Abstract
Although GLP-1 (glucagon like peptide-1) based therapies (GLP-1 agonists and dipeptidyl peptidase-4 inhibitors) is currently playing a cornerstone role in the treatment of type 2 diabetes, dilemma does exist about some of its basic physiology. So far, we know that GLP-1 is secreted by the direct actions of luminal contents on the L cells in distal jejunum and proximal ileum. However, there is growing evidence now, which suggest that other mechanism via “neural” or “upper gut” signals may be playing a second fiddle and could stimulate GLP-1 secretion even before the luminal contents have reached into the proximities of L cells. Therefore, the contribution of direct and indirect mechanism to GLP-1 secretion remains elusive. Furthermore, no clear consensus exists about the pattern of GLP-1 secretion, although many believe it is monophasic. One of the most exciting issues in incretin science is GLP-1 level and GLP-1 responsiveness. It is not exactly known as to what happens to endogenous GLP-1 with progressive worsening of dysglycemia from normal glucose tolerance to impaired glucose to frank diabetes and furthermore with increasing duration of diabetes. Although, conventional wisdom suggests that there may be a decrease in endogenous GLP-1 level with the worsening of dysglycemia, literature showed discordant results. Furthermore, there is emerging evidence to suggest that GLP-1 response can vary with ethnicity. This mini review is an attempt to put a brief perspective on all these issues.
Keywords: Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 agonist, glucagon like peptide-1 level, impaired fasting glucose, impaired glucose tolerance, type 2 diabetes
INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is the incretin hormone secreted from intestinal L cell in two major forms: GLP-1 (7-36) and GLP-1 (7-37 amide) often termed “active” GLP-1. The main biological action of GLP-1 depends on their two N-terminal amino acid. This two N-terminal amino acid is primarily removed by an enzyme dipeptidyl peptidase-4 (DPP-4) to truncated “inactive” GLP-1 (9-36, 9-37 amide). Active GLP-1 is responsible for glucose-dependent insulin secretion, suppression of glucagon secretion and delayed gastric emptying. Both glucose-dependent intestinal polypeptide (GIP) and GLP-1 are together termed “incretins” and account for approximately 70% of beta cell insulin secretion. It is now increasingly clear that both peptides are necessary for normal glucose tolerance (NGT). However, ubiquitous distribution of enzyme DPP-4 in human bodies quickly metabolizes active GLP-1 that results in its half-life of only approximately 1 min in the circulation.[1] To exploit this gluco-metabolic benefit of GLP-1, two approaches were considered. The first approach included the development of GLP-1 receptor agonist (GLP-1RA) with closest possible homology to native GLP-1 structure, but resistant to DPP-4 and therefore capable of binding and stimulating GLP-1 receptor for a longer time. Second approach included the development of a molecule that can inhibit DPP-4 and thereby increases endogenous GLP-1 in circulation for a longer time.[2]
Consequently, it is obvious that GLP-1RA will be an effective agent irrespective of endogenous GLP-1 levels whereas DPP-4 inhibitors will depend upon endogenous GLP-1. Therefore, it is necessary to ascertain the effectiveness of DPP-4 inhibitors with the changes in endogenous GLP-1. It is generally perceived that DPP-4 inhibitors would be more effective during the early stages of diabetes considering the progressive GLP-1 decline; however, DPP-4 inhibitors have been found be effective even in later stage. Interestingly, it is not yet clear as to what happens to GLP-1 level with increasing duration of diabetes. This mini review is an endeavor to search the existing literature, to clarify primarily as to what happens to endogenous GLP-1 levels with worsening degree of glycaemia.
Glucagon like peptide-1 secretion
Controversies exist about the pattern of GLP-1 secretion. The temporal pattern of GLP-1 following oral administration of nutrients (carbohydrates and lipids in particular) seems to begin with a rather early rise starting approximately 10-15 min after eating, peaks during the 2nd h and then slowly declines to baseline over several hours.[3] Subsequently, few other studies in humans also describe “monophasic” secretory responses.[4,5,6] However, some studies suggested a classical “biphasic” pattern, with an early peak followed by a nadir and a second rise in GLP-1 concentration.[7,8,9] This biphasic pattern consist of the first or early phase which takes place within a few minutes after nutrient load and is supposed to lasts for approximately 30-60 min, whereas the second or delayed phase continues for approximately 60-180 min.[7,8,9] Therefore, further studies are required to conclude anything concrete regarding GLP-1 pattern of secretion.
