Abstract
Stress impairs gastric emptying, reduces stomach compliance and induces early satiety via vagal actions. We have shown recently that the ability of the anti-stress neuropeptide oxytocin (OXT) to modulate vagal brainstem circuits undergoes short-term plasticity via alterations in cAMP levels subsequent to vagal afferent fibre-dependent activation of metabotropic glutamate receptors. The aim of the present study was to test the hypothesis that the OXT-induced gastric response undergoes plastic changes in the presence of the prototypical stress hormone, corticotropin releasing factor (CRF). Whole cell patch clamp recordings showed that CRF increased inhibitory GABAergic synaptic transmission to identified corpus-projecting dorsal motor nucleus of the vagus (DMV) neurones. In naive brainstem slices, OXT perfusion had no effect on inhibitory synaptic transmission; following exposure to CRF (and recovery from its actions), however, re-application of OXT inhibited GABAergic transmission in the majority of neurones tested. This uncovering of the OXT response was antagonized by pretreatment with protein kinase A or adenylate cyclase inhibitors, H89 and di-deoxyadenosine, respectively, indicating a cAMP-mediated mechanism. In naive animals, OXT microinjection in the dorsal vagal complex induced a NO-mediated corpus relaxation. Following CRF pretreatment, however, microinjection of OXT attenuated or, at times reversed, the gastric relaxation which was insensitive to l-NAME but was antagonized by pretreatment with a VIP antagonist. Immunohistochemical analyses of vagal motoneurones showed an increased number of oxytocin receptors present on GABAergic terminals of CRF-treated or stressed vs. naive rats. These results indicate that CRF alters vagal inhibitory circuits that uncover the ability of OXT to modulate GABAergic currents and modifies the gastric corpus motility response to OXT.
Key points
The prototypical stress hormone, corticotropin releasing factor (CRF), and the prototypical anti-stressor hormone, oxytocin (OXT), are known to modulate brainstem neurocircuits involved in visceral reflexes.
We demonstrated recently that the brainstem neurocircuits through which OXT exerts its actions are modulated by vagal afferent fibres; however, it is unknown whether the OXT-induced modulation of brainstem vagal neurocircuits is also regulated differentially by CRF.
Here we elucidate the cellular mechanisms and the effects on gastric tone of OXT following exposure of vagal brainstem neurones to CRF and report that CRF induces short-term plastic changes in OXT-sensitive vagal neurocircuits.
The results presented may represent a possible mechanism through which stress alters the central regulation of gastrointestinal functions and may reflect the vagal dysregulation occurring as a consequence of stress-exacerbated functional dyspepsia.
Introduction
Neuroactive peptides play an important role in the regulation of gastrointestinal (GI) functions and many studies have reported the central role of both the prototypical stress hormone, corticotropin releasing factor (CRF), as well as the prototypical anti-stress hormone, oxytocin (OXT), in the modulation of GI tone and motility (Richar et al. 1991; Fujimiya & Inui, 2001; Lewis et al. 2002; Stengel & Tache, 2008; Babygirija et al. 2010b, 2012; Buckinx et al. 2011; Llewellyn-Smith et al. 2012; Holmes et al. 2013). Specifically, many laboratories have shown that, in experimental animals, injection of CRF in the cisterna magna, lateral ventricles, dorsal vagal complex (DVC; i.e. nucleus tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMV) and area postrema) or paraventricular nucleus of the hypothalamus (PVN) diminishes gastric acid secretion, slows gastric emptying and small intestine transit but accelerates large bowel transit (Tache et al. 1983, 1987; Garrick et al. 1988; Heymann-Monnikes et al. 1991; Monnikes et al. 1992; Martinez et al. 1997; Lewis et al. 2002; Tsukamoto et al. 2006). This wide range of GI responses to CRF resembles those induced by stress; indeed, both CRF-induced and stress-related GI effects are blocked by pretreatment with CRF receptor antagonists (Stengel & Tache, 2008). Interestingly, CRF does not seem to play a role in controlling basal GI activity since CRF receptor antagonists do not modulate GI functions in non-stressed animals, suggesting that the effects of CRF are restricted to a central modulation of GI functions in time of stress only (Lenz et al. 1988; Martinez et al. 1997).
Conversely, intracerebroventricular administration of OXT antagonists increases gastric motility, suggesting that oxytocinergic pathways are active tonically and regulate upper GI motility (Flanagan et al. 1992; Fujimiya & Inui, 2001). OXT is released from PVN neurones following ingestion of a meal and, upon activating oxytocin receptors (OXT-Rs) in the DVC, stimulates gastric secretion and decreases gastric motility via a NO-dependent, l-NAME-sensitive vagal pathway; OXT activation in the caudal brainstem also modulates cardiorespiratory and feeding functions (Swanson & Kuypers, 1980; Richar et al. 1991; Rinaman, 1998; Peters et al. 2008; Veening et al. 2010; Llewellyn-Smith et al. 2012; Onaka et al. 2012; Holmes et al. 2013). Interestingly, hypothalamic OXT plays a major role in the recovery of gastric and colonic motility following stress adaptation (Babygirija et al. 2010b, 2012; Zheng et al. 2010), indicating an anti-stress action of this neuropeptide in the regulation of GI functions.
We have shown recently that DVC application of OXT induces a vagally dependent relaxation of the gastric corpus that is blocked by pretreatment with the nitric oxide synthase antagonist, l-NAME (Holmes et al. 2013). Following surgical or pharmacological blockade of vagal afferent fibres impinging onto DVC neurones, however, the response of the gastric corpus to OXT application was attenuated or, in many cases, reversed, i.e. OXT microinjected in the DVC now increased corpus tone (Holmes et al. 2013). We also demonstrated that these effects were most likely due to trafficking of OXT-Rs resulting in modulation of the GABAergic synapse; we further demonstrated that a key feature of this process was the modulation of cAMP levels by group II metabotropic glutamate receptors (mGluRs) (Holmes et al. 2013). This GABAergic synapse, by regulating the firing rate of DMV neurones (Browning & Travagli, 2001; Babic et al. 2011), plays an essential role in the vagally dependent modulation of gastric motility (Sivarao et al. 1998). We then hypothesized that these vagal afferent inputs may be part of a conditional control mechanism that regulates the sensory-motor integration of visceral functions in a metabolically inexpensive manner by allowing relatively rapid adaptations of the gastric motility response to environmental and/or pathophysiological conditions (Holmes et al. 2013). Indeed, it is well recognized that afferent vagal fibres are activated following food ingestion and, through adenylate-cyclase-coupled neurotransmitters and/or neuromodulators, prime the vagal neurocircuitry to respond appropriately to digestive processes (Mayo et al. 2003; Berthoud et al. 2006; Drucker, 2006; Dufresne et al. 2006; Tache & Million, 2006).
The aim of the present study was to investigate whether (i) CRF pretreatment alters selectively the effects of OXT on the NTS–DMV GABAergic synapse, (ii) pretreatment with CRF alters the OXT-mediated decrease in gastric corpus tone, and (iii) the mechanism of action of OXT was altered following CRF pretreatment.
Methods
All in vivo and in vitro procedures were conducted in accordance with the National Institutes for Health guidelines, with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee and according to the policies and regulations of journal policy on animal experimentation.
Retrograde tracing
Gastric-projecting DMV neurones were identified following application of the fluorescent neuronal tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) to discrete regions of the stomach as described previously (Browning et al. 1999; Holmes et al. 2009). Fourteen-day-old Sprague–Dawley rat pups of either sex were anaesthetised deeply (2.5% isoflurane, 600 ml min−1), an abdominal laparotomy was performed and crystals of DiI were applied to the gastric major curvature at the level of the corpus. The dye was embedded in place with a fast-hardening epoxy resin, the surgical area was flushed with warmed saline and the wound closed in layers with 5/0 vicryl suture and staples. Rats were allowed to recover for 10–15 days prior to removal of the brainstem for electrophysiological recordings.
Electrophysiology
As described previously (Browning et al. 1999; Holmes et al. 2009), rats were anaesthetised with isoflurane followed by bilateral pneumothorax before removal of the brainstem which was placed immediately in chilled, oxygenated Krebs solution (see below for composition). Four to six coronal slices (300 μm thick) were cut spanning the entire rostro-caudal extent of the DVC and brainstem slices were incubated in oxygenated Krebs solution at 30 ± 1°C for at least 90 min prior to use.
