Abstract
Physical inactivity that accompanies ageing and disease may hasten disability by reducing skeletal muscle contractility. To characterize skeletal muscle functional adaptations to muscle disuse, we compared contractile performance at the molecular, cellular and whole-muscle levels in healthy active older men and women (n = 15) and inactive older men and women with advanced-stage, symptomatic knee osteoarthritis (OA) (n = 16). OA patients showed reduced (P < 0.01) knee extensor function. At the cellular level, single muscle fibre force production was reduced in OA patients in myosin heavy chain (MHC) I and IIA fibres (both P < 0.05) and differences in IIA fibres persisted after adjustments for fibre cross-sectional area (P < 0.05). Although no group differences in contractile velocity or power output were found for any fibre type, sex was found to modify the effect of OA, with a reduction in MHC IIA power output and a trend towards reduced shortening velocity in women, but increases in both variables in men (P < 0.05 and P = 0.07, respectively). At the molecular level, these adaptations in MHC IIA fibre function were explained by sex-specific differences (P ≤ 0.05) in myosin–actin cross-bridge kinetics. Additionally, cross-bridge kinetics were slowed in MHC I fibres in OA patients (P < 0.01), attributable entirely to reductions in women with knee OA (P < 0.05), a phenotype that could be reproduced in vitro by chemical modification of protein thiol residues. Our results identify molecular and cellular functional adaptations in skeletal muscle that may contribute to reduced physical function with knee OA-associated muscle disuse, with sex-specific differences that may explain a greater disposition towards disability in women.
Key points
Muscle disuse that accompanies ageing and chronic disease may hasten physical disability by impairing skeletal muscle contractility.
We compared skeletal muscle contractile function at the various anatomic levels between two groups of older men and women matched for sex, health status and body size, of which one group was habitually active and the other inactive.
Muscle disuse reduced force generation, power output and contractile velocity in single muscle fibres, with differential adaptations in some parameters in men and women. Sex-specific cellular phenotypes were explained by differential adaptations in molecular muscle function. Moreover, aspects of the molecular functional phenotype apparent in inactive women could be recapitulated in vitro by chemical modification of protein thiols.
Our results identify molecular and cellular contractile dysfunction in skeletal muscle that may contribute to reduced physical function with muscle disuse, with sex-specific differences that may explain a greater disposition towards disability in women.
Introduction
Both ageing and the development of chronic disease are associated with increased rates of physical disability, which are thought to partially reflect adaptations in skeletal muscle (Fielding et al. 2011). One such adaptation is the diminishing of skeletal muscle mechanical function, which includes decreases in static and dynamic contractile performance and yields a reduction in muscle power output (Reid & Fielding, 2012). Adaptations in whole-muscle function are undoubtedly multi-factorial, but are likely to reflect, in part, functional changes intrinsic to skeletal muscle because age- and disease-related contractile deficits are apparent in experimental preparations that remove the influence of neural activation and calcium cycling (D'Antona et al. 2003; Yu et al. 2007; Miller et al. 2010, 2013). Numerous factors have been put forth as mediators of maladaptive changes in skeletal muscle, including inflammation, altered endocrine hormone levels, mitochondrial dysfunction and impaired neural activation (Fielding et al. 2011). Although these factors may play a role, many skeletal muscle changes may simply evolve secondary to the muscle disuse that accompanies ageing and many chronic diseases (Toth et al. 1997; Hughes et al. 2004; Ferriolli et al. 2012). Muscle disuse has long been considered as a contributing factor to muscle functional decline, but the extent to which it contributes to the reductions in muscle contractility observed in ageing and chronic disease is unclear.
Disuse impairs skeletal muscle performance. In human studies, reductions in whole-muscle force production and power output have been noted (Narici & Boer, 2011). These adaptations are similarly observed in isolated single muscle fibre preparations, with reductions in force production, both absolute and normalized per fibre cross-sectional area (CSA) or tension (Widrick et al. 1997, 2002; Yamashita-Goto et al. 2001; Trappe et al. 2004; Hvid et al. 2011), and contractile velocity (Yamashita-Goto et al. 2001; Widrick et al. 2002; Trappe et al. 2004), although some studies have shown increased velocity (Widrick et al. 1997, 2002). Regardless, the net effect of disuse is a decrease in muscle power output (Narici & Boer, 2011). Given the similarities between the adaptations that occur with disuse and those that occur with ageing and chronic disease, it is tenable to posit that some of the functional adaptations observed in healthy and diseased older adults may derive from muscle disuse.
Evidence to test the above postulate is limited because of the paucity of studies examining muscle disuse in older adults. More recently, laboratories have begun to experimentally impose muscle disuse on older human volunteers (Kortebein et al. 2007; Hvid et al. 2011). Although these experimental paradigms mimic the type of disuse that occurs with acute disease and convalescence, they do not model the less dramatic and more protracted disuse that accompanies ageing or disease in ambulatory humans. There are, however, clinical conditions which may serve as models of this type of protracted muscle disuse. Advanced knee osteoarthritis (OA) is one such condition, in which weight-bearing activity is substantially curtailed because of knee joint pain (Thomas et al. 2003) to a level comparable with that in ageing and disease (Toth et al. 1997; Hughes et al. 2004; Ferriolli et al. 2012). If knee OA patients are selectively recruited to be free of acute and chronic disease, and other conditions that may affect skeletal muscle structure or function, they can serve as a clinical model in which to study the effects of muscle disuse on skeletal muscle biology.
The overall goal of the present study was to investigate the effects of muscle disuse on contractile function at the molecular, cellular and whole-tissue levels. To do this, we recruited two groups of healthy, older men and women. One group consisted of patients with advanced-stage knee OA, who reported very low levels of habitual physical activity, and the other of individuals who self-reported having recreationally active lifestyles, but no symptoms or clinical evidence of significant knee OA. All individuals underwent tests of whole-muscle contractile function, as well as muscle biopsies to investigate cellular- and molecular-level contractile function. The resulting data were used to test the hypothesis that muscle disuse associated with advanced-stage knee OA reduces skeletal muscle contractility. In light of recent observations that age-related functional adaptations differ between men and women (Miller et al. 2013), and that younger men and women adapt differently to acute muscle disuse (Yasuda et al. 2005), we further examined whether the effects of disuse are modified by sex.
Methods
Ethical approval
Written informed consent was obtained from all volunteers prior to their participation. The protocol was approved by the Committee on Human Research at the University of Vermont and conformed to the requirements of the Declaration of Helsinki.
Subjects
Sixteen (eight men, eight women) older adults with symptomatic knee OA were recruited from the Adult Reconstruction Clinic of the Department of Orthopaedics and the surrounding community. All participants self-reported a prior diagnosis of knee OA. Six of these patients were recruited within 2 months of total knee arthroplasty surgery (bilateral or staged-bilateral in three patients and unilateral in three patients). Volunteers selected for study had symptomatic [based on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Bellamy et al. 1988)] and radiographic [Kellgren and Lawrence (K&L) grade 3 or 4 (Kellgren & Lawrence, 1957)] evidence of advanced knee OA in at least one knee and a body mass index (BMI) of <30 kg m−2. Additionally, patients reported being inactive or participating in light-intensity activities, based on the Stanford Brief Activity Survey (Taylor-Piliae et al. 2006), which corresponds to activity levels in the metabolic equivalent (MET) range of 1.0–1.5. To ensure that muscle function in knee OA patients reflected the effects of muscle disuse and not the effects of other chronic diseases or health conditions, volunteers were excluded if they had: (i) a current or past history, clinical signs or symptoms of diabetes, heart failure, pulmonary disease, thyroid disease, peripheral arterial disease, neurological or neuromuscular disease or autoimmune disease; (ii) a current or past (within 10 years) history of smoking; (iii) a current or past (within 10 years) history of malignancy, excluding non-melanoma skin cancer, or (iv) prior knee replacement in either knee. All volunteers had normal blood counts and chemistry, and renal, liver and thyroid function, based on standard blood tests. No patients were taking sex steroid replacement therapy (oestrogen or oestrogen/progestin therapy in women or androgen replacement in men), oral or inhaled corticosteroids or any other medication that might affect muscle function. Three OA volunteers (19%; two women, one man) were on stable regimens of HMG-CoA reductase inhibitors (statins). Plasma creatine kinase levels were within the normative range in these volunteers and none had symptoms or signs of statin-induced myopathy. Additionally, six participants (38%; four men, two women) had hypertension and were on stable anti-hypertensive therapy, consisting of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (50%), diuretics (33%) and adrenergic blocking agents (33%). None had received an intra-articular injection (hyaluronan or corticosteroid) or participated in any rehabilitation programme during the 6 months prior to testing.
Active controls (eight men, seven women) were selected to match the OA patient group for age and sex and to be healthy and free from disease or medications that might affect muscle size and function. The inclusion and exclusion criteria applied to this group were identical to those enumerated for knee OA patients, with some notable exceptions. Control subjects did not have symptoms consistent with knee OA [according to WOMAC scores (Bellamy et al. 1988)] or radiographic evidence of significant knee OA (K&L grades of > 2), and they self-reported being recreationally active and participating in activities of moderate to very hard intensity, based on the Stanford Brief Activity Survey (Taylor-Piliae et al. 2006). We chose to include individuals with activity patterns in the ‘very hard intensity’ category because results from our laboratories show that reference data on the MET level of activities overestimates energy requirements by ∼35% in older adults (Savage et al. 2007). In other words, many of the activities in the ‘very hard intensity’ category actually reflect activities of moderate intensity (5–7 METs) because the MET data used to grade these activities is artefactually high. This provided a control population with activity levels in the moderate to high range, but excluded individuals who were sedentary or engaged in light activities. Additionally, we did not recruit any individual who was currently training for athletic competition (e.g. masters athletes). Five individuals (33%; three men, two women) were on stable regimens of statins. Plasma creatine kinase levels were within the normative range in these volunteers and none had symptoms or signs of statin-induced myopathy. Additionally, six controls (40%; four men, two women) had hypertension and were on stable anti-hypertensive therapy consisting of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (67%), diuretics (33%) and adrenergic blocking agents (17%).
