Abstract
Amyloidosis is the term for diseases caused by the extracellular deposition of insoluble polymeric protein fibrils in tissues and organs. Insulin-derived amyloidosis is a rare, yet significant complication of insulin therapy. Insulin-derived amyloidosis at injection site can cause poor glycemic control and increased insulin dose requirements because of the impairment in insulin absorption, which reverse on change of injection site and/or excision of the mass. This entity should be considered and assessed by histopathology and immunohistochemistry, in patients with firm/hard local site reactions, which do not regress after cessation of insulin injection at the affected site. Search strategy: PubMed was searched with terms “insulin amyloidosis”. Full text of articles available in English was reviewed. Relevant cross references were also reviewed. Last search was made on October 15, 2014.
Keywords: Diabetes, insulin, insulin injection, insulin resistance, localized amyloidosis
INTRODUCTION
Amyloidosis is the term for diseases caused by the extracellular deposition of insoluble polymeric protein fibrils in tissues and organs.[1] The term amyloid was coined by the pathologist Rudolf Virchow, who thought such deposits were cellulose-like under the microscope.[1] Amyloid diseases can be systemic or localized. The accepted nomenclature is AX, where A indicates amyloidosis and X represents the protein in the fibril.[1] For example: AL is amyloid composed of immunoglobulin light chains, and has been called primary systemic amyloidosis; AF groups the familial amyloidoses, most commonly due to mutations in the transthyretin, the transport protein for thyroid hormone and retinol-binding protein.[1] Localized amyloidosis may be a local site reaction of insulin injection, and the amyloid at the insulin injection site is identified as amyloid insulin type (A-Ins).[2] Localized A-Ins was first described in a human subject in 1983.[3] Though <100 cases have been described in literature, report of 50 cases from one institution, and seven from another indicate that insulin-derived amyloidosis may be a more common complication of insulin therapy than previously thought.[4,5] It is possible that misdiagnosis of local site reactions as lipohypertrophy and lack of awareness/availability of histopathological investigations, may lead to underreporting of insulin-derived amyloidosis. Interestingly, insulin and enfuvirtide are the only two drugs that have been reported to cause iatrogenic amyloidosis.[4] This review will cover the pathophysiology, diagnostic features, clinical consequences and treatment aspects of insulin derived amyloidosis.
Definition
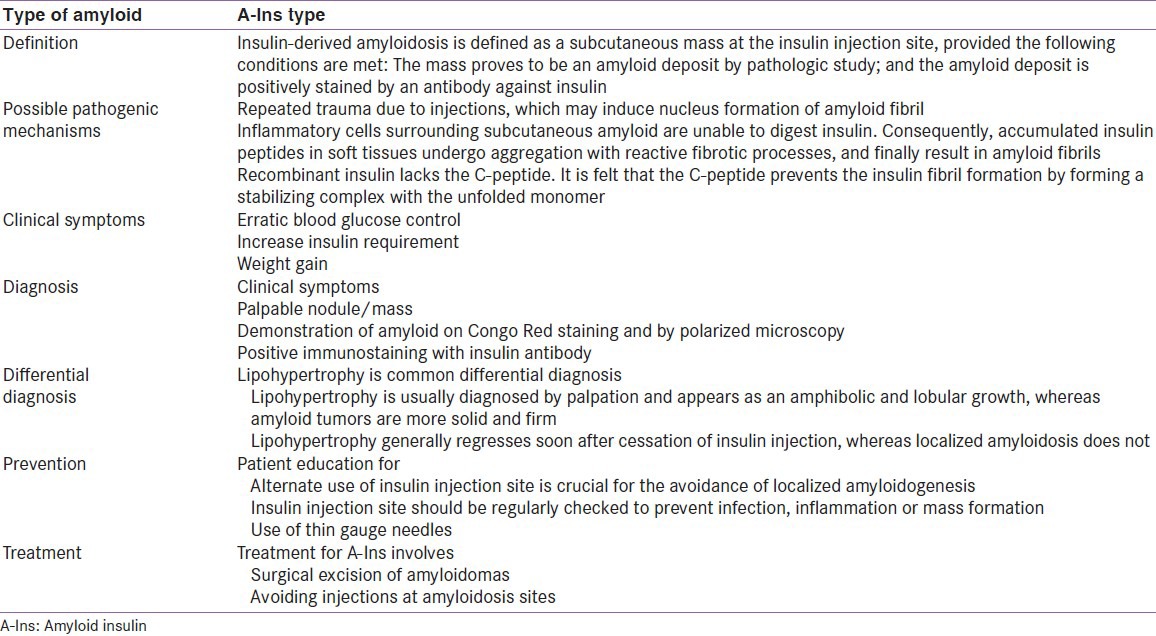
Insulin-derived amyloidosis is defined as a subcutaneous mass at the insulin injection site, provided the following conditions are met: The mass proves to be an amyloid deposit by pathologic study; and the amyloid deposit is positively stained by an antibody against insulin.[5]
Pathogenesis
Pancreas is the most common site to have localized amyloidosis in diabetes mellitus. But that seem to be a disease process related, unlike localized amyloidosis that is related to insulin therapy per se.[2] The nature of amyloid in the insulin injection site is considered to be insulin itself and is identified as A-Ins type. Although the mechanism by which amyloid fibrils are elaborated has been explored extensively, the precise process in insulin-derived amyloidosis is still poorly understood due to the rarity of published cases.[2] One factor may be the repeated trauma due to injections, which may induce nucleus formation of amyloid fibril.[5]
On histology, chronic active inflammatory changes as well as foreign body reactions with multinucleated giant cells in and around the amyloid depositions are seen. Inflammatory cells surrounding subcutaneous amyloid are unable to digest insulin. Consequently, accumulated insulin peptides in soft tissues undergo aggregation with reactive fibrotic processes and finally result in amyloid fibrils.[2]