Mechanism of glucagon like peptide-1 secretion
Currently, conflicting evidence exist about the mechanisms of GLP-1 early phase release. It is assumed that GLP-1 has a prior stimulatory effect on insulin secretion as an intestinal signal, even when nutrients are far away and have not yet reached to duodenum (K cell) or jejunum (L cell). This phase of GLP-1 release is presumably mediated by “upper gut signals” either via GIP or gastrin-releasing peptide or neural signals.[4,5] The second phase or delayed phase of GLP-1 secretion takes place due to direct actions of luminal contents on L cells in distal jejunum and proximal ileum.[4,5] A study by Schirra and Miholic et al. suggested an existence of L cell in the proximal duodenum. Possibly, direct stimulation of these proximal L-cells of duodenum may be accounted for early or first phase GLP-1 secretion.[10,11] Therefore, relative contributions of direct and indirect mechanisms prompting the biphasic secretion of GLP-1 in humans still remain to be fully elucidated.
Glucagon like peptide-1 level in dysglycemia
There is no substantial clarity yet, as to what happens to GLP-1 level on varying degree of dysglycemia [Table 1]. Literature also varies a lot on this issue. One of the earliest and the largest cross-sectional study by Toft-Nielsen et al. demonstrated that the postprandial GLP-1 levels, the area under the curve (AUC) and the GLP-1 increments, following a 4-h mixed meal tolerance tests were significantly lower in type 2 diabetes, when compared to impaired glucose tolerance (IGT) or NGT groups. Although, fasting GLP-1 were normal in all groups including type 2 diabetes.[7] This study suggested a highly significant (53%) reduction in incremental GLP-1 concentrations and overall (19%) reduction in the AUC in type 2 diabetes compared to healthy controls.[7] Subsequently, several other investigators also supported this finding and suggested a progressive decrease in GLP-1 level and GLP-1 responsiveness with the worsening degree of hyperglycemia starting from NGT to IGT to frank type 2 diabetes.[8,12,13,14,15,16,39] However, some recent studies challenge those findings and point to no changes in GLP-1 levels in either IGT or type 2 diabetes.[9,17,18,19] Furthermore, two meta-analysis currently available also suggested no changes in GLP-1 level.[19,20] Therefore, any conclusion regarding GLP-1 level on varying degree of worsening the glycaemia, remains elusive.
Table 1.
GLP-1 levels in dysglycaemia
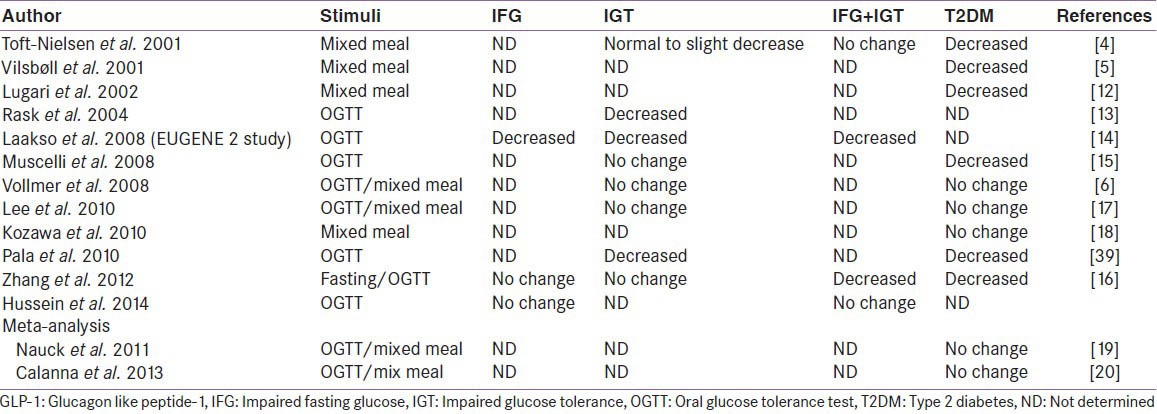
Although the mechanism for these discrepancies is far from clear, following factors are suggested to be responsible for giving diverging results:
Diagnostic criteria of pre-diabetes or diabetes across the studies
Criteria to define impaired fasting glucose (IFG), IGT and diabetes, vary in different studies. Earlier studies included IFG plus IGT under IGT groups, whereas newer study followed current ADA definition laid down in 2006 and had separate group for IFG, IGT, and both.
Course of disease
Different stages of glucose-metabolic disorders can complicate the impact of GLP-1 secretion.
