Abstract
The normal diaphragm has an inspiratory action on the lower ribs, but subjects with chronic obstructive pulmonary disease commonly have an inward displacement of the lateral portions of the lower rib cage during inspiration. This paradoxical displacement, conventionally called ‘Hoover's sign’, has traditionally been attributed to the direct action of radially oriented diaphragmatic muscle fibres. In the present study, the inspiratory intercostal muscles in all interspaces in anaesthetized dogs were severed so that the diaphragm was the only muscle active during inspiration. The displacements of the lower ribs along the craniocaudal and laterolateral axes and the changes in pleural pressure (ΔPpl) and transdiaphragmatic pressure were measured during occluded breaths and mechanical ventilation at different lung volumes between functional residual capacity (FRC) and total lung capacity. From these data, the separate effects on rib displacement of ΔPpl and of the force exerted by the diaphragm on the ribs were determined. Isolated spontaneous diaphragm contraction at FRC displaced the lower ribs cranially and outward, but this motion was progressively reversed into a caudal and inward motion as lung volume increased. However, although the force exerted by the diaphragm on the ribs decreased with increasing volume, it continued to displace the ribs cranially and outward. These observations suggest that Hoover's sign is usually caused by the decrease in the zone of apposition and, thus, by the dominant effect of ΔPpl on the lower ribs, rather than an inward pull from the diaphragm.
Key points
Subjects with chronic obstructive pulmonary disease and hyperinflation commonly have an inward displacement of the lateral walls of the lower rib cage during inspiration. This paradoxical displacement, conventionally called ‘Hoover's sign’, is traditionally attributed to the pull by radially oriented diaphragmatic muscle fibres.
In this study in anaesthetized dogs, we measured the displacement of the lower ribs during isolated spontaneous diaphragm contraction at different lung volumes, and determined the separate effects on rib displacement of pleural pressure and of the force exerted by the diaphragm.
The results show that diaphragm contraction at low lung volumes causes an inspiratory displacement of the lower ribs, but this is progressively reversed into an expiratory displacement as lung volume increases. However, the effect of the force exerted by the diaphragm on the ribs remains inspiratory at all lung volumes.
These observations suggest that Hoover's sign is usually caused by the dominant expiratory effect of pleural pressure on the lower ribs, rather than an inward pull from the diaphragm.
Introduction
Measurements of thoracoabdominal motion in subjects with quadriplegia caused by the complete traumatic transection of the cervical cord (Mortola & Sant’Ambrogio, 1978; Danon et al. 1979; Strohl et al. 1984; Estenne & De Troyer, 1985; Urmey et al. 1986) and studies of rib displacements in dogs (De Troyer, 2011, 2012) have established that isolated contraction of the diaphragm during breathing produces an inward displacement of the upper portion of the rib cage and an expansion of the lower portion of the rib cage. The expiratory action of the diaphragm on the upper rib cage is primarily caused by the fall in pleural pressure, whereas its inspiratory action on the lower rib cage is the result of two components of the force developed by the muscle (De Troyer & Boriek, 2011). The first component, denoted the ‘insertional force’, is the direct cranial force applied by the muscle fibres of the costal portion of the diaphragm on the ribs into which they insert. The second component, the ‘appositional force’, is the lateral force caused by the transmission of abdominal pressure to the lower rib cage in the zone of apposition (Mead, 1979).
Healthy individuals also have an expansion of the lower rib cage during inspiration. By contrast, subjects with chronic obstructive pulmonary disease (COPD) and hyperinflation commonly have an inspiratory inward displacement of the lateral portions of the lower rib cage (Gilmartin & Gibson, 1984; Binazzi et al. 2008; Aliverti et al. 2009). This paradoxical displacement, conventionally called ‘Hoover's sign’, has traditionally been attributed to the direct action of the diaphragm on the lower ribs (Hoover, 1920; Gilmartin & Gibson, 1986). Accordingly, because the diaphragm is low in these subjects and the zone of apposition of the muscle to the lower rib cage is small, the muscle fibres of the diaphragm would become radially oriented during inspiration and would, therefore, pull the lower ribs inward, rather than cranially, during contraction.
However, studies using ultrasonography have shown that the zone of apposition in subjects with COPD is reduced, but not eliminated (Gorman et al. 2002; Priori et al. 2013). In addition, a recent study of the mechanics of the canine diaphragm has challenged the notion that the muscle exerts an inward force on the lower ribs at high lung volumes (Leduc et al. 2012). The diaphragm in that study was selectively activated by supramaximal stimulation of the phrenic nerves in the neck, and the configuration of the muscle and the position of the lower ribs were assessed by computed tomography (CT). When lung volume before stimulation was increased by applying a transrespiratory pressure of +30 cmH2O or more at the airway opening, so that the zone of apposition of the diaphragm was reduced to zero, stimulation of the phrenic nerves did not produce any lower rib displacement. However, these findings are complicated by the fact that stimulation of the phrenic nerves in the neck elicits non-uniform contraction of the diaphragm with excessive shortening of the muscle fibres of the costal portion of the muscle, so that the lower rib displacements may differ from those that occur during spontaneous isolated diaphragm contraction (De Troyer, 2011). Consequently, the question of whether the diaphragm at high lung volumes can have an expiratory action on the lower ribs remains.
The objective of the present study was to answer that question. The inspiratory intercostal muscles in dogs were severed in all interspaces so that the diaphragm was the only muscle active during inspiration, and displacements of the lower ribs along the craniocaudal and laterolateral axes of the body were measured during single inspiratory efforts against an occluded airway and during mechanical passive lung inflation at different lung volumes. From these data, the separate effects on rib displacement of pleural pressure and of the force developed by the diaphragm were determined. The results show that the displacement of the lower ribs is the net result of the inward force exerted by pleural pressure and the cranial and outward force exerted by the diaphragm. At functional residual capacity (FRC), the effect of the diaphragmatic force dominates and the net rib displacement is cranial and outward. With increasing volume, the effect of the diaphragmatic force remains inspiratory, but this effect decreases relative to that of pleural pressure so that the net rib displacement becomes caudal and inward.
