Abstract
Recent studies in rat muscle fibres show that repetitive firing of action potentials causes changes in fibre resting membrane conductance (Gm) that reflect regulation of ClC-1 Cl− and KATP K+ ion channels. Methodologically, these findings were obtained by inserting two microelectrodes at close proximity in the same fibres enabling measurements of fibre input resistance (Rin) in between action potential trains. Since the fibre length constant (λ) could not be determined, however, the calculation of Gm relied on the assumptions that the specific cytosolic resistivity (Ri) and muscle fibre volume remained constant during the repeated action potential firing. Here we present a three-microelectrode technique that enables determinations of multiple cable parameters in action potential-firing fibres including Rin and λ as well as waveform and conduction velocities of fully propagating action potentials. It is shown that in both rat and mouse extensor digitorum longus (EDL) fibres, action potential firing leads to substantial changes in both muscle fibre volume and Ri. The analysis also showed, however, that regardless of these changes, rat and mouse EDL fibres both exhibited initial decreases in Gm that were eventually followed by a ∼3-fold, fully reversible increase in Gm after the firing of 1450–1800 action potentials. Using this three-electrode method we further show that the latter rise in Gm was closely associated with excitation failures and loss of action potential signal above −20 mV.
Key points
Current methods for determination of the resting membrane conductance in active, action potential-firing muscle fibres are based on the assumptions that the volume and the cytosolic composition of the fibres remain constant during activity.
Here, we present a microelectrode-based method that without such assumptions measures a range of cable parameters including the resting membrane conductance together with action potential characteristics of individual fibres during intermittent action potential firing.
The method can be used to study the role of activity-induced regulation of the resting membrane conductance for action potential excitation and propagation in active muscles.
Introduction
The resting membrane conductance (Gm) of skeletal muscle fibres reflects the function of the K+ and Cl− ion channels that are open at the resting membrane potential. Classically, Gm of intact fibres has been assessed using a current clamping technique with two intracellular microelectrodes: one electrode is used for injecting currents (current electrode) while the other electrode records the membrane potential (recording electrode) at two or more inter-electrode distances by consecutively penetrating the fibre at different locations. The fibre input resistance (Rin) and length constant (λ) are then obtained from fits of the cable equation to plots showing steady membrane potential change induced by constant current injection against inter-electrode distances (Hodgkin & Rushton, 1946). From these values, Gm can be calculated assuming a constant that characterizes the cytosolic resistivity (Ri) (Hodgkin & Rushton, 1946; Boyd & Martin, 1959; Pedersen et al. 2005). However, the time needed to perform the multiple, consecutive penetrations of the fibre with the recording microelectrode (minutes) does not allow for determinations of more rapid changes in Gm during ongoing muscle activity. To circumvent this problem, Gm in active muscle fibres has in some studies (Fink & Lüttgau, 1976; Pedersen et al. 2009a,b) been estimated by a method where two microelectrodes were placed in the muscle fibre at close proximity, making it possible to determine Rin from a single current injection lasting just 25 ms. The obtained values for Rin during the experiment could then be used to estimate Gm in the active fibre.
While this approach did reveal novel regulation of the muscle specific Cl− channel (ClC-1) and ATP-sensitive K+ (KATP) channels in active muscle fibres that is important for normal muscle function, the studies by Pedersen et al. (2009a,b) had three important methodological limitations. First, because the electrodes were placed at close proximity in order to directly measure Rin, λ could not be determined and estimates of Gm during the ongoing muscle activity therefore had to assume that resistance of the intracellular space (ri) and its constituents of Ri and muscle fibre cross-sectional area (CSA) or, equivalently, muscle fibre volume, remained constant during activity. In conflict with this, several studies report that muscle activity is associated with muscle fibre swelling (Fink & Lüttgau, 1976; Sejersted & Sjøgaard, 2000; Usher-Smith et al. 2006, 2009). If fibre volume was actually increasing during the activity the assumption of constant ri would have introduced overestimates of Gm. Second, since Gm estimates were solely based on observed Rin changes, absolute values of Gm were not obtained directly. Instead Rin changes during activity had to be multiplied by a Gm value determined in resting fibres under similar experimental conditions in order to obtain absolute values of Gm during activity. Third, due to the close proximity of the electrodes, an evaluation of whether the elicited action potentials propagated and determination of their propagation velocities were excluded.
The present study aimed to develop a method for measuring cable parameters in active muscle fibres that did not have these methodological limitations. Here we introduce a three-microelectrode technique that permits multiple cable parameters to be measured in 200 ms, enabling the measurements to be completed between repeated action potential trains. This method which yields absolute values for Rin, λ, ri and the membrane conductance per unit length of fibre (gm) in individual muscle fibres, has previously been applied to effectively resting muscles from frogs and rats (Adrian et al. 1970; Adrian & Marshall, 1977) but here we use the method in fibres firing repeated trains of action potentials. We furthermore present a method for describing to what extent Ri and CSA contribute to observed ri changes during activity. The three-electrode method then provided measurement of Gm during activity without having to rely on the assumptions of a constant Ri and CSA, or values of Gm from resting fibres. Additionally, the three-electrode technique permitted simultaneous determinations of action potential waveform and conduction velocity during the experiments. This made it possible to examine the action potential excitation, waveform characteristics and velocity of fully propagating action potentials.