Experimental protocol
Eligibility was determined during a screening visit, at which medical history, physical examination, blood samples and bilateral whole-muscle strength testing were performed, the latter to determine which leg would be studied, as well as to familiarize patients with the strength-testing procedure. Additionally, all volunteers underwent bilateral knee X-rays to grade the knee joints for signs of OA. Following the screening visit, volunteers were provided with an accelerometer and asked to wear the device for 10 days to assess their free-living, weight-bearing activity levels. Volunteers who met the entry criteria and who were enrolled underwent a more comprehensive battery of whole-muscle strength testing during an outpatient visit. At least 5 days later, muscle tissue was obtained via percutaneous biopsy of the vastus lateralis. Body composition was assessed during an additional outpatient visit.
Knee extensor muscle function
Screening assessment consisted of bilateral measurements of isometric knee extensor torque at 55 deg, as described previously (Toth et al. 2010). Briefly, after they had been familiarized with the equipment and completed warm-up trials, volunteers performed five trials. The highest torque value during each contraction was considered as the peak isometric torque and the highest peak torque from the five trials represented the peak torque for that leg. In OA patients, the leg with the lower peak isometric torque was studied throughout the remainder of testing, whereas, in controls, the leg with the higher peak isometric torque was studied. This decision was intended to further dichotomize the population for skeletal muscle use.
Following enrolment, volunteers underwent both isometric and isokinetic strength testing in the leg chosen for study, as described in Toth et al. (2010). For isometric measurements, three trials were performed with the lever arm fixed at 70 deg. Isokinetic measurements were obtained at extensions of 60 deg s−1 and 180 deg s−1, in that order, with two trials of five consecutive repetitions at each speed (each separated by 2 min of rest). Data are not available for two OA patients for the isometric condition because of the severity of knee pain and for two patients at the 180 deg s−1 speed because they could not reach the target velocity.
Accelerometry
Free-living physical activity energy expenditure above basal metabolism was estimated using a single-plane accelerometer (Toth et al. 2010). Briefly, volunteers were instructed to wear the accelerometer on their waistline during waking hours for as many days as possible over a 10 day period.
Total and regional body composition
Total and regional fat mass and fat-free mass were measured by dual-energy X-ray absorptiometry (GE Lunar Corp., Madison, WI, USA). Arm and leg fat-free tissue mass was used to calculate appendicular skeletal muscle, as described previously by Miller et al. (2009). Mid-thigh skeletal muscle CSA was measured by computed tomography (Miller et al. 2009). Mid-thigh muscle CSA was not assessed in five OA patients.
Solutions for muscle biopsy acquisition and single-fibre studies
Dissection solution contained 20 mm N,N-bis[2-hydroxyethyl]-2-aminoethanesulphonic acid, 5 mm ethylene glycol-bis(2-aminoethylether)-N,N,N, N-tetraacetic acid (EGTA), 5 mm MgATP, 1 mm free Mg2+, 1 mm dithiothreitol (DTT) and 0.25 mm inorganic phosphate (Pi) with an ionic strength of 175 mEq, pH 7.0, and at pCa 8 (pCa = −log10 [Ca2+]). Skinning solution contained 170 mm potassium proprionate, 10 mm imidazole, 5 mm EGTA, 2.5 mm MgCl2, 2.5 mm ATP-Na2H2, and an EDTA-free protease inhibitor (cOmplete Mini Protease Inhibitor EDTA free; Roche Diagnostics Corp., Indianapolis, IN, USA) at pH 7.0. The storage solution was identical to the skinning solution, but included 1 mm sodium azide and excluded the protease inhibitor cocktail. The relaxing solution was identical to the dissecting solution with the addition of 15 mm creatine phosphate (CP), 5 mm Pi and 300 units ml−1 of creatine phosphokinase (CPK). The pre-activating solution was identical to the relaxing solution, except at an EGTA concentration of 0.5 mm. The activating solution was the same as the relaxing solution, except at pCa 4.5. All solutions used for mechanical experiments were adjusted to an ionic strength of 175 mEq using sodium methane sulphate.
Muscle biopsy and processing of tissue samples
Biopsies of the vastus lateralis were performed as described in detail in Toth et al. (2012). For single-fibre mechanical assessments, tissue was placed immediately into cold (4°C) dissecting solution. Thereafter, muscle fibre bundles were carefully dissected and processed for single-fibre measurements (Miller et al. 2009), with longterm storage at −20°C in storage solution with 50% glycerol (v/v). Any remaining tissue deemed to be unusable in mechanical experiments was frozen in liquid nitrogen and stored at −80°C.
Processing of muscle fibres for mechanical measurements
Fibres were allowed to chemically skin for 24 h (±2 h) prior to experiments. Segments (∼2.5 mm) of chemically skinned single fibres were isolated and processed for assessment of cellular- and molecular-level functional parameters. Fibres for molecular-level assessments were processed as described in Miller et al. (2009), including fixation with glutaraldehyde of the ends of fibres to which aluminium foil t-clips were attached for mounting on the motor and force gauge. These procedures are intended to ensure the stiffness of the fibre attachment to the measurement rig far exceeds the stiffness of the activated fibre, thus allowing the accurate measurement of viscoelastic properties of the fibre. Experiments in which the compliance of our fixation system was compared between an activated fibre and a Silastic rod (Dow Corning Corp., Midland, MI, USA) of similar dimensions indicate that at least 99.95% of measured compliance derives from the activated fibre, and not from the t-clip, length control motor or force gauge (data not shown). Following fixation, isolated single-fibre segments were skinned a second time in dissecting solution containing 1% Triton (v/v) for 30 min to ensure the permeabilization of the sarcolemma and sarcoplasmic reticulum. Fibres for cellular-level mechanical assessments were processed in a manner similar to those for molecular-level measurements, with the exception that the ends of these fibres were not fixed with glutaraldehyde. Notably, two separate sets of fibres were analysed: one for molecular-level and the other for cellular-level contractile function. All mechanical experiments were performed within 3 weeks of the biopsy procedure.
Cellular-level contractile function measurements
A commercially available apparatus (Aurora Scientific, Inc., Aurora, ON, Canada) was adapted for the use of isotonic load clamps on single, chemically skinned, skeletal muscle fibres. This system is similar to that previously described by our research group for sinusoidal analysis in human single muscle fibres (Miller et al. 2010), but differs in several respects. Briefly, chemically skinned, single muscle fibres were dissected from a bundle, trimmed to length (∼2000–2500 μm) and attached to the force gauge and length motor using the Moss clamp approach (Moss, 1979). This system was suspended over a glass-bottomed, aluminium bath plate containing eight wells (∼100 μl) holding experimental solutions. A single larger chamber (∼450 μl) was used to load the fibre and measure its dimensions once mounted. The temperature was set at 15°C by a thermo-electric cooler built into the plate and maintained at this level unless otherwise noted. The plate moved independently of the force transducer and length control motor, and was activated by two stepper motors. One motor moved the plate vertically and the other moved it longitudinally, allowing for the rapid transfer of the fibre from one solution bath to another. The bath plate and trough were mounted to an inverted microscope (Carl Zeiss GmbH, Jena, Germany) so that it tracked vertically with the optics. Fibre length was adjusted to achieve a sarcomere length of 2.65 μm, as measured by Fourier transformation of optical density viewed longitudinally through the microscope (IonOptix Corp., Milton, MA, USA). Fibre dimensions were measured using micrometers built into the microscope stage and the microscope eyepiece (Lasico, Inc., Los Angeles, CA, USA). Five pairs of measurements (from the top and side views) of fibre width were taken at regular intervals along the fibre length. Side views were obtained through a right-angled, mirrored prism built into the larger chamber of the bath plate. The average fibre CSA was calculated using these diameters, assuming an elliptical cross-section.
Fibres were suspended in relaxing solution during mounting and measuring procedures. Prior to all activations, fibres were transferred to pre-activating solution for 30 s to allow for more rapid and uniform Ca2+ activation upon subsequent transfer to activating solution. When isometric tension reached a maximum (Tmax), three mechanical load steps, expressed as a fraction of Tmax, were imposed by feedback control of progressively shortening muscle length (ML) (Igor Pro; WaveMetrics, Inc., Portland, OR, USA). Thereafter, the fibre was returned to the relaxing solution and re-lengthened. These procedures were repeated to obtain at least 15 isotonic contractions per fibre. Force was maintained over the duration of each step (∼180 ms) and steady-state velocity (V) was calculated as the slope of the length change during the final 25–50 ms of each step. Tension–velocity relationships were fit to the Hill equation (Palmer et al. 2013). From these fits, various parameters were derived (Palmer et al. 2013), including: maximum unloaded shortening velocity (Vmax), velocity at maximum power (Vopt), tension at maximum power (Topt) and maximum power production (Pmax). If fibre force decreased by >10% from the first to the last activation or if sarcomere patterns were skewed or disrupted, fibres were discarded from subsequent analysis.
Slack tests were performed following isotonic load clamps, as described previously (Edman, 1979). Following activation and attainment of Tmax, varying degrees of rapid, negative length changes (expressed as a percentage of fibre length) were introduced. The duration between the introduction of the length change and tension redevelopment was assessed. The slack distance (ΔL) for each step was plotted against the time required to take up the slack (Δt) and a line fit to the plot of ΔL versus Δt using least squares regression analysis in at least four length changes per fibre, with the slope representing shortening velocity (in mm s−1), which was also expressed relative to fibre length. If fibre force decreased by >10% from the first to the last activation or if sarcomere patterns were skewed or disrupted, fibres were discarded from subsequent analysis.