Methods
The studies were carried out in seven adult bred-for-research dogs (22–32 kg) anaesthetized with pentobarbital sodium (initial dose: 30 mg kg−1 i.v.), as approved by the Animal Ethics and Welfare Committee of the Brussels School of Medicine. The animals were placed in the supine position and intubated with a cuffed endotracheal tube. A venous cannula was inserted in the forelimb to maintain the delivery of anaesthetic (3–5 mg kg−1 h−1 i.v.). The abdomen was then opened using a 4 cm mid-line incision cranial to the umbilicus, and a balloon catheter system filled with 1.0 ml of air was placed between the stomach and the liver and connected to a differential pressure transducer (Validyne Engineering Corp., Northridge, CA, USA) to measure the changes in abdominal pressure (ΔPab). After the abdomen had been closely sutured, the rib cage and intercostal muscles were exposed on both sides of the chest from the first to the 11th rib by reflection of the skin and superficial muscle layers, and the inspiratory intercostal muscles were eliminated from the act of breathing using a method described previously (De Troyer, 2011, 2012). Thus the external intercostal and levator costae muscles in interspaces 1–8 were severed bilaterally from the chondrocostal junctions to the spine, and the internal intercostal nerves in interspaces 2–8 on the right side and in interspaces 3–8 on the left side were sectioned at the chondrocostal junctions to denervate the corresponding parasternal intercostal muscles. The parasternal intercostal muscle in the first interspace was also sectioned bilaterally from the sternum to the chondrocostal junction. Finally, the vagi were isolated through a mid-line incision of the neck and sectioned in order to avoid the apnoea and prominent expiratory muscle activation that lung inflation would otherwise elicit (Bishop, 1967; Kelsen et al. 1977; Gilmartin et al. 1987).
Measurements
After completion of the surgical procedure, a hook was implanted in the right 10th rib in the mid-axillary line (i.e. in one of the ribs into which the diaphragm inserts in the dog) (Evans & Christensen, 1979) and connected by long, inextensible threads to two linear displacement transducers (Schaevitz Engineering, Inc., Pennsauken, NJ, USA) to measure rib displacement along the craniocaudal and laterolateral axes. This technique has been previously described in detail (De Troyer & Kelly, 1982). In addition, a differential pressure transducer was connected to a side port of the endotracheal tube to measure changes in airway opening pressure (ΔPao), a balloon-catheter system filled with 0.5 ml of air was placed in the mid-oesophagus to measure changes in pleural pressure (ΔPpl), and a pair of silver hook electrodes spaced 3–4 mm apart were inserted in the intact parasternal intercostal muscle of the second left interspace to quantify neural inspiratory drive and to provide a time reference for pressure and rib displacement. The position of the oesophageal balloon was adjusted by the occlusion technique (Baydur et al. 1982) and the parasternal electrodes were implanted in parallel fibres in the vicinity of the sternum (i.e. in the muscle area known to receive the greatest inspiratory drive) (De Troyer et al. 2005). The electromyography (EMG) signal was processed with an amplifier (model 830/1; CWE, Inc., Ardmore, PA, USA), bandpass-filtered from 100 Hz to 2000 Hz, and rectified before its passage through a leaky integrator with a time constant of 0.2 s.
Protocol
Baseline parameters were allowed to stabilize for 30 min following completion of the instrumentation, after which measurements of rib displacements, ΔPao, ΔPpl, ΔPab and parasternal intercostal EMG activity were obtained. The animal breathed spontaneously throughout. Every five to 10 breaths, however, lung volume was increased above FRC during the expiratory pause, and the endotracheal tube was occluded for a single inspiratory effort. At least 20–40 different levels of inflation taken in random order were applied in each animal; five or six occluded breaths were also obtained at FRC.
After these measurements were completed, the animal was connected to a mechanical ventilator (Harvard Apparatus, Inc., Holliston, MA, USA) and hyperventilated until the disappearance of parasternal intercostal EMG activity. The rate of the ventilator was then set slightly above the frequency previously observed during spontaneous diaphragmatic breathing, and measurements of rib displacement, ΔPao, ΔPpl and ΔPab were made for five or six breaths. While the animal was still connected to the ventilator, lung volume was then passively increased to ∼5, 10, 15, 20 and 25 cmH2O transrespiratory pressure in random order, and measurements were obtained for five or six breaths after each volume increase. The procedure was performed twice in each animal.
The animals were maintained at a constant, rather deep level of anaesthesia throughout the study. They had no corneal reflex and made no spontaneous movements other than respiratory movements during either the surgery or the measurement procedures. Rectal temperature was maintained at 36–38°C with infrared lamps. At the end of the experiment, the animals were killed with pentobarbital sodium (30–40 mg kg−1 i.v.).
Data analysis
Data for each animal were analysed in three stages. Firstly, phasic inspiratory electrical activity in the second left parasternal intercostal muscle during each occluded breath was quantified by measuring the peak height of the integrated EMG signal in arbitrary units. The peak inspiratory displacement of rib 10 along the craniocaudal axis (X) and the laterolateral axis (Y), and ΔPao (or ΔPpl) and ΔPab were measured relative to the onset of the parasternal inspiratory burst. Consequently, the values of rib displacement and pressure changes considered in the study's calculations resulted exclusively from the contraction of the diaphragm (and the single parasternal intercostal of the second left interspace) and were not corrupted by the relaxation of the abdominal muscles and the internal interosseous intercostals at the end of expiration. By convention, inspiratory rib displacements in the cranial or outward direction were given positive signs, and inspiratory rib displacements in the caudal or inward direction were given negative signs. The change in transdiaphragmatic pressure (ΔPdi) during each occluded breath was also calculated (ΔPdi = ΔPab − ΔPpl).