Sample size
Previous study had very small patient number (n < 50) and not all of them took all three subgroups of pre-diabetes (IFG, IGT, IFG plus IGT) and type 2 diabetes in to the consideration.
Treatments influence
In most of the previous studies, the subjects could have taken the hypoglycemic therapies, which can influence GLP-1 release. Recent studies have shown that metformin and alpha-glucosidase inhibitor increases GLP-1 level.
Sampling time
Possible variations in duration of GLP-1 second phase release and timing of GLP-1 measurement following glucose load or mixed meal could be responsible.
Detection methods
On secretion, GLP-1 and GIP undergo rapid processing catalyzed by DPP-4 and lose their ability to stimulate insulin secretion. It is therefore of great importance to measure not only intact but also a total (i.e. intact plus DPP-4-processed) forms of incretin hormones to study their secretion and processing in vivo. Although, assay for intact GLP-1 and GIP require specific antibodies that have not been widely available. These differences in the methodology and sensitivity of techniques used over time in measuring total GLP-1 or intact GLP-1 concentrations can sometimes produce divergent results.
Racial differences
Variance may be attributed to a significant difference in fasting and glucose-stimulated GLP-1 levels among different races.[17,21]
Other factors determining glucagon like peptide-1 response
A univariate and multivariate regression analysis suggested that age, body weight, non-esterified fatty acid (NEFA) and glucagon level can influence the GLP-1 secretion. While increasing age and higher NEFA can increase GLP-1, higher BMI and high glucagon suppresses GLP-1.[19]
Glucagon like peptide-1 levels in Asians
From Asians perspective, data are even more conflicting as diverging results in total GLP-1, intact GLP-1 and GIP have been observed:
“Total” GLP-1 level in Asian studies varied from low to normal.[16,17,18,22] A study by Yabe et al. showed negligible GLP-1 response after meal ingestion despite robust GIP response in both healthy and diabetic Japanese subjects.[22] The reason for this reduced GLP-1 response is not exactly clear but could be explained by meal size and meal composition (nutrient-induced), sometimes critically responsible for GLP-1 response.[23,24,25,26] South-Asians may be different from Japanese in their GLP-1 response. Study by Sleddering et al. suggested higher GLP-1 and higher insulin level after a glucose load in young healthy South-Asians living in UK, compared to Caucasian counterparts.[27] However, it remains to be elucidated whether higher GLP-1 response in South-Asians was due to a compensatory increased secretion or due to GLP-1 resistant state.[27] This study also suggested that the peak GLP-1 levels preceded the peak insulin response and paralleled with insulinogenic index, thereby suggesting a direct relation between the increased GLP-1 response and the insulin secretion by the β-cell.[27] These finding stimulates further research to ascertain the intra-ethnic difference among East-Asians versus South-Asians
“Intact” GLP-1 level was found to be considerably low in both Japanese type 2 diabetes and healthy controls, compared to Caucasians.[22,28,29] The very low levels of intact GLP-1 can occur due to either impaired secretion from the gut or accelerated metabolism by DPP-4, or both. Logically, any finding of low “intact” GLP-1 despite a significant peak of “total” GLP-1 following a glucose load would hint towards a possible enhanced GLP-1 metabolism mediated by DPP-4. Interestingly, the study by Yabe et al. also showed a higher “intact” GIP: “total” GIP ratio compared to GLP-1, thereby implying that enhanced DPP-4 activity was selective to GLP-1. Although, it appears that GLP-1 is more liable to DPP-4 processing compared to GIP, further studies are really required to understand the basis of the selective reduction of intact GLP-1 in Japanese.[30] Moreover, these findings must be interpreted with caution in the light of different assay and methodology used to measure “total” GLP-1 or “intact” GLP-1 in these studies[31]
“Total” GIP level following a glucose load or mixed meal were higher in Japanese but levels of “intact” GIP were similar compared to Caucasians.[8,22,28,29] This might suggest a possible increase in processing of GIP by DPP-4 in Japanese. It should be noted that although the GIP response are enhanced in both Caucasians and Japanese type 2 diabetes (compared to healthy controls), the GLP-1 response in Japanese Type 2 diabetes is significantly reduced. The reason for perceived enhanced GIP response in diabetic patients is not fully clear currently.
