Abstract
The prevalence of gestational diabetes mellitus (GDM) is increasing because of the worldwide obesity/diabetes epidemic. The complications of untreated GDM affect both the mother and baby and include complications during pregnancy as well as increased risk of subsequent type-2 diabetes in mothers and offspring. Standard tests for hyperglycemia in diabetes, such as fasting glucose and hemoglobin (HbA1c), are currently not recommended for GDM screening. Instead, an oral glucose tolerance test is specified, which is invasive, time-consuming, and not easily accessible to many at-risk populations. In this study, we describe a multi-analyte maternal serum profile test that incorporates novel glycoprotein biomarkers and previously described GDM-associated markers. In screening for GDM by multi-analyte panel, the detection rate was 87% at a false-positive rate of 1%.
Keywords: Gestational diabetes, glycosylated fibronectin, maternal serum biomarker
INTRODUCTION
The rates of obesity and diabetes have increased rapidly over the last 20 years in the US as well as globally.[1] It is not surprising that the incidence of gestational diabetes mellitus (GDM) is also increasing in parallel to the overall rise in obesity and type-2 diabetes.[2] The adoption of new diagnostic criteria based on the recent HAPO study will increase the prevalence of GDM to approximately 18% of all pregnancies.[3] In light of the fact that 80-90% of women with GDM can be managed with lifestyle therapy alone,[4] universal screening for GDM is increasingly considered justified.[5]
GDM is a serious complication of pregnancy that can increase the risks of several maternal-fetal disorders, including macrosomia, shoulder dystocia or birth injury, premature delivery, and preeclampsia.[6] In addition to the increased risk of complications associated with gestation and delivery, there are also serious post-natal complications of GDM. About 5-10% of women with GDM are found to have diabetes immediately after pregnancy, and women who had GDM have a 10-fold higher chance of developing diabetes within the next 10-20 years.[7] It is now apparent that children of mothers with GDM have an 8-fold higher risk of developing type-2 diabetes mellitus in later life.[8] Thus, untreated GDM contributes to the overall diabetic population in both the short and long term.
Universal or even widespread GDM screening is hampered by the fact that the standard assessments of diabetes and pre-diabetes, such as fasting insulin/glucose and HbA1c, are not recommended for screening of GDM. Instead, the recommended parameter is an oral glucose tolerance test (OGTT),[4] which is expensive and invasive, requiring a hospital visit and multiple blood draws. Therefore, improved methods and analytes for GDM screening are needed to increase diagnosis rates and prevent maternal and child risk of future diabetes. Specifically, the development of minimally invasive testing with robust analyte combinations will greatly aid in the identification of GDM and the institution of appropriate interventions, especially in at-risk and under-served populations.[9]
RESEARCH DESIGN AND METHODS
Study recruitment and methods were approved by the Institutional Ethics Committee, and informed consent was obtained from each participant. The details of this study population have been described previously.[10] Specifically, 1463 consecutive women within the second and third trimesters of pregnancy underwent a 75-g OGTT followed by a 2-hour plasma glucose determination. GDM was diagnosed as a 2-hour plasma glucose ≥7.8 mmol/l (140 mg/dl), consistent with WHO criteria. All remaining women were categorized as non-diabetic. The current study employed a case-control design, in which 14 non-diabetic and 15 GDM serum samples were randomly selected from the described population. Clinical characteristics of study subjects are described in Table 1. Serum samples were analyzed to obtain measures of sex-hormone binding globulin (SHBG), adiponectin, human chorionic gonadotropin (hCG), placental lactogen, C-reactive protein (CRP), pregnancy-specific glycoprotein-1 (PSG-1), and fibronectin, as well as specific glycosylated forms of fibronectin and PSG-1 [Table 2]. Two-dimensional differential in-gel electrophoresis (2D-DIGE) and immunoassays (ELISA) were performed as previously described.[11,12,13,14,15] Differential glycosylation of fibronectin and PSG-1 was determined by direct lectin-binding immunoassays.
Table 1.
