Abstract
As the prevalence of these endocrine dysfunctions increases, the association of polycystic ovary syndrome (PCOS) and autoimmune thyroid disease is increasingly being recognised. While the causality of this association is still uncertain, the two conditions share a bidirectional relationship. The exact nature of this link has not been elucidated yet. Both syndromes share certain common characteristics, risk factors, and pathophysiological abnormalities. Simultaneously, certain etiopathogenetic factors that operate to create these dysfunctions are dissimilar. Polycystic appearing ovaries are a clinical feature of hypothyroidism, though hypothyroidism should be excluded before diagnosing PCOS. Adiposity, increased insulin resistance, high leptin, evidence of deranged autoimmunity, all of which are present in both disease states, seem to play a complex role in connecting these two disorders. This brief communication explores the nature of the relationship between PCOS and hypothyroidism. It reviews current data and analyses them to present a unified pathophysiological basis, incorporating these complex relationships, for the same.
Keywords: Autoimmunity, leptin, obesity, polycystic ovary syndrome, thyroid
INTRODUCTION
Thyroid disorders and polycystic ovary syndrome (PCOS) are two of the most common endocrine disorders in the general population. Although the etiopathogenesis of hypothyroidism and PCOS is completely different, these two entities have many features in common. An increase in ovarian volume and cystic changes in ovaries have been reported in primary hypothyroidism. In the other direction, it is increasingly realized that thyroid disorders are more common in women with PCOS as compared to the normal population.[1,2,3,4] Whether this is due to some common factors predisposing an individual to both disorders, or due to a pathophysiological connection between the two disorders has not been established until now. The purpose of descriptive and exploratory review is to explore the relationship between these two disorders. To generate a hypothesis linking these disorders, terms “PCOS,” “autoimmunity,” “subclinical hypothyroidism,” “thyroid autoimmunity,” “thyroid autoantibodies,” “leptin,” obesity” and “thyroiditis” were searched in various combination in PubMed, Google scholar and Embase. Relevant articles from this search as well as from cross references were retrieved and included. Elucidation of this relationship between thyroid disorders and PCOS requires answers to two questions: (1) What happens to ovaries in thyroid disorders? (2) What happens to thyroid in PCOS?
WHAT HAPPENS TO OVARIES IN THYROID DISORDERS?
This question is relatively easier to answer, and pathways leading to change in ovarian morphology in hypothyroidism are well-known. In the presence of hypothyroidism, ovarian morphology becomes poly-cystic. Hence, thyroid disorders are one of the exclusion criteria before making a diagnosis of PCOS in any women. The underlying pathophysiology has been outlined in Figure 1. Rise in thyrotropin-releasing hormone (TRH) in primary hypothyroidism leads to increased prolactin and thyroid stimulating hormone (TSH). Prolactin contributes toward polycystic ovarian morphology by inhibiting ovulation as a result of the change in the ratio of follicle stimulating hormone (FSH) and luteinizing hormone and increased dehydroepiandrosterone from the adrenal gland. Increased TSH also contributes due to its spill-over effect on FSH receptors. Increased collagen deposition in ovaries as a result of hypothyroidism has also been suggested.
Figure 1.
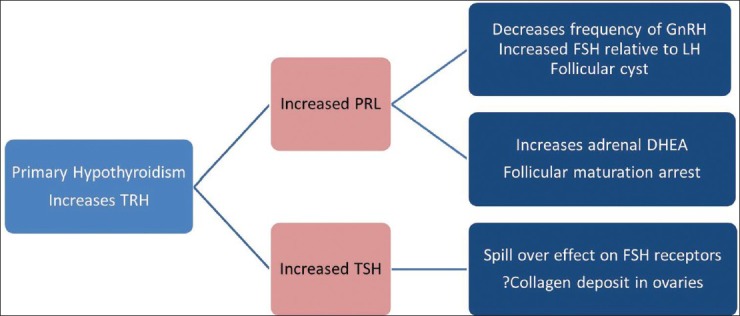
Pathophysiology of polycystic ovaries in patients with primary hypothyroidism
The severity of ovarian morphology also depends on duration and severity of underlying primary hypothyroidism. In most severe cases like long standing untreated cases of congenital hypothyroidism ovarian morphology can be very striking and can even be mistaken for ovarian malignancies. These cases have been given an eponym Van Wyk and Grumbach syndrome, after the scientist who first described the case.[5]
In a study, on somewhat less severe primary hypothyroidism, by Muderris et al., 26 treatment naïve females with primary hypothyroidism, with mean TSH 57.1 mcg/dl, underwent evaluation of ovarian volume before and after replacement with thyroxine.[6] Twenty-six healthy normal controls were also recruited. Ten of 26 hypothyroid females had polycystic appearing ovaries on ultrasound sonography test at baseline. All women with primary hypothyroidism had significantly higher ovarian volumes than controls. Even the subgroup without polycystic appearing ovaries had significantly higher ovarian volumes. However, there was no correlation of TSH levels with cyst formation. The most remarkable finding of this study was normalization of ovarian volume in all patients, with or without polycystic appearing ovaries, after replacement of thyroxine.