Methods
Ethical approval, dissection of muscles and solutions
In all experiments extensor digitorum longus (EDL) muscles from adult C57BL/6 mice (12- to 14-week-old, ∼25 g, males and females) or adult Wistar rats (12- to 14-week-old, ∼230 g, females) were used. EDL contains almost exclusively fast-twitch fibres (Edström et al. 1982; Smith et al. 2013). Animals were fed ad libitum and kept under 12 h light/dark conditions at 21°C. To obtain muscles, the animals were anaesthetized with isoflurane, then killed by cervical dislocation. Such handling, killing and use of animals complied with Danish Animal Welfare regulations. No experiments were performed on live animals. After dissection, muscles were placed in an experimental chamber that was perfused (∼25 ml min−1) with standard Krebs–Ringer bicarbonate solution containing (mm): 122 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2, 5.0 d-glucose. To avoid problems with breakage or dislocation of the microelectrodes used for electrophysiological measurements, the contractile activity of the muscles was in all experiments reduced by addition of 50 μm of the myosin II inhibitor N-benzyl-p-toluene sulphonamide (BTS) to the incubation solutions (Cheung et al. 2002). Other studies have shown that this has minimal effect on muscle excitability (Pedersen et al. 2009a) and, furthermore, only reduces energy consumption during activity by ∼20% (Zhang et al. 2006). All experiments were carried out at 30°C and the re-cycled solution was continuously gassed with a mixture of 95% O2 and 5% CO2 (pH ∼7.4) throughout the experiments.
Electrophysiological set-up
Individual muscle fibres in intact EDL muscles and the electrodes used for impalements were visualized on a TV screen using a Nikon DS-Vi1 camera and a Nikon upright microscope (Eclipse FN1, DFA, Glostrup, Denmark). The glass pipettes were filled with 2 m potassium citrate resulting in electrode resistances around 15 MΩ. Two electrodes were connected to a TEC-05X two-electrode clamp system (NPI, Tamm, Germany) and the third electrode was connected to a SEC-05X single electrode amplifier system (NPI, Tamm, Germany). Experimental protocols for current injection and sampling of data (>30 kHz) were performed using Signal 4 or 5 and a Power1401 interface (Cambridge Electronic Design Ltd, Cambridge, UK). Retrieval of data values and fitting of data to the cable equation (eqn (1)) to obtain Rin and λ were done using scripts written in Signal.
Two- and three-electrode-based measurements
When Gm of intact fibres is assessed using current clamping techniques the most common approach is to experimentally determine Rin and λ and then calculate Gm from these parameters assuming a constant Ri. Two electrodes have typically been used to determine the steady membrane potential responses to constant current injections at a minimum of three inter-electrode distances. By dividing the magnitude of the membrane responses (ΔV) with the current injected (I) the transfer resistances at the three inter-electrode distances are obtained. These are then plotted against inter-electrode distance and fitted to the cable equation (eqn (1)) that applies to cells of infinite length with a membrane represented by a parallel resistor–capacitor (RC) circuit (Hodgkin & Rushton, 1946). Rin and λ are then obtained from such fits:
| (1) |
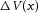 represents the steady state change in membrane potential at position x in response to constant current injected at x = 0. From Rin and λ the membrane resistance per unit length of fibre (rm), ri and the membrane conductance per unit length of fibre (gm) can be calculated:
represents the steady state change in membrane potential at position x in response to constant current injected at x = 0. From Rin and λ the membrane resistance per unit length of fibre (rm), ri and the membrane conductance per unit length of fibre (gm) can be calculated:
| (2) |
| (3) |
Gm can then be calculated by dividing gm with the surface area per unit length of fibre (FSA). In resting muscle fibres a common approach for estimating FSA is to first determine the cross-sectional area of the fibre (CSA) from ri assuming a constant value for the specific sarcoplasmic resistivity (Ri), here taken to be 180 Ω cm as determined in resting fibres (Albuquerque & Thesleff, 1968), using eqn (4):
| (4) |
Next, by assuming the muscle fibre to be a perfect cylinder, FSA can be calculated from CSA as:
| (5) |
and Gm can then be calculated as:
| (6) |
The present study introduces a three-electrode method that enables measurements of Rin and λ in between trains of action potentials without having to move electrodes. In these experiments, three electrodes (V1–V3) were inserted into the same fibre with inter-electrode distances of around 0.3–0.5 mm (V1 to V2) and 1.1–1.8 mm (V1 to V3). By switching the current injection between V1 and V3 it was then possible to obtain the membrane potential response to the current injections at three inter-electrode distances, because the V2–V3 distance was kept at approximately twice the V1–V2 distance. Note that, with this approach for alternating the current injections between the V1 and the V3 electrodes, the response at the longest distance was determined twice. This allowed for a comparison of the two voltage responses at the longest distances. These were almost identical in all fibres examined, a finding that is compatible with the application of cable theory for infinite cable structures. The current injections that were used for determination of cable parameters were 50 ms long (−20 or –30 nA) and the time between the current injections from V1 and V3 was 600 ms. This time between current injections could, however, easily be reduced to allow all the measurements that are required for determination of cable parameters to be performed in less than 200 ms. Trains of action potentials were triggered by injecting large-amplitude currents (300 nA, 1 ms) through V1. Fibres, in which the resting membrane potential recorded by any of the three electrodes depolarized beyond −60 mV during the experimental protocol, were omitted.
Intracellular water content
Contralateral pairs of rat EDL muscles were dissected out and equilibrated at 4 or 40 mm K+ for 90 min in Krebs–Ringer buffer to obtain groups of muscles with normal and increased volume, respectively. To determine the extracellular space of the muscles, the incubation solution contained [14C]sucrose (0.1 μCi ml–1 and 1 mm non-labelled sucrose). After incubation, the tendons were cut off and the muscles were blotted, weighed for determination of wet weight and subsequently dried overnight at 60°C. After determination of dry weights, the muscles were soaked in 0.12 m trichloroacetic acid (TCA) for 20 h, after which the content of [14C]sucrose in the supernatant was determined by scintillation counting. Intracellular water content, expressed per gram dry weight, was calculated from the total water content by subtracting the extracellular water as determined from the muscle content of [14C]sucrose.