An additional series of experiments were conducted on a subset of fibres [n = 47 myosin heavy chain (MHC) I fibres from three female OA patients and one female control] to explore the effect of temperature on cellular contractile properties. Isotonic load clamps were performed on two separate sets of fibres at 15°C and 25°C. We did not assess these variables serially in the same fibres because the fibres became inherently unstable at higher temperatures in response to repeated isotonic load clamps (e.g. sarcomere disorganization and catastrophic failures), as others have observed (Bottinelli et al. 1996).
Molecular-level contractile function measurements
The experimental apparatus and procedures used in molecular-level measurements of contractile function were similar to those described previously (Miller et al. 2010), except that measurements were conducted at a single temperature (25°C). Briefly, fibres were attached to a piezoelectric motor and a strain gauge in relaxing solution, the sarcomere length set to 2.65 μm and fibre length measured. Each fibre underwent an initial activation (pCa 4.5) to verify the integrity of the fibre (maintenance of normal sarcomeric register), followed by a second activation for performance of sinusoidal analysis. Sinusoidal analysis was performed under maximal Ca2+-activated conditions (pCa 4.5; 25°C and 5 mm Pi) to estimate myosin–actin cross-bridge mechanics and kinetics, as described previously (Miller et al. 2010).
An additional series of experiments was performed to evaluate the effects of chemical modification of protein thiol groups using N-ethylmaleamide (NEM) in MHC I fibres. The solutions used were identical to those described above, except that all solutions used for single-fibre experiments were devoid of DTT. Fibres were studied at 25°C prior to and following the addition of a single concentration of NEM. Following sinusoidal length perturbation under baseline maximal Ca2+-activated conditions (i.e. no NEM), fibres were incubated in NEM (0 μm, 12.5 μm, 25 μm or 50 μm; n = 9, n = 11, n = 6 and n = 5, respectively) for 20 min at 5°C. Fibres were then washed in relaxing solution (5 × 2 min) at 25°C to remove any residual NEM and a second round of sinusoidal length perturbations imposed under maximal Ca2+-activated conditions. Data are expressed as a fractional change, whereby the change in tension or cross-bridge kinetic parameter at each NEM concentration is expressed relative to the change observed without NEM (i.e. ‘0 μm’ NEM condition).
To characterize myosin–actin cross-bridge mechanics and kinetics using sinusoidal analysis, complex modulus data [Y(ω); elastic and viscous moduli, derived as described previously (Miller et al. 2010)] at peak calcium activation were fitted to the following mathematical expression:
| (1) |
where ω = 2πf in s−1, A, B and C are magnitudes expressed in mn mm−2, 2πb and 2πc are characteristic frequencies expressed in s−1, i = −11/2, α = 1 s−1, and k = a unitless exponent. This equation yields three characteristic processes, A, B and C, which relate to various mechanical (A, B, C and k) and kinetic [2πb and (2πc)−1] properties of the cross-bridge cycle (Miller et al. 2010). Briefly, 2πb, the frequency portion of the B-process, is interpreted as the apparent rate of myosin force production or the rate of myosin transition between the weakly and strongly bound states. The inverse of the frequency portion of the C-process, or (2πc)−1, represents the average myosin attachment time (ton) to actin. The magnitudes of the B- and C-processes (parameters B and C) are proportional to the number of myosin heads strongly bound to actin and the cross-bridge stiffness. Finally, the A-process has no kinetic or enzymatic dependence and therefore reflects the viscoelastic properties of the non-enzymatic, passive elements in the myofilaments. Under Ca2+-activated conditions, the A-process represents the underlying stiffness of the lattice structure and the attached myosin heads in series. The parameter A indicates the magnitude of the viscoelastic modulus and k represents the angle at which the A-process lies relative to the x-axis, which reflects the viscous to elastic modulus relationship of the A-process (k = 0 purely elastic vs. k = 1 purely viscous).
MHC protein isoform distribution
Following all cellular and molecular mechanical assessments, single fibres were placed in gel loading buffer for determination of MHC isoform composition by SDS-PAGE to identify fibre type, as described above. We restricted our analysis to MHC I, IIA and IIA/X fibres, as the numbers of IIX and hybrid (I/IIA, I/IIA/IIX) fibres were too few to permit analysis. Additionally, for NEM and temperature experiments, we restricted our analysis to those fibres that were determined by gel electrophoresis to be MHC I fibres and thus MHC II fibre data are not presented. For MHC IIA/X fibres, the relative proportion of IIA and IIX isoforms was determined by densitometry (Toth et al. 2013).
MHC and actin protein content
Myosin and actin protein content were evaluated via gel electrophoresis in tissue homogenates (∼10 mg) as described in Miller et al. (2009), with the minor modification that the tissue was homogenized in 300 mm KCl, 150 mm potassium phosphate, 10 mm sodium pyrophosphate, 5 mm dithiothreitol, 5 mm magnesium chloride (pH 6.8), to which a protease inhibitor cocktail (Sigma-Aldrich Corp., St Louis, MO, USA) was added, which should solubilize the vast majority of myosin and actin (Cosper & Leinwand, 2012). These measures were not performed in one female control because of lack of tissue.
Statistics
Differences in means between groups were determined using unpaired t tests (IBM spss Statistics for Windows Version 19.0; IBM Corp., Armonk, NY, USA) and by analysis of variance (ANOVA) with sex as an additional factor in the model to evaluate whether the effects of OA differ between men and women (i.e. group × sex interaction effect). Group differences in whole-muscle strength measures were also examined after controlling for muscle size measures using analysis of covariance (ANCOVA). For variables in which multiple observations were performed within the same individual (e.g. single-fibre mechanical indices and CSA), a linear mixed model (sas Version 9.3; SAS Institute, Inc., Cary, NC, USA) was used, with group assignment as the between-subject factor (i.e. control group vs. OA group). A linear mixed model was used because the general linear model assumes that each measurement is independent, which is not the case for multiple fibres evaluated within each volunteer (i.e. fibres from the same subject are related). Accordingly, we have included a repeated effect in the model to account for variations in fibre characteristics within each individual. By accounting for this within-subject variance, this approach can be considered more conservative than the simple process of taking an average value for each fibre characteristic for each subject, which yields a single value for each individual with no estimate of variance. The additional between-subject factor of sex was included in the model to evaluate whether OA effects might differ between men and women. To examine the temperature effects, the mixed model was used with temperature (15°C vs. 25°C) and group (control vs. OA) as fixed effects. In examinations of the effects of protein thiol modification, ANOVA was used to examine the effects of NEM concentration on relative changes in mechanical parameters. Finally, relationships between variables were determined by Pearson correlation coefficients, with the normality of dependent and independent variables confirmed by the Shapiro–Wilk test (IBM spss Version 19.0). All data are reported as means ± s.e.m. Statistical significance was set at P < 0.05 and a trend towards significance was considered as indicated by a P-value of < 0.10.
Results
Physical characteristics
The physical characteristics of men and women in the knee OA and control groups are shown in Table 1. No group differences in age, body mass, height, BMI or total or regional body composition were observed, although group × sex interaction effects were noted for BMI and absolute and relative fat mass. As expected, accelerometer-derived activity level was lower (45%; P < 0.001) in knee OA patients than in controls, but there was no group × sex interaction effect. OA subjects and controls wore the accelerometer for a similar average number of days (9.2 ± 1.0 days vs. 8.8 ± 1.7 days; P = 0.36). In keeping with their clinical diagnosis and radiographic evidence, OA subjects reported a significantly lower score on the WOMAC questionnaire than controls (61 ± 3 vs. 97 ± 2; P < 0.001), indicating a much higher burden of knee OA symptoms, with no group × sex interactions.
Table 1.
Physical characteristics of volunteers with knee osteoarthritis (OA) and age- and sex-matched controls
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| Controls | OA group | Controls | OA group | Controls | OA group | |
| n | 15 | 16 | 8 | 8 | 7 | 8 |
| Age (years) | 67.5 ± 1.4 | 71.2 ± 1.5 | 68.0 ± 2.0 | 70.4 ± 1.7 | 67.0 ± 2.2 | 72.1 ± 2.6 |
| Body mass (kg) | 69.8 ± 4.1 | 70.3 ± 2.6 | 81.3 ± 4.5 | 77.2 ± 3.0 | 56.8 ± 2.2 | 63.5 ± 2.4 |
| Height (cm) | 168 ± 2 | 166 ± 3 | 174 ± 2 | 175 ± 1 | 162 ± 1 | 158 ± 3 |
| BMI (kg m−2) | 24.5 ± 1.0 | 25.3 ± 0.6 | 26.9 ± 1.1 | 25.1 ± 0.9 | 21.7 ± 1.0 | 25.6 ± 0.8 ‡ |
| Fat mass (kg) | 20.7 ± 2.2 | 22.2 ± 1.6 | 24.0 ± 3.4 | 19.4 ± 2.7 | 17.0 ± 2.0 | 25.0 ± 1.5 † |
| Fat mass (%) | 29.7 ± 1.8 | 33.2 ± 2.5 | 29.0 ± 2.7 | 25.4 ± 2.9 | 30.4 ± 2.5 | 41.0 ± 1.1 ‡ |
| Fat-free mass (kg) | 47.2 ± 2.4 | 45.6 ± 2.8 | 55.3 ± 1.2 | 55.6 ± 2.0 | 37.9 ± 0.8 | 35.7 ± 1.3 |
| Appendicular skeletal muscle mass (kg) | 27.9 ± 1.7 | 26.6 ± 1.8 | 33.5 ± 0.9 | 32.8 ± 1.4 | 21.5 ± 0.7 | 20.3 ± 1.0 |
| Activity level (kcal day−1) | 494 ± 46 | 270 ± 30* | 542 ± 62 | 331 ± 45 | 439 ± 66 | 210 ± 27 |
Data are means ± s.e.m. Abbreviation: BMI, body mass index. *, group effect (P < 0.01); †, group × sex effect (P < 0.05); ‡, group × sex effect (P < 0.01).
