; CG. cloacal gland. Female, IS. isthmus; SG. shell gland; V. vagina; C. cloaca. (b), The copulatory behavior of Japanese quail showing the cloacal contact movement. (c), Bioactivity of the crude extract derived from the cloacal gland (CG) or testis (T) on spontaneous contractions of the isolated female vagina (V). The upward arrow indicates application of the extract to the tissue. (d–f), Microscopic observation of the entrance of sperm storage tubules (SST). Mature females were intra-vaginally injected with (d), 30 μl phosphate-buffered saline (PBS), (e), cloacal gland secretion (CGS) or (f), 2 × 10−9 M prostaglandin F2α (PGF2α). After 10 min injection, the mucosa of utero-vaginal junction was isolated and observed under light microscope. Arrows indicate the entrance of SST. Bar = 50 μm.
In this study, we first identified PGF2α in the CG of male quail and subsequently demonstrated unique mechanisms of PGF2α for successful fertilization after transportation with sperm to female vagina by copulation in quail. We further found that the CG contains glucose that acts as energy source for the intrinsic mobility of sperm after transportation to female vagina in this domestic bird.
Results and Discussion
In the first experiment, we found that a crude extract derived from the CG of two males potentiates spontaneous contractions of the female vagina (Fig. 1c). In contrast, the testicular extract did not induce such a significant change (Fig. 1c). Likewise, injections of cloacal gland secretion (CGS) into the vagina caused the opening of the entrance of sperm storage tubules (SST) (compare Fig. 1, d and e), the specialized simple tubular invaginations responsible for the long-term sperm storage and fertilization in birds18. These results prompted us to identify the active components responsible for the stimulation of vaginal contractions. By using a series of C-18 reversed-phase columns (Fig. 2a) and a cation-exchange column (Fig. 2b) of the high-performance liquid chromatography (HPLC) system (Supplementary Fig. S3), we purified a bioactive substance from the extract of the CG of 100 males showing a single peak on the final reversed-phase column under an isocratic condition (Fig. 2c). The spectroscopic data of the isolated bioactive substance (Supplementary information) identified prostaglandin F2α (PGF2α) as compared with the spectra of authentic specimens, such as PGF2α, (15R)-PGF2α and (13Z, 15R)-PGF2α19. The calculated amount of the isolated substance (PGF2α) from the tissues of 100 males was 0.21 μmols. An excitatory effect of this substance (2.5 × 10−11 M) was detected in the vaginal bioassay (Fig. 2d). In addition, intra-vaginal injection of 2 × 10−9 M PGF2α, which is equivalent to the PGF2α concentration in the CGS (2.1 ± 0.25 × 10−9 M, mean ± SEM, n = 3), successfully opened the entrance of SST (Fig. 1f). Because the effective dose of PGF2α coincided with that in the CGS, the observed effects of PGF2α are considered as physiological actions. Effects of PGF2α on the spontaneous contractions of the female vagina was inhibited by pretreatment of female vagina with AL 8810, a selective antagonist for the receptor of PGF2α (PGF2α-R)20 (Supplementary Fig. S1).
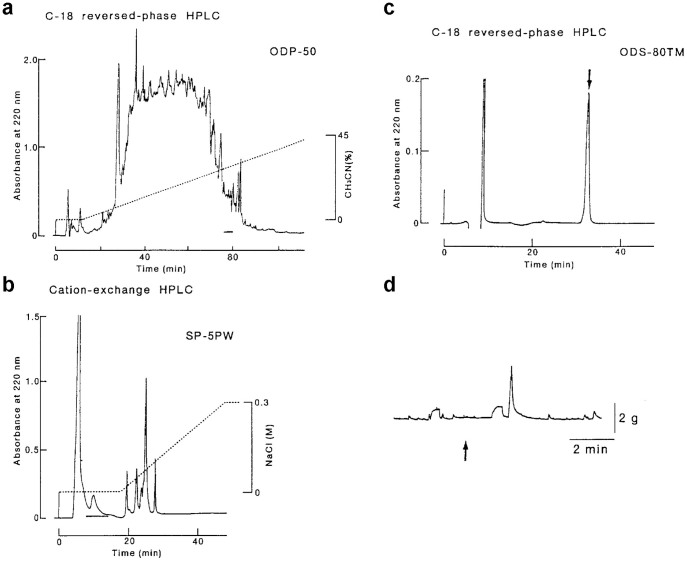
Figure 2. HPLC profile of the retained material (RM) of disposable C-18 reversed-phase cartridges on a reversed-phase column.