Chemicals and isotopes
All chemicals were of analytical grade. BTS was purchased from Toronto Research Chemicals, isoflurane from Baxter Medical and [14C]sucrose from Perkin Elmer.
Statistics
Average data are presented as mean ± SEM. Statistical difference between groups was obtained using Student's two-tailed t test.
Results
The experimental approach with the three electrodes is illustrated in Fig. 1: Three electrodes (V1, V2, V3) were inserted in a fibre to give three different inter-electrode distances (X1, X2 and X3) (Fig. 1A). To rapidly determine cable parameters, small-amplitude currents (−30 nA, 50 ms) were injected first through V1 and then through V3. This made it possible to obtain the resulting membrane potential responses at the three inter-electrode distances: V2 recorded the voltage responses at the shortest distance (V1 to V2 = X1) during the V1 current injection and at the middle distance (V3 to V2 = X2) during the V3 current injection. The voltage response at the longest distance (between V1 and V3 = X3) was recorded twice, firstly by V3 when injecting current through V1 and secondly by V1 when injecting current through V3. For each run of current injections, V1 was additionally used to inject large-amplitude currents for triggering a 3.5 s train of 50 action potentials (300 nA, 1 ms). Figure 1B illustrates the current injections during one run and Fig. 1C and D show typical membrane potential recordings from V2 and V3 during such a run of current injections.
Figure 1. Three-electrode technique for determination of cable parameters in between intermittently fired trains of action potentials.
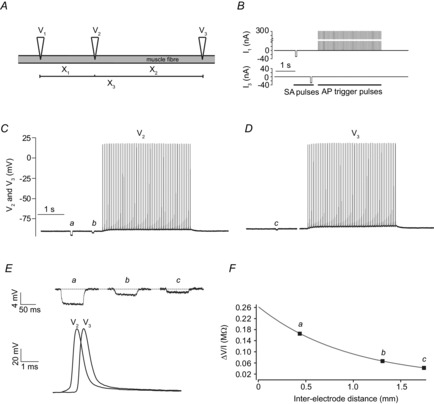
A, illustration of the three microelectrodes (V1, V2, V3) when inserted in the same muscle fibre of an intact muscle. The positions of the inserted electrodes allowed three inter-electrode distances to be identified (X1, X2, X3). Two electrodes were used for current injections (V1, V3) and recording of the membrane potential while the third electrode (V2) only recorded the membrane potential. B shows one run of current injections (I1, I3) through the V1 and V3 electrodes consisting of small-amplitude hyperpolarizing pulses (−30 nA, 50 ms duration) used for determination of the cable parameters (SA pulses) and a train of large-amplitude depolarizing current pulses (15 Hz, 50 pulses, 1 ms duration, 300 nA) that were used to trigger action potentials (AP trigger pulses). C and D, representative recordings of membrane potential from V2 and V3 in a rat EDL muscle fibre during a run of the current injections. Because the voltage recording by electrode V3 was unreliable during its current injection, the part of the trace in D when current was injected through V3 has been removed for clarity. E, shows the deflections of the membrane potential (a, b, c) at the three inter-electrodes distances in response to the small hyperpolarizing currents that were injected prior to the train of action potentials. Shown below is an overlay of the first action potential in the trains recorded by V2 and V3. F, by dividing the magnitude of the deflections (a, b, c) with the current injected (−30 nA) the transfer resistance was obtained. This was plotted against inter-electrode distance and fitted to the cable equation (eqn (1)) to obtain Rin and λ of the fibre.
It can be seen that the V2 electrode recorded small deflections in the membrane potential resulting from the small-amplitude currents injected from both V1 and V3 (indicated by a and b). The membrane potential response to current injection from V1 is larger than that observed during current injection by V3 (a vs. b) reflecting the placing of V2 at ∼half the distance from V1 as from V3. Similarly, the membrane potential response at V3 to the current injection through V1 (indicated by c) is smaller than the responses at V2 reflecting the longest distance between electrodes. Figure 1E shows enlargements of the membrane potential deflections (a, b, c) in response to the small-amplitude current injections (upper panel). It further shows the first action potential of the V2 and V3 trains in Fig. 1C and D (lower panel). The action potential was clearly delayed in the V3 recording when compared to the V2 recording reflecting the conduction delay between the two electrodes. Also, when comparing the foot of the action potentials at the two electrodes it is clear that the electrotonic membrane potential response to the current through V1 that triggered the action potential had dissipated almost completely in the V3 recording confirming that at this electrode the action potential was fully propagating. Figure 1F shows how the membrane potential responses (a, b, c) to the small-amplitude current pulses were used to extract Rin and λ. The magnitudes of the responses were divided by the injected current, and the resulting transfer resistances were plotted against inter-electrode distance and fitted to the steady state cable equation (eqn (1)).