Knee extensor muscle function
Data on knee extensor contractile function measured during screening visits to determine the study leg, as well as the tissue composition of the study leg, are shown in Table 2. Peak knee extensor isometric torque was lower in the weaker leg in the OA group than in the stronger leg of controls (P < 0.001). Leg fat-free tissue mass did not differ between groups (P = 0.27), although there was a trend (P = 0.08) towards a lower mid-thigh muscle CSA in the OA group. Peak isometric torque deficits in the OA group persisted following statistical adjustment for leg fat-free tissue mass and thigh muscle CSA (P < 0.001 for both). No group × sex interactions were noted for any variables.
Table 2.
Knee extensor isometric torque production and tissue composition of the leg chosen for study in the initial screening evaluation of muscle function in subjects with osteoarthritis (OA) and age- and sex-matched controls
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| Controls | OA group | Controls | OA group | Controls | OA group | |
| Leg studied (left/right) | 7/8 | 8/8 | 3/5 | 3/5 | 4/3 | 5/3 |
| Knee extensor isometric torque at 55 deg (Nm) | 182.9 ± 8.9 | 96.5 ± 9.9* | 205.3 ± 8.6 | 120.4 ± 14.0 | 157.2 ± 9.6 | 72.9 ± 23.0 |
| Difference from non-studied leg (%) | 9.9 ± 2.2 | −22.2 ± 3.7* | 8.8 ± 4.3 | −26.0 ± 4.3 | 11.2 ± 4.6 | −18.4 ± 4.3 |
| Leg fat-free mass (kg) | 7.85 ± 0.43 | 7.37 ± 0.49 | 9.22 ± 0.33 | 8.94 ± 0.33 | 6.28 ± 0.35 | 5.80 ± 0.33 |
| Thigh muscle cross-sectional area (cm2) | 70.9 ± 4.0 | 59.3 ± 7.5 | 81.9 ± 4.3 | 80.7 ± 5.5 | 58.4 ± 4.6 | 41.5 ± 5.0 |
| DEXA-adjusted knee extensor isometric torque at 55 deg (Nm) | 179 ± 6.3 | 100 ± 6.1* | 179 ± 12.2 | 99 ± 11.3 | 179 ± 11.7 | 102 ± 12.9 |
| CT-adjusted knee extensor isometric torque at 55 deg (Nm) | 177 ± 7.4 | 109 ± 8.7* | 195 ± 12.3 | 121 ± 14.2 | 162 ± 11.0 | 93 ± 16.2 |
Data are means ± s.e.m. Adjusted knee isometric torque data presents group-adjusted means following covariance for either leg fat-free mass from dual-energy X-ray absorptiometry (DEXA) or thigh muscle cross-sectional area from computed tomography (CT).
Data on more in-depth assessment of knee extensor contractile function in the study leg following enrolment, under both isometric and isokinetic conditions, are shown in Table 3. Isometric peak torque was reduced in OA subjects (P < 0.001), as was peak torque under isokinetic conditions at both 60 deg s−1 and 180 deg s−1 (P < 0.001 for both). Statistical control for either leg fat-free tissue mass or mid-thigh muscle CSA did not diminish group differences in peak isometric (both P < 0.01) or isokinetic knee extensor torque (60 deg s−1: both P < 0.01; 180 deg s−1: P < 0.01 and P < 0.02, respectively). No group × sex interactions were noted for any variables.
Table 3.
Knee extensor peak torque production under isometric and isokinetic conditions in subjects with osteoarthritis (OA) and age- and sex-matched controls
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | OA group | % | Control | OA group | % | Control | OA group | % | |
| Isometric | |||||||||
| 70 deg (Nm) | 176.9 ± 13.2 | 103.1 ± 10.8 | −42* | 209.5 ± 12.1 | 130.0 ± 13.0 | −38 | 139.6 ± 13.0 | 76.7 ± 13.0 | −45 |
| Isokinetic | |||||||||
| 60 deg s−1 (Nm) | 125.9 ± 9.3 | 70.6 ± 6.5 | −44* | 143.7 ± 9.3 | 87.1 ± 9.3 | −39 | 105.4 ± 10.0 | 54.1 ± 9.3 | −49 |
| 180 deg s−1 (Nm) | 79.5 ± 6.5 | 52.3 ± 5.1 | −34* | 91.3 ± 6.3 | 67.4 ± 6.7 | −26 | 65.9 ± 6.7 | 37.1 ± 6.7 | −44 |
Data are means ± s.e.m. Percentage data represent the relative difference between OA patients and controls, or sex-specific controls, for absolute data (%). No significant group × sex interactions were noted for any strength measure when sex was included in the statistical model. *, effect of OA (P < 0.001).
Cellular-level contractile properties
Single-muscle fibre force production, CSA and tension (force/CSA) measured at 15°C are shown for MHC I, IIA and IIA/X fibres in Fig. 1. Single-fibre force production was reduced in MHC I (−21%) and IIA (−28%) fibres from knee OA subjects compared with those from controls (both P < 0.05). Although group average force was reduced similarly (−28%) in MHC IIA/X fibres from the OA group, the difference did not reach statistical significance
Figure 1. Single skeletal muscle fibre force production, cross-sectional area (CSA) and tension (force/CSA).
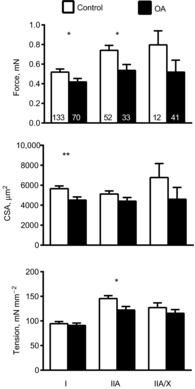
Single muscle fibre force production, CSA and tension in myosin heavy chain (MHC) I, IIA and IIA/X fibres from healthy, active controls and volunteers with knee osteoarthritis (OA) in fibres evaluated for cellular contractile function. The number of fibres analysed in each group for each fibre type is indicated at the base of each bar on the top panel. Data were acquired under maximal Ca2+-activated conditions (pCa 4.5) at 15°C and represent means ± s.e.m. *P < 0.05; **P = 0.01.
(P = 0.16). The average CSA was lower (−20%; P = 0.01) in MHC I fibres from OA subjects, whereas the decreases in average values in MHC IIA (−14%) and IIA/X (−32%) fibres from the OA group did not reach significance (P = 0.15 and P = 0.26, respectively). The expression of single-fibre force relative to fibre CSA (i.e. tension) minimized group differences in all fibre types, but a significantly lower tension (−16%) persisted in MHC IIA fibres (P = 0.02) from the OA group. Of note, variations in tension between the groups in MHC IIA/X fibres did not reflect differences in the fractional expression of the two isoforms as there were no differences (P = 0.58 for both) in the proportion of MHC IIA (53 ± 3% vs. 55 ± 2%) or MHC IIX (47 ± 3% vs. 45 ± 2%). Finally, to interrogate whether the effects of knee OA differed between men and women, we included sex as an additional factor in the statistical model. We observed no group × sex interaction effects for force, fibre CSA or tension in MHC I (P-values: 0.73–0.84), IIA (P-values: 0.36–0.90) or IIA/X fibres (P-values: 0.32–0.66), and no group × sex effect for the relative proportion of MHC IIA or IIX expressed (both P = 0.35).
In addition to isometric conditions, we evaluated single-fibre contractile function under isotonic conditions (Fig. 2). No group differences were observed in Vmax, Vopt, Topt or Pmax in any of the fibre types. Similarly, unloaded shortening velocity (Vo) determined from slack tests (Fig. 3) did not differ between groups.
Figure 2. Maximal shortening velocity, velocity and tension at which peak power is obtained and peak power output.
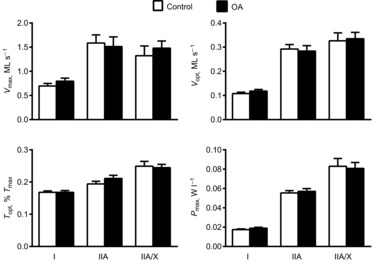
Maximal shortening velocity (Vmax), velocity (Vopt) and tension (Topt) at which peak power is obtained and peak power output (Pmax), in myosin heavy chain (MHC) I, IIA and IIA/X fibres from healthy, active controls and volunteers with knee osteoarthritis (OA). Numbers of fibres analysed are identical to those in Fig. 1. Data were acquired under maximal Ca2+-activated conditions (pCa 4.5) at 15°C and represent means ± s.e.m.
Figure 3. Unloaded shortening velocity obtained from slack tests.

Unloaded shortening velocity was obtained from slack tests conducted under maximal Ca2+-activated conditions (pCa 4.5) at 15°C in myosin heavy chain (MHC) I, IIA and IIA/X fibres from healthy, active controls and volunteers with knee osteoarthritis (OA). The number of fibres analysed in each group for each fibre type is indicated at the base of each bar. Data are means ± s.e.m.
To evaluate whether sex might modify the effects of OA, we again included sex as a factor in the statistical model (Fig. 4). MHC IIA fibres showed a group × sex effect (P < 0.04) for Pmax, with OA being associated with lower power in women and higher power in men. Post hoc pairwise comparisons showed that differences within each sex were not significant, although there was a trend for Pmax in OA men to be higher than in control men (P = 0.07). Thus, the opposing direction of differences in men and women with OA explained the group × sex effect. In addition, there was a strong trend (P = 0.07) for a group × sex effect for Vmax. Moreover, there was a group × sex effect for Vopt (P = 0.05) of similar character, with no pairwise differences between men and women in either group. By contrast with MHC IIA fibres, no group × sex effects were found for any variable for MHC I (P-values: 0.19–0.99) or MHC IIA/X fibres (P-values: 0.35–0.74). Lastly, there were no group × sex interactions for Vo for any fibre type (P-values: 0.40–0.78).
Figure 4. Sex modifies the effects of osteoarthritis (OA) on myosin heavy chain (MHC) IIA contractile measurements.