(a), The RM loaded onto the column was eluted with a linear gradient of acetonitrile (ACN) and collected in 50 fractions of 2 ml each. Aliquots (20 μl) of each fraction were evaporated to dryness, dissolved in the physiological saline (pH 7.3) and applied to the bioassay. The bioactive fractions were indicated by the horizontal bar. (b), HPLC profile of the bioactive fractions (in panel a) on a cation-exchange column. Elution was performed in a linear gradient of NaCl in PB (pH 6.8) and eluents were collected in 1-ml fractions. The bioactivity was detected in the flow through fractions (horizontal bar). (c), Final purification of the bioactive substance (0.21 μmoles) by HPLC using a reversed-phase column. Isocratic elution with 36% ACN at a flow rate of 0.3 ml/min. (d), Bioactivity of the isolate on spontaneous contractions of the isolated female vagina. The upward arrow indicates application of an aliquot (2.5 × 10−11 M) of the isolate.
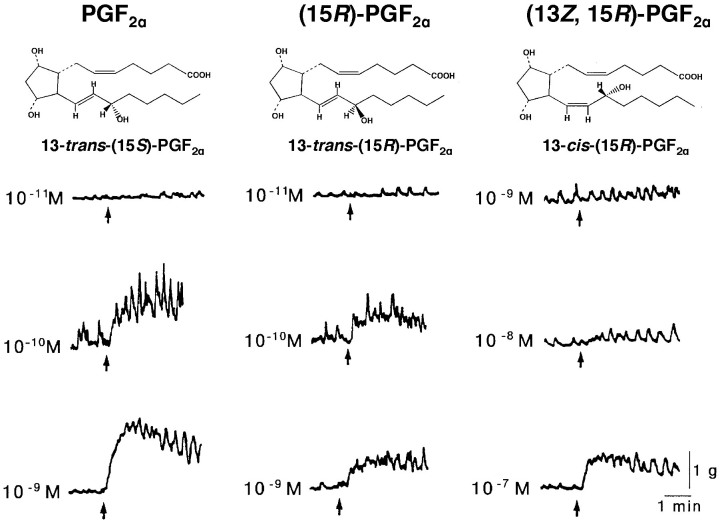
The bioactivity of an isolated substance that results in vaginal contractions was compared with those of PGF2α analogues. Although all of the stereoisomers resulted in vaginal contractions, PGF2α caused the greatest effect (Fig. 3). From these results, we hypothesized that PGF2α in the CGS supports sperm uptake into the SST by opening the SST entrance and vaginal contractions. To test this hypothesis, we measured sperm filling rate in the SST of quail, which were received intra-vaginal injection of CGS or PGF2α before artificial insemination (AI) of the washed sperm. As the results, both CGS and PGF2α treatment increased SST sperm filling (Fig. 4, a, b, c). These results indicated that the male bird transports PGF2α into the vagina at the time of mating, and this substance supports the sperm uptake into the SST in quail. Presence of PGF2α-R in the utero-vaginal junction (UVJ) where SST exists was also confirmed by gene-specific in situ hybridization (Supplementary Fig. S2a). Our semi-quantitative RT-PCR analysis indicated that PGF2α-R mRNA was expressed not only in the vagina and UVJ, but was also present in the uterus, isthmus, magnum and infundibulum of the oviduct (Supplementary Fig. S2b). This observation coincides with previous findings that PGF2α derived from preovulatory follicles induced contractions of the oviduct and might also be involved in the process of egg transport and oviposition21. We predicted that paternal PGF2α, transported into the female vagina at the time of copulation does not reach to the uterus beyond the UVJ. This is because our previous findings in which Hoechst 33342 DNA dye injected into the vagina was able to stain the nucleus of the surface epithelium of the vagina and UVJ, but not those of the uterus22. From these findings, we predicted that PGF2α-R expressed in the vagina and UVJ would only be involved in sperm transport and uptake into the SST. The elucidation of the roles of PGF2α-R expressed in the uterus or other parts of the oviduct for sperm transport should be examined in the future studies.