Figure 2 summarizes experiments applying the three-electrode method to determine cable parameters in rat EDL fibres during repeated action potential firing. The experimental protocol consisted of 78 repetitive runs of the current injections shown in Fig. 1B. After this the action potential triggering was stopped allowing the cable parameters to be measured during recovery from the activity. Figure 2A and B shows membrane potential recordings from V2 and V3 during the first, the 20th and the 78th run. Responses to the small-amplitude current injections were larger in the 20th run and, contrastingly, smaller in the 78th run when compared to observations in the first run. This is most clearly seen in the enlarged membrane potential responses from the three runs (upper panel of Fig. 2C). In the lower panel of Fig. 2C the transfer resistances from these three runs have been plotted and fitted to the cable equation (eqn (1)). Compared to the fit from the first run, the fit from the 20th run yielded a larger ordinate intercept, demonstrating an increased Rin, and its shallower slope reflects an increased λ. In contrast, the 78th run gave a lower ordinate intercept equivalent to a lower Rin and a steeper slope reflecting a reduced λ. This type of analysis to extract Rin and λ during action potential firing was repeated for all runs of current injection in 14 rat EDL fibres. Figure 2D shows that on average Rin and λ both rose with the onset of action potential firing and remained elevated for around 200–250 s, which corresponds to firing 1450–1800 action potentials (hereafter referred to as Phase 1). With continued action potential firing, Rin and λ both showed marked reductions with the decline in Rin slightly preceding the decline in λ (hereafter referred to as Phase 2). The changes in Rin and λ were reversible with both parameters returning to their respective control levels prior to action potential firing within 100 s after cessation of the activity.
Figure 2. Determination of Rin and λ in action potential-firing rat EDL fibres using the three-electrode technique.
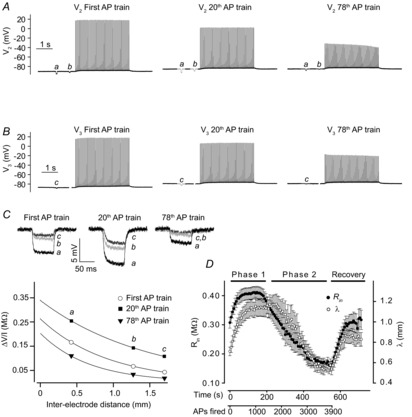
A and B show membrane potential recordings from electrodes V2 (A) and V3 (B) during the first, the 20th and the 78th run of current injections (see Fig. 1B) in a representative fibre. C, upper panel shows enlargements of the membrane potential deflections from the three runs of current injections in A and B. The lower panel shows plots and fits for the three runs of current injections from which Rin and λ could be obtained. This approach was performed on all 78 runs in the protocol. D shows average Rin and λ from 14 fibres as a function of time and number of action potentials excited. Average data shown as mean ± SEM.
The three-electrode approach for measuring cable parameters during action potential firing also proved useful when studying mouse EDL fibres. As in the rat fibres, the mouse fibres showed initial significant increases in Rin and λ during repeated action potential firing, from 0.30 ± 0.06 MΩ and 0.53 ± 0.03 mm in the first run to 0.35 ± 0.06 MΩ and 0.93 ± 0.04 mm in the 20th run (Phase 1, P = 0.01 and 0.0002, t test, n = 7 fibres). With continued action potential firing, both Rin and λ decreased significantly, with Rin and λ dropping to 0.19 ± 0.06 MΩ and 0.35 ± 0.04 mm after the 76th run (Phase 2, P = 0.01 and 0.003, t test, n = 7 fibres). As in rat fibres, the decline in Rin with the onset of Phase 2 slightly preceded the decline in λ. When the action potential firing was ceased, Rin and λ both recovered to their levels prior to action potential firing within 100 s. These data illustrate that action potential firing in mouse fibres also elicited dramatic changes in cable parameters and, furthermore, that the three-electrode method is applicable when studying the smaller mouse fibres.
Determination of gm and ri
Since the three-electrode method presented here determines both Rin and λ, it is possible to calculate gm and ri using eqns (2) and (3). Figure 3A shows that in fibres from both rat and mouse, the average gm was roughly halved over the first 200 s of action potential firing (Phase 1) whereas a fully reversible ∼3-fold increase took place at the beginning of Phase 2. While the previous method for determining Gm during muscle activity (Pedersen et al. 2009a,b) assumed a constant ri throughout the activity, the three-electrode technique revealed that ri actually underwent clear changes during the action potential activity (Fig. 3B). Since ri is determined by the specific sarcoplasmic resistivity (Ri) and the cross-sectional area of the muscle fibres (CSA) (see eqn (4)), this shows that either Ri, CSA or both were changing during the activity.
Figure 3. Cable parameters during repeated firing of action potentials in rat and mouse EDL fibres calculated from observations of Rin and λ.
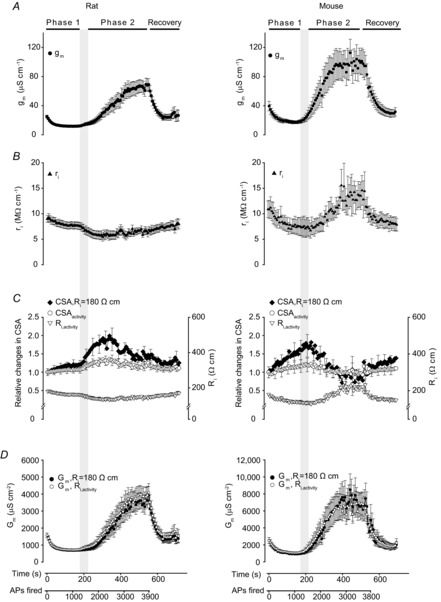
All plots are plotted against the abscissa displayed below D. The shaded vertical bar indicates the time during the experiments when Phase 2 started to develop. A, membrane conductance per centimetre of fibre length, gm. B, resistance of the intracellular space per unit length, ri. The observed changes in ri during the experiments could theoretically be explained from changes in muscle fibre cross-sectional area (CSA), or a combination of changes in CSA and the specific intracellular resistivity (Ri). Thus, C shows both how CSA would change if Ri remained constant at 180 Ω cm (CSA,Ri = 180 Ω cm) and how CSA and Ri would change during the experiments if the increases in CSA represent an additional intracellular cross-sectional area with a resistivity of 65 Ω cm (CSAactivity and Ri,activity; see Results for calculations). For clarity, only every fifth SEM is shown. D, Gm, the resting membrane conductance per square centimetre of surface membrane was calculated either on the assumption of a constant Ri of 180 Ω cm (Gm,Ri = 180 Ω cm) or by using CSAactivity and Ri,activity (Gm,Ri,activity). n = 7 or 14 (mouse and rat, respectively). Average data shown as mean ± SEM.