Participant characteristics by GDM status
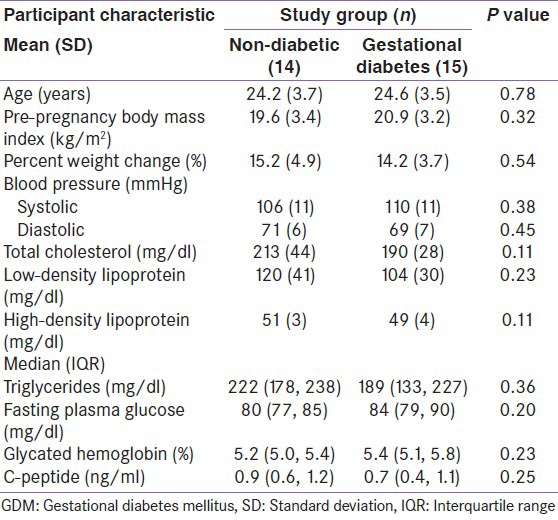
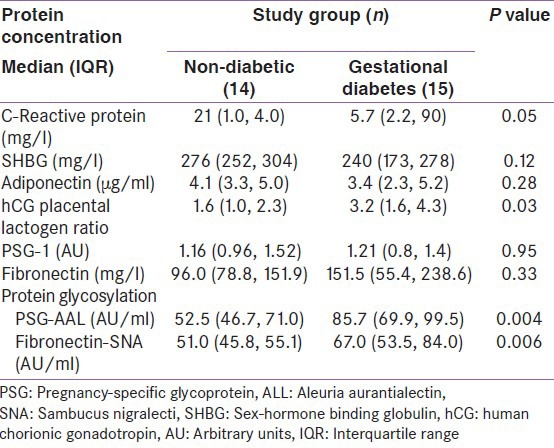
Table 2.
Differences in serum analyte levels between normal and women with gestational diabetes
T-tests were used for analysis of normally distributed continuous variables and the Wilcoxon nonparametric equivalent for variables with skewed distribution. Chi-square and Fisher's Exact tests were used for categorical variables. Parametric and Wilcoxon nonparametric t-tests were used to test differences across study groups for variables with normal and skewed distributions, respectively. Ratios of proteins were computed and tested across study groups using Wilcoxon nonparametric t-tests.
Receiver Operating Characteristic (ROC) curves generated from predicted probabilities from logistic regression modeling were used to evaluate the classification ability of individual and multiple analyte combinations.[16] The area under the ROC curve (AUROC) was computed from simple logistic regression to describe the classification ability of each protein, ratio, and glycosylated protein individually. Based on the AUROC results, individual proteins, ratios, and glycosylated proteins were added sequentially to build a multi-analyte model for improved classification performance. All statistical analyses were performed using SAS software version 9.22 (SAS Institute Inc., Cary, NC).
RESULTS AND DISCUSSION
We previously reported the discovery and validation of novel biomarkers for intra-amniotic infection, Down syndrome, pre-term birth, preeclampsia, diabetic nephropathy, and type-2 diabetes in multiple body fluids using combinations of 2D-DIGE and tandem mass spectroscopy.[11,12,13,14,15]
Subsequent proteomic studies on maternal serum demonstrated increased glycosylation of serum proteins in GDM. This is illustrated in Figure 1, which shows a 2D-DIGE comparison of the total glycoprotein fraction of pooled control and GDM maternal serum, in which protein spots that were differentially abundant in control compared with GDM samples appear as green or red spots, while proteins present at similar levels appear as yellow. The arrows point to individual protein spots that correspond to differentially abundant putative biomarkers.
Figure 1.