A single brainstem slice was placed on a custom-made perfusion chamber (volume 0.5 ml) held in place with a nylon mesh, on the stage of a Nikon E600FN microscope equipped with tetramethylrhodamine isothiocyanate (TRITC) epifluorescent filters. Slices were maintained at 32 ± 1°C by continuous perfusion with Krebs solution. DiI-filled neurones were identified under TRITC epifluorescence and electrophysiological recordings made under brightfield illumination using differential interference contrast (Nomarski) optics.
Whole cell patch clamp recordings were made using pipettes (2–5 MΩ tip resistance) filled with a potassium gluconate solution (see below for composition) and a single electrode voltage clamp amplifier (Axoclamp 1D, Molecular Devices, Union City, CA, USA). Data were filtered at 2 kHz, digitized via a Digidata 1320 interface and analysed utilizing pCLAMP 9 software (Molecular Devices). Recordings with a series resistance >20 MΩ were eliminated from the study.
Electrical stimulation
A bipolar stimulating electrode with a tip separation of ∼125 μm (WPI, Sarasota, FL, USA) was placed in the NTS subnucleus centralis or medialis and used to electrically evoke either inhibitory or excitatory postsynaptic currents (eIPSCs or eEPSCs, respectively). Electrical stimuli (10–500 μA, 0.05–1.0 ms) were applied every 20 s throughout the recording to evoke submaximal currents. When recording eIPSCs, the perfusing Krebs solution contained the non-selective ionotropic glutamate antagonist kynurenic acid (1 mm) while the GABAA receptor antagonist picrotoxin (50 μm) was added to the perfusate when recording eEPSCs.
Miniature synaptic currents
Miniature IPSCs and EPSCs (mIPSCs and mEPSCs, respectively) were recorded from DMV neurones perfused with 1 μm tetrodotoxin and 1 mm kynurenic acid or 50 μm picrotoxin, respectively, and, when recording mIPSCs, the recording pipettes were filled with a potassium chloride solution (see below for composition). Recordings were conducted in the voltage clamp configuration at −60 mV (mEPSC) or −50 mV (mIPSC). Miniature events were analysed with Mini Analysis Program Software (Synaptosoft, Leonia, NJ, USA).
Drug application and statistical analysis
All drugs were dissolved in the perfusing Krebs solution at concentrations described previously as being effective; OXT and CRF were used at 100 nm unless specified otherwise (Browning & Travagli, 2001; Lewis et al. 2002; Holmes et al. 2013). Agonists were applied for a period of time sufficient for the response to reach a plateau; neurones were allowed to recover for at least 5 min prior to drug re-application. Antagonists were applied for at least 5 min prior to agonist re-application. Each neurone served as its own control, that is, the responses of any neurone was assessed before and after drug application using ANOVA and paired Student's t test; inter-group comparisons were analysed using the χ2 test. A minimum variation of ±10% in eIPSC or eEPSC amplitude and a minimum variation of ±40% in mIPSC or mEPSC frequency or amplitude was arbitrarily taken as indication of a response. Only responding neurones are included in the statistical analyses; results are expressed as mean ± SEM with significance defined as P < 0.05.
In vivo studies: surgical preparations and agonist applications
Male Sprague–Dawley rats weighing 200–350 g were fasted overnight (16 h maximum; water ad libitum) and anaesthetized with thiobutabarbital (Inactin; 120–150 mg kg−1 i.p.). Once a deep plane of anaesthesia was achieved (absence of the palpebral reflex), rats were intubated with a tracheal catheter and the anterior gastric corpus was exposed via a midline laparotomy. A miniature strain gauge (RB Products, Minneapolis, MN, USA) was sutured to the serosal surface of the anterior gastric corpus in alignment with the circular smooth muscle. Animals were then placed in a stereotaxic frame; rectal temperature was monitored and maintained at 37 ± 1°C with a heating pad. The strain gauge signal was amplified (QuantaMetrics EXP CLSG-2, Newton, PA, USA), filtered (low pass filter cutoff = 0.5 Hz), recorded on a polygraph (Grass model 79, Quincy, MA, USA) and on a computer using Axotape 10 software (Molecular Devices).
The 4th ventricle was exposed via blunt dissection, the meningeal membranes above the vagal trigone were dissected and the exposed brainstem was covered with pre-warmed saline during a 60 min period of stabilization. A dual-barrelled micropipette (20–40 μm tip diameter) was lowered into the left DVC (in mm: +0.1–0.3 rostro-caudal from calamus scriptorius, +0.1–0.3 medio-lateral and −0.5 dorso-ventral). After an additional 30 min of stabilization, baseline values of gastric tone were determined by calculating the mean value of the 5 min period immediately preceding drug application. Drugs were microinjected in 60 nl volumes by picospritzer (Toohey Co., Fairfield, NJ, USA) or applied to the surface of the 4th ventricle (2 μl). All drugs were dissolved in isotonic phosphate-buffered saline (PBS; see composition below). The drug-induced effects on the gastric corpus were measured as the average tone displacement over a baseline of the 30 s period centred around the peak effect. The basal strain gauge output was monitored for any changes for a minimum of 10 min following drug infusion. At the conclusion of the experiment, rats were killed with a bilateral pneumothorax and perfused transcardially with ∼200 ml of saline followed by ∼200 ml of 4% paraformaldehyde (PFA) in PBS. The brainstem was removed and postfixed in 4% PFA + 20% sucrose for 24–48 h, after which the brainstem was frozen, sliced in 40 μm-thick coronal sections throughout the rostro-caudal extent of the DVC and counterstained with cresyl-violet. Injection sites were identified on a Nikon E400 microscope.
In vivo data and statistical analysis
The gain of individual strain gauges was adjusted to produce a 0.1 V/100 mg signal with a 1.0 g load applied externally. The drug-induced effects on corpus tone were calculated through the interpolation value of the calibration measures. Basal corpus tone was not preset to a fixed value, although a small degree of tension (∼500 mg) was applied to the corpus smooth muscle when suturing the strain gauge; the data reported are thus absolute values of corpus tone displacement. Data were evaluated by comparing the change in response between pre- and post-treatment values within each group by ANOVA or paired t test (SPSS Inc, Chicago, IL, USA) and are reported as mean ± SEM. In all instances, significance was set at P < 0.05.
Immunohistochemistry
Rats were divided into three groups, one group was used as control (n = 5 rats) while one of the other groups (n = 5) was restraint-stressed for 120 min after which rats were anaesthetised, perfused transcardially with saline followed by a 4% PFA solution in PBS and the brainstem was extracted, placed in PFA fixative and stored at 4°C overnight. Brainstems were then transferred into PBS containing 20% sucrose, stored overnight at 4°C and sliced at 50 μm thickness.
The third set of rats (n = 4) was anaesthetized, placed in the stereotaxic frame and the fourth ventricle was exposed as described above prior to 2 μl of PBS containing CRF (50 pmol) being applied to the floor of the fourth ventricle. Twenty minutes later the rats were perfused transcardially with saline followed by a 4% PFA solution in PBS and the brainstem was extracted, placed in PFA fixative and stored at 4°C overnight. Brainstems were then transferred into PBS containing 20% sucrose, stored overnight at 4°C and sliced at 50 μm thickness.
Brainstem slices from all sets of experiments were processed for double-labelling immunofluorescence using rabbit antisera directed against the oxytocin receptor (OXT-R; 1:500 dilution; Santa Cruz Biotech, Dallas, TX, USA) and mouse monoclonal antibodies against glutamic acid decarboxylase (GAD-67; 1:500 dilution; Millipore, Billerica, MA, USA).
The diluent used for all antibodies was 0.1 m PBS (pH 7.4) containing 10% normal horse serum, 0.3% Triton X-100 and 0.05% Merthiolate. Sections were incubated with primary antibodies for 3 days at room temperature (RT), washed in PBS–0.3% Triton X-100 (15 min × 3) at RT and incubated for 4 h at RT with secondary antibodies (goat anti-rabbit conjugated with Alexa 568 1:500, OXT-R staining; and goat anti-mouse conjugated with Alexa 488 1:500, GAD-67 staining). Slices were then rinsed with PBS and mounted with Fluoromount G (Southern Biotechnology, Birmingham, AL, USA).
Confocal microscopic images were collected by using an Olympus Fluoview confocal scanning laser microscope equipped with a Kr/Ar ion laser equipped with filters for the selective visualization of Alexa 488 and Alexa 568.