In summary, as there is no significant difference in either GLP-1 or GIP levels between T2DM and healthy control, incretin deficiency does not seem to be accountable for the reduced insulin response in Japanese. However, findings of low “intact” GLP-1 levels and low GLP-1 response after meal might have special implications for reduced insulin secretory capacity and exaggerated response to incretin-based therapy in the Asians in particular with East-Asians diabetic cohorts.[32,33,34]
Some data does exist in the literature that suggests differential incretin response in different ethnic groups. A randomized, double-blind, placebo-controlled, 18-week trial (n = 530) conducted by Mohan et al., evaluating efficacy and safety of Sitagliptin among Asian population (Korea, China and India) revealed significant glucose lowering (placebo-subtracted, −1.0%; P < 0.001) with Sitagliptin. Although, similar HbA1c reduction were noted in all three subpopulation relative to baseline, Indians and Koreans exhibited better HbA1c lowering (−1.4% each) compared with Chinese (−0.7%) against placebo.[35] However, this seems to have occurred primarily due to increase HbA1c in placebo arm of Indians (+0.7%) and Koreans (+0.6%) patients and decrease HbA1c in placebo arm of Chinese (−0.2%) patients. Few other individual studies and a meta-analysis primarily conducted in Asian subjects also hinted at better HbA1c reduction with incretin-based therapies, when indirectly compared with the results from phase 3 global trials primarily done in Caucasian, African-American and Hispanic populations.[36]
A 24-week, real-life observational study (n = 14) conducted by Kesavadev et al. evaluating efficacy and safety of liraglutide in Indian patients showed remarkable lowering of HbA1c (−2.26%, P < 0.001), which looked quite higher from what had been observed in six phase 3 global randomized liraglutide effect and action in diabetes study (maximum − 1.5% in LEAD-4 study).[37] However, these results should be interpreted in the light of the biases associated with any smaller, observational studies. In contrast, a 16-week double blind randomized study (n = 929) by Yang et al. suggested a similar glucose-lowering with liraglutide among all Asians (Chinese, Koreans and Indians).[38]
Finally, a meta-analysis done from 62 randomized controlled trial by Park et al. suggested a significant better glucose lowering effect of DPP-4 inhibitor in Asians compared to non-Asians (Asians: −1.67%; 95%CI, −1.89 to − 1.44 vs. nonAsians: −0.65%; 95% CI, −0.71 to − 0.60; P < 0.05).[32] Another recent meta-analysis done by Kim et al. also suggested significantly exaggerated incretin response in Asians. Asian-dominant studies (studies with ≥ 50% Asians participants) clearly showed a greater HbA1c lowering than non-Asian dominant studies (between-group difference for DPP-4 inhibitors: −0.18%, P = 0.006; between-group difference for GLP-1 agonist: −0.32%, P = 0.04).[33,34]
It should be noted however that, Asian studies included in these meta-analysis mainly consisted of East-Asians. Whether, these results can be extrapolated to South-Asians is a subject of speculation in the light of significantly different etio-patho-physiological features between South-Asians versus East-Asians.
CONCLUSION
Although conventional wisdom suggests that the GLP-1 level progressively decreases with increasing duration of dysglycemia, meta-analysis of various studies suggests no significant changes in GLP-1 level. Nevertheless, the final conclusion can only be derived from prospective or longitudinal studies, which will measure GLP-1 level along the entire course of diabetes starting from normoglycemia to IGT to frank diabetes and its further course over the years.
Possible ethnic differences in GLP-1 level and GLP-1 responsiveness after meal challenge might also exist among East-Asians, in particular in Japanese and Koreans. However, this cannot be generalized to South-Asians. South-Asians found to have higher GLP-1 and higher insulin levels, and they could be completely divergent from East-Asians counterparts. Consequently, further data would be required to understand the difference in GLP-1 response among East-Asians versus South-Asians. Nevertheless, it is likely that these differences in GLP-1 response may impact the effectiveness of incretin-based therapies among certain ethnic groups. As, beta cell secretory dysfunctions and poor GLP-1 reserve are two main primary defects observed in East-Asians, they may perhaps respond better with incretin-based therapies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–8. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 4.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 5.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–87. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 7.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 8.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–63. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 9.Rask E, Olsson T, Söderberg S, Johnson O, Seckl J, Holst JJ, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–5. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 10.Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miholic J, Orskov C, Holst JJ, Kotzerke J, Meyer HJ. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci. 1991;36:1361–70. doi: 10.1007/BF01296800. [DOI] [PubMed] [Google Scholar]