Naturally occurring human pro-insulin is composed of an A-chain and B-chain connected by a C-peptide. Recombinant insulin lacks the C-peptide. The amyloid deposits from insulin derived amyloidosis show the complete presence of only the A and B chains without the C-peptide (exogenous insulin). It is felt that the C-peptide prevents the insulin fibril formation by forming a stabilizing complex with the unfolded monomer.[6] This may be one of the factors in the pathogenesis of insulin derived amyloidosis.
Though initially described with insulin of animal source, insulin-derived amyloidosis can be seen with recombinant human insulin and analogs as well. It may present with acanthosis of overlying skin.[7,8,9]
Clinical characteristics
Insulin-derived amyloidosis forms a hard subcutaneous mass at the injection site.[5] The amyloid mass so formed has also been referred as “amyloidoma” and “insulin ball”.[4,10] Patients may present to the clinician as a direct result of noticing local site reactions or symptoms thereof.[4]
Blood glucose levels
Patients may have associated brittle diabetes as a result of unpredictable release of insulin from these nodules[2,11] Insulin-derived amyloidosis can result in episodes of severe hypoglycemia due to erratic absorption from the affected site.[5,12]
Insulin dose requirement
In one of the case series, the mean daily insulin dose was 57 units when the insulin-derived amyloidosis was discovered. After changing injection sites to avoid amyloidosis affected sites, glycemic control improved, and the mean daily insulin dose was reduced by 53%.[5] In another case, within 14 days of changing the injection site, insulin dose decreased from 132 to 42 units/day, with improved glycemic control.[13]
Insulin absorption
Nagase et al. studied insulin kinetics, by comparing serum insulin levels after injections into insulin-derived amyloidosis sites and normal sites in four patients. In their study, a large portion of insulin injected into insulin-derived amyloidosis sites did not appear in the serum. They speculated an increased local degradation of insulin in amyloid or trapping of insulin in amyloid fibrils after injection.[5]
Weight gain
A dramatic increase in weight has been reported in persons with insulin-derived amyloidosis because of an increase in insulin dose, which results from impaired absorption.[2]
Diagnosis
Clinical
The typical physical appearance of insulin-derived amyloidosis is a nodule/hard mass, palpated at the sites where the patients repeatedly injected insulin.[2,4,5]
Histopathology and immunohistochemistry
Amyloidosis is typified by extracellular deposition of fibrillary proteins with homogenous eosinophilic appearance on routine staining. Congo-red staining demonstrates positive red color and yields apple green birefringence by polarized microscopy.[2,4,5] Immunostaining with insulin antibody will be positive for this deposition.[2]
Confirmation of apple green birefringence as well as immunohistochemistry is important for the identification of A-Ins type amyloid.[2] Proteomic analysis of the amyloid deposits is a useful adjunct in making an accurate diagnosis, as well as in elucidating the composition of amyloid.[4]
Differential diagnosis
Lipohypertropy is the commonest differential diagnosis of local site reactions in persons on insulin therapy. Lipohypertrophy is usually diagnosed by palpation and appears as an amphibolic and lobular growth, whereas amyloid tumors are more solid and firm. Lipohypertrophy generally regresses soon after cessation of insulin injection, whereas localized amyloidosis does not. If a subcutaneous mass does not regress after cessation of insulin injection, histological examination for an accurate diagnosis should be done.[2]
Insulin-derived amyloidosis should also be differentiated from systemic amyloidosis. Long-standing diabetics can have hypertrophic cardiomyopathy, proteinuria, peripheral, and autonomic neuropathy, symptoms and signs can be mistaken for systemic amyloidosis. Presence of monoclonal gammopathy may confound the situation. Mass spectrometry-based proteomic amyloid typing can help in definitive diagnosis.[14] Immunostaining with insulin antibody can be done in the absence of mass spectrometry.