Determination of Gm
To calculate Gm it was necessary to determine whether the changes in ri during activity were caused by changes in Ri, CSA or both. Initially, calculating CSA under the assumption of a constant value for Ri of 180 Ω cm (Albuquerque & Thesleff, 1968) using eqn (4) showed that both mouse and rat fibres underwent a marked and reversible increase in CSA of 80–100% with the highest values obtained early in Phase 2 (filled symbols, Fig. 3C). As expected the muscle fibre length remains constant during the experiments, and the increments in CSA during activity would therefore suggest a corresponding 80–100% increase in fibre volume. Although the transient nature of this swelling may to some extent have escaped detection in other studies, where the approaches for measuring fibre swelling had lower temporal resolution, the magnitude of the swelling appeared unreasonably large. However, if swelling-associated water entry caused a decline in Ri then the above CSA calculations would have overestimated the fibre swelling during activity (see eqn (4)). It was therefore possible that the above increments in CSA and volume estimates of up to 100% did at least in part reflect swelling-induced declines in Ri. Prompted by this analysis, an experiment was made to evaluate whether electrophysiological recordings overestimate fibre swelling. Thus, rat EDL muscles were exposed to an elevation in extracellular K+ from 4 to 40 mm, an experimental manoeuvre that is known to cause swelling of frog muscle fibres (Usher-Smith et al. 2006). The intracellular water content was then compared between muscles at 4 and 40 mm K+ to directly determine muscle swelling and this could then be compared to estimates of swelling obtained in electrophysiological recordings. These experiments showed that while intracellular water content increased by 16.2 ± 0.4% (n = 6 vs. 6) at 40 mm K+, the electrophysiological approach relying on a constant Ri of 180 Ω cm suggested a volume increase of 45% (CSA from 2037 ± 108 μm2 at 4 mm K+ to 2956 ± 123 μm2 at 40 mm K+, n = 8 vs. 27, P = 0.0005, t test). This confirmed that electrophysiological recordings overestimate fibre swelling, a finding that must reflect a reduction in Ri during swelling. Formalizing this idea, entry of water introduces a lower resistance pathway for intracellular current flow, and the intracellular conductivity at 40 mm K+
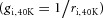 after swelling then becomes:
after swelling then becomes:
| (7) |
where 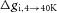 reflects the change in intracellular conductivity by fibre swelling and
reflects the change in intracellular conductivity by fibre swelling and 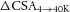 represents the actual volume expansion as determined from the water content measurements. From these two parameters the resistivity of the added intracellular volume (Rs) can be calculated to be ∼65 Ω cm using eqn (8):
represents the actual volume expansion as determined from the water content measurements. From these two parameters the resistivity of the added intracellular volume (Rs) can be calculated to be ∼65 Ω cm using eqn (8):
| (8) |
This value is close to the resistivity of 300 mm NaCl saline (55 Ω cm, Geddes & Baker, 1967) as might be expected for an isosmotic accumulation of water in the swelling fibres. Having extracted Rs for volume expansion it became possible to give a more realistic determination of CSA during muscle activity. Again the intracellular conductivity is considered to consist of a conductivity from the resting fibre and a conductivity introduced by activity-induced swelling:
| (9) |
From this the area introduced by swelling (ΔCSAs) can be isolated to:
| (10) |
where gi,activity is the intracellular conductivity during muscle activity, which consist of the conductivity from a resting fibre (gi,rest) and the conductivity added by swelling (Δgi,s); adding the area introduced by swelling to the area of the fibre prior to activity (CSArest) the cross-sectional area of the fibre during activity (CSAactivity) can be calculated:
| (11) |
From this the average Ri during activity, Ri,activity, can in turn be calculated using eqn (4):
| (12) |
This analysis showed that with the lower resistivity of the added intracellular volume, the resistivity of the fibres declined during activity with the largest drop occurring during the transition from Phase 1 to Phase 2 (Fig. 3C, triangles). It further showed that CSAactivity, and therefore the fibre volume, only rose ∼35%.
Having determined Ri and CSA during activity Gm was next calculated (open circles in Fig. 3D). This was compared to the limiting case of calculating Gm under the assumption of a constant Ri of 180 Ω cm during the activity (filled circles in Fig. 3D). Comparison of the two scenarios shows that Gm during activity was largely unaffected by the assumption of constant Ri (compare filled and open circles). Thus, under the assumption of a constant Ri of 180 Ω cm the reduction in Gm during Phase 1 in rats as estimated from the values recorded after 1000 action potentials (APs) was only 4% larger than the values obtained using CSAactivity. Likewise, after 2000 APs corresponding to Phase 2, the assumption of a constant Ri of 180 Ω cm only led to an underestimation of 20%. Figure 3D further demonstrates that very similar magnitudes and timings of Gm changes were observed in rat and mouse fibres. Thus, in both species Gm went through an initial reduction of around 60% during Phase 1, and after firing around 1450–1800 action potentials, it transitioned to its elevated state in Phase 2 of ∼3 times the resting level.