- 12.Lugari R, Dei Cas A, Ugolotti D, Finardi L, Barilli AL, Ognibene C, et al. Evidence for early impairment of glucagon-like peptide 1-induced insulin secretion in human type 2 (non insulin-dependent) diabetes. Horm Metab Res. 2002;34:150–4. doi: 10.1055/s-2002-23199. [DOI] [PubMed] [Google Scholar]
- 13.Rask E, Olsson T, Söderberg S, Holst JJ, Tura A, Pacini G, et al. Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism. 2004;53:624–31. doi: 10.1016/j.metabol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stancáková A, et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;51:502–11. doi: 10.1007/s00125-007-0899-2. [DOI] [PubMed] [Google Scholar]
- 15.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–8. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Tang X, Cao H, Lü Q, Li N, Liu Y, et al. Impaired secretion of total glucagon-like peptide-1 in people with impaired fasting glucose combined impaired glucose tolerance. Int J Med Sci. 2012;9:574–81. doi: 10.7150/ijms.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Yabe D, Nohtomi K, Takada M, Morita R, Seino Y, et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J. 2010;57:119–26. doi: 10.1507/endocrj.k09e-269. [DOI] [PubMed] [Google Scholar]
- 18.Kozawa J, Okita K, Imagawa A, Iwahashi H, Holst JJ, Yamagata K, et al. Similar incretin secretion in obese and non-obese Japanese subjects with type 2 diabetes. Biochem Biophys Res Commun. 2010;393:410–3. doi: 10.1016/j.bbrc.2010.01.134. [DOI] [PubMed] [Google Scholar]
- 19.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia. 2011;54:10–8. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 20.Calanna S, Christensen M, Holst JJ, Laferrère B, Gluud LL, Vilsbøll T, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–72. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasquez-Mieyer PA, Umpierrez GE, Lustig RH, Cashion AK, Cowan PA, Christensen M, et al. Race affects insulin and GLP-1 secretion and response to a long-acting somatostatin analogue in obese adults. Int J Obes Relat Metab Disord. 2004;28:330–3. doi: 10.1038/sj.ijo.0802561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yabe D, Kuroe A, Lee S, Watanabe K, Hyo T, Hishizawa M, et al. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: Comparison of type 2 diabetes patients and healthy controls. J Diabetes Investig. 2010;1:56–9. doi: 10.1111/j.2040-1124.2010.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, et al. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E779–84. doi: 10.1152/ajpendo.90233.2008. [DOI] [PubMed] [Google Scholar]
- 24.Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkänen HM. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr. 2002;75:254–62. doi: 10.1093/ajcn/75.2.254. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, et al. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69:1135–43. doi: 10.1093/ajcn/69.6.1135. [DOI] [PubMed] [Google Scholar]
- 26.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sleddering MA, Bakker LE, Janssen LG, Meinders AE, Jazet IM. Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism. 2014;63:226–32. doi: 10.1016/j.metabol.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 29.Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, et al. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes. 2004;53:654–62. doi: 10.2337/diabetes.53.3.654. [DOI] [PubMed] [Google Scholar]
- 30.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–81. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 31.Deacon CF, Holst JJ. Immunoassays for the incretin hormones GIP and GLP-1. Best Pract Res Clin Endocrinol Metab. 2009;23:425–32. doi: 10.1016/j.beem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: Meta-analysis. Ann Pharmacother. 2012;46:1453–69. doi: 10.1345/aph.1R041. [DOI] [PubMed] [Google Scholar]
- 33.Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–9. doi: 10.1111/dom.12293. [DOI] [PubMed] [Google Scholar]
- 35.Mohan V, Yang W, Son HY, Xu L, Noble L, Langdon RB, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83:106–16. doi: 10.1016/j.diabres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Louisa M, Takeuchi M, Takeuchi M, Nafrialdi, Setiabudy R. Incretin-based therapies for type 2 diabetes mellitus in Asian patients: Analysis of clinical trials. Med J Indones. 2010;19:205–12. [Google Scholar]
- 37.Kesavadev J, Shankar A, Krishnan G, Jothydev S. Liraglutide therapy beyond glycemic control: An observational study in Indian patients with type 2 diabetes in real world setting. Int J Gen Med. 2012;5:317–22. doi: 10.2147/IJGM.S27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: A 16-week, randomized, double-blind, active control trial. Diabetes Obes Metab. 2011;13:81–8. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 39.Pala L, Ciani S, Dicembrini I, Bardini G, Cresci B, Pezzatini A, et al. Relationship between GLP-1 levels and dipeptidyl peptidase-4 activity in different glucose tolerance conditions. Diabet Med. 2010;27:691–5. doi: 10.1111/j.1464-5491.2010.03010.x. [DOI] [PubMed] [Google Scholar]