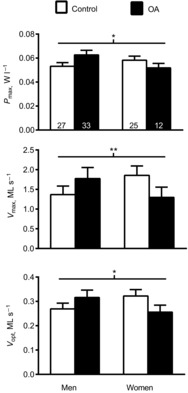
Influence of sex on the effects of OA on MHC IIA contractile measurements, including maximal power output (Pmax), maximal shortening velocity (Vmax) and velocity at which maximal power is obtained (Vopt) in healthy, active controls and volunteers with knee OA. The number of fibres analysed in each group for each fibre type is indicated at the base of each bar in the top panel. Data were acquired under maximal Ca2+-activated conditions (pCa 4.5) at 15°C and represent means ± s.e.m. *P ≤ 0.05; **P = 0.07 for group × sex effect.
Molecular-level contractile function
To further characterize skeletal muscle function at the myosin–actin cross-bridge level and to interrogate potential mechanisms underlying group and group × sex differences in cellular-level contractile function, we assessed myosin–actin cross-bridge mechanics and kinetics under maximal Ca2+-activated conditions (pCa 4.5) at 25°C in separate sets of MHC I, IIA and IIA/X fibres. In agreement with fibres in which cellular-level measurements were performed (Fig. 1), reduced force production was observed in MHC I (−18%; P < 0.01) and IIA (−26%; P < 0.05) fibres from OA patients, whereas the decrease in force production in MHC IIA/X fibres from OA patients was not significant (−24%; P = 0.16). Also similarly, single-fibre CSA was reduced in MHC I fibres from OA patients (−21%; P < 0.01), whereas reductions were not significant in MHC IIA (−25%; P = 0.18) and IIA/X (−19%; P = 0.43) fibres. However, we did not observe differences in single-fibre tension in MHC IIA fibres (−7%; P = 0.25). Moreover, tension did not differ in MHC I (−1%; P = 0.85) or IIA/X fibres (−16%; P = 0.15).
Myosin–actin cross-bridge mechanics and kinetics are shown in Fig. 5. In MHC I fibres, there was a slowing of cross-bridge kinetics in OA patients, as evidenced by a 19% increase in ton (P < 0.05). Other cross-bridge kinetic and mechanic parameters in MHC I fibres did not differ between groups (P-values: 0.18–0.82), although there were trends towards greater B and C values for OA patients versus controls (P = 0.09 and P = 0.10, respectively). In MHC IIA fibres, the parameter k was 17% lower in the OA group than in the control group (P < 0.05), although none of the other myosin–actin cross-bridge mechanic or kinetic parameters differed between groups (P-values: 0.13–0.58). In MHC IIA/X fibres, there was a trend towards a lower A value in the OA group compared with controls (P = 0.06), but no other parameters differed (P-values: 0.11–0.65).
Figure 5. Sinusoidal analysis parameters in myosin heavy chain (MHC) I, IIA and IIA/X fibres.
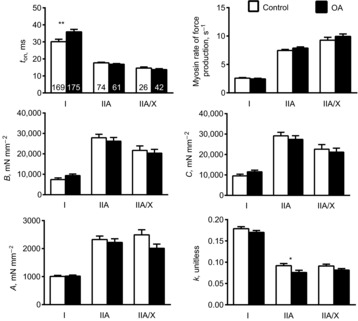
Sinusoidal analysis parameters in MHC I, IIA and IIA/X fibres from healthy, active controls and volunteers with knee osteoarthritis (OA). Details of the physiological interpretation of each parameter are provided in Methods, under Molecular-level contractile function measurements. The number of fibres analysed in each group for each fibre type is indicated at the base of each bar in the top left panel. Functional data were acquired under maximal Ca2+-activated conditions (pCa 4.5) at 25°C. Data are means ± s.e.m. *P < 0.05; **P < 0.01.
To determine if sex modified the effect of OA on myosin–actin cross-bridge mechanic and kinetic parameters in MHC I and IIA fibres, we included sex as a factor in the statistical model (Fig. 6). In MHC I fibres, a group × sex interaction effect (P < 0.05) was observed for ton. Pairwise comparisons showed longer attachment times in women with OA than in control women (P < 0.01), whereas those in men with OA did not differ from those in control men (P = 0.66), indicating that the increased ton in OA subjects was explained by increases in women with knee OA. In MHC IIA fibres, there were strong trends (both P = 0.06) for group × sex interaction effects for ton and myosin rate of force production. Pairwise comparisons showed that OA was associated with a reduction in ton in men compared with controls (P = 0.05) and an increase in myosin rate of force production in OA men compared with controls (P < 0.05). No other group × sex interaction effects were noted for any other mechanic or kinetic parameter in either muscle fibre type (P-values: 0.19–0.92).
Figure 6. Sex modifies the effects of osteoarthritis (OA) on sinusoidal analysis parameters.
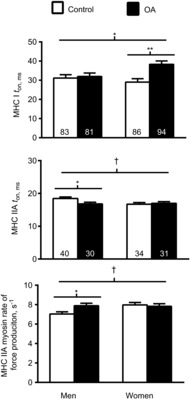
Effect of sex on OA effects on sinusoidal analysis parameters in myosin heavy chain (MHC) I and IIA fibres from healthy, active controls and volunteers with knee OA. The number of fibres analysed in each group is indicated at the base of each bar for each fibre type, with the middle and lower panel having the same number of fibres for the MHC IIA fibre type. Functional data were acquired under maximal Ca2+-activated conditions (pCa 4.5) at 25°C. Data are means ± s.e.m. *P ≤ 0.05; **P < 0.01; †P = 0.06.
Myosin and actin protein content
Alterations in cellular and molecular muscle function may be related to loss of myosin or actin protein content, each of which has been shown to occur in experimental models of acute disuse and clinical conditions (Riley et al. 1998; Larsson et al. 2000; Miller et al. 2009). However, we did not observe reduced levels of either myofilament protein in tissue homogenates from OA patients (Fig. 7) and no group × sex interaction effect was noted (P-values: 0.10–0.35). Note that the trend towards a group × sex interaction effect reflected a slightly higher myosin content in women with OA (9%), which is unlikely to explain reduced cross-bridge kinetics (Miller et al. 2010).
Figure 7. Myosin and actin protein expression.
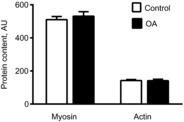
Myosin and actin protein expression in tissue homogenates from the control (n = 14) and knee osteoarthritis (OA) (n = 16) groups. Data are means ± s.e.m.
Effects of chemical modification of protein thiols on molecular muscle function
The group × sex effects in both muscle fibre types prompted us to explore the potential role of oxidative modification of myofilament proteins. To test this possibility, we treated chemically skinned MHC I muscle fibres from healthy controls with the thiol-specific alkylating agent NEM, which irreversibly modifies protein thiol groups. We found a concentration-dependent increase (P < 0.01) in ton with increasing NEM (Fig. 8). As NEM concentration increased, tension began to decrease in a concentration-dependent manner, albeit that this effect only trended towards statistical significance (P = 0.07).
Figure 8. Chemical modification of protein thiol residues with N-ethylmaleamide (NEM).
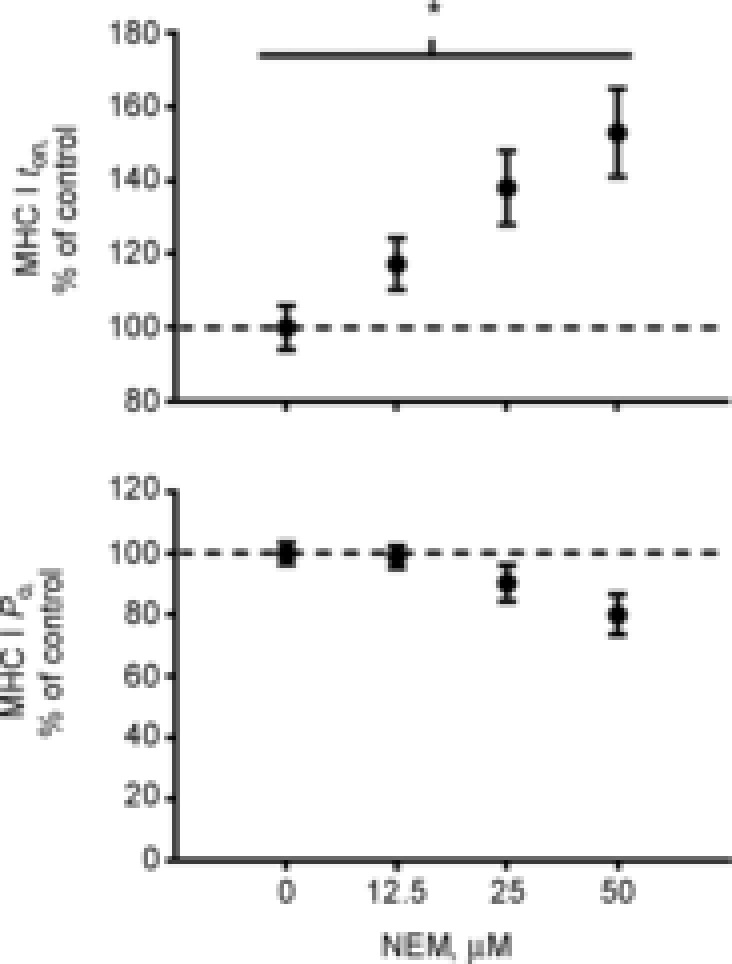
Effect of chemical modification of protein thiol residues with NEM on myosin attachment time and tension in myosin heavy chain (MHC) I fibres from control volunteers. Fibres were serially assessed for tension and myosin–actin cross-bridge kinetics before and following treatment with a single concentration of NEM. Numbers of fibres analysed under 0 μm, 12.5 μm, 25 μm and 50 μm were 9, 11, 6 and 5, respectively. The change in tension or ton at each NEM concentration is expressed relative to the change under control conditions (i.e. no NEM added). *P < 0.01 concentration-dependent effect of NEM.