The inspiratory rib displacements, ΔPao and ΔPdi obtained during occluded breaths at the different lung volumes were then plotted against the values of Pao before inspiration (i.e. the precontractile transrespiratory pressures), and the relationships were fitted by quadratic regression equations. Rib displacements and pressures at fixed transrespiratory pressures at 2.5 cmH2O increments were determined from these equations by interpolation.
In the second stage of analysis, rib displacements and pressure changes produced by mechanical ventilation at the different lung volumes were measured. Thus, for each lung volume (i.e. each transrespiratory pressure), the values of the cranial (X) and outward (Y) displacement of rib 10 and ΔPpl and ΔPdi during mechanical passive lung inflation obtained over four or five consecutive breaths in the two trials were averaged.
In the third stage of analysis, the rib displacements driven by ΔPdi alone during occluded breaths at the different lung volumes were determined by using the following equations, where the coefficients a and c describe, respectively, the craniocaudal and laterolateral rib displacement produced by a unit change in ΔPpl alone, and the coefficients b and d describe, respectively, the craniocaudal and laterolateral rib displacement produced by a unit change in ΔPdi alone:
| (1) |
| (2) |
Thus, for a given lung volume, the values of rib displacements and pressure changes measured during mechanical ventilation and those obtained from the relationships during occluded breaths provided one set of two equations for X and one set of two equations for Y, and the values of the coefficients a, b, c and d for that particular lung volume were obtained by solving these equations.
Finally, data were averaged across the animal group and are presented as means ± s.e.m. Statistical assessments of the effects of increasing lung volume on rib displacement and pressure changes during occluded breaths and on the values of the coefficients a–d were made by analysis of variance (ANOVA) with repeated measures, and multiple comparison testing of the mean values was performed, when appropriate, using Tukey honest significance (HS) tests. The criterion for statistical significance was taken as P < 0.05.
Results
Rib displacement during occluded breaths
The records of Pao, Pab, rib 10 displacement and parasternal intercostal EMG activity in the second left interspace obtained in a representative animal during an occluded breath at FRC and during an occluded breath at a higher lung volume are shown in Fig. 1. During the occluded breath at FRC (Fig. 1A), ΔPao and ΔPab at peak inspiration were −10.0 cmH2O and +2.0 cmH2O, respectively, and rib 10 moved cranially and outward as it did during the preceding unimpeded breath. By contrast, when lung volume before occlusion was increased to a transrespiratory pressure of +20.0 cmH2O (Fig. 1B), ΔPao and ΔPab during the occluded breath were only −2.6 cmH2O and +0.4 cmH2O, respectively, and rib 10 moved caudally and inward. The inspiratory EMG activity recorded from the second left parasternal intercostal during occlusion at the elevated lung volume, however, was similar to that recorded during occlusion at FRC.
Figure 1. Records of rib 10 displacement and pressure changes during isolated spontaneous diaphragm contraction in a representative animal.
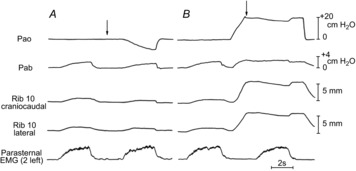
A, traces of rib 10 displacement along the craniocaudal and laterolateral axes, airway opening pressure (Pao), and abdominal pressure (Pab) during an unimpeded breath and an occluded breath (arrow) at resting end-expiration [functional residual capacity (FRC)]; the trace of electromyographic (EMG) activity in the parasternal intercostal of the second left interspace is also shown. B, an unimpeded breath at FRC followed by an occluded breath; between the two breaths, however, the lungs were passively inflated to a transrespiratory pressure of +20 cmH2O. Note that the rib moved cranially and outward (upward deflections) during both the unimpeded breath and the occluded breath at FRC. However, during the occluded breath at the higher lung volume, the rib moved caudally and inward (downward deflections), and the changes in Pao and Pab were much smaller. Inspiratory EMG activity in the parasternal intercostal, however, was unchanged.
The values of ΔPao and ΔPab obtained in the seven animals during the occluded breaths at the different lung volumes are shown in Fig. 2, and the displacements of rib 10 along the craniocaudal and laterolateral axes are shown in Fig. 3. During occluded breaths at FRC, rib 10 moved cranially and outward in six of seven animals, and caudally in one animal. For the animal group, therefore, the displacement of the rib along the craniocaudal and laterolateral axes at peak inspiration was +0.93 ± 0.42 mm and +1.04 ± 0.29 mm, respectively; the corresponding values for ΔPao and ΔPab were −10.2 ± 1.4 cmH2O and +1.9 ± 0.1 cmH2O. As transrespiratory pressure before occlusion was increased from zero to +25 cmH2O, however, ΔPao gradually and continuously decreased from −10.2 ± 1.4 cmH2O to −2.0 ± 0.3 cmH2O (P < 0.001), and ΔPab decreased from +1.9 ± 0.1 cmH2O to +0.2 ± 0.1 cmH2O (P < 0.001). The inspiratory cranial and outward displacement of the rib also decreased rapidly as transrespiratory pressure was increased to +8 cmH2O and 10 cmH2O (P < 0.01), at which point it was reversed into a caudal and inward displacement. The caudal and inward rib displacement then progressively increased in magnitude as transrespiratory pressure was increased to +17.5 cmH2O, and decreased as pressure was increased further to +25 cmH2O.
Figure 2. Effect of gradual lung inflation on the pressure changes produced by isolated spontaneous diaphragm contraction.