Figure 3. Bioactivities of PGF2α, (15R)-PGF2α and (13Z, 15R)-PGF2α on spontaneous contractions of the isolated female vagina.
The upward arrow indicates application of each stereoisomer.
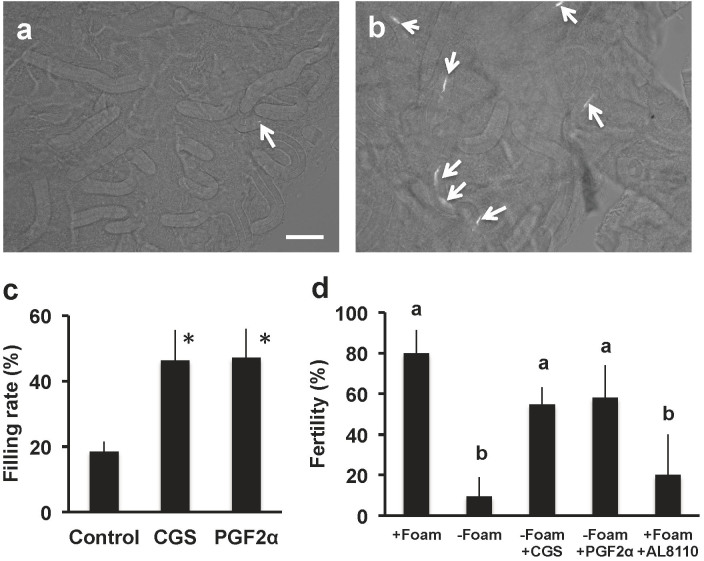
Figure 4. Effects of cloacal gland secretion (CGS) and PGF2α on the fertilization of Japanese quail.
Female quail were treated with (a), vehicle alone, or (b), CGS. After 10 min, the Hoechst 33342-stained sperm were artificially inseminated. The utero-vaginal junction mucosa was isolated from the bird 1 h after the artificial insemination (AI), and SST was observed under fluorescence microscope. Arrows indicate the resident sperm in the SST. Bar = 100 μm. (c), The percentage of the SST containing sperm was calculated. The data are expressed as the mean ± SEM of 3 replicates. An asterisk indicates a significant difference, P < 0.05. (d), The female was mated with the male without treatment (+Foam), or the male in which foam was manually removed (−Foam). The female was intra-vaginally injected with CGS (−Foam +CGS) or 2 × 10−9 M PGF2α (−Foam +PGF2α) and were allowed to mate with the male, which had his foam manually removed. The female of which was intra-vaginally injected 1 μM AL 8810 was mated with intact male (+Foam +AL8810). Eggs were collected daily for 8 days and examined for embryo development in order to confirm fertilization. Fertility was calculated and expressed as the mean ± SEM. 3–5 birds were used for each treatment. Values with different letters are significantly different, P < 0.05.
Constitutive expression of PGF2α-R in the UVJ throughout the ovulatory cycle is advantageous because females can copulate with a male at any time (Supplementary Fig. S2c). In agreement with this assumption, sperm filling rate of the SST was constant irrespective of the timing of mating during the ovulatory cycle (Supplementary Fig. S2d). Our results demonstrated the fertility of the male whose foam was manually removed before mating was significantly decreased when compared to that of the intact male (Fig. 4d). Notably, the intra-vaginal injection of CGS or PGF2α before mating recovered fertility of the subfertile male (Fig. 4d). The hatchability of the fertilized eggs obtained from the CGS- or PGF2α-treated animals was 100% (4 or 3 birds were used for CGS or PGF2α treatment, respectively). These results indicated that this treatment had no adverse effects on embryo development. In addition, intact male fertility was decreased when AL 8110 was intra-vaginally injected prior to copulation (Fig. 4d). These results indicate the physiological importance of PGF2α in the CGS for successful fertilization. The inability of the male to fertilize without foam appears to be related to the absence of PGF2α as the biological basis of sustained fertility in domestic birds is in their capacity for the sperm to reside in the SST23. The concentrations of PGF2α found in the SP of turkey and rooster are reported to be 100–140 pg/ml and less than 100 pg/ml, respectively24,25. Those values are approximately 7 times lower than that of quail CG. Although we did not perform vaginal bioassay in turkeys or chickens, their SP PGF2α levels were sufficient to induce the vaginal contraction of quail. The question of whether this unique mechanism is also functional in other birds remains to be solved.