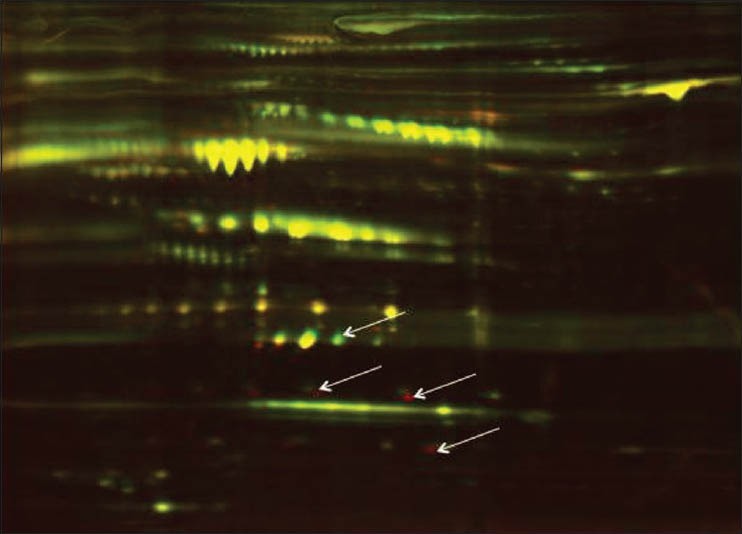
2D-DIGE analysis of control and GDM maternal serum. Pooled samples were first adsorbed on multi-lectin columns to purify the total glycoprotein fraction, this fraction was then eluted and subjected to 2- D DIGE. Differentially abundant proteins (arrows) appear as red or green spots depending on the extent of under- or over-abundance
For the present study, we selected two specific maternal serum glycoproteins, fibronectin and PSG-1 for assessment of potential changes in glycosylation status. Lectin reactivity profiling revealed that fibronectin glycosylation associated with Sambucus nigralectin (SNA) binding and PSG-1 glycosylation associated with Aleuria aurantialectin (AAL) binding were significantly elevated in GDM maternal serum compared with control serum. Therefore, these two protein-lectin pairs, fibronectin-SNA and PSG-AAL, were selected for inclusion in a multi-analyte panel with additional biomarkers previously demonstrated to exhibit differential abundance in GDM, including adiponectin,[17,18,19,20] sex hormone binding globulin (SHBG),[18,21,22,23] and CRP[24,25] as well as the ratio of hCG to placental lactogen. These analytes were evaluated singly and in combination in a set of control and GDM maternal serum samples from the cohort described in Table 1.
The mean participant age and pre-pregnancy BMI were 24.4 ± 3.5 years and 20.3 ± 3.3 kg/m2, respectively. Glycated hemoglobin measures did not differ between non-diabetic (5.2%; IQR: 5.0-5.4%) and GDM participants (5.4%; IQR: 5.1-5.8%). Fasting plasma glucose measures were also similar between groups 80 mg/dl (IQR: 77-85 mg/dl) and 84 mg/dl (IQR: 79-90 mg/dl); P = 0.20]. In addition, no statistically significant difference was observed between study groups for any other clinical parameter that was measured.
Although fasting plasma glucose and glycated HbA1c measures were not different between groups, the levels of PSG-AAL, fibronectin-SNA and the hCG/placental lactogen ratio were significantly elevated in the GDM group (P = 0.004, P = 0.006 and P = 0.03, respectively), as shown in Table 2. The difference in maternal serum CRP levels demonstrated borderline significance (P = 0.05), with a median concentration of 2.1 mg/l in non-diabetics and 5.7 mg/l in GDM participants. Placental lactogen and hCG exhibited altered levels in GDM maternal serum in previous studies.[26,27] These data suggest that combining these proteins in a ratio may improve discrimination ability. ROC curves utilizing fibronectin-SNA and PSG-AAL and the combination of these two analytes are shown in Figure 2. While the ability to detect GDM using both analytes is good (AUROC: 0.85), their use in conjunction with the other analytes described Table 2 within a multi-analyte model [Figure 3] demonstrated clearly superior performance (AUROC: 0.97). Specifically, the combination of fibronectin-SNA and PSG-AAL alone had a detection rate of 74% at a false positive rate of 6%, while the multi-analyte model had a marked increase in the detection rate (87%) at a false positive rate <1% [Figure 3]. Alternately, a single marker test with fibronectin-SNA (AUROC: 0.81) is likely to be cost-effective in preventing GDM and in reducing the increased costs associated with its complications.