Quantitative analysis was conducted on a minimum of one and up to six tissue sections (150–200 μm apart) for each of the three levels of the DMV (i.e. caudal to area postrema, at the level of the area postrema and rostral to area postrema) to identify labelled terminals apposing DMV neurons. Profiles were defined as double-labelled for OXT-Rs and GAD-67 if both labels appeared in profiles similar in size and geometry that overlap upon the merging of the images resulting in a yellow colour. Profiles double-labelled for OXT-Rs and GAD-67 were counted manually on single optical layer sections by an investigator unaware of the treatment and expressed as OXT-R/GAD-67 co-localized profiles in a 200 μm × 200 μm area (i.e. 0.04 mm2). Digital images were contrast-enhanced using Olympus Fluoview FV1000 software. Data were evaluated by comparing the values within each group by ANOVA or paired t test (SPSS Inc., Chicago, IL, USA) and are reported as mean ± SEM. In all instances, significance was set at P < 0.05.
Drugs and solutions
Krebs solution (mm): 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4 and 11 dextrose, maintained at pH 7.4 by bubbling with 95%–5% O2–CO2. Potassium gluconate intracellular solution (mm): 128 potassium gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 Hepes, 1 EGTA, 2 Na2ATP, 0.25 NaGTP, adjusted to pH 7.36 with KOH. Potassium chloride intracellular solution (mm): 140 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 10 EGTA, 2 Na2ATP, 0.25 NaGTP, adjusted to pH 7.36 with HCl. PBS (mm): 115 NaCl, 75 Na2HPO4, 7.5 KH2PO4.
DiI, Alexa 488 and Alexa 568 were purchased from Invitrogen Corp. (Eugene, OR, USA); tetrodotoxin was purchased from Alomone Labs (Jerusalem, Israel); all other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Results
Oxytocin did not modulate inhibitory synaptic transmission to gastric-projecting DMV neurons
As described previously (Holmes et al. 2013), perfusion of brainstem slices with OXT (100 nm) had no effect on the amplitude of eIPSCs in any of the 22 neurones tested (169 ± 15.8 pA in control vs. 170 ± 15.8 pA in the presence of OXT, i.e. 102 ± 2% of control amplitude, P > 0.05). Similarly, perfusion of brainstem slices with OXT had no effect on mIPSC frequency (0.77 ± 0.18 events s−1 in control vs. 0.75 ± 0.15 events s−1 in the presence of OXT; P > 0.05) or amplitude (68 ± 9.6 pA in control vs. 68 ± 8.4 pA in the presence of OXT; P > 0.05; n = 6; Fig. 1).
Figure 1. Corticotropin-releasing factor uncovers the oxytocin-mediated decrease of the evoked IPSC amplitude via a cAMP/PKA pathway.
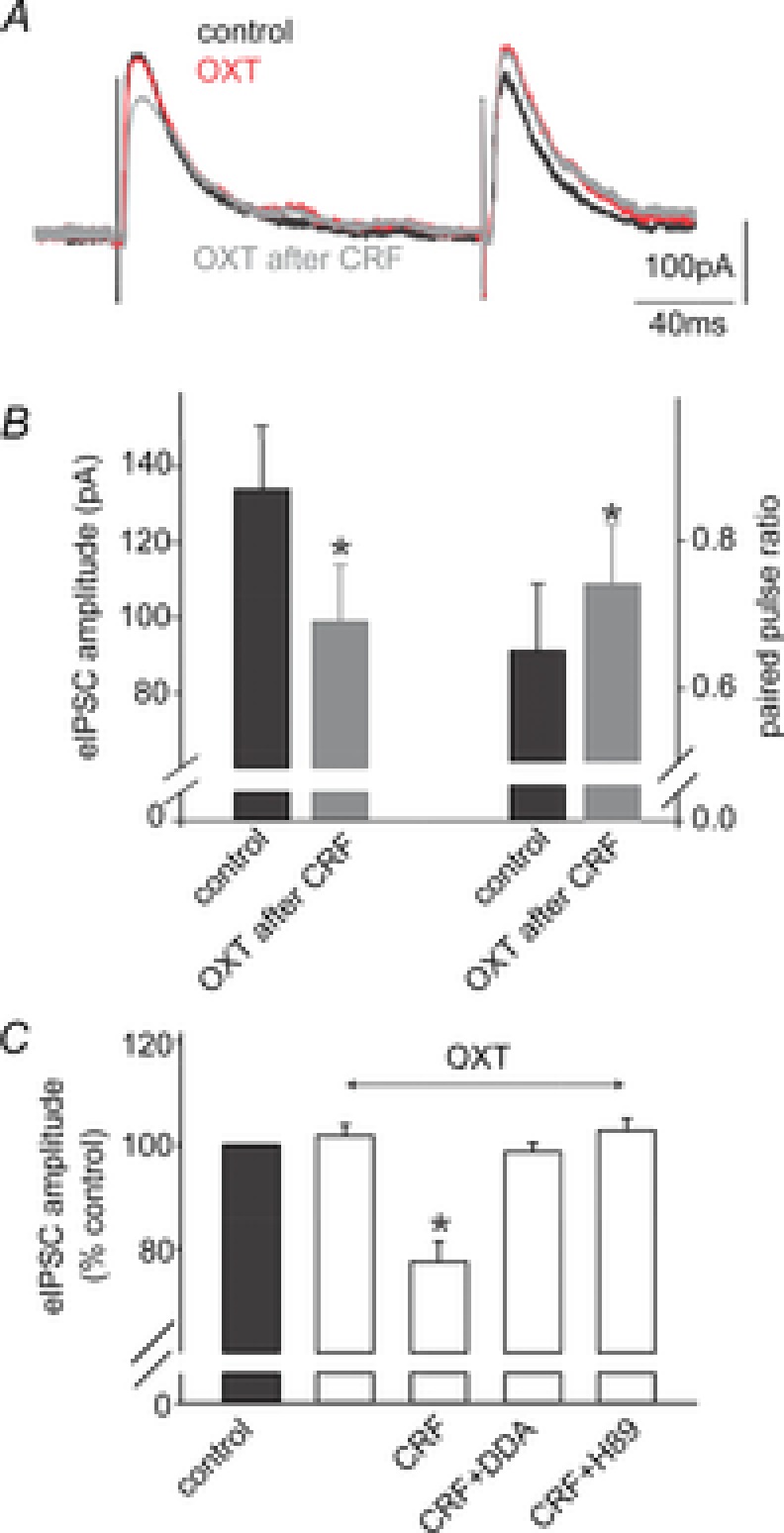
A, representative traces of evoked IPSCs (eIPSCs) obtained upon electrical stimulation of the adjacent NTS in a gastric-projecting DMV neurone voltage clamped at −50 mV. Perfusion with OXT (100 nm) had no effect upon eIPSCs; however, following perfusion with CRF (100 nm), a second perfusion with OXT reduced the eIPSC amplitude. B, summary graph of the effects of OXT after CRF perfusion on the amplitude of the eIPSC (left) and on the paired pulse ratio (ratio of the amplitude of paired eIPSCs evoked in rapid succession; right). Note that OXT perfusion alters both the eIPSC amplitude as well as the paired pulse ratio. C, summary graph of the normalized effects of OXT on eIPSC amplitude either alone, or after exposure to CRF, CRF + DDA or CRF + H89. Note that the ability of CRF to uncover the effects of OXT were prevented by pretreatment with the adenylate cyclase inhibitor DDA, or the PKA inhibitor H89. *P < 0.05.
Activation of CRF receptors uncovers presynaptic actions of oxytocin to inhibit GABAergic synaptic transmission
In eight neurones in which perfusion with OXT failed to modulate inhibitory synaptic transmission (131 ± 18.2 in control vs. 130 ± 13.4 pA in the presence of OXT; P > 0.05), the ability of CRF to modulate eIPSCs was assessed. After wash-out and recovery from the initial OXT application, gastric-projecting DMV neurones were perfused with CRF, which itself increased eIPSC amplitude (135 ± 16 pA in control vs. 162 ± 16.0 pA in the presence of CRF, i.e. 123 ± 11% of control; P < 0.05). Following wash-out and recovery, neurones were re-exposed to OXT; in six of these neurones, OXT now decreased eIPSC amplitude by 23 ± 3.9% (P < 0.05). OXT had no effect on eIPSC amplitude in the remaining two neurones (Fig. 1).
Alterations in the paired pulse ratio, i.e. the ratio of the amplitude of two evoked postsynaptic currents evoked a few milliseconds apart, is often used to indicate whether a drug is acting at pre- or postsynaptic sites (Grudt et al. 1995). In neurones in which CRF uncovered inhibitory actions of OXT, the paired pulse ratio increased from 0.65 ± 0.09 to 0.74 ± 0.08 (P < 0.05), suggesting a presynaptic site of action.