Prevention
Patient education for the alternate use of insulin injection site is crucial for the avoidance of localized amyloidogenesis.[2,4,5] Insulin injection site should be regularly checked to prevent infection, inflammation or mass formation.[2] Use of thin gauge needles, proper site rotation and self-examination by patient and by health care practitioner can avoid this complication.
Treatment
Treatment for A-Ins involves surgical excision of amyloidomas or avoiding injections at amyloidosis sites. The presence of amyloid mass at the site of injection site may cause poor penetration of insulin, and this may probably contribute to the insulin resistance.[2,4,5]
SUMMARY
Insulin-derived amyloidosis causes poor glycemic control and increase insulin dose requirements, because of impaired insulin absorption and may result in erratic control, including hypoglycemic episodes and weight gain. A palpable nodule/mass at injection sites should raise suspicion of insulin derived amyloidosis, especially if it firm/hard and does not regress after cessation of insulin injection at the affected site. Treatment for A-Ins involves surgical excision of amyloidomas or avoiding administration of over affected sites. In addition, patient education for site rotation is crucial for the avoidance of localized amyloidogenesis. The important points are summarized in Table 1.
Table 1.
Insulin derived amyloidosis
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Seldin DC, Skinner M. Amyloidosis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. USA: McGraw-Hill Companies, Inc; 2012. pp. 945–50. [Google Scholar]
- 2.Shikama Y, Kitazawa J, Yagihashi N, Uehara O, Murata Y, Yajima N, et al. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49:397–401. doi: 10.2169/internalmedicine.49.2633. [DOI] [PubMed] [Google Scholar]
- 3.Störkel S, Schneider HM, Müntefering H, Kashiwagi S. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108–11. [PubMed] [Google Scholar]
- 4.D’souza A, Theis JD, Vrana JA, Dogan A. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71–5. doi: 10.3109/13506129.2013.876984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagase T, Iwaya K, Iwaki Y, Kotake F, Uchida R, Oh-i T, et al. Insulin-derived amyloidosis and poor glycemic control: A case series. Am J Med. 2014;127:450–4. doi: 10.1016/j.amjmed.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Landreh M, Stukenborg JB, Willander H, Söder O, Johansson J, Jörnvall H. Proinsulin C-peptide interferes with insulin fibril formation. Biochem Biophys Res Commun. 2012;418:489–93. doi: 10.1016/j.bbrc.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 7.Yumlu S, Barany R, Eriksson M, Röcken C. Localized insulin-derived amyloidosis in patients with diabetes mellitus: A case report. Hum Pathol. 2009;40:1655–60. doi: 10.1016/j.humpath.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127–9. doi: 10.4103/0377-4929.130920. [DOI] [PubMed] [Google Scholar]
- 9.Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25–9. doi: 10.1111/j.1365-2230.2012.04373.x. [DOI] [PubMed] [Google Scholar]
- 10.Nagase T, Katsura Y, Iwaki Y, Nemoto K, Sekine H, Miwa K, et al. The insulin ball. Lancet. 2009;373:184. doi: 10.1016/S0140-6736(09)60041-6. [DOI] [PubMed] [Google Scholar]
- 11.Albert SG, Obadiah J, Parseghian SA, Yadira Hurley M, Mooradian AD. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374–6. doi: 10.1016/j.diabres.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Endo JO, Röcken C, Lamb S, Harris RM, Bowen AR. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: Sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:e113–4. doi: 10.1016/j.jaad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Lonsdale-Eccles AA, Gonda P, Gilbertson JA, Haworth AE. Localized cutaneous amyloid at an insulin injection site. Clin Exp Dermatol. 2009;34:e1027–8. doi: 10.1111/j.1365-2230.2009.03711.x. [DOI] [PubMed] [Google Scholar]
- 14.D’souza A, Theis JD, Vrana JA, Buadi F, Dispenzieri A, Dogan A. Localized insulin-derived amyloidosis: A potential pitfall in the diagnosis of systemic amyloidosis by fat aspirate. Am J Hematol. 2012;87:E131–2. doi: 10.1002/ajh.23334. [DOI] [PubMed] [Google Scholar]