Action potential waveform and conduction velocity during the repetitive action potential firing
The three-electrode technique permitted characterization of the waveform of fully propagating action potentials from the V3 recording, and, from the latency in the action potential between the V2 and V3 recordings, the conduction velocity could be determined. Figure 4A shows overlays of V2 and V3 recordings of the first action potential in the first, the 20th and the 78th action potential train in a representative rat fibre. Through the protocol, the propagation delay between the V2 and V3 recordings rose progressively as indicated by the lengthening of the horizontal lines above the recordings in Fig. 4A that span the gaps between the peaks in the V2 and V3 action potentials. From such delays the conduction velocity was calculated for the first action potential in all runs. In both species, this analysis showed that the conduction velocity fell gradually throughout the protocol. In rat fibres it was 2.14 ± 0.19, 1.75 ± 0.10, 1.62 ± 0.11, 1.43 ± 0.15 and 1.24 ± 0.15 m s−1 for the first, the 1001th, 2001th, 3001th and 3801th action potential, respectively (n = 14 fibres), and in mouse fibres it was 2.02 ± 0.21, 1.50 ± 0.14, 1.10 ± 0.14, 1.02 ± 0.1 and 0.76 ± 0.15 m s−1 for the first, the 1001th, 2001th, 3001th and 3601th action potential (n = 5 fibres), respectively. When compared to the changes in cable parameters during activity, the conduction velocity appeared to fall gradually during Phase 1 and Phase 2 with only a few fibres showing an accelerated decline in the transition to Phase 2. Contrastingly, Phase 2 was associated with abrupt excitation failure in 5 out of 14 rat fibres and in 2 out of 7 mouse fibres. Excitation failure was categorized as lack of an action potential in both V2 and V3 while propagation failure was identified from the presence of an action potential in the V2 recording but its absence in the V3 recording. Propagation failure with Phase 2 was observed in 4 of the 14 rat fibres and in 2 of the 7 mouse fibres.
Figure 4. Analysis of action potential waveform and resting membrane potential during activity in rat and mouse fibres.
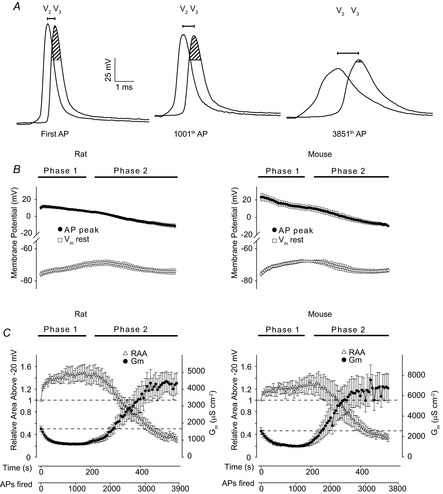
A shows overlays of V2 and V3 recordings of the first action potential in the first (First AP), 20th (1001th AP) and the 78th (3851th AP) run of current injections in a representative rat EDL fibre. The shaded area in the V3 recording indicates the part of the action potential waveform above −20 mV. B, action potential peak (AP peak) and resting membrane potential (Vm rest) measured by V3 during the experiments using rat (n = 14) and mouse (n = 7) EDL fibres. C shows the changes in relative waveform area above −20 mV (RAA) of the first action potential recorded by V3 in each run during the experiments as illustrated in A, together with the calculated Gm. n = 7 or 14 (mouse and rat, respectively). Plots in B and C have been shown on the same time axis displayed below C. Average data shown as mean ± SEM.
Comparing the three pairs of V2 and V3 recordings in Fig. 4A, a clear drop in the action potential peak is seen to develop during activity. Also, a slight depolarization of the resting membrane potential is noticed in the middle recordings that contrasts the hyperpolarization/repolarization in the right recordings. The action potential peaks and the resting membrane potential were determined in all fibres and the average observations are shown in Fig. 4B with observations from rat in the left panel and mouse observations in the right panel. In both species a depolarization of around 8 mV occurred during Phase 1. With the transition to Phase 2 the resting membrane potential repolarized to its value prior to activity. Figure 4B further shows that the V3 action potential peaks declined throughout the protocol. At the transition to Phase 2 the decline in the average recordings of peaks was somewhat accelerated in both species and during this transition the peaks became negative. Importantly, because the onset of Phase 2 occurred with some variation in the different fibres, the averaging tended to smooth the accelerated decline in peaks that was observed in several fibres during transition to Phase 2. Thus, a clear decline in action potential peak during the transition to Phase 2 was observed in 7 out of 11 rat fibres and in 5 out of 7 mouse fibres.
The sarcolemmal action potential triggers the t-system action potential which in turn triggers sarcoplasmic reticulum (SR) Ca2+ release by activating voltage sensors in the t-system membrane. Voltage sensor charge movements show that these sensors have a half-activation voltage of around −20 mV (Hollingworth & Marshall, 1981; Chua & Dulhunty, 1988). Provided that the sarcolemmal and t-system action potentials undergo similar changes during repetitive firing, a decline in action potential waveform above −20 mV might reduce SR Ca2+ release. The area above −20 mV (illustrated by shaded area in Fig. 4A) of the first action potential in all V3 trains in the protocol were therefore measured and related to the first V3 action potential in the first run. Figure 4C shows this relative area of the V3 action potentials together with Gm during the activity. In both rat and mouse fibres, the area above −20 mV increased during Phase 1 while, contrastingly, during the transition to Phase 2 the area above −20 mV fell precipitously to less than 50% of the value in the first action potential.