Figure 2.
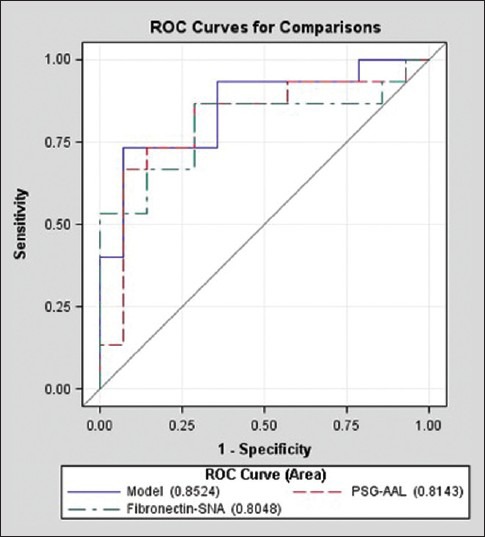
Receiver operating characteristic (ROC) curves illustrating the ability of fibronectin and PSG glycosylation to distinguish pregnant women with and without gestational diabetes
Figure 3.
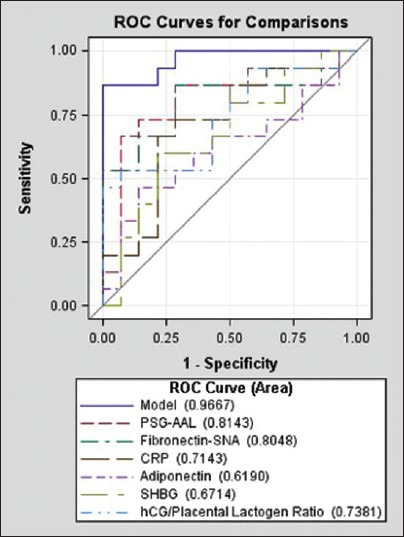
Receiver operating characteristic (ROC) curves illustrating the classification performance of each protein and protein glycosylation pattern as individual analytes and as a multi-analyte model.
CONCLUSIONS
These studies demonstrate that a multi-analyte test profile, comprising individual proteins, their ratios, and specific protein glycosylation patterns in maternal serum, can identify GDM patients independently classified by OGTT. These analytes are all amenable to analysis in dried bloodspots, which will enable the development of a minimally invasive, convenient, and cost-efficient screening test for GDM that will be particularly useful for evaluation of underserved populations that suffer significant disparities in diabetes care. Fibronectin-SNA is an early predictor before clinical hyperglycemia sets in. For cost considerations, a single marker fibronectin-SNA can also predict early GDM, and women positive for fibronectin-SNA can monitor fasting blood glucose and follow medical nutritional intervention to prevent GDM.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Smyth S, Heron A. Diabetes and obesity: The twin epidemics. Nat Med. 2005;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 3.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basevi V, Di Mario S, Morciano C, Nonino F, Magrini N. Comment on: American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34:11–S61. doi: 10.2337/dc11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moses RG, Cheung NW. Point: Universal screening for gestational diabetes mellitus. Diabetes Care. 2009;32:1349–51. doi: 10.2337/dc09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitanchez D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Med. 2010;36:617–27. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 8.Damm P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int J Gynecol Obstet. 2009;104(Suppl 1):S25–6. doi: 10.1016/j.ijgo.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Nagalla SR, Canick JA, Jacob T, Schneider KA, Reddy AP, Thomas A, et al. Proteomic analysis of maternal serum in Down syndrome: Identification of novel protein biomarkers. J Proteome Res. 2007;6:1245–57. doi: 10.1021/pr060539h. [DOI] [PubMed] [Google Scholar]
- 10.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravett MG, Thomas A, Schneider KA, Reddy AP, Dasari S, Jacob T, et al. Proteomic analysis of cervical-vaginal fluid: Identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res. 2007;6:89–96. doi: 10.1021/pr060149v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao PV, Lu X, Standley M, Pattee P, Neelima G, Girisesh G, et al. Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care. 2007;30:629–37. doi: 10.2337/dc06-2056. [DOI] [PubMed] [Google Scholar]
- 13.Pereira L, Reddy AP, Jacob T, Thomas A, Schneider KA, Dasari S, et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res. 2007;6:1269–76. doi: 10.1021/pr0605421. [DOI] [PubMed] [Google Scholar]