After natural mating, the ejaculated sperm are deposited into the vagina; however, it is reported that more than 80% of the sperm are ejected from the vagina following mating18. This suggests that the vagina is the primary sperm selection site in avian species. It is also reported that less than 1% of the sperm that are inseminated into the vagina are able to enter the SST26, and the resident sperm in the SST are thought to participate in the subsequent process of fertilization. In addition to the vaginal rejection of the sperm, the oviductal immune system may also affect the survivability of the sperm since the sperm are recognized as foreign bodies in the oviduct. Immunocompetent cells for acquired immunity (i.e. macrophages, antigen-presenting cells expressing MHC class II, CD4+, CD8+ T cells and premature B and plasma cells) are reported to localize in the oviduct mucosal tissue in sexually matured chickens to protect the tissues from infection27,28. A significant increase in the number of lymphocytes as well as antigen-presenting cells expressing MHC class II in the stroma of UVJ in low fertility hens were observed29. Das et al. demonstrated that the resident sperm in the SST of a fertile bird are protected from immune responses by TGFβ-mediated suppression of anti-sperm immunoreactions presumably by suppressing the proliferation of T- and B-lymphocytes30. Thus, the elimination of anti-sperm immune responses is one of the factors responsible for sperm maintenance in the SST. Although prostaglandin E was reported to induce oviposition in quail25, the immune-tolerance systems against male antigens that have been reported in the mammalian SP2 are not found in birds. Similarly, evidence for another sperm protection system in mice was recently published by Kawano et al. who observed that mice lacking seminal vesicle secretion 2 (SVS2) protein are infertile and their sperm are killed by uterus-derived cytotoxic factors31. They showed that SVS2 protein coats sperm surface and protects the sperm from the attack by the uterus. These views suggest that the SST provides a shelter for the sperm from the female attack. Importantly, the transport of PGF2α at the time of mating is considered as a natural mechanism protecting the sperm from the rejection or killing by the female reproductive tract. Thus, the successful sperm uptake into the SST with the aid of PGF2α action appears to be a key event for the achievement of fertilization in birds.
It is reported that CGS possesses the potency to augment sperm motility32,33, however, we failed to detect such stimulatory effects when the physiological concentration of PGF2α was added to the sperm extender, indicating that PGF2α does not participate in the enhancement of sperm motility in quail. To explore the machinery of how CGS contributes to the enhancement of sperm motility, free sugar analysis of a water-soluble phase of tissue homogenates of the CG was further carried out with the HPLC and gas chromatography (GLC) systems. The water-soluble extract of the CG contained free sugars, such as glucose, galactose and fructose (Supplementary Table S1). However unlike the CG, the testicular extract was completely devoid of glucose. Both the total amount and the concentration of fructose were lower than those of glucose and galactose in the CG (Supplementary Table S1). The present determination showing a significant level of glucose in the CG is in agreement with previous findings in which the production of the foamy material results from the production of carbon dioxide and hydrogen through bacterial action on glucose34. Accordingly, it is probable that glucose in the CGS is ejected as a foamy material accompanying with sperm, which may be utilized as energy source for sperm mobility. This mechanism, in concert with the excitatory effects of PGF2α, enables successful fertilization in quail. Our results provide a novel biological insight into the understanding of how the CG contributes to fertilization in a domestic bird.
Methods
Animals
Male and female Japanese quail, Coturnix japonica, 8–20 weeks of age (Motoki Corporation) maintained individually under a photoperiod of 14-h light (L): 10-h dark (D) (lights went on at 05:00) with ad libitum access to water and a commercial diet (Motoki Corporation) were used for experiments. All experimental procedures for the care and use of animals were carried out in accordance with approved guidelines of the Animal Care Committees of Shizuoka University and Waseda University.