Confirmation of a presynaptic site of action was obtained from four neurones in which the actions of CRF to uncover inhibitory actions of OXT on mIPSCs were assessed. Initially, OXT had no effect upon either mIPSC frequency (82 ± 14.0% of control) or amplitude (103 ± 7.97% of control). Following perfusion with CRF, which itself did not induce a significant increase in mIPSC frequency (from 0.55 ± 0.15 events s−1 to 0.65 ± 0.15 events s−1; P > 0.05) or amplitude (64 ± 14.8 pA in control vs. 55 ± 4.7 pA in the presence of CRF; P > 0.05), re-application of OXT decreased mIPSC frequency in all four neurones tested (0.53 ± 0.09 events s−1 following CRF wash-out vs. 0.33 ± 0.06 events s−1 in the presence of OXT; P < 0.05). The amplitude of mIPSCs was unaffected by the re-application of OXT (51 ± 6.0 pA following CRF wash-out vs. 49 ± 5.5 pA in the presence of OXT; P > 0.05; Fig. 2).
Figure 2. Oxytocin-mediated inhibition of miniature inhibitory currents is uncovered by pretreatment with CRF.
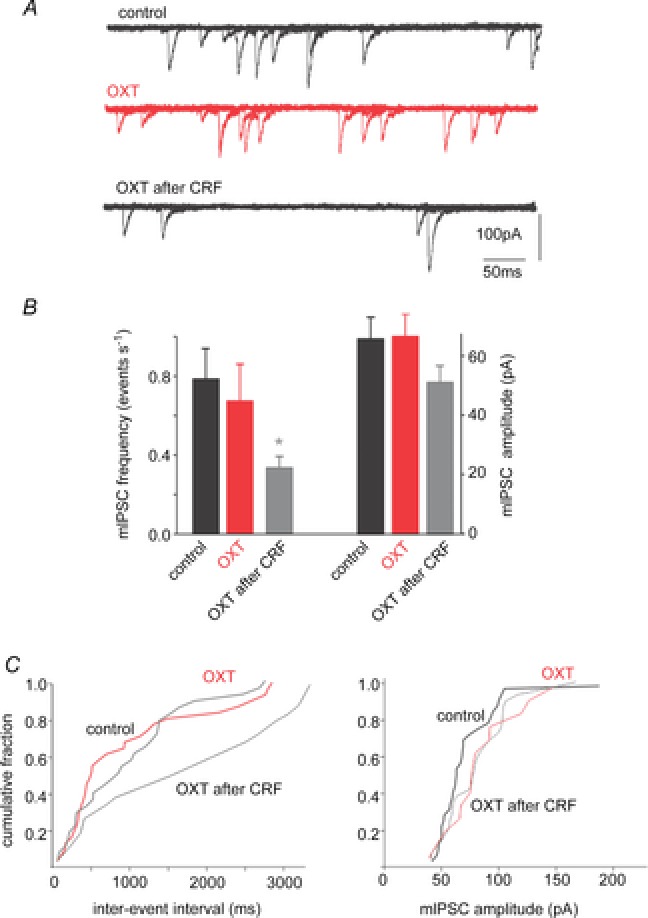
A, in a gastric-projecting DMV neurone voltage clamped at −50 mV, miniature IPSCs (mIPSCs) were unaffected by perfusion with OXT (100 nm) unless the slice was pretreated with CRF (100 nm). B, summary graph of the effects of OXT, alone and after CRF pretreatment, on the frequency and amplitude of mIPSCs. Note that OXT perfusion reduced the frequency, but did not alter the amplitude significantly, of mIPSCs. *P < 0.05. C, cumulative histograms of the effects of perfusion of OXT, alone and after CRF pretreatment, on the frequency (left) and amplitude (right) of mIPSCs from the neurone illustrated in A.
These results suggest that exposure to CRF uncovers presynaptic actions of OXT to inhibit GABAergic synaptic transmission.
The actions of CRF to uncover presynaptic inhibitory actions of oxytocin involve activation of the adenylate cyclase–protein kinase A pathway
The role of adenylate cyclase activation in the actions of CRF to uncover presynaptic inhibitory actions of OXT was investigated using the adenylate cyclase inhibitor, di-deoxyadenosine (DDA; 10 μm). In six neurones in which OXT failed to inhibit eIPSC amplitude (136 ± 17 pA in control vs. 135 ± 17 pA in OXT, P > 0.05), brainstem slices were perfused with DDA, which itself had no effect on eIPSC amplitude (152 ± 23.3 pA in control vs. 141 ± 22.2 pA in the presence of DDA, P > 0.05). In the continued presence of DDA, brainstem slices were then perfused with CRF; in these neurones, CRF no longer increased eIPSC amplitude (141 ± 22 pA in DDA vs. 147 ± 19 pA in the presence of DDA and CRF, P > 0.05). OXT was re-applied to the same brainstem slices following wash-out of CRF. In 5 of these 6 neurones, OXT had no effect on eIPSC amplitude (153 ± 20.2 pA in DDA vs. 152 ± 21.5 pA in the presence of DDA and OXT, P > 0.05; P < 0.05 vs. effects of OXT in the absence of DDA).
To further investigate the involvement of the adenylate cyclase–protein kinase A (PKA) pathway in the actions of CRF to uncover OXT modulation of inhibitory transmission, the ability of the cAMP-dependent PKA inhibitor, H89 (1 μm) was assessed. In four neurons in which OXT had no effect on eIPSC amplitude (254 ± 33.1 pA in control vs. 266 ± 25.2 pA in the presence of OXT, P > 0.05), brainstem slices were then perfused with H89, which itself had no effect upon eIPSC amplitude (225 ± 25.4 pA in control vs. 257 ± 23.9 pA in the presence of H89; P > 0.05). In the continued presence of H89, CRF no longer increased eIPSC amplitude (257 ± 23.9 pA in DDA vs. 259 ± 35.6 pA in the presence of DDA plus CRF, P > 0.05). After wash-out of CRF, re-application of OXT, in the continued presence of H89, had no effect on eIPSC amplitude in any of the four neurones tested (221 ± 45.1 pA in H89 vs. 226 ± 45.1 pA in H89 plus OXT; P > 0.05; P > 0.05 vs. effects of OXT in the absence of H89; Fig. 1).
These results suggest that, as indicated previously (Holmes et al. 2013), low levels of cAMP within GABAergic brainstem terminals prevent OXT from modulating inhibitory synaptic transmission in brainstem neurocircuits. Exposure to CRF activates the adenylate cyclase–PKA pathway, uncovering the ability of OXT to inhibit GABAergic synaptic transmission to gastric-projecting DMV neurones.
CRF does not modulate the oxytocin inhibition of excitatory synaptic transmission to gastric-projecting DMV neurones
As reported previously (Holmes et al. 2013), OXT perfusion decreases excitatory synaptic transmission to a sub-population of gastric-projecting DMV neurones. In the present study, the ability of CRF to modulate the ability of OXT to decrease glutamatergic transmission was assessed in seven neurones. In three of these neurones, OXT decreased eEPSC amplitude by 28 ± 1.9% (168 ± 15.9 pA in control vs. 104 ± 9.8% in the presence of OXT; P < 0.05). Upon OXT wash-out, these three neurones were unresponsive to CRF, i.e. eEPSC amplitude was 158 ± 16.4 pA in control vs. 167 ± 12.7 pA in the presence of CRF; P > 0.05. Re-application of OXT decreased eEPSC amplitude by 31 ± 1.5% (168 ± 14.9 pA in control vs. 117 ± 12.3 pA in the presence of OXT; P < 0.05; P > 0.05 vs. effects of OXT in the absence of CRF).
In the remaining four neurones, OXT had no effect on eEPSC amplitude (185 ± 62.7 pA in control vs. 185 ± 65.4 pA in the presence of OXT; P > 0.05). Following perfusion with CRF, which had no effect on eEPSC amplitude (186 ± 64.8 pA in the presence of CRF; P > 0.05), upon CRF wash-out re-application of OXT failed to inhibit eEPSC amplitude (147 ± 39.1 pA in control vs. 151 ± 35.5 pA in the presence of OXT; P > 0.05; data not shown).
These results suggest that CRF neither uncovered nor augmented the presynaptic actions of OXT to decrease glutamatergic synaptic transmission to gastric-projecting DMV neurones.