Effects of temperature on myofilament function
Cellular and molecular functional measurements were carried out at different temperatures. There were disagreements between cellular and molecular functional measures; most notably, molecular functional deficits found in MHC I fibres at 25°C in women OA patients were not evident as diminished cellular function at 15°C. To further examine the nature of these discrepancies, we measured cellular function at these two different temperatures in MHC I fibres in women. As expected, there were marked effects of a reduction in temperature from 25°C to 15°C (all P < 0.001) as evidenced in decreases in MHC I fibre tension (134 ± 7.8 mN mm−2 vs. 94.9 ± 5.4 mN mm−2), Vmax (1.44 ± 0.17 ML s−1 vs. 0.66 ± 0.16 ML s−1), Vopt (0.42 ± 0.03 ML s−1 vs. 0.10 ± 0.03 ML s−1) and Pmax (0.120 ± 0.008 W l−1 vs. 0.017 ± 0.007 W l−1). Of particular interest, there was a trend towards a temperature × disease interaction (P = 0.08) for Vmax, with a more pronounced decline in Vmax with reduction in temperature in control fibres (2.7-fold) compared with OA fibres (1.8-fold). Other variables did not show temperature × group effects (P-values: 0.27–0.95).
Other mediators of sex effects
To evaluate whether differences in adiposity (Table 1) explain group × sex interaction effects in cellular and molecular function, we examined the relationships between these parameters and adiposity indices. There were no relationships between adiposity measures (BMI, fat mass, percentage fat mass) and single-fibre tension or mechanical properties from isotonic load clamps in the total cohort (r-values: −0.20 to 0.26; P-values: 0.18–1.00). Moreover, there were no relationships between any adiposity index and cross-bridge kinetic parameters for MHC I or IIA fibres (r-values: −0.20 to 0.03; P-values: 0.25–0.86).
Discussion
The present data suggest that, at the single muscle fibre (i.e. cellular) level, muscle disuse in older adult humans associated with knee OA is characterized by reductions in force production in both MHC I and IIA fibres, primarily as a result of muscle atrophy. In MHC IIA fibres, sex modified the effect of disuse on muscle fibre power and velocity, with increases in these variables in men and reductions in women. This cellular contractile phenotype, in turn, was explained by sex-specific differences in molecular-level function. We also observed an effect of disuse to reduce cross-bridge kinetics in MHC I fibres, which was entirely attributable to a reduction in cross-bridge kinetics in women with OA. This molecular contractile phenotype in MHC I fibres could be recapitulated in vitro by chemical modification of protein thiol residues. In summary, although both men and women with knee OA showed reduced force-generating capacity in single muscle fibres primarily as a result of atrophy, only men exhibited an increase in velocity that might serve to offset losses in skeletal muscle power output. Thus, our results highlight sex as a modifier of cellular- and molecular-level adaptations in skeletal muscle contractile performance with knee OA-associated disuse and suggest potential mechanisms underlying functional deficits in older inactive women that may predispose them to higher rates of disability.
Some effects of disuse on muscle mechanical function were not evident until the additional influence of sex was considered; most notably, reciprocal changes in Pmax, Vmax and Vopt in MHC IIA fibres, with increases in men and reductions in women. These cellular-level functional adaptations were reinforced and explained by differential molecular adaptations in myosin–actin cross-bridge kinetics, particularly in an increase in cross-bridge kinetics in men. For instance, the reduced ton in men would be expected to increase Vmax (and, in turn, Pmax) as there is an inverse relationship between these two variables (Tyska & Warshaw, 2002; Piazzesi et al. 2007). Additionally, an increase in men in the myosin rate of force production, which reflects the sum of the forward and backward rate constants for the transition of myosin from the weakly to the strongly bound state (Kawai et al. 1993), would also increase Vmax if the increase reflected a greater forward rate constant (i.e. transition from the weakly to the strongly bound state) (Piazzesi et al. 2007). Thus, our results identify distinct sex-specific adaptations in skeletal muscle function at the level of the myosin–actin cross-bridge interaction that scale to the cellular level to influence power output. As fast-twitch fibres disproportionately influence contractile properties of the whole muscle during maximal isokinetic/tonic assessments (Gür et al. 2003) because of their greater power output (Fig. 2), these changes are likely to have the most profound impact on maximal muscle performance measures of all the adaptations we observed.
The phenotype observed in MHC IIA fibres in men, in which disuse is associated with a greater velocity of contraction in the face of reduced force-generating capacity, is comparable with observations in preclinical models of muscle disuse at the single-fibre level (Reiser et al. 1987), and is in keeping with whole-muscle adaptations in humans and animals of fibre type switching towards faster-contracting fibres (i.e. MHC II), with concurrent reductions in whole-muscle torque (Narici & Boer, 2011). These increases in velocity may serve to diminish reductions in muscle power output in the face of reduced force-producing capacity. Because of the cross-sectional nature of our study, we cannot infer that adaptations in men were related to changes over time in these parameters, but this notion is supported by recent longitudinal studies which found increased single-muscle fibre Pmax and Vo over a 3 year follow-up in mobility-limited and healthy older adults (Reid et al. 2014). The authors hypothesized that these changes may represent a compensatory adaptation to minimize age-related functional loss. If this is indeed the case, our current work suggests increased cross-bridge kinetics as a potential molecular mechanism underlying such compensatory functional adaptations, which, upon the clinical background of knee OA-associated muscle disuse, would limit functional deterioration in men, whereas women appear to lack this adaptation. Together with our recent findings showing reduced cross-bridge kinetics with ageing in women compared with men (Miller et al. 2013), these results identify lower myosin–actin cross-bridge kinetics as a potential molecular mechanism underlying higher disability rates in older women (Jette & Branch, 1981).
By contrast with MHC IIA fibres, MHC IIA/X fibres did not exhibit differences between groups or group × sex effects. Given the uneven distribution of this fibre type across OA and control participants, with relatively few fibres in controls, our inability to detect differences for this fibre type may relate to Type II error. We were compelled to present these data, however, because of their greater representation in OA patients, which suggests, as has been observed in experimental models of muscle disuse in humans (Andersen et al. 1999), that OA-associated muscle disuse may provoke an increased expression of MHC IIA/X hybrid fibres and, in turn, a greater contribution of these fibres to the whole-muscle functional phenotype.
In addition to lower power output in MHC IIA fibres, force production was reduced in MHC I and MHC IIA fibres with disuse. In MHC I fibres, atrophy fully accounted for force decrements (i.e. no differences in force/fibre CSA), whereas in MHC IIA fibres atrophy was non-significant and only partially explained group differences in force production. Unlike muscle fibre power output, velocity and cross-bridge kinetics, however, the effects of muscle disuse on force production and fibre CSA were similar in men and women, suggesting a general role for muscle fibre atrophy in contributing to diminished muscle performance with disuse in older adults, a phenomenon that has been well characterized in models of acute muscle disuse in healthy, young and older volunteers (Hvid et al. 2011; Narici & Boer, 2011). Taken together with the aforementioned adaptations in MHC IIA fibres, these results suggest that both quantitative and qualitative (i.e. function per unit size) changes at the cellular and molecular levels contribute to impaired skeletal muscle performance with knee OA-associated muscle disuse.
Marked effects of knee OA on molecular muscle function in MHC I fibres were also observed, specifically a reduction in myosin–actin cross-bridge kinetics. In fact, these differences were the most pronounced of all simple group effects observed. As with MHC IIA fibres, these differences were sex-specific, with effects in OA women driving the lower cross-bridge kinetics. Reduced cross-bridge kinetics, and a greater ton in particular, would be expected to diminish muscle function primarily by decreasing fibre-shortening velocity, as velocity is commonly assumed to be reciprocally related to ton (Tyska & Warshaw, 2002). However, this was not borne out at the cellular level as no group or group × sex effects were noted for Vmax. An issue in extrapolating from molecular- to cellular-level functional assessments is that each was carried out at different temperatures (i.e. 15°C for cellular and 25°C for molecular assessments). This is especially true for MHC I fibres, which are particularly sensitive to the modifying effects of temperature within this range (Ranatunga, 1984). Additional experiments suggested that the effects of temperature on Vmax in MHC I fibres may differ between OA and controls, with more pronounced reductions in Vmax from 25°C to 15°C in control versus OA women, which may explain the failure of the molecular phenotype to become evident in cellular functional measures. Regardless of the explanation for differences between molecular and cellular functional measures, sexually dimorphic adaptations to disuse in MHC I fibres may have functional significance. MHC I fibres comprise approximately 60% of the total quadriceps muscle cross-section (Lexell et al. 1988) and represent the first fibre type to be activated in the orderly recruitment process of voluntary activation (Henneman et al. 1965). Thus, the aggregate functional response for any activity comprises at least some contribution from MHC I fibres during both submaximal and maximal efforts. As most daily activities rarely involve maximum voluntary muscle contraction, the function of MHC I fibres is probably important to functional independence in older adults.