Extraction and purification of bioactive substance
Excised cloacal gland (CG) tissues (ca. 70 g wet weight) of 100 male quail were immediately frozen in liquid nitrogen. The frozen tissues were pulverized, boiled for 15 min in 4% acetic acid and homogenized by a Polytron. The homogenate was centrifuged at 10,000 × g for 40 min at 4°C and the supernatant fluid was evaporated to dryness. The dried extract was dissolved in 0.1% trifluoroacetic acid (TFA) and then forced through disposable C-18 cartridges (Mega Bond-Elut, Varian). The retained material (RM) eluted with 50% methanol was concentrated and filtered through a membrane (0.45 μm; Dismic-13cp, Advantec). The filtrate was subjected to the HPLC system (TRY ROTAR VI, Jasco) according to our previous methods35,36,37. In brief, the RM of disposable C-18 cartridges was loaded onto a C-18 reversed-phase column and eluted with a 120-min linear gradient of 0–60% acetonitrile (ACN) in 0.1% TFA (pH 2.2) at a flow rate of 1 ml/min. The bioactive fractions were then applied onto a cation-exchange column and eluted with a 70-min linear gradient of 0–0.7 M NaCl in 10 mM phosphate buffer (PB; pH 6.8) at a flow rate of 0.5 ml/min. The bioactive fractions were re-chromatographed by the other C-18 reversed-phase column with a linear gradient of 28–43% ACN in 0.1% TFA. Final purification was performed with the same column under an isocratic condition with 36% ACN at a flow rate of 0.3 ml/min. The purification scheme is presented in supplementary Fig. S3.
Bioassay for vaginal contractions
The bioactivity of an extract and each HPLC fraction was examined by monitoring the effects on spontaneous contractions of the vaginal oviduct. For the bioassay, the vaginal oviduct of female quail was excised and cut transversely with 5–10 mm length. Both ends of the excised vagina were tied with two cotton threads, one being connected to the bottom of an experimental chamber and the other to a force-displacement transducer (NEC San-ei Instruments). The chamber was filled with 10 ml physiological saline (pH 7.3), which was bubbled with air and incubated at 37–39°C. The test substance dissolved in 0.1 ml saline was injected into the chamber.
Structure determination of bioactive substance
Mass spectrometry (MS) analyses were performed on a JEOL JMS SX-102 equipped with a xenon beam generating system as a FAB-ion source at 10 kV of accele voltage by use of glycerol as a matrix. 1H NMR spectra were observed with a JEOL GSX-500 spectrometer at 500 MHz in CDCl3 using tetramethylsilane as an internal standard. The spectroscopic data of the isolated substance were compared with those of authentic specimens. Based on the results of structure analyses, the substance was identical with PGF2α. Therefore, the characterized native substance was further compared with PGF2α with regard to behavior on HPLC.
Semen collection and artificial insemination
Ejaculated semen was obtained from male quail during mating prior to ejaculation according to the procedure of Kuroki and Mori38. Semen obtained from two males was suspended in Hanks' balanced salt solution (HBSS) supplemented with 0.5 μM Hoechst 33342. The concentrations of sperm were measured with a hemocytometer. The ejaculates were washed two times with HBSS with repeated centrifugation at 800 × g for 3 min, and the final pellet was used for artificial insemination (AI).
Fertility assay
The CGS was obtained by centrifugation of isolated foam at 20,000 × g for 10 min. PGF2α level in the CGS was measured by ELISA kit (Cayman). The CGS or PGF2α dissolved in PBS (1 ng/ml) was intra-vaginally injected (30 μl/bird). To observe the effects of AL8110 (Cayman), the chemical dissolved in dimethyl sulfoxide was diluted with PBS (1 μM), and intra-vaginally injected before copulation. The concentration of AL8810 was adopted from Griffen et al.20. After 10 min, the female was received AI or allowed to copulate with the male. Eggs were collected everyday and fertility was confirmed by the development of a blastoderm. In addition, some eggs were incubated at 37°C with rocking at a 30° angle until hatching to assess the hatchability.
Observation of SST
The UVJ was isolated as described previously39. After washing with PBS, the specimens were fixed with 3.7% formaldehyde solution, and the fixed UVJ was cut into small pieces with scissors, mounted in glycerol and examined under a fluorescence microscope with a 20 x objective39 (BX 51, Olympus Optics). The total number of SST and the number of SST filled with sperm were counted, and the filling rate (%) of SST was calculated.