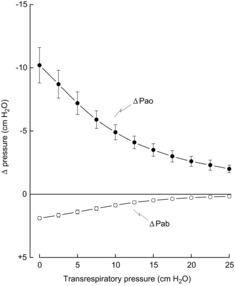
Mean ± s.e.m. changes in airway opening pressure (ΔPao) and abdominal pressure (ΔPab) obtained in seven animals during occluded breaths at different lung volumes. The values in the different animals were determined at fixed transrespiratory pressures at 2.5 cmH2O increments by interpolation.
Figure 3. Effects of gradual lung inflation on rib 10 displacement during isolated spontaneous diaphragm contraction.
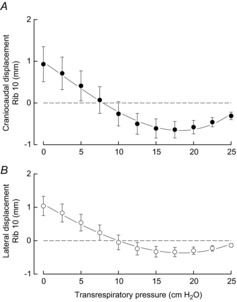
Mean ± s.e.m. values of rib displacement along the craniocaudal (A) and laterolateral (B) axes obtained in seven animals during occluded breaths at different lung volumes. Positive values correspond to displacements in the cranial and outward direction; negative values correspond to displacements in the caudal and inward direction. The cranial and outward displacement of the rib produced by diaphragm contraction at functional residual capacity (transrespiratory pressure: 0 cmH2O) progressively decreased as lung volume before contraction increased and was reversed into a caudal and inward displacement when transrespiratory pressure was +8 cmH2O to +10 cmH2O.
Rib displacement driven by ΔPdi
Mechanical ventilation produced a cranial and outward displacement of rib 10 with an increase in Ppl and a decrease in Pdi at all lung volumes; the values of the coefficients a, b, c and d obtained at the different lung volumes are shown in Fig. 4. In agreement with our previous observation (Wilson & De Troyer, 2013), the values of a and b at FRC were nearly equal and averaged, respectively, 0.80 ± 0.06 and 0.74 ± 0.06 for the animal group. The values of c and d at FRC were also nearly equal and averaged 0.50 ± 0.05 and 0.50 ± 0.05, respectively. The values of a remained essentially unchanged as transrespiratory pressure was increased from zero to +15 cmH2O and then decreased as transrespiratory pressure was increased further (P ≤ 0.05); the values of c remained unaltered as transrespiratory pressure was increased from zero to +25 cmH2O. By contrast, the values of b and d decreased progressively (P ≤ 0.01) as transrespiratory pressure was increased from +10 cmH2O to +25 cmH2O. In one animal, both coefficients were found to reverse from positive to slightly negative values when transrespiratory pressure was set at +25 cmH2O.
Figure 4. Effect of gradual lung inflation on the coefficients of rib displacement during isolated spontaneous diaphragm contraction.
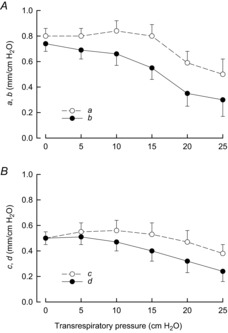
Mean ± s.e.m. values of a and b (A) and c and d (B) obtained in seven animals. a and b are the coefficients in the equation X = aΔPpl + bΔPdi, where X is the craniocaudal displacement of rib 10 in mm, ΔPpl is the change in pleural pressure in cmH2O, and ΔPdi is the change in transdiaphragmatic pressure in cmH2O. c and d are the coefficients in the equation Y = cΔPpl + dΔPdi, where Y is the laterolateral displacement of the rib in mm. Note that b and d gradually decrease as transrespiratory pressure increases from +10 cmH2O to +25 cmH2O.
The contributions of ΔPpl and ΔPdi to total rib displacement during occluded breaths at the different lung volumes were obtained from the values of these coefficients. That is, for each lung volume in each animal, the craniocaudal and lateral displacements of the rib produced by ΔPpl and ΔPdi were computed by using the values of the coefficients and those of the pressure changes. The lateral rib displacements were then plotted against the corresponding craniocaudal displacements and the total rib displacements were measured from the plots.
The plots obtained at the different lung volumes for the animal group are shown in Fig. 5. The filled circle in each panel of the figure corresponds to the rib displacement measured during occluded breaths at the transrespiratory pressure indicated at the top left margin of the panel, and the open circle represents the position of the rib that would have occurred if ΔPdi were zero. The dotted line, therefore, shows rib displacement attributable to ΔPpl alone, and the continuous line shows rib displacement produced by ΔPdi. The total rib displacement produced by ΔPpl was caudal and inward at all lung volumes and progressively decreased in magnitude (P < 0.001) from −9.37 ± 0.98 mm to −1.22 ± 0.27 mm as transrespiratory pressure was increased from zero to +25 cmH2O. The total rib displacement produced by ΔPdi also decreased gradually and continuously with increasing lung volume; in one animal, displacement at +25 cmH2O transrespiratory pressure was caudal and inward and amounted to −0.2 mm. In the other six animals, however, ΔPdi displaced the ribs in the cranial and outward direction at all lung volumes. For the animal group as a whole, therefore, the total rib displacement driven by ΔPdi at FRC was +10.74 ± 1.16 mm, but displacement at +25 cmH2O transrespiratory pressure was still +0.88 ± 0.31 mm (P < 0.001).
Figure 5. Trajectories of rib displacement produced by pleural and transdiaphragmatic pressure during isolated spontaneous diaphragm contraction at different lung volumes.