CRF pretreatment alters the corpus tone response to oxytocin microinjection
We then conducted a series of in vivo experiments to investigate whether CRF pretreatment altered the OXT-mediated decrease of tone in the gastric corpus.
In vivo recordings of corpus tone were conducted in anaesthetised rats in which microinjections in the DVC of PBS (60 nl) did not induce any significant variation in corpus tone. Microinjection of 150 pmol of OXT in the DVC induced a −154 ± 13.9 mg decrease in corpus tone, which returned to baseline values after approximately 15 min. In the same group of rats, 4th ventricle application of CRF (50 pmol) induced a −70 ± 18.2 mg decrease in corpus tone which returned to baseline values within 10 min (n = 24 rats).
Pretreatment with CRF was also found to modulate the response to microinjection of OXT (summarized in Table 1). In one group of rats (n = 8), microinjection of 150 pmol of OXT induced a −126 ± 12.7 mg decrease in corpus tone. Following a 30 min recovery, CRF was applied to the floor of the 4th ventricle followed 15 min later by microinjection of OXT which now induced a +139 ± 42.2 mg increase over baseline in corpus tone (P < 0.05 vs. OXT alone). In another group of rats, microinjection of 150 pmol of OXT induced a −174 ± 18.8 mg decrease in corpus tone in naive rats which was reduced to −43 ± 12.6 mg following pre-treatment with CRF (n = 16 rats; P < 0.05 vs. OXT alone; Fig. 3; Table 1).
Table 1.
Corpus tone with oxytocin alone and following CRF treatment
| Oxytocin alone | Oxytocin after CRF | |
|---|---|---|
| (mg) | (mg) | |
| All groups (N = 24) | −154 ± 13.9 | 6.5 ± 16.7* |
| CRF decreases the effect of OXT alone (N = 16) | −174 ± 18.8 | −43 ± 12.6* |
| CRF reverses the effect of OXT alone (N = 8) | −126 ± 12.7 | 139 ± 42.2* |
The first microinjection of oxytocin (Oxytocin alone) in the DVC decreases corpus tone. Following CRF pretreatment, a second microinjection of oxytocin (Oxytocin after CRF) in the DVC modulates differentially the corpus tone.*P < 0.05 vs. Oxytocin alone.
Figure 3. CRF pretreatment alters the corpus tone response to microinjection of oxytocin in the DVC.
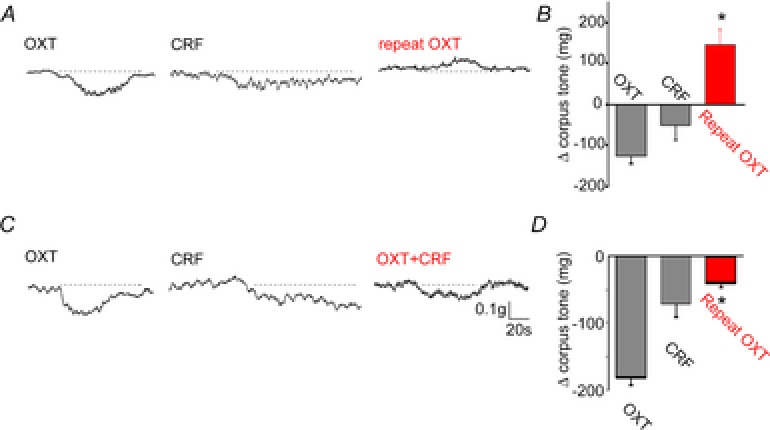
Representative trace showing that microinjection of OXT in the DVC decreases corpus tone (left panel). Upon recovery from the OXT-induced corpus relaxation, application of CRF to the floor of the 4th ventricle induced a decrease in gastric tone (middle panel); upon recovery of baseline tone, a second microinjection of OXT increased corpus tone (right panel). The data are summarized in a graphic representation in B. C, representative trace showing that microinjection of OXT in the DVC decreases corpus tone (left panel). Upon recovery from the OXT-induced corpus relaxation, application of CRF to the floor of the 4th ventricle induced a decrease in gastric tone (middle panel); upon recovery of baseline tone, a second microinjection of OXT induced a decrease in corpus tone that was significantly smaller than that obtained upon the first microinjection of OXT (right panel). The data are summarized in a graphic representation in D. *P < 0.05.
These data indicate that application of CRF alters the corpus tone response to a subsequent microinjection of OXT in the DVC.
Following CRF, the oxytocin-induced decrease in gastric tone does not involve a nitric oxide pathway
We then conducted a series of experiments aimed at investigating the mechanism of the corpus relaxation induced by OXT following application of CRF.
We reported recently that, in naive rats, the response to OXT microinjection is antagonized completely by systemic administration of l-NAME (Holmes et al. 2013). In five rats, microinjection of OXT induced a −103 ± 8.9 mg decrease in corpus tone. After a 30 min recovery period, CRF was applied to the floor of the 4th ventricle and followed, 15 min later, by i.v. infusion of l-NAME, which increased gastric tone by 398 ± 136.8 mg. In the presence of l-NAME, re-application of OXT induced a similar decrease in gastric tone to that in naive rats, i.e. −178 ± 31.6 mg; P > 0.05 vs. OXT alone (Fig. 4; Table 2).
Figure 4. Following CRF pretreatment, the oxytocin-induced effects on corpus tone are mediated by VIP.
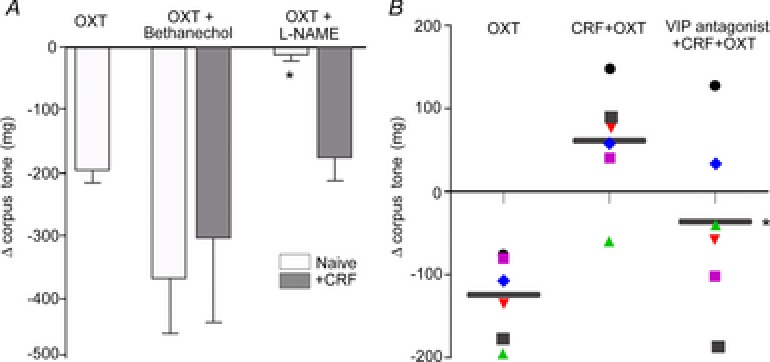
A, bar graphs showing the effects of OXT on the corpus tone in different conditions. Microinjection of OXT in the DVC decreased corpus tone in naive animals (white bar); the effect was prevented by administration of the NO synthase inhibitor l-NAME, but not by the non-selective muscarinic agonist bethanecol. Following pretreatment with CRF, the corpus response to OXT was not inhibited by l-NAME or bethanechol (grey bar). *P < 0.05 vs. OXT alone in naive rats or vs. oxytocin + CRF. B, data points showing the response of individual animals to microinjection of OXT alone (left column), the response to the second administration of OXT conducted after CRF pretreatment (middle column) and the administration of OXT preceded by CRF and VIP antagonist administration (right column). Black bar represents the mean response; P < 0.05 vs. CRF + OXT.
Table 2.
The response of the gastric corpus to microinjection of oxytocin engages different pathways in naive and CRF-exposed rats
| Naive rats | Rats exposed to CRF | ||
|---|---|---|---|
| Oxytocin (mg) | Repeat oxytocin (mg) | Oxytocin (mg) | Repeat oxytocin after CRF (mg) |
| −173 ± 52.5 | l-NAME (N = 4) −8 ± 7.7* | −103 ± 8.9 | l-NAME (N = 5) −178 ± 31.6 |
| −131 ± 21.3 | Bethanechol (N = 4) −375 ± 88.4 | −108 ± 6.5 | Bethanechol (N = 4) −311 ± 129.6 |
| −138 ± 20.5 | VIP antagonist (N = 7) −64 ± 20.9 | −138 ± 20.5 | VIP antagonist (N = 7) −23 ± 39.6* |
P < 0.05 vs. Oxytocin alone.
These data indicate that, following CRF application, the OXT-mediated decrease in corpus tone is not mediated by release of NO by the non-adrenergic non-cholinergic (NANC) pathway.
We have shown previously that following application of the group II mGluR antagonist EGLU the OXT-mediated effect was due to withdrawal of a tonically active cholinergic pathway (Holmes et al. 2013). To investigate whether the oxytocin-induced decrease in corpus tone following CRF was similarly due to an effect on the vagal cholinergic pathway, we conducted the following series of experiments. In four rats, microinjection of OXT in the DVC induced a −108 ± 6.5 mg decrease in corpus tone. Following CRF administration, i.v. infusion of bethanechol increased gastric tone by 985 ± 394.1 mg. If OXT perfusion reduced corpus tone despite the exogenous stimulation by supramaximal doses of the muscarinic cholinergic agonist, bethanecol, then we could infer that the effects of OXT are not mediated by a postganglionic cholinergic pathway to the stomach but rather by postganglionic NANC pathways. Indeed, in the presence of bethanechol, the second microinjection of OXT induced a –311 ± 129.6 mg decrease in corpus tone (P > 0.05 vs. OXT alone; Fig. 4 and Table 2).