Discussion
The present study introduces a three-electrode technique that can be used to determine how a range of cable parameters are affected by repetitive action potential firing. By utilizing this new approach it was possible to test the assumption of a constant ri that previously had to be made in order to allow Gm changes during muscle activity to be estimated from Rin measurements in active fibres (Pedersen et al. 2009a,b). The method also allowed the determination of whether changes in cable parameters were temporally associated with changes in fibre excitability, action potential waveform and action potential conduction velocity.
Comparison of the three-electrode technique with previous methods for measuring Gm in active fibres
Application of the three-electrode method to measure ri directly during activity revealed that ri actually falls during action potential firing in both rat and mouse fibres. The ri is directly proportional to Ri and inversely related to CSA (see eqn (4)). Since, however, neither Ri nor CSA can be determined directly from electrophysiological measurements of cable parameters, the evaluation of their respective contributions to the recorded changes in ri required additional information. Here, two different approaches were used to circumvent this problem. In the first approach, Gm was calculated under the assumption that Ri did not change during muscle activity but remained constant at the value of 180 Ω cm determined in resting muscles (Albuquerque & Thesleff, 1968). From this, CSA (eqn (4)) could be calculated, and this could be used to calculate a value for Gm (eqn (6)). In the second approach we reasoned that muscle fibre swelling must reflect the entry of extracellular water to the fibres, and by measuring the increase in water content in muscles swollen at 40 mm K+ we estimated that such entry of extracellular water corresponds to adding additional space for intracellular current flow in the form of isosmotic solution. This additional water was determined to have a resistivity of 65 Ω cm approximately corresponding to saline containing 300 mm of NaCl (Geddes & Baker, 1967). Since this resistivity is considerably lower than that of the cytosol in resting fibres (180 Ω cm), an increase in muscle fibre volume during activity will cause a concomitant decrease of Ri (Ri,activity, see eqn (12)). At the same time, the calculated increase in CSA (CSAactivity, see eqn (11)) of the active muscle fibres will be smaller than when calculated from a constant Ri of 180 Ω cm. Thus, if Ri stayed constant at 180 Ω cm throughout the action potential firing activity, the muscle fibre had to swell by around 80–100% to account for the measured decrease in ri. Contrastingly, given that any increase in cell volume represents additional space for intracellular current flow with a resistivity of 65 Ω cm, the swelling only needed to be around 35% to explain the decrease in ri. The latter increase in cell volume is in much closer agreement with previous studies employing other methods for volume determination in active muscle. Thus, in a study using XZ-plan confocal scanning of amphibian fibres that allows fast measurements of fibre cross-sectional area during electrical stimulation, it was observed that with prolonged stimulation the fibre volume increased by 20% (Usher-Smith et al. 2006). Using both in vivo and in vitro methods, similar increases in intracellular volume of muscle fibres have been demonstrated in humans during intense exercise (for review, see Usher-Smith et al. 2009). On this basis it seems that the three-electrode technique, with the inclusion of an Ri,activity that depended on cell volume, reliably detects actual changes in muscle volume during repeated action potential firing. An attractive feature of this approach for determination of muscle fibre swelling during activity is the high temporal resolution that the method provides. In addition, because ri is dependent on both Ri and CSA, and we here demonstrate that Ri falls concomitantly with an increase in CSA, it can be speculated that electrophysiogical determination of volume changes has higher sensitivity than techniques that measure volume directly such as imaging techniques.
Importantly, however, although the calculated volume of the active fibres depended significantly on whether Ri was assumed constant or, more realistically, changed during the experiment (Fig. 3C), the two methods led to almost identical values for Gm (Fig. 3D). Moreover, the observed development in Gm during action potential firing (Fig. 3D) fully confirmed the marked changes in Gm during repeated action potential firing that was originally observed in experiments in rats where the calculations of Gm were based on measurements of Rin using just two electrodes at close proximity and the assumption of constant ri (Pedersen et al. 2009a,b). This conclusion suggests that the changes in ri observed in the present study need not preclude the use of Rin measurements to estimate changes in Gm in fibres during activity. Thus, if only Gm is to be measured during action potential firing, the previously presented method, where Gm is evaluated from single measurements of Rin using just two electrodes at close proximity (Pedersen et al. 2009a,b), provides reliable values with very high temporal resolution requiring only around 50–75 ms per measurement. If a more detailed examination of cable parameters is needed together with surface action potential waveforms and conduction velocities, however, the three-electrode method presented here provides a technique for obtaining these measurements with a minimal loss of time resolution requiring around 200 ms per measurement.
Co-temporal changes in cable parameters and propagating action potentials
Analysis of fully propagating action potentials during Phase 1 showed that while the conduction velocity and the peaks of the action potentials tended to drop, there were no observations of excitation or propagation failures. Furthermore, the area of the action potential waveform above −20 mV increased during this part of the activity. These features demonstrate that muscle excitability was well maintained during the first phase of the action potential firing. With continued action potential firing both rat and mouse fibres transitioned into the state of high Gm in Phase 2. The most notable changes in muscle excitability and action potential characteristics that were associated with this transition were the appearance of excitation and propagation failures and marked reduction in the area of the action potential waveform above −20 mV. In contrast, the changes in the conduction velocity during the experiments showed no obvious temporal relation to the changes in Gm.