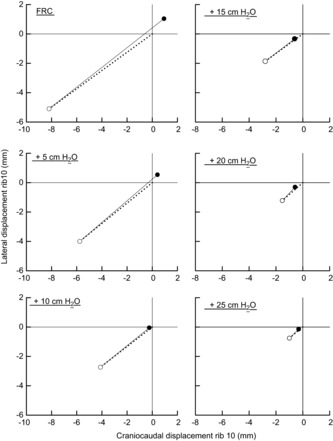
The different panels correspond to the different lung volumes. Numbers at the top left refer to transrespiratory pressure before diaphragm contraction. In each panel, the displacement of rib 10 along the craniocaudal axis is shown on the abscissa, and the displacement of the rib along the laterolateral axis is shown on the ordinate; positive values correspond to displacements in the cranial and outward direction, and negative values correspond to displacements in the caudal and inward direction. Filled circles represent the mean rib displacements measured during occluded breaths in seven dogs; open circles represent the mean position of the rib that would have occurred if the change in transdiaphragmatic pressure (ΔPdi) were zero. The dotted lines, therefore, represent rib displacements produced by the change in pleural pressure (ΔPpl) alone, and the continuous lines represent rib displacements caused by ΔPdi alone. Note that the rib displacement produced by ΔPdi decreased with increasing lung volume but remained cranial and outward at all lung volumes.
Discussion
The action of the diaphragm on the lower ribs at different lung volumes has been previously assessed in dogs using supramaximal stimulation of the phrenic nerves in the neck (D'Angelo & Sant’Ambrogio, 1974; De Troyer et al. 2009; Leduc et al. 2012). This technique is useful to study the mechanics of the diaphragm because it produces a constant, well-defined level of muscle activation and avoids the simultaneous contraction of other muscles. It allows, therefore, for a precise evaluation of the response of the diaphragm to different conditions, such as lung inflation. In the dog, however, stimulation of the phrenic nerves in the neck involves the C5 and C6 but not the C7 nerve roots. Because the C7 roots supply a limited part of the costal portion of the canine diaphragm and a large dorsal part of the crural portion (Landau et al. 1962), this implies that such stimulation causes nearly complete activation of the costal portion of the muscle but only partial activation of the crural portion. In addition, isolated stimulation of the phrenic nerves produces extreme shortening of the muscle fibres of the costal portion of the diaphragm and a large descent of the dome, so that most of the lower portion of the rib cage becomes exposed to pleural pressure, including when stimulation is performed at low lung volumes. As a result, whereas the lower ribs move cranially and outward during isolated spontaneous contraction of the diaphragm at FRC (De Troyer, 2011, 2012; Wilson & De Troyer, 2013), they move caudally and inward during phrenic nerve stimulation.
To determine the effect of lung inflation on the physiological action of the diaphragm on the lower ribs, it was therefore essential to ensure: (i) an isolated spontaneous contraction of the muscle at different lung volumes; (ii) a constant level of muscle activation, and (iii) a method to distinguish the rib displacement produced by muscle force from that produced by pleural pressure. In the following paragraphs, we first discuss the extent to which these requirements were met in the present study. We then analyse the results of the study and evaluate the impact of lung volume on the determinants of the action of the diaphragm on the lower ribs. Finally, we discuss the limitations of the study and its implications for the mechanism of Hoover's sign.
Methodological considerations
In the present experiments, the external intercostal muscles in all interspaces were severed, and the parasternal intercostal muscles in all but one interspace were denervated. In addition, all measurements were obtained while the animals were breathing spontaneously. Consequently, the diaphragm was the only muscle active during inspiration and the normal spatial distribution of neural drive among the different portions of the muscle, as described by Brancatisano et al. (1991) and Johnson et al. (2002), was maintained.
No attempt was made to record diaphragmatic EMG activity in these animals because moving viscera in the upper abdominal cavity and implanting electrodes in the diaphragmatic muscle fibres may induce inhibition of the diaphragm (Road et al. 1984; Ford et al. 1988). In addition, when recorded with i.m. electrodes, diaphragmatic EMG activity shows artefactual alterations with changes in muscle length, including during increases in lung volume (Kim et al. 1985; Gandevia & McKenzie, 1986). However, similarly to neural drive to the diaphragm, neural drive to the canine parasternal intercostals is primarily governed by supraspinal control mechanisms (De Troyer 1991, 1997). Measurements of motoneuron synchronization in cats have also shown that phrenic motoneurons and parasternal intercostal motoneurons receive common monosynaptic inputs (Vaughan & Kirkwood, 1997). Because our animals showed no alteration in inspiratory EMG activity in the parasternal intercostal of the second left interspace with increasing lung volume, it is reasonable to assume that neural drive to the diaphragm was also unaltered.
When the diaphragm is the only muscle active, the only forces acting on the lower ribs are the force generated by pleural pressure and the force exerted by the muscle fibres, which is proportional to ΔPdi. Equations (1) and (2) follow directly from this fact, and the only assumption embodied in these equations is that at any given lung volume, the relationship between rib displacement and ΔPpl and ΔPdi is linear. By measuring rib displacement and pressure changes during both occluded breaths and passive mechanical ventilation at different lung volumes, we were able to determine the coefficients in these equations and, thus, to assess the magnitude of the separate effects of ΔPpl and ΔPdi on rib displacement.
Impact of lung volume on diaphragmatic action on the lower ribs
In agreement with our previous observations (De Troyer, 2011; Wilson & De Troyer, 2013), contraction of the diaphragm at FRC displaced the ribs cranially and outward, but this displacement decreased rapidly in magnitude as lung volume increased. When transrespiratory pressure before contraction was set at +8 cmH2O, the inspiratory cranial displacement of the rib was suppressed (Fig. 3A). The outward rib displacement was also abolished when transrespiratory pressure was set at +10 cmH2O (Fig. 3B). When transrespiratory pressure was increased further, the displacements became caudal and inward.
As Fig. 5 shows, the displacement of the ribs is the small net result of large, but nearly equal and opposite, effects of ΔPpl and ΔPdi. The balance between these two effects changes with increasing lung volume so that net rib displacement decreases and then changes sign. This shift is caused by decreases in coefficients b and d relative to a and c, and these decreases are most likely attributable to the decrease in the zone of apposition of the diaphragm, as observed on radiographs (Petroll et al. 1990; Boriek et al. 1996) and CT scans (Pettiaux et al. 1997) of the chest.