In situ hybridization
The frozen sections of the isolated UVJ were prepared for in situ hybridization, as described elsewhere40. The antisense 45-mer oligonucleotide probes for the receptor for PGF2α (5′- CATGGTTAGGAGACGAGAGGCAGCGCTCAACACATTTCAGCAGCC -3′, 5′- GCGATGGCTCCGTTGATGAGGTGGCCAAAGAGATCAGTGATGACC -3′, 5′- GGCTTGGTGACTCCAATGCAACGTTCAACAGCCATCACGCTGCCC -3′) were labeled with [33P] dATP (NEN Life Science Products) using terminal deoxyribonucleotidyl transferase (Gibco, Frederick). Hybridization was performed overnight at 42°C. Washing was performed twice at room temperature for 30 min and at 55°C for 40 min. After washing, the slides were exposed imaging plate (Fujifilm) and detected with BAS-2000 (Fujifilm).
RT-PCR
Total RNA was extracted from the oviductal tissues with a commercial kit, RNAiso (Takara Biomedicals), according to the manufacturer's instructions. Messenger RNA was isolated using an oligotex-dT30 mRNA purification kit (Takara Biomedicals), according to the manufacturer's instructions, and was reverse transcribed using a ReverTra Ase kit (Toyobo). PCR amplification was performed using specific primer sets for the receptor for PGF2α (sense: 5′- CCAACAGTCTCGCAATAGCA - 3′; antisense, 5′- AAAGTGGGCACAAACCAAAG- 3′). In parallel, primers (sense: 5′- GACGAAGACGGTGAAGAAGG-3′; antisense, 5′-CTTGGTGTCTGGGTCCACTT-3′) for quail S17 ribosomal protein41 were used as an internal control. For non-RT control, mRNA of UVJ was treated in a same way except to replace the reverse transcriptase with water. The products were analyzed on a 1.0% agarose gel stained with ethidium bromide and visualized under UV transillumination. The intensity of the bands was measured with NIH Image ver. 1.61.
Free sugar analysis
For the measurement of free sugar contents, 1 ml homogenate of the CG was first boiled in deionized water for 10 min, and then 4 ml ethanol was added to the homogenate. Supernatant was obtained by centrifugation (1,000 × g, 10 min) and dried at 50°C with a stream of filtered air. Dried materials were dissolved in 1 ml distilled water, and then filtered through a membrane (0.45 μm; Gelman). Sample solution (0.5 ml) was introduced into the HPLC system (model 307, Gilson) equipped with a pulsed amperometric detector (Dionex). The column (CarboPac PA-1, Dionex) was eluted with 0.16 M NaOH at a flow rate of 1 ml/min. Fructose was eluted at 4.3 min. The other half sample solution was reduced for 1 h at room temperature by the addition of 0.5 ml NaBH4 (20 mg/ml) in 2 M NH4OH containing 300 μg inositol as an internal standard. Reduced monosaccharides were acetylated for 15 min at room temperature by the addition of 200 μl acetic anhydride and 50 μl 1-methylimidazole as a catalyst42. Acetylated monosaccharides were analyzed by gas chromatography (GLC; GC-7A, Shimadzu) with a capillary column (SP-2440, Supelco).
Statistical analysis
Results were expressed as the mean ± SEM. The significance of differences between the groups was evaluated by one-way ANOVA followed by the Duncan's Multiple Range test or Student's t test (only Table S1). The differences were considered significant if P < 0.05.
Author Contributions
T.S. and K.T. conceived and designed the study. T.S., S.I., N.S., T.H., S.M., M.M., G.H., E.Y., T.Y., K.U. and K.T. performed the experiments and wrote the paper. All authors approved the final manuscript.
Supplementary Material
Supplementary information
Supplementary movie
Acknowledgments
The authors thank Drs Y. Muneoka (Hiroshima University) and T. Ubuka (Waseda University) for their valuable discussions, T. Kotegawa (Hiroshima University), M. Niikawa (Shizuoka University), T. Aoya (Shizuoka University) and Dr. Y. Tobari (Waseda University) for their technical assistances and Dr. S. Meddle (Roslin Institute) for critical reading and English editing of our manuscript. This work was supported in part by Grants-in-Aid for Scientific Research (22132004 and 22227002 to K.T.; 24380153 and 24112710 to T.S.) and for Challenging Exploratory Research (25660211 to T.S.).