These data indicate that following CRF pretreatment, the effects on corpus tone of OXT microinjection in the DVC occur via a mechanism that is unrelated to NO or cholinergic pathways.
Since vagal inhibitory actions are mediated also by peripheral release of vasointestinal peptide (VIP) (Goyal & Rattan, 1980; Chang et al. 2003), we tested the effects of CRF and OXT following pretreatment with VIP antagonist (50 μg kg−1 i.v.; Krowicki & Hornby, 1996).
In seven rats microinjection of OXT in the DVC induced a −138 ± 20.5 mg decrease in corpus tone. Following CRF administration i.v. infusion of VIP antagonist increased gastric tone by 69 ± 14.6 mg; in the presence of the VIP antagonist, the second microinjection of OXT induced a −23 ± 39.6 mg decrease in corpus tone (P < 0.05 vs. OXT alone) (Fig. 4).
These data indicate that following CRF pretreatment, the effects on corpus tone of OXT microinjection in the DVC occur via a mechanism that appears to involve the peripheral release of VIP. Data are summarized in Table 2.
Immunohistochemistry
Since pretreatment with the prototypical stress hormone CRF appears to uncover an effect of OXT on otherwise unresponsive GABAergic vagal neurocircuits and alters the response of corpus tone to a second microinjection of OXT, we conducted a series of experiments aimed at testing the hypothesis that CRF pretreatment induces the expression of OXT-Rs, on GABAergic terminals impinging onto DMV neurones.
In control conditions very few, if any, instances of profiles double-labelled for OXT-Rs and GAD-67 apposing DMV neurones were observed (2.7 ± 0.32 OXT-R/GAD-67 profiles/0.04 mm2, n = 5 rats). Following administration of CRF to the floor of the 4th ventricle, the number of appositions of profiles with co-localized OXT-Rs and GAD-67-IR increased to 10.7 ± 3.25, i.e. 402 ± 122.4% of control (n = 4 rats; P < 0.05; not shown).
These data indicate that CRF administration induces the expression of OXT-Rs on GABAergic terminals apposing DMV neurones.
We then conducted a series of experiments aimed at testing the hypothesis that stress itself induces the expression of OXT receptors on GABAergic terminals impinging onto DMV neurones. Following 2 h of restraint stress, the number of appositions of profiles with co-localized OXT-Rs and GAD-67-IR increased to 20.4 ± 3.57, i.e. 769 ± 134.8% of control (N = 5; P < 0.05; Fig. 5).
Figure 5. Restraint stress increases the expression of OXT-R on GABAergic terminals apposing DMV neurones.
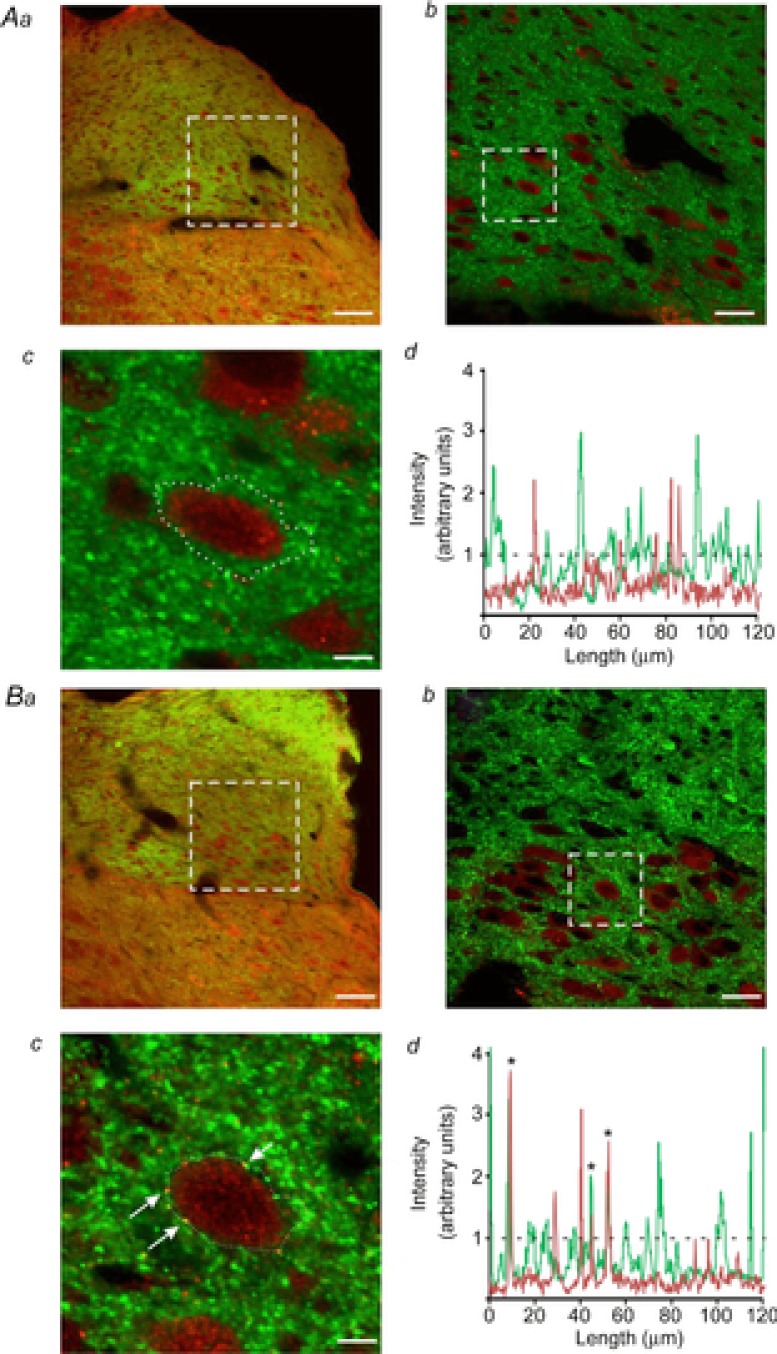
Aa, representative micrograph from a control rat showing GAD-67-IR-positive terminals (green staining) and OXT-R-positive receptors (red staining) in the dorsal vagal complex. Ab, higher magnification of the dotted area in a. Note that DMV neurones are seen as dark areas surrounded by the green immunofluorescence. Ac, single DMV neurone captured at a higher magnification from the area highlighted in b shows no co-localization of GAD-67 puncta (green) with OXT-Rs (red). Ad, graphical representation of colour intensity around the DMV neurone traced in Ac. Metamorph software analysis confirms no overlapping intensity signals between GAD-67 (green line) and OXT-Rs (red line). The dashed line represents the threshold of signal co-localization. Ba, representative micrograph showing GAD-67-IR-positive terminals (green staining) and OXT-R-positive receptors (red staining) in the dorsal vagal complex from a rat that has undergone 2 h of acute restraint stress. Bb, higher magnification of the dotted area in a. Note DMV neurones are seen as dark areas surrounded by green immunofluorescence. Bc, single DMV neurone captured at a higher magnification from the area highlighted in b shows several instances of co-localization of GAD-67 puncta (green) with OXT-Rs (red). Bd, graphical representation of colour intensity around the DMV neurone traced in c. Metamorph software analysis confirms the presence of overlapping intensity signals between GAD-67 (green line) and OXT-Rs (red line). Asterisks in the graph indicate the same co-localized puncta shown in panel c (arrows). The dashed line represents the threshold of signal co-localization. Scale bar: 75 μm for a; 25 μm for b; 5.625 μm for c.
These data indicate that acute stress, similar to exogenous application of CRF, increases the expression of OXT-Rs on GABAergic terminals apposing DMV neurones.