A quantitative estimate of this decrease can be obtained from the values of the coefficients. Thus, as shown in Fig. 2, the ratio of ΔPdi to ΔPpl at FRC was, on average, −1.2.
Substituting for ΔPdi in eqns (1) and (2), therefore, yields
| (3) |
| (4) |
It follows that X and Y values will become zero when, respectively, a = 1.2 b and c = 1.2 d (i.e. when b/a and d/c are 0.83). As Fig. 6 shows, b/a in our animals decreased from 0.93 to 0.83 as transrespiratory pressure was increased from zero to +7 cmH2O, and d/c decreased from 1.02 to 0.83 as transrespiratory pressure was increased from zero to +11.5 cmH2O. These two values of transrespiratory pressure fit well with the values for zero rib displacements (Fig. 3). Furthermore, b and d are the sum of the effect of the appositional force and the effect of the insertional force, and a recent study has shown that, in the dog, the appositional contribution to b and d at FRC is ∼40% (Wilson & De Troyer, 2013). Therefore, we can conclude that b/a and d/c are 0.83 and that cranial and outward rib displacement is suppressed when the appositional force is approximately 60% of its value at FRC. The force exerted by the diaphragm still drives the ribs cranially and outward at this point (Fig. 5), but this force is exactly balanced by the effect of ΔPpl.
Figure 6. Effect of lung inflation on the ratios b/a and d/c during isolated spontaneous diaphragm contraction.
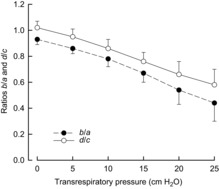
Mean ± s.e.m. values of the ratios of the coefficients of the change in transdiaphragmatic pressure (ΔPdi) (b and d) over the coefficients of change in pleural pressure (ΔPpl) (a and c) obtained in seven dogs. Both b/a and d/c decreased progressively and continuously as lung volume before contraction increased. FRC, functional residual capacity.
It is worth noting that the ratio of ΔPdi to ΔPpl remained nearly constant with increasing lung volume, which implies that the ratio of abdominal compliance to rib cage compliance was independent of lung volume. If these ratios had changed with lung volume, the value of transrespiratory pressure at which X and Y become zero would differ. For example, if the ratio of abdominal compliance to rib cage compliance decreased and ΔPdi/ΔPpl increased with increasing lung volume, the value of b/a and d/c at which rib displacement became zero would be smaller and the decrease in the zone of apposition required to suppress rib displacement would be larger.
As transrespiratory pressure before contraction was increased further from +10 cmH2O to +15 cmH2O and beyond, diaphragm contraction displaced the ribs caudally and inward (Fig. 3). In fact, both ΔPdi and the values of the coefficients b and d decreased with increasing pressure, so that the magnitude of the rib displacement driven by ΔPdi progressively and continuously decreased (Fig. 5). One of the key results of this study, however, is that in all but one animal, the direction of this displacement continued to be cranial and outward until total lung capacity (TLC; transrespiratory pressure of +25 cmH2O) was approached. Thus, as the zone of apposition was reduced to <60% of its FRC value, the inspiratory effect of ΔPdi decreased further and was outweighed by the expiratory effect of ΔPpl.
In one animal, the coefficients of ΔPdi were reversed from positive to slightly negative values when transrespiratory pressure before diaphragm contraction was set at +25 cmH2O. This reversal implies that the zone of apposition in this animal totally disappeared during the course of inspiration and that the insertional force of the diaphragm pulled the ribs caudally and inward. The diaphragm muscle fibres at this point, however, were very short and developed little force, so that ΔPdi was very small. In addition, the values of b and d were also small. As a result, the rib displacement resulting from the force exerted by the diaphragm was only 2% of the displacement produced at FRC.
Implications for the mechanism of Hoover's sign
The model investigated in this study represents a context that differs from that in human subjects with COPD. Firstly, the canine and human rib cages differ in shape and whether this difference affects the action of the diaphragm on the ribs may be subject to question. However, as we have indicated, isolated contraction of the diaphragm at FRC in dogs causes both an expansion of the lower rib cage and an inward displacement of the upper rib cage (De Troyer, 2011, 2012), as it does in humans with quadriplegia (Mortola & Sant’Ambrogio, 1978; Danon et al. 1979; Strohl et al. 1984; Estenne & De Troyer, 1985; Urmey et al. 1986). Previous studies (Wilson & De Troyer, 2013) have also shown that the lower rib cage expansion produced by the canine diaphragm is the result of the insertional and appositional forces developed by the muscle, as in humans (Loring & Mead, 1982).
The second difference between the context of the present study and that in subjects with COPD is that inflation in the animals was produced acutely, whereas emphysema develops slowly over many years and may induce remodelling in the diaphragm. Studies in emphysematous hamsters have shown in particular that chronic inflation causes a loss of sarcomeres in series along the diaphragmatic muscle fibres (Farkas & Roussos, 1983; Kelsen et al. 1983), and measurements of the pressure changes produced by phrenic nerve stimulation in emphysematous subjects have suggested that such a length adaptation also occurs in humans (Similowski et al. 1991). It is possible therefore that in such subjects, the adverse effects of muscle length on the force developed by the diaphragm are, in part, compensated for by a smaller number of sarcomeres. However, an increase in diaphragmatic force with chronic inflation would affect both pleural pressure and transdiaphragmatic pressure and thus the balance between the two forces would be unchanged. Consequently, although the difference in rib cage shape and the remodelling of the diaphragm in subjects with emphysema might alter the magnitude of the lower rib displacements produced by the diaphragm, they are unlikely to affect the direction of the displacements.