The mechanism underlying the observed differences in molecular function is not clear, but we have similarly observed reduced cross-bridge kinetics in heart failure (Miller et al. 2010), ageing (Miller et al. 2013) and cancer (Toth et al. 2013). One potential pathoaetiological factor common to all of these conditions, including muscle disuse (Powers et al. 2007), is increased oxidative stress (Barreiro et al. 2006; Vescovo et al. 2008; Ramamoorthy et al. 2009). To test this possibility, we treated chemically skinned MHC I muscle fibres from healthy controls with the thiol-specific alkylating agent NEM, which irreversibly modifies protein thiol groups (Perkins et al. 1997). We chose MHC I fibres because they displayed the greatest reductions in cross-bridge kinetics and most prominent modifying effects of sex (Fig. 7), but also because of their known greater structural stability during repeated activations at high temperatures (25°C in the current experiments) (Bottinelli et al. 1996). In support of a potential involvement of oxidative stress in the current phenotype observed in OA patients, we found that NEM showed a concentration-dependent effect to reduce cross-bridge kinetics (i.e. increase ton) in MHC I fibres. In fact, when we down-titrated the NEM concentration, we produced a functional phenotype (17% increase in ton, with no change in tension) (Fig. 8) that bears a striking resemblance to the differences observed in our study in MHC I fibres (19% increase in ton, with no change in tension) (Figs 5 and 1, respectively). Interestingly, others have suggested that cellular oxidants have more profound effects on MHC I fibres (Dutka et al. 2011), which may explain the more robust effects of OA on molecular function in MHC I fibres. As thiol residues are highly reactive to oxidants and biological oxidants have functional effects similar to those of NEM (Galler et al. 1997; Perkins et al. 1997), oxidative modification of myofilament proteins may partially explain the disuse phenotype observed in this study. Whether similar functional effects are apparent in MHC IIA fibres was not investigated, owing to the lability of these fibres to repeated activations at high temperatures (Bottinelli et al. 1996), but is suggested by data from preclinical models (Galler et al. 1997; Perkins et al. 1997). The reasons why such adaptations in molecular function might differ by sex are also unclear, but they may relate to a tendency for inactive, older women to suffer enhanced oxidant production, diminished oxidant defences and/or greater sensitivity to oxidant damage, as some studies suggest (Barreiro et al. 2006), although we acknowledge that others do not (Pansarasa et al. 2000). Nevertheless, as numerous myofilament proteins contain functionally relevant thiol residues (Williams & Swenson, 1982; Kim et al. 2001; Evangelista et al. 2010; Mollica et al. 2012), differential adaptations in men and women may be explained by modifications to one or more of these proteins, specific residues within each protein(s) and/or the complex interaction of protein thiol modification with modification of other residues by different cellular oxidants or other post-translational modifications (Miller et al. 2013). The identification and resolution of the complex effects of oxidative modification of myofilament proteins will require a considerable amount of further study. To our knowledge, however, the present results represent the first evidence in humans, albeit correlative, to support the long-held notion that post-translational modification of myofilament proteins mediates deterioration in contractile function in response to muscle disuse.
Although OA patients showed marked reductions in both static and dynamic knee extensor contractile performance independent of reductions in muscle size (Table 3), in agreement with prior work (Slemenda et al. 1997), we found no sex differences in the extent of muscle weakness. This may relate to our small sample size as larger studies have found diminished muscle strength in women with advanced knee OA (Petterson et al. 2007). Indeed, on a relative basis, both isometric and isokinetic muscle strength were more impaired in women than in men, albeit that these differences did not reach significance. Unfortunately, variations in neural adaptations to knee OA, such as those in pain-induced inhibition (Petterson et al. 2007), antagonist co-contraction (Hortobagyi et al. 2005) and reduced motor unit discharge rates (Farina et al. 2005), obscure our ability to discern an effect of cellular and molecular contractile deficiencies on whole-muscle in vivo performance. In populations unencumbered by this caveat, we have demonstrated that cellular and molecular contractile deficiencies are associated with reduced whole-muscle and body function (Miller et al. 2013; Toth et al. 2013), which further suggests that these contractile deficits should scale to the tissue level to contribute to functional disability with muscle disuse.
Building on this last point, there are several caveats to our studies that deserve discussion. The utility of a healthy population of patients with advanced OA as a clinical model of muscle disuse is not without limitation. The neural adaptations that accompany advanced OA may alter muscle fibre physiology through numerous mechanisms. Alterations in the frequency of motor neuron impulses may elicit fibre type shifts in skeletal muscle (Pette & Vrbova, 1985). However, the extent of pain-induced modulation of firing frequency is quite small (Farina et al. 2005) and prior studies in preclinical models suggest that the isolated effect of neurally mediated fibre type shifts does not alter the underlying functional properties of the resultant fibre (Sweeney et al. 1986). Additionally, joint inflammation and/or pain can modulate reflex motor neuron activation (He et al. 1988), which theoretically can alter muscle tone and stiffness. However, even conditions characterized by chronic muscle spasticity, which is thought to be attributable in part to excessive reflex activation, do not alter skeletal muscle structure, function or molecular composition in human single MHC I and IIA fibres (Olsson et al. 2006). Recent studies have shown increased inflammation in skeletal muscle in OA patients (Levinger et al. 2011), suggesting that our results may reflect the influence of both disuse and muscle inflammation. Inflammatory signalling upregulates atrophic mediators (Cai et al. 2004) and is associated with diminished myofilament function secondary to increased oxidant stress (Hardin et al. 2008), suggesting that muscle inflammation may mediate the structural and contractile phenotypes observed in the present study. Whether inflammation accompanies the primary disease process or occurs secondary to the ensuing muscle disuse (Powers et al. 2007), however, is difficult to discern. Studies in preclinical models argue for activation of classical inflammatory signalling pathways in skeletal muscle with disuse (Jackman et al. 2013). Moreover, in humans the loss of central neural drive caused by stroke is associated with upregulation of muscle inflammatory markers (Hafer-Macko et al. 2005), which further argues that muscle disuse may drive local muscle inflammation. Unlike in knee OA, in which the primary pathology is adjacent to the muscle of interest, the effector of muscle disuse in stroke is sufficiently distal to the skeletal muscle to argue against an effect of the primary pathology to promote muscle inflammation. For these reasons, we believe that the inflammatory state in the muscle is likely to represent a consequence of muscle disuse, rather than of the primary joint pathology. Finally, our single-fibre experimental approach has several caveats. Firstly, we are limited in our ability to assess molecular and cellular contractile function at physiological temperatures because of the instability of the skinned fibre preparation (Bottinelli et al. 1996). Secondly, the different temperatures used in these evaluations may complicate the extrapolation of results across anatomic levels (e.g. MHC I fibres), as discussed above. Thirdly, we are limited to assessing molecular functional measures under isometric conditions. However, we have recently shown that estimates of cross-bridge kinetics derived under these conditions closely approximate the parameters measured under dynamic conditions (Tanner et al. 2011). Finally, we cannot discount the possibility that inhomogeneities in sarcomere length may have influenced our functional measures. However, sarcomere inhomogeneities have been observed in the most basic myofibril preparations (Rassier et al. 2003) and in in vivo conditions in humans (Llewellyn et al. 2008), suggesting that some degree of sarcomere inhomogeneity is an inherent property of normal muscle contraction.
In summary, our results demonstrate novel cellular and molecular adaptations in muscle function to knee OA-associated disuse in older adult humans and suggest the possible involvement of oxidative modification of myofilament proteins in some of these adaptations. The sex-specific nature of these adaptations may represent a molecular mechanism underlying the greater rates of disability in older women.
Acknowledgments
We thank all the volunteers who dedicated their valuable time to these studies.
Glossary
- A
A-process magnitude
- B
B-process magnitude
- BMI
body mass index
- C
C-process magnitude
- CP
creatine phosphate
- CPK
creatine phosphokinase
- CSA
cross-sectional area
- DTT
dithiothreitol
- f
frequency of length perturbations
- k
A-process unitless exponent
- MHC
myosin heavy chain
- ML
muscle length
- NEM
N-ethylmaleamide
- OA
osteoarthritis
- Pi
inorganic phosphate
- Pmax
maximum power production
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- ton
average myosin attachment time
- Topt
tension at maximum power
- Vmax
maximum shortening velocity
- Vopt
velocity at maximum power
- Y(ω)
complex modulus
- 2πb
B-process characteristic frequency
- 2πc
C-process characteristic frequency
- ΔL
slack distance
- Δt
time required to take up slack
Additional information
Competing interests
None declared.
Author contributions
D.W.M., B.D.B., P.A.A. and M.J.T. were involved in the conception and design of the experiments. D.M.C., M.S.M., A.P.S., T.W.T., J.R.S., P.D.S. and M.J.T. were involved in the collection, analysis and interpretation of data. All of the authors were involved in the drafting or critical revision of the paper and all approved the final manuscript for submission.
Funding
This study was funded by grants from the National Institutes of Health (AG-033547 to M.J.T.), an Institutional National Research Service Award (HL-007647 to D.M.C.) and a Mentored Research Scientist Development Award (AG-031303 to M.S.M.).