Discussion
In the present study we report the following findings. (i) Oxytocin did not modulate inhibitory GABAergic neurotransmission to gastric-projecting DMV neurones unless the brainstem slice was pretreated with CRF. Under these conditions, oxytocin inhibited eIPSC amplitude and decreased mIPSC frequency in DMV neurones. (ii) The CRF-mediated uncovering of the oxytocin response was due to activation of a cAMP–PKA pathway within the GABAergic NTS–DMV synapse. (iii) CRF had no effect upon the ability of oxytocin to modulate glutamatergic synaptic transmission to gastric-projecting DMV neurones. (iv) Application of oxytocin to the DVC decreased gastric corpus tone. This action was attenuated or reversed by pretreatment with CRF. (v) In naive rats, l-NAME pretreatment decreased the oxytocin-induced corpus relaxation. Following CRF pretreatment, however, the effects of oxytocin to modulate gastric corpus tone no longer involved a NO-dependent pathway but rather a VIP-dependent pathway. (vi) Following CRF pretreatment or acute stress, there was an increased expression of oxytocin receptors in GABAergic puncta apposing DMV neurones.
Although the pathophysiological changes that occur in the oxytocinergic hypothalamic-vagal-gastric corpus pathway as a consequence of stress remain to be elucidated fully, the present study provides additional information by presenting new insights into the organization and plasticity of this neurocircuitry. It is well accepted that the GABAergic synapse between NTS and DMV neurones provides the most robust influence over the spontaneous activity of vagal preganglionic DMV neurones; indeed, pharmacological blockade of this ongoing GABAergic activity increases the DMV neuronal firing rate dramatically and, by consequence, increases gastric motility (Sivarao et al. 1998; Babic et al. 2011). We have shown in recent years that the level of activity within this GABAergic synapse is finely controlled by cAMP levels within the brainstem, such that when cAMP levels are low, the tonically active GABAergic synapse is unavailable for modulation. When cAMP levels are increased, however, either by pharmacological (e.g. forskolin, CRF, or agents that increase adenylate cyclase activity), physiological or pathophysiological (e.g. feeding in previous studies; Mayo et al. 2003; Berthoud et al. 2006; Drucker, 2006; Dufresne et al. 2006; Tache & Million, 2006; stress in the present study) or surgical (e.g. deafferentation) manipulations, this previously unresponsive synapse can be modulated by neuroactive agents that influence vagal outflow to the stomach (Browning & Travagli, 2001, 2009; Browning et al. 2004, 2006; Holmes et al. 2013). While all vagal preganglionic neurones of the DMV are cholinergic and, as of now, no readily available tool can be used to distinguish their functions, gastric postganglionic parasympathetic neurones can be separated in distinct pathways, an inhibitory non-adrenergic, non-cholinergic (NANC) pathway and an excitatory cholinergic pathway. Neuroactive substances such as OXT which inhibit gastric tone and motility may do so, therefore, either by activation of the inhibitory NANC pathway or by inhibition of the tonic excitatory cholinergic pathway (Travagli et al. 2006). Our data indicate that a high level of plasticity exists in the neurocircuits that control preganglionic neurones and, by consequence, the final vagal output to the GI tract. The data presented herein suggest that different vagal pathways are involved in the OXT-mediated effects on the gastric corpus tone. In fact, in naive rats corpus relaxation is achieved via activation of a NO-sensitive NANC pathway (Holmes et al. 2013). Since pretreatment with the NO synthase inhibitor l-NAME did not prevent the corpus relaxation induced by OXT microinjection in CRF-pretreated rats, the NO-sensitive pathway appears not to play a role in these animals. This type of plasticity in response to OXT suggests a shift in the brainstem vagal pathways engaged after CRF pretreatment; indeed, this shift in the OXT response of brainstem vagal pathways is supported by our electrophysiological data.
The electrophysiological data presented herein demonstrate that the GABAergic synaptic response of vagal central circuits to OXT is determined by the prototypical stress hormone CRF. This modulation of motor outflow to the gastric corpus occurs via cAMP–PKA signalling, in a manner similar to the recently reported actions of OXT following blockade of vagal afferent fibre activity (Holmes et al. 2013). Here we show that in contrast to the lack of effect of OXT in naive neurones, after exposure to CRF, OXT inhibited GABAergic synaptic transmission to gastric-projecting DMV neurones via actions at presynaptic sites. It is of interest to note that CRF did not modulate the ability of OXT to affect glutamatergic synaptic transmission onto DMV neurones, suggesting once more that excitatory NTS–DMV neurotransmission may play a distinctly different role in the regulation and control of gastric corpus activity or may not be involved in the regulation of corpus motility under these circumstances.
Similar to that observed previously in rats that had undergone vagal afferent fibre removal (Holmes et al. 2013), rats that had undergone either CRF pretreatment in the 4th ventricle or acute restraint stress showed a significant increase in the co-localization of oxytocin receptors on GABAergic puncta apposing DMV neurones. These data suggest that the acute release of CRF, such as occurs in response to a stressful event, induces plasticity within OXT-responsive vagal brainstem neurocircuits which has the potential to alter vagal output to the GI tract. This plasticity is most likely determined by translocation of OXT-Rs to the synaptic membrane of GABAergic terminals. The electrophysiological data reported herein suggest that CRF pretreatment activates a cAMP–PKA-dependent pathway which induces the translocation of OXT-Rs on the membrane terminals of GABAergic neurons. This translocation of the OXT-Rs on GABAergic neurons impinging onto DMV neurones allows oxytocin to inhibit the GABAergic current and, by consequence, increase the vagal output to the stomach, in a manner similar to that which we described previously for μ-opioid receptors (Browning et al. 2004, 2006) A similar mechanism of action, i.e. receptor translocation upon increase in cAMP–PKA activity, appears to be a common mechanism to adapt the response of brainstem vagal neurocircuitry to different pathophysiological situations and to different circulating neurotransmitters and neurohormones, such as was also indicated by our reports on 5-HT and NPY (Browning & Travagli, 2001, 2009) or a recent paper by Smith's group (Blake & Smith, 2014). A consequence of this OXT-R translocation is that GABAergic inputs to a different subset of DMV neurones are inhibited by OXT resulting in disinhibition of vagal outputs to the corpus. Indeed, our in vivo data show that, following brainstem CRF pretreatment, the corpus relaxation in response to OXT is either reduced or, in a subset of animals, reversed, such that brainstem OXT microinjection increases corpus tone.
While, at first instance, the data reported in this paper may be seen as merely a minor variation of the same phenomenon reported previously (Holmes et al. 2013), a fundamental difference is apparent: the CRF-induced modulation of OXT-responsive brainstem vagal neurocircuits appears to engage distinctly different vagal neurocircuits such that the OXT-induced corpus relaxation no longer involves either a postganglionic NO or cholinergic muscarinic-dependent pathway. Specifically, the response of the gastric corpus to OXT following brainstem CRF administration was not antagonized by pretreatment with l-NAME or by supramaximal doses of bethanechol. Since neither NO synthase blockade nor muscarinic receptor activation overcame the response to OXT, we tested whether the gastric effects of OXT were mediated by VIP neurotransmission (Chang et al. 2003). Our data indicate that pretreatment with the VIP antagonist attenuates the OXT-induced effects on the gastric corpus significantly.
Our data thus suggest that, despite the similarities in the brainstem responses to OXT following vagal deafferentation and stress, there appear to be fundamental differences in the neural output initiating a functional response of the gastric corpus. This observation further strengthens our long-held hypothesis that underlying differences exist not only in the organization of excitatory and inhibitory central vagal neurocircuits but also in their gastric output, such that patterns of specialization or segregated lines of neuronal specificity are devoted to delivering the appropriate motor gastric response in physiological as well as in pathophysiological conditions (Browning & Travagli, 2007; Babic et al. 2011).
This interaction between CRF and OXT has clear potential to explain some of the observed GI physiological responses to stress. OXT inputs to the DVC arise exclusively from the paraventricular nucleus of the hypothalamus and previous studies have demonstrated that central application of OXT antagonists increases gastric motility, suggesting that these oxytocinergic pathways are active tonically (Swanson & Kuypers, 1980; Voorn & Buijs, 1983; Richar et al. 1991; Flanagan et al. 1992). Hypothalamic OXT is important in the normalization and recovery of gastric and colonic motility following adaptation to stress (Babygirija et al. 2010a,2010b, 2011; Bulbul et al. 2010; Zheng et al. 2010). The present paper provides a potential mechanism by which this adaptation may occur: stress is known to induce the release of CRF, which in turn decreases gastric tone and motility via central brainstem actions (Lewis et al. 2002; Stengel & Tache, 2008). In the present study we have confirmed that brainstem microinjection of CRF decreases corpus tone but, interestingly, we have also shown that the subsequent actions of OXT are attenuated or even reversed. This suggests that, following exposure to CRF, the endogenous, and tonically active, oxytocinergic pathway is no longer able to inhibit gastric motility and tone. Thus, the actions of CRF to decrease gastric tone and motility are not exacerbated but, rather, self-limited.