However, the animals in this study were breathing with the diaphragm alone, whereas in subjects with COPD the inspiratory inward displacement of the lateral walls of the lower rib cage is seen during coordinated contraction of the diaphragm and the inspiratory muscles of the rib cage. In addition, the present measurements of rib displacement were obtained during occluded breaths, whereas subjects with COPD exhibit Hoover's sign during breathing with the airway open (albeit with elevated airway resistance). The impact of these differences on the displacements of the lower rib cage is uncertain. Yet the present finding that isolated contraction of the canine diaphragm at high lung volumes displaces the lower ribs caudally and inward while the force exerted by the muscle on the ribs remains inspiratory is consistent with the observations of Gorman et al. (2002) and Priori et al. (2013). Using ultrasonography, these investigators assessed the size of the zone of apposition of the diaphragm in two groups of subjects with COPD and hyperinflation, including several subjects with Hoover's sign. As anticipated, the zone of apposition in these subjects was smaller than that in healthy individuals. However, in none of the subjects was the zone reduced to zero at end-inspiration, which implies that Hoover's sign was not related to an inward pull of the ribs by radially oriented diaphragm muscle fibres. The findings of the present study, combined with these clinical observations, suggest that instead the sign is usually produced by the dominant effect of pleural pressure as a consequence of a decrease in the zone of apposition.
Acknowledgments
None declared
Glossary
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- EMG
electromyography
- FRC
functional residual capacity
- TLC
total lung capacity
- ΔPab
change in abdominal pressure
- ΔPao
change in airway opening pressure
- ΔPdi
change in transdiaphragmatic pressure
- ΔPpl
change in pleural pressure
Additional information
Competing interests
None declared.
Author contributions
A.D.T. contributed to all aspects of the study. T.A.W. contributed to the study design and the writing of the manuscript. Both authors approved the final version of the manuscript for submission.
Funding
None.
References
- Aliverti A, Quaranta M, Chakrabarti B, Albuquerque ALP, Calverley PM. Paradoxical movement of the lower rib cage at rest and during exercise in COPD patients. Eur Respir J. 2009;33:49–60. doi: 10.1183/09031936.00141607. [DOI] [PubMed] [Google Scholar]
- Baydur A, Bechrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the oesophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- Binazzi B, Bianchi R, Romagnoli I, Lanini B, Stendardi L, Gigliotti F, Scano G. Chest wall kinematics and Hoover's sign. Respir Physiol Neurobiol. 2008;160:325–333. doi: 10.1016/j.resp.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Bishop B. Diaphragm and abdominal muscle responses to elevated airway pressures in the cat. J Appl Physiol. 1967;22:959–965. doi: 10.1152/jappl.1967.22.5.959. [DOI] [PubMed] [Google Scholar]
- Boriek AM, Rodarte JR, Margulies SS. Zone of apposition in the passive diaphragm of the dog. J Appl Physiol. 1996;81:1929–1940. doi: 10.1152/jappl.1996.81.5.1929. [DOI] [PubMed] [Google Scholar]
- Brancatisano A, Amis TC, Tully A, Kelly WT, Engel LA. Regional distribution of blood flow within the diaphragm. J Appl Physiol. 1991;71:583–589. doi: 10.1152/jappl.1991.71.2.583. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Sant'Ambrogio G. Direct action of contracting diaphragm on the rib cage in rabbits and dogs. J Appl Physiol. 1974;36:715–719. doi: 10.1152/jappl.1974.36.6.715. [DOI] [PubMed] [Google Scholar]
- Danon J, Druz WS, Goldberg NB, Sharp JT. Function of the isolated paced diaphragm and the cervical accessory muscles in C1 quadriplegics. Am Rev Respir Dis. 1979;119:909–919. doi: 10.1164/arrd.1979.119.6.909. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Differential control of the inspiratory intercostal muscles during airway occlusion in the dog. J Physiol. 1991;439:73–88. doi: 10.1113/jphysiol.1991.sp018657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A. Role of joint receptors in modulation of inspiratory intercostal activity by rib motion in dogs. J Physiol. 1997;503:445–453. doi: 10.1111/j.1469-7793.1997.445bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A. The action of the canine diaphragm on the lower ribs depends on activation. J Appl Physiol. 2011;111:1266–1271. doi: 10.1152/japplphysiol.00402.2011. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Respiratory effect of the lower rib displacement produced by the diaphragm. J Appl Physiol. 2012;112:529–534. doi: 10.1152/japplphysiol.01067.2011. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Mechanics of the respiratory muscles. In: Sieck G, Boriek A, editors; Fredberg J, editor. Comprehensive Physiology. The Respiratory System: Mechanics of Breathing. Oxford: Wiley-Blackwell; 2011. pp. 1273–1300. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kelly S. Chest wall mechanics in dogs with acute diaphragm paralysis. J Appl Physiol. 1982;53:373–379. doi: 10.1152/jappl.1982.53.2.373. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Leduc D, Cappello M, Mine B, Gevenois PA, Wilson TA. Mechanisms of the inspiratory action of the diaphragm during isolated contraction. J Appl Physiol. 2009;107:1736–1742. doi: 10.1152/japplphysiol.00753.2009. [DOI] [PubMed] [Google Scholar]
- Estenne M, De Troyer A. Relationship between respiratory muscle electromyogram and rib cage motion in tetraplegia. Am Rev Respir Dis. 1985;132:53–59. doi: 10.1164/arrd.1985.132.1.53. [DOI] [PubMed] [Google Scholar]