References
- Florman H. M. & Ducibella T. Physiology of Reproduction, 3 edition (eds Neill, J.D.) 55–145 (Academic Press, San Diego, 2006). [Google Scholar]
- Robertoson S. A. Seminal plasma and male factor signaling in the female reproductive tract. Cell Tissue Res. 322, 43–52 (2005). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez H. et al. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 66, 11–22 (2011). [DOI] [PubMed] [Google Scholar]
- Juyena N. S. & Stelletta C. Seminal plasma: An essential attribute to spermatozoa. J. Androl. 33, 536–551 (2012). [DOI] [PubMed] [Google Scholar]
- Lengerova A. & Vojtiskova M. Prolonged survival of syngenic male skin grafts in parous C57 B1 mice. Folia Biol. 9, 72–74 (1963). [PubMed] [Google Scholar]
- Gwathmey T. M. et al. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol. Reprod. 75, 501–507 (2006). [DOI] [PubMed] [Google Scholar]
- Bergström S. Prostaglandins: members of a new hormonal system. These physiologically very potent compounds of ubiquitous occurrence are formed from essential fatty acids. Science 157, 382–391 (1967). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez H. & Einarsson S. Influence of prostaglandins on the spontaneous motility of pig oviducts. Anim. Reprod. Sci. 8, 259–279 (1985). [Google Scholar]
- Waberski D. Effects of semen components on ovulation and fertilization. J. Reprod. Fertil. Suppl. 52, 105–109 (1997). [PubMed] [Google Scholar]
- Horvat G. & Bilkei G. Exogenous prostaglandin F2α at time of ovulation improves reproductive efficiency in repeat breeder sows. Theriogenology 59, 1479–1484 (2003). [DOI] [PubMed] [Google Scholar]
- Claus R. Physiological role of seminal components in the reproductive tract of the female pig. J. Reprod. Fertil. Suppl. 40, 117–131 (1990). [PubMed] [Google Scholar]
- Troedsson M. H. T. et al. Components inseminal plasma regulating sperm transport and elimination. Anim. Reprod. Sci. 89, 171–186 (2005). [DOI] [PubMed] [Google Scholar]
- Maes D. G. D. et al. Motility characteristics of boar spermatozoa after addition of prostaglandin F2α. Theriogenology 60, 1435–1443 (2003). [DOI] [PubMed] [Google Scholar]
- Follett B. K. Marshall's Physiology of Reproduction (eds Lamming, G. E.) 283–350 (Churchill-Livingstone, New York, 1984). [Google Scholar]
- King A. S. Form and Function in Birds, (eds King, E. S. & McLelland, J.) 63–105 (Academic Press, New York, 1981). [Google Scholar]
- Coil W. & Wetherbee D. K. Observations on the cloacal gland of the Eurasian quail, Coturnix coturnix. Ohio J. Sci. 59, 268–270 (1959). [Google Scholar]
- Fujihara N. Biomechanism of the Gonads, (eds Iwasawa, H.) 56–71 (IPC, Tokyo, 1990). [Google Scholar]
- Bakst M. R. Role of the oviduct in maintaining sustained fertility in hens. J. Anim. Sci. 89, 1323–1329 (2011). [DOI] [PubMed] [Google Scholar]
- Andersen N. H. & Lin B. S. 500-MHz proton NMR studies of the medium-dependent conformational preference of prostaglandin F2 alpha analogs. Biochemistry 24, 2338–2347 (1985). [DOI] [PubMed] [Google Scholar]
- Griffen B. W. et al. AL-8810: A novel prostaglandin F2α analog with selective antagonist effects at the prostaglandin F2α (FP) receptor. J. Pharm. Exp. Ther. 290, 1278–1284 (1999). [PubMed] [Google Scholar]
- Shimada K., Olson D. M. & Etches R. J. Follicular and uterine prostaglandin levels in relation to uterine contraction and the first ovulation of a sequence in the hen. Biol. Reprod. 31, 76–82 (1984). [DOI] [PubMed] [Google Scholar]
- Hiyama. et al. Sperm activation by heat shock protein 70 supports the migration of sperm released from sperm storage tubules in Japanese quail (Coturnix japonica). Reproduction 147, 167–178 (2014). [DOI] [PubMed] [Google Scholar]
- Bakst M. R. et al. Comparisons of sperm storage tubule distribution and number in 4 strains of mature broiler breeders and in turkey hens before and after the onset of photostimulation. Poult. Sci. 89, 986–992 (2010). [DOI] [PubMed] [Google Scholar]