It is important to recognize that the physiological responses to CRF and OXT investigated in the current manuscript are most likely an in vivo and in vitro representation of acute, rather than chronic, stress. Acute stress may be best considered as a necessary, indeed vital, homeostatic adaptation to an internal or external adverse event that is essentially a reflexive protective mechanism and results in modulation of neural pathways in a time-limited adaptive manner. In this regard, we have demonstrated previously that the activation of the cAMP–PKA pathway (such as following CRF exposure) modulates GABAergic synaptic transmission to gastric-projecting DMV neurones for approximately 30–60 min before returning to pre-stimulation levels (Browning et al. 2004, 2006; Browning & Travagli, 2009). Thus, the functional GI outcomes of acute exposure to CRF are relatively temporally restricted and do not exacerbate the inhibitory gastric actions of the tonically active oxytocinergic pathway, so as not to result in a prolonged, and potentially damaging, decrease in gastric functions.
Chronic or prolonged stress, in contrast, presents a more serious challenge to allostasis and the ability to adapt to ongoing internal or external adverse events that frequently results in GI dysfunction. Given the short-term adaptations in brainstem vagal neural signalling observed in response to acute exposure to the stress-peptide CRF, it is intriguing to speculate that prolonged exposure to stress or CRF results in more permanent alterations in these neurocircuits including dysregulation of the tonic oxytocinergic pathway. Studies in rats have demonstrated adaptation in the hypothalamic oxytocinergic system in response to chronic homotypic stress whereas rats exposed to heterotypic stress fail to adapt (Babygirija et al. 2010b, 2011, 2012; Bulbul et al. 2010; Zheng et al. 2010). Such a potential dichotomy in neural rewiring presents a means by which adaptation to stress may be investigated which may be of importance in understanding why some humans, but not others, show GI dysfunctions, such as functional dyspepsia, in response to ongoing stressful conditions.
Acknowledgments
We thank Cesare M. and Zoraide Travagli, and W. Nairn Browning for support and encouragement.
Glossary
- CRF
corticotropin releasing factor
- DDA
di-deoxyadenosine
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DMV
dorsal motor nucleus of the vagus
- DVC
dorsal vagal complex
- eEPSCs
evoked excitatory postsynaptic currents
- eIPSCs
evoked inhibitory postsynaptic currents
- GAD-67
glutamic acid decarboxylase
- GI
gastrointestinal
- IR
immunoreactivity
- l-NAME
l-NG-nitroarginine methyl ester
- mEPSCs
miniature EPSCs
- mGluR
metabotropic glutamate receptor
- mIPSCs
miniature IPSCs
- NANC
non-adrenergic non-cholinergic
- NTS
nucleus tractus solitarius
- OXT
oxytocin
- OXT-R
oxytocin receptor
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- VIP
vasointestinal peptide
Additional information
Competing interests
None of the authors have any conflict of interest.
Author contributions
K.N.B., T.B. and R.A.T.: conception and design of experiments, and collection, analysis and interpretation of electrophysiological, in vivo and immunohistochemical data. T.B., G.M.H. and R.A.T.: collection of in vivo data. L.T. and F.H.C.: collection of immunohistochemical data. K.N.B., T.B. and R.A.T.: drafting the article or revising it critically for important intellectual content. All authors approved the final version of the manuscript.
Funding
This work was supported by NIH grant DK-55530.
References
- Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2011;300:G21–G32. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci Lett. 2011;495:77–81. doi: 10.1016/j.neulet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Babygirija R, Bulbul M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci. 2012;167:56–60. doi: 10.1016/j.autneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Babygirija R, Zheng J, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J Neuroendocrinol. 2010a;22:1181–1186. doi: 10.1111/j.1365-2826.2010.02069.x. [DOI] [PubMed] [Google Scholar]
- Babygirija R, Zheng J, Ludwig K, Takahashi T. Central oxytocin is involved in restoring impaired gastric motility following chronic repeated stress in mice. Am J Physiol Regul Integr Comp Physiol. 2010b;298:R157–R165. doi: 10.1152/ajpregu.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav. 2006;89:517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Blake CB, Smith BN. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2014 doi: 10.1152/ajpregu.00138.2014. (in press; DOI: 10.1152/ajpregu.00138.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. μ-Opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:9344–9352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–8988. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil. 2009;21:1309–e126. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol. 2006;575:761–776. doi: 10.1113/jphysiol.2006.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckinx R, Adriaensen D, Nassauw LV, Timmermans JP. Corticotrophin-releasing factor, related peptides, and receptors in the normal and inflamed gastrointestinal tract. Front Neurosci. 2011;5:54. doi: 10.3389/fnins.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbul M, Babygirija R, Ludwig K, Takahashi T. Central oxytocin attenuates augmented gastric postprandial motility induced by restraint stress in rats. Neurosci Lett. 2010;479:302–306. doi: 10.1016/j.neulet.2010.05.085. [DOI] [PubMed] [Google Scholar]
- Chang HY, Mashimo H, Goyal RK. Musings on the Wanderer: What's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256–260. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2001;21:1565–1582. doi: 10.1016/s0196-9781(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Garrick T, Veiseh A, Sierra A, Weiner H, Tache Y. Corticotropin-releasing factor acts centrally to suppress stimulated gastric contractility. Reg Pept. 1988;21:173–181. doi: 10.1016/0167-0115(88)90101-2. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Rattan S. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurons. Nature. 1980;288:378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Williams JT, Travagli RA. Inhibition by 5-hydroxytryptamine and noradrenaline in substantia gelatinosa of guinea-pig spinal trigeminal nucleus. J Physiol. 1995;485:113–120. doi: 10.1113/jphysiol.1995.sp020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann-Monnikes I, Tache Y, Trauner M, Weiner H, Garrick T. CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain Res. 1991;554:139–144. doi: 10.1016/0006-8993(91)90181-t. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol. 2013;591:3081–3100. doi: 10.1113/jphysiol.2013.253732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol. 2009;587:4749–4759. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowicki ZK, Hornby PJ. Contribution of acetylcholine, vasoactive intestinal polypeptide and nitric oxide to CNS-evoked vagal gastric relaxation in the rat. Neurogastroenterol Motil. 1996;8:307–317. doi: 10.1111/j.1365-2982.1996.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterol. 1988;95:1510–1517. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–146. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Kellett DO, Jordan D, Browning KN, Travagli RA. Oxytocin-immunoreactive innervation of identified neurons in the rat dorsal vagal complex. Neurogastroenterol Motil. 2012;24:e136–e146. doi: 10.1111/j.1365-2982.2011.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Rivier J, Wang L, Tache Y. Central injecton of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Therap. 1997;280:754–760. [PubMed] [Google Scholar]
- Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Schmidt BG, Raybould HE, Tache Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol Gastrointest Liver Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- Onaka T, Takayanagi Y, Yoshida M. Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. J Neuroendocrinol. 2012;24:587–598. doi: 10.1111/j.1365-2826.2012.02300.x. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci. 2008;28:11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richar P, Moos F, Freund-Mercier M-J. Central effects of oxytocin. Physiol Rev. 1991;71:331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Stengel A, Tache Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2008;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tache Y, Goto Y, Gunion MW. Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science. 1983;222:935–937. doi: 10.1126/science.6415815. [DOI] [PubMed] [Google Scholar]
- Tache Y, Maeda-Hagiwara M, Turkelson CM. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol Gastrointest Liver Physiol. 1987;253:G241–G245. doi: 10.1152/ajpgi.1987.253.2.G241. [DOI] [PubMed] [Google Scholar]
- Tache Y, Million M. Physiology of the Gastrointestinal Tract. Academic Press; 2006. Central corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis in gastrointestinal physiology; pp. 791–816. [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K, Nakade Y, Mantyh C, Ludwig K, Pappas TN, Takahashi T. Peripherally administered CRF stimulates colonic motility via central CRF receptors and vagal pathways in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1537–R1541. doi: 10.1152/ajpregu.00713.2005. [DOI] [PubMed] [Google Scholar]
- Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiol Behav. 2010;101:193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Voorn P, Buijs RM. An immuno-electronmicroscopical study comparing vasopressin, oxytocin, substance P and enkephalin containing nerve terminals in the nucleus of the solitary tract of the rat. Brain Res. 1983;270:169–173. doi: 10.1016/0006-8993(83)90809-0. [DOI] [PubMed] [Google Scholar]
- Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G946–G953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