- Evans HE, Christensen GC. Miller's Anatomy of the Dog. 2nd edn. Philadelphia, PA: Saunders; 1979. [Google Scholar]
- Farkas GA, Roussos C. Diaphragm in emphysematous hamsters: sarcomere adaptability. J Appl Physiol. 1983;54:1635–1640. doi: 10.1152/jappl.1983.54.6.1635. [DOI] [PubMed] [Google Scholar]
- Ford GT, Grant DA, Rideout KS, Davison JS, Whitelaw WA. Inhibition of breathing associated with gallbladder stimulation in dogs. J Appl Physiol. 1988;65:72–79. doi: 10.1152/jappl.1988.65.1.72. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK. Human diaphragmatic EMG: changes with lung volume and posture during supramaximal phrenic stimulation. J Appl Physiol. 1986;60:1420–1428. doi: 10.1152/jappl.1986.60.4.1420. [DOI] [PubMed] [Google Scholar]
- Gilmartin JJ, Gibson GJ. Abnormalities of chest wall motion in patients with chronic airflow obstruction. Thorax. 1984;39:264–271. doi: 10.1136/thx.39.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin JJ, Gibson GJ. Mechanisms of paradoxical rib cage motion in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;134:683–687. doi: 10.1164/arrd.1986.134.4.683. [DOI] [PubMed] [Google Scholar]
- Gilmartin JJ, Ninane V, De Troyer A. Abdominal muscle use during breathing in the anaesthetized dog. Respir Physiol. 1987;70:159–171. doi: 10.1016/0034-5687(87)90047-8. [DOI] [PubMed] [Google Scholar]
- Gorman RB, McKenzie DK, Pride NB, Tolman JF, Gandevia SC. Diaphragm length during tidal breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 2002;166:1461–1469. doi: 10.1164/rccm.200111-087OC. [DOI] [PubMed] [Google Scholar]
- Hoover CF. The diagnostic significance of inspiratory movements of the costal margins. Am J Med Sci. 1920;159:633–646. [Google Scholar]
- Johnson RL, Jr, Hsia CCW, Takeda SI, Wait JL, Glenny RW. Efficient design of the diaphragm: distribution of blood flow relative to mechanical advantage. J Appl Physiol. 2002;93:925–930. doi: 10.1152/japplphysiol.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kelsen SG, Altose MD, Cherniack NS. Interaction of lung volume and chemical drive on respiratory muscle EMG and respiratory timing. J Appl Physiol. 1977;42:287–294. doi: 10.1152/jappl.1977.42.2.287. [DOI] [PubMed] [Google Scholar]
- Kelsen SG, Wolanski T, Supinski GS, Roesmann U. The effect of elastase-induced emphysema on diaphragmatic muscle structure in hamsters. Am Rev Respir Dis. 1983;127:330–334. doi: 10.1164/arrd.1983.127.3.330. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Druz WS, Sharp JT. Effect of muscle length on electromyogram in a canine diaphragm strip preparation. J Appl Physiol. 1985;58:1602–1607. doi: 10.1152/jappl.1985.58.5.1602. [DOI] [PubMed] [Google Scholar]
- Landau BR, Akert K, Roberts TS. Studies on the innervation of the diaphragm. J Comp Neurol. 1962;119:1–10. [Google Scholar]
- Leduc D, Cappello M, Gevenois PA, De Troyer A. Mechanism of the lung-deflating action of the canine diaphragm at extreme lung inflation. J Appl Physiol. 2012;112:1311–1316. doi: 10.1152/japplphysiol.01422.2011. [DOI] [PubMed] [Google Scholar]
- Loring SH, Mead J. Action of the diaphragm on the rib cage inferred from a force-balance analysis. J Appl Physiol. 1982;53:756–760. doi: 10.1152/jappl.1982.53.3.756. [DOI] [PubMed] [Google Scholar]
- Mead J. Functional significance of the area of apposition of diaphragm to rib cage. Am Rev Respir Dis. 1979;119:31–32. doi: 10.1164/arrd.1979.119.2P2.31. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Sant’Ambrogio G. Motion of the rib cage and the abdomen in tetraplegic patients. Clin Sci Mol Med. 1978;54:25–32. doi: 10.1042/cs0540025. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Knight H, Rochester DF. Effect of lower rib cage expansion and diaphragm shortening on the zone of apposition. J Appl Physiol. 1990;68:484–488. doi: 10.1152/jappl.1990.68.2.484. [DOI] [PubMed] [Google Scholar]
- Pettiaux N, Cassart M, Paiva M, Estenne M. Three-dimensional reconstruction of human diaphragm with the use of spiral computed tomography. J Appl Physiol. 1997;82:998–1002. doi: 10.1152/jappl.1997.82.3.998. [DOI] [PubMed] [Google Scholar]
- Priori R, Aliverti A, Albuquerque AL, Quaranta M, Albert P, Calverley PMA. The effect of posture on asynchronous chest wall movement in COPD. J Appl Physiol. 2013;114:1066–1075. doi: 10.1152/japplphysiol.00414.2012. [DOI] [PubMed] [Google Scholar]
- Road JD, Burgess KR, Whitelaw WA, Ford GT. Diaphragm function and respiratory response after upper abdominal surgery in dog. J Appl Physiol. 1984;57:576–582. doi: 10.1152/jappl.1984.57.2.576. [DOI] [PubMed] [Google Scholar]
- Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325:917–923. doi: 10.1056/NEJM199109263251304. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Mead J, Banzett RB, Lehr J, Loring SH, O'Cain CF. Effect of posture on upper and lower rib cage motion and tidal volume during diaphragm pacing. Am Rev Respir Dis. 1984;130:320–321. doi: 10.1164/arrd.1984.130.2.320. [DOI] [PubMed] [Google Scholar]
- Urmey W, Loring S, Mead J, Slutsky AS, Sarkarati M, Rossier A, Brown R. Upper and lower rib cage deformation during breathing in quadriplegics. J Appl Physiol. 1986;60:618–622. doi: 10.1152/jappl.1986.60.2.618. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Kirkwood PA. Evidence from motoneurone synchronization for disynaptic pathways in the control of inspiratory motoneurones in the cat. J Physiol. 1997;503:673–691. doi: 10.1111/j.1469-7793.1997.673bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TA, De Troyer A. Effects of the insertional and appositional forces of the canine diaphragm on the lower ribs. J Physiol. 2013;591:3539–3548. doi: 10.1113/jphysiol.2013.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]