- Kennedy J. H., Korn N. & Thurston R. J. Prostaglandin levels in seminal plasma and sperm extracts of the domestic turkey, and the effects of cyclooxygenase inhibitors on sperm motility. Reprod. Biol. Endocrinol. 1, 74 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertelendy F. Effects of prostaglandins, cyclic AMP, seminal plasma, indomethacin, and other factors on oviposition in the Japanese quail, Coturnix Coturnix japonica. J. Reprod. Fertil. 40, 87–93 (1974). [DOI] [PubMed] [Google Scholar]
- Bakst M. R., Wishart G. & Brullard J. P. Oviductal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 5, 117–143 (1994). [Google Scholar]
- Zheng W. M., Yoshimura Y. & Tamura T. Effects of age and gonadal steroids on the localization of antigen-presenting cells, T and B cells in the chicken oviduct. J. Reprod. Fertil. 114, 45–54 (1998). [DOI] [PubMed] [Google Scholar]
- Zheng W. M. & Yoshimura Y. Localization of macrophages in the chicken oviduct: effects of age and gonadal steroids. Poult. Sci. 78, 1014–1018 (1999). [DOI] [PubMed] [Google Scholar]
- Das S. C., Nagasaka N. & Yoshimura Y. Changes in the localization of antigen presenting cells and T cells in the utero-vaginal junction after repeated artificial insemination in laying hens. J. Reprod. Dev. 51, 683–687 (2005). [DOI] [PubMed] [Google Scholar]
- Das S. C., Isobe N., Nishibori M. & Yoshimura Y. Expression of transforming growth factor-β isoforms and their receptors in utero-vaginal junction of the hen oviduct in presence or absence of resident sperm with reference to sperm storage. Reproduction 132, 781–790 (2006). [DOI] [PubMed] [Google Scholar]
- Kawano N. et al. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc. Natl. Acad. Sci. U. S. A. 111, 4145–4150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P. et al. Cloacal gland foam enhances motility and disaggregation of spermatozoa in Japanese quail (Coturnix japonica). Theriogenology 75, 563–569 (2011). [DOI] [PubMed] [Google Scholar]
- Cheng K. M., Hickman A. R. & Nichols C. R. Role of the proctodeal gland foam of male Japanese quail in natural copulations. Auk 106, 279–285 (1989). [Google Scholar]
- McFarland L. Z., Warner R. L., Wilson W. O. & Mather F. B. The cloacal gland complex of the Japanese quail. Experientia 15, 941–943 (1968). [DOI] [PubMed] [Google Scholar]
- Li D. et al. An oviposition-inducing peptide: isolation, localization, and function of avian galanin in the quail oviduct. Endocrinology 137, 1618–1626 (1996). [DOI] [PubMed] [Google Scholar]
- Ubuka T. et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 153, 373–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osugi T. et al. Evolutionary origin of the structure and function of gonadotropin-inhibitory hormone: insights from lampreys. Endocrinology 153, 2362–2374 (2012). [DOI] [PubMed] [Google Scholar]
- Kuroki M. & Mori M. Binding of spermatozoa to the perivitelline layer in the presence of a protease inhibitor. Poult. Sci. 76, 748–752 (1997). [DOI] [PubMed] [Google Scholar]
- Ito T. et al. Progesterone is a sperm-releasing factor from the sperm-storage tubules in birds. Endocrinology 152, 3952–3962 (2011). [DOI] [PubMed] [Google Scholar]
- Yoshimura T. et al. Molecular analysis of avian circadian clock genes. Mol. Brain Res. 78, 207–215 (2000). [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Tsukada A., Saito N. & Shimada K. Profiles of mRNA expression of genes related to sex differentiation of the gonads in the chicken embryo. Poult. Sci. 82, 1462–1467 (2003). [DOI] [PubMed] [Google Scholar]
- Blakeney A. B., Harris P. J., Henry R. J. & Stone B. A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 113, 291–299 (1983). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary movie