Recently, in a study by Ganie et al., 175 girls with euthyroid chronic lymphocytic thyroiditis (CLT) and 46 age-matched non-CLT girls underwent evaluation for diagnosis of PCOS.[7] These girls were all 13-18 years old (mean age 14.7 years). In girls with euthyroid CLT prevalence of PCOS was significantly higher when compared to their control counterparts (46.8 vs. 4.3%, P < 0.001). The CLT girls had higher body mass index (BMI), waist circumference, and systolic blood pressure (P < 0.001). These girls also had higher hirsutism score, the lower number of annual menstrual cycles as well as higher insulin resistance score. However, two factors need to be considered before drawing any conclusion from this study. First, there seem to be a co-segregation of PCOS and CLT cases, leading to the classic “Which comes first, the chicken or the egg?” dilemma: Whether autoimmune thyroiditis predisposes subjects to develop characteristics suggestive of PCOS or whether PCOS is a forerunner of autoimmune thyroiditis, remains speculation. Second, this study recruited adolescents with a mean age of 14.7 years. In this age group, the diagnosis of PCOS is not always tenacious, as normal girls of this age may have polycystic appearing ovaries, acne, and menstrual abnormalities.
In summary, therefore, hypothyroidism can lead to polycystic morphology of the ovaries. While this morphology can vary with severity and duration of hypothyroidism, there is no evidence to suggest that primary hypothyroidism can lead to PCOS.
WHAT HAPPENS TO THYROID IN POLYCYSTIC OVARY SYNDROME?
The prevalence of subclinical thyroid dysfunction in the general population has been estimated around 10%, but in reproductive years this prevalence is considerably low at 4-6%.[8,9] In recent years, a number of publications have reported increased incidence of thyroid disorders in females with PCOS. Sinha et al. compared 80 PCOS females with 80 controls and found significant higher prevalence of goiter (27.5% vs. 7.5%) and subclinical hypothyroidism (22.5% vs. 8.75%) in PCOS patients as compared to controls.[1] An another study conducted in young women with PCOS found prevalence of subclinical hypothyroidism (defined as TSH > 4.5 μIU/ml) to be 11.3% (mean TSH level of 6.1 ± 1.2 mIU/L). There was no difference in two groups (with or without subclinical hypothyroidism) with respect to BMI, waist circumference or Ferriman–Gallwey score. Low-density lipoprotein cholesterol (LDL-C) was found to be significantly higher in the cohort with subclinical hypothyroidism.[2]
The pathophysiological pathway connecting these two disorders has not been clearly delineated as of now. The most obvious connection, perhaps, is the increased BMI and insulin resistance common to both conditions. Increase in BMI is an integral part of PCOS and is seen in a large majority (54-68%) of these cases.[10] The link between thyroid functions and obesity is again an interesting one, with unclear pathophysiological mechanisms; there is, however, enough evidence to say that TSH is higher in people with high BMI.[11,12] The proposed link is depicted in Figure 2. Obesity is associated with an altered milieu with increase in pro-inflammatory markers and increase in insulin resistance. This, through undefined mechanisms, leads to decreased deiodinase-2 activity at pituitary level resulting in relative T3 deficiency and increase in TSH levels.[12] Another pathway, based upon leptin, has been hypothesized to explain this observation. Increased leptin in obesity has been proposed to act directly on the hypothalamus resulting in increased TRH secretion.[13] Raised TSH levels, with any of these two pathways, act on adipocytes to increase their proliferation. In culture studies, TSH has been shown to increase proliferation of adipocytes as well as increase in production of pro-inflammatory markers from adipocytes, acting on TSH receptors present on adipocytes. Muscogiuri et al. recently studied 60 euthyroid subjects to find a correlation of TSH (with in normal range) to either adipose tissue or insulin resistance. On univariate analysis, both adiposity and insulin resistance were significantly associated with raised TSH, but after multivariate regression, visceral adipose tissue volume was found to be the only predictor of TSH (P = 0.01).[12] The another interesting observation about TSH lowering effect of metformin has been reported in both PCOS and non-PCOS population.[14,15] Metformin has been shown