Since the voltage sensor in the t-system has a half-activation voltage of around −20 mV (Hollingworth & Marshall, 1981; Chua & Dulhunty, 1988), the reduction in action potential waveform area above −20 mV could reduce the efficiency with which action potentials induce SR Ca2+ release. Provided that similar changes are induced by the Gm rise on the action potential waveform in the t-system, this would cause failure of voltage sensor activation and, hence, reduced SR Ca2+ release. The Gm rise during Phase 2 could represent a cellular mechanism underlying fatigue in fast-twitch fibres by inducing excitation failures and by reducing the action potential waveform in the voltage range that triggers SR Ca2+ release.
Conclusion
The three-electrode method presented here allows for detailed electrophysiological measurements of multiple cable parameters including Rin, λ, ri and gm together with conduction velocity and waveform characteristics of action potentials with a time resolution of around 200 ms. When combined with inhibitors of the contractile filaments these measurements can be performed repeatedly in action potential-firing muscle fibres. This method makes it possible to study the regulation of ion channels that contributes to the resting membrane conductance and their importance for the excitability of active muscles without the assumptions of a constant volume and constant Ri that were required with previous methods.
Acknowledgments
We thank T. L. Andersen, V. Uhre and M. Stürup-Johansen for technical assistance.
Glossary
- AP
action potential
- BTS
N-benzyl-p-toluene sulphonamide
- CSA
muscle fibre cross-sectional area
- EDL
extensor digitorum longus
- FSA
surface membrane area per unit length of the muscle fibre
- gi
intracellular conductivity
- gm
membrane conductance per unit length of fibre
- Gm
membrane conductance per square centimetre of surface membrane
- I
injected current
- ri
resistance of the intracellular space per unit length
- Ri
specific cytosolic resistivity
- Rin
input resistance
- rm
membrane resistance per unit length of fibre
- SR
sarcoplasmic reticulum
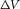
magnitude of the membrane voltage response to constant current injections
- λ
length constant
Additional information
Competing interests
None declared.
Author contributions
All authors contributed to the conception and/or design of the experiments. A.R., R.D. and T.H.P. performed the experiments, and they collected and analysed the data. All experiments were carried out at Aarhus University. Data interpretation was done by A.R., R.D., O.B.N., C.L.H. and T.H.P. The article was drafted by A.R., O.B.N and T.H.P. and it was revised critically for important intellectual content by R.D., O.B.N. and C.L.H. All authors approved the final manuscript.
Funding
This work was supported by the Danish Medical Research Council (T.H.P. and O.B.N.), the Lundbeck Foundation (O.B.N.), the Carlsberg Foundation (T.H.P), the A. P. Møller foundation (T.H.P) and the Faculty of Health Science, Aarhus University (A.R.).
References
- Adrian RH, Chandler WK, Hodgkin AL. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970;208:607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian RH, Marshall MW. Sodium currents in mammalian muscle. J Physiol. 1977;268:223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968;73:471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Martin AR. Membrane constants of mammalian muscle fibres. J Physiol. 1959;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A, Dantzig J, Hollingworth S, Baylor S, Goldman Y, Mitchison T, Straight A. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Chua M, Dulhunty AF. Inactivation of excitation-contraction coupling in rat extensor digitorum longus and soleus muscles. J Gen Physiol. 1988;91:737–757. doi: 10.1085/jgp.91.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L, Hultman E, Sahlin K, Sjöholm H. The contents of high-energy phosphates in different fibre types in skeletal muscles from rat, guinea-pig and man. J Physiol. 1982;332:47–58. doi: 10.1113/jphysiol.1982.sp014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R, Lüttgau HC. An evaluation of the membrane constants and the potassium conductance in metabolically exhausted muscle fibres. J Physiol. 1976;263:215–238. doi: 10.1113/jphysiol.1976.sp011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes LA, Baker LE. The specific resistance of biological material–a compendium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967;5:271–293. doi: 10.1007/BF02474537. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Rushton WA. The electrical constants of a crustacean nerve fibre. Proc R Soc Med. 1946;134:444–479. doi: 10.1098/rspb.1946.0024. [DOI] [PubMed] [Google Scholar]
- Hollingworth S, Marshall MW. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol. 2005;125:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli FV, Flatman JA, Nielsen OB. Regulation of ClC-1 and KATP channels in action potential-firing fast-twitch muscle fibers. J Gen Physiol. 2009a;134:309–322. doi: 10.1085/jgp.200910290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Macdonald WA, de Paoli FV, Gurung IS, Nielsen OB. Comparison of regulated passive membrane conductance in action potential-firing fast- and slow-twitch muscle. J Gen Physiol. 2009b;134:323–337. doi: 10.1085/jgp.200910291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Smith IC, Gittings W, Huang J, McMillan EM, Quadrilatero J, Tupling AR, Vandenboom R. Potentiation in mouse lumbrical muscle without myosin light chain phosphorylation: is resting calcium responsible? J Gen Physiol. 2013;141:297–308. doi: 10.1085/jgp.201210918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher-Smith JA, Huang CL, Fraser JA. Control of cell volume in skeletal muscle. Biol Rev Camb Philos Soc. 2009;84:143–159. doi: 10.1111/j.1469-185X.2008.00066.x. [DOI] [PubMed] [Google Scholar]
- Usher-Smith JA, Skepper JN, Fraser JA, Huang CL. Effect of repetitive stimulation on cell volume and its relationship to membrane potential in amphibian skeletal muscle. Pflugers Arch. 2006;452:231–239. doi: 10.1007/s00424-005-0022-9. [DOI] [PubMed] [Google Scholar]
- Zhang S, Andersson DC, Sandström ME, Westerblad H, Katz A. Cross bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol. 2006;291:C147–C154. doi: 10.1152/ajpcell.00578.2005. [DOI] [PubMed] [Google Scholar]