References
- Andersen JL, Gruschy-Knudsen T, Sandri C, Larsson L, Schiaffino S. Bed rest increases the amount of mismatched fibers in human skeletal muscle. J Appl Physiol. 1999;86:455–460. doi: 10.1152/jappl.1999.86.2.455. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Coronell C, Laviña B, Ramírez-Sarmiento A, Orozco-Levi M, Gea J. Aging, sex differences, and oxidative stress in human respiratory and limb muscles. Free Radic Biol Med. 2006;41:797–809. doi: 10.1016/j.freeradbiomed.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh B-C, Lidov HGW, Hasselgren P-O, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cosper PF, Leinwand LA. Myosin heavy chain is not selectively decreased in murine cancer cachexia. Int J Cancer. 2012;130:2722–2727. doi: 10.1002/ijc.26298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Mollica JP, Lamb GD. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol. 2011;110:705–716. doi: 10.1152/japplphysiol.00739.2010. [DOI] [PubMed] [Google Scholar]
- Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibers. J Physiol. 1979;291:143–150. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista AM, Rao VS, Filo AR, Marozkina NV, Doctor A, Jones DR, Gaston B, Guilford WH. Direct regulation of striated muscle myosins by nitric oxide and endogenous nitrosothiols. PLoS ONE. 2010;5:e11209. doi: 10.1371/journal.pone.0011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Arendt-Nielsen L, Graven-Nielsen T. Experimental muscle pain reduces initial motor unit discharge rates during sustained submaximal contractions. J Appl Physiol. 2005;98:999–1005. doi: 10.1152/japplphysiol.01059.2004. [DOI] [PubMed] [Google Scholar]
- Ferriolli E, Skipworth RJE, Hendry P, Scott A, Stensteth J, Dahele M, Wall L, Greig C, Fallon M, Strasser F, Preston T, Fearon KCH. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage. 2012;43:1025–1035. doi: 10.1016/j.jpainsymman.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S, Hilber K, Gobesberger A. Effects of nitric oxide on force-generating proteins of skeletal muscle. Pflugers Arch. 1997;434:242–245. doi: 10.1007/s004240050391. [DOI] [PubMed] [Google Scholar]
- Gür H, Gransberg L, vanDyke D, Knutsson E, Larsson L. Relationship between in vivo muscle force at different speeds of isokinetic movements and myosin isoform expression in men and women. Eur J Appl Physiol. 2003;88:487–496. doi: 10.1007/s00421-002-0760-8. [DOI] [PubMed] [Google Scholar]
- Hafer-Macko CE, Yu S, Ryan AS, Ivey FM, Macko RF. Elevated tumor necrosis factor-α in skeletal muscle after stroke. Stroke. 2005;36:2021–2023. doi: 10.1161/01.STR.0000177878.33559.fe. [DOI] [PubMed] [Google Scholar]
- Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-α acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. 2008;104:694–699. doi: 10.1152/japplphysiol.00898.2007. [DOI] [PubMed] [Google Scholar]
- He X, Proske U, Shawible H-G, Schmidt RF. Acute inflammation of the knee joint in the cat alters responses of flexor motorneurons to leg movements. J Neurophysiol. 1988;59:326–340. doi: 10.1152/jn.1988.59.2.326. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motorneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Westerkamp L, Beam S, Moody J, Garry J, Holbert D, Devita P. Altered hamstring–quadriceps muscle balance in patients with knee osteoarthritis. Clin Biomech. 2005;20:97–104. doi: 10.1016/j.clinbiomech.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Ørtenblad N, Aagaard P, Kjaer M, Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol. 2011;589:4745–4757. doi: 10.1113/jphysiol.2011.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman RW, Cornwell EW, Wu C-L, Kandarian SC. Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol. 2013;98:19–24. doi: 10.1113/expphysiol.2011.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette AM, Branch LG. The Framingham Disability Study: II. Physical disability among the aging. Am J Public Health. 1981;71:1211–1216. doi: 10.2105/ajph.71.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Saeki Y, Zhao Y. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res. 1993;73:35–50. doi: 10.1161/01.res.73.1.35. [DOI] [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Bobkova E, Hegyi G, Muhlrad A, Reisler E. Actin cross-linking and inhibition of the actomyosin motor. Biochemistry. 2001;41:86–93. doi: 10.1021/bi0113824. [DOI] [PubMed] [Google Scholar]
- Kortebein P, Ferranddo A, Lombeida J, Wolfe RR, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Edstrom L, Eriksson LI, Zackrisson H, Argentini C, Schiafino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28:34–45. doi: 10.1097/00003246-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, McKenna MJ, Cameron-Smith D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum. 2011;63:1343–1348. doi: 10.1002/art.30287. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fibre types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Llewellyn ME, Barretto RPJ, Delp SL, Schnitzer MJ. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature. 2008;454:784–788. doi: 10.1038/nature07104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol. 2013;115:1004–1014. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, Palmer BM, Toth MJ. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibers from humans. J Physiol. 2010;588:4039–4053. doi: 10.1113/jphysiol.2010.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, VanBuren P, LeWinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2:700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD. S-Glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol. 2012;590:1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length–tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Boer MD. Disuse of the musculo-skeletal system in space and on earth. Eur J Appl Physiol. 2011;111:403–420. doi: 10.1007/s00421-010-1556-x. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Kruger M, Meyer LH, Ahnlund L, Gransberg L, Linke WA, Larsson L. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol. 2006;577:339–352. doi: 10.1113/jphysiol.2006.116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BM, Schmitt JP, Seidman CE, Seidman JG, Wang Y, Bell SP, LeWinter MM, Maughan DW. Elevated rates of force development and MgATP binding in F764L and S532P myosin mutations causing dilated cardiomyopathy. J Mol Cell Cardiol. 2013;57:23–31. doi: 10.1016/j.yjmcc.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: role of reactive oxygen species. Free Radic Res. 2000;33:287–293. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- Perkins WJ, Han YS, Sieck GC. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol. 1997;83:1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985;8:676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Petterson SC, Raisis L, Bodenstab A, Snyder-Mackler L. Disease-specific gender differences among total knee arthroplasty candidates. J Bone Joint Surg Am. 2007;89:2327–2333. doi: 10.2106/JBJS.F.01144. [DOI] [PubMed] [Google Scholar]
- Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Donohue M, Buck M. Decreased Jun-D and myogenin expression in muscle wasting of human cachexia. Am J Physiol Endocrinol Metab. 2009;297:E392–E401. doi: 10.1152/ajpendo.90529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW. The force–velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassier DE, Herzog W, Pollack GH. Dynamics of individual sarcomeres during and after stretch in activated single myofibrils. Proc Biol Sci. 2003;270:1735–1740. doi: 10.1098/rspb.2003.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K, Pasha E, Doros G, Clark D, Patten C, Phillips E, Frontera W, Fielding R. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fibre contractile properties. Eur J Appl Physiol. 2014;114:29–39. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Kasper CE, Moss RL. Myosin subunits and contractile properties of single fibers from hypokinetic rat muscles. J Appl Physiol. 1987;63:2293–2300. doi: 10.1152/jappl.1987.63.6.2293. [DOI] [PubMed] [Google Scholar]
- Riley DA, Bain JLW, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Disproportionate loss of thin filaments in human soleus muscle after 17-day bed rest. Muscle Nerve. 1998;21:1280–1289. doi: 10.1002/(sici)1097-4598(199810)21:10<1280::aid-mus6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Savage PD, Toth MJ, Ades PA. A re-examination of the metabolic equivalent concept in individuals with coronary heart disease. J Cardiopulm Rehabil Prev. 2007;27:149–150. doi: 10.1097/01.HCR.0000270693.16882.d9. [DOI] [PubMed] [Google Scholar]
- Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinsky FD. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Kushmerick MJ, Mabuchi K, Gergely J, Sreter FA. Velocity of shortening and myosin isozymes in two types of rabbit fast-twitch muscle fibers. Am J Physiol Cell Physiol. 1986;251:C431–C434. doi: 10.1152/ajpcell.1986.251.3.C431. [DOI] [PubMed] [Google Scholar]
- Tanner BCW, Wang Y, Maughan DW, Palmer BM. Measuring myosin cross-bridge attachment time in activated muscle fibers using stochastic vs. sinusoidal length perturbation analysis. J Appl Physiol. 2011;110:1101–1108. doi: 10.1152/japplphysiol.00800.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Piliae RE, Norton LC, Haskell WL, Mahbouda MH, Fair JM, Iribarren C, Hlatky MA, Go AS, Fortmann SP. Validation of a new brief physical activity survey among men and women aged 60–69 years. Am J Epidemiol. 2006;164:598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- Thomas SG, Pagura SM, Kennedy D. Physical activity and its relationship to physical performance in patients with end stage knee osteoarthritis. J Orthop Sports Phys Ther. 2003;33:745–754. doi: 10.2519/jospt.2003.33.12.745. [DOI] [PubMed] [Google Scholar]
- Toth MJ, Gottlieb SS, Goran MI, Fisher ML, Poehlman ET. Daily energy expenditure in free-living heart failure patients. Am J Physiol Endocrinol Metab. 1997;272:E469–E475. doi: 10.1152/ajpendo.1997.272.3.E469. [DOI] [PubMed] [Google Scholar]
- Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, Der-Torossian H, Couch ME, Dittus K. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin–actin cross-bridge formation and kinetics. J Appl Physiol. 2013;114:858–868. doi: 10.1152/japplphysiol.01474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol. 2012;590:1243–1259. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan D, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol. 2010;143:276–282. doi: 10.1016/j.ijcard.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe SW, Trappe TA, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol. 2004;557:501–513. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Warshaw DM. The myosin power stroke. Cell Motil Cytoskeleton. 2002;51:1–15. doi: 10.1002/cm.10014. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Ravara B, Dalla Libera L. Skeletal muscle myofibrillar protein oxidation and exercise capacity in heart failure. Basic Res Cardiol. 2008;103:285–290. doi: 10.1007/s00395-007-0692-x. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Romatowski JG, Bain JLW, Trappe SW, Trappe TA, Thompson JL, Costill DL, Riley DA, Fitts RH. Effect of 17 days of bed rest on peak isometric force and unloaded shortening velocity of human soleus fibers. Am J Physiol Cell Physiol. 1997;273:C1690–C1699. doi: 10.1152/ajpcell.1997.273.5.c1690. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Trappe SW, Romatowski JG, Riley DA, Costill DL, Fitts RH. Unilateral lower limb suspension does not mimic bed rest or spaceflight effects on human muscle fiber function. J Appl Physiol. 2002;93:354–360. doi: 10.1152/japplphysiol.01245.2001. [DOI] [PubMed] [Google Scholar]
- Williams DL, Swenson CA. Disulfide bridges in tropomyosin: effect on ATPase activity of actomyosin. Eur J Biochem. 1982;127:495–499. [PubMed] [Google Scholar]
- Yamashita-Goto K, Okuyama R, Honda M, Kawasaki K, Fujita K, Yamada T, Nonaka I, Ohira Y, Yoshioka T. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol. 2001;91:417–424. doi: 10.1152/jappl.2001.91.1.417. [DOI] [PubMed] [Google Scholar]
- Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol. 2005;99:1085–1092. doi: 10.1152/japplphysiol.00247.2005. [DOI] [PubMed] [Google Scholar]
- Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiologica. 2007;190:229–241. doi: 10.1111/j.1748-1716.2007.01699.x. [DOI] [PubMed] [Google Scholar]