- 14.Rao PV, Reddy AP, Lu X, Dasari S, Krishnaprasad A, Biggs E, et al. Proteomic identification of salivary biomarkers of type 2 diabetes. J Proteome Res. 2009;8:239–45. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 15.Rasanen J, Girsen A, Lu X, Lapidus JA, Standley M, Reddy A, et al. Comprehensive maternal serum proteomic profiles of preclinical and clinical preeclampsia. J Proteome Res. 2010;9:4274–81. doi: 10.1021/pr100198m. [DOI] [PubMed] [Google Scholar]
- 16.Hosmer DL, Lemeshow S. 2nd ed. New York, NY: John Wiley and Sons; 2001. Applied Logistic Regression. [Google Scholar]
- 17.Thyfault JP, Hedberg EM, Anchan RM, Thorne OP, Isler CM, Newton ER, et al. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig. 2005;12:41–5. doi: 10.1016/j.jsgi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31:135–41. doi: 10.1002/pd.2636. [DOI] [PubMed] [Google Scholar]
- 19.Vitoratos N, Valsamakis G, Mastorakos G, Boutsiadis A, Salakos N, Kouskouni E, et al. Pre- and early post-partum adiponectin and Interleukin-1beta levels in women with and without gestational diabetes. Hormones (Athens) 2008;7:230–6. doi: 10.14310/horm.2002.1202. [DOI] [PubMed] [Google Scholar]
- 20.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: A potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 21.Bartha JL, Comino-Delgado R, Romero-Carmona R, Gomez-Jaen MC. Sex hormone binding globulin in gestational diabetes. Acta Obstet Gynecol Scand. 2000;79:839–45. [PubMed] [Google Scholar]
- 22.Thadhani R, Wolf M, Hsu-Blatman K, Sandler L, Nathan D, Ecker JL. First-trimester sex hormone binding globulinand subsequent gestational diabetes mellitus. Am J Obstet Gynecol. 2003;189:171–6. doi: 10.1067/mob.2003.343. [DOI] [PubMed] [Google Scholar]
- 23.Morisset AS, Dubé MC, Drolet R, Robitaille J, Weisnagel SJ, Tchernof A. Sex hormone-binding globulin levels and obesity in women with gestational diabetes: Relationship with infant birth weight. Gynecol Endocrinol. 2011;11:905–9. doi: 10.3109/09513590.2011.569602. [DOI] [PubMed] [Google Scholar]
- 24.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: The central role of maternal obesity. J Clin Endorinol Metabol. 2003;88:3507–12. doi: 10.1210/jc.2003-030186. [DOI] [PubMed] [Google Scholar]
- 25.Wolf M, Sandler L, Hsu K, Vossen-Smirnakis K, Ecker JL, Thadhani R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003;26:819–24. doi: 10.2337/diacare.26.3.819. [DOI] [PubMed] [Google Scholar]
- 26.Räty R, Anttila L, Virtanen A, Koskinen P, Laitinen P, Mörsky P, et al. Maternal midtrimester free beta-hCG and AFP serum levels in spontaneous singleton pregnancies complicated by gestational diabetes mellitus, pregnancy- induced hypertension or obstetric cholestasis. Prenat Diagn. 2003;23:1045–8. doi: 10.1002/pd.751. [DOI] [PubMed] [Google Scholar]
- 27.Olszewski J, Szczurowicz A, Wójcikowski C. Changes in levels of human placenta lactogen (hPL), progesterone, and estriol in blood serum and estrogens in urine during gestational diabetes mellitus. Ginekol Pol. 1995;66:145–50. [PubMed] [Google Scholar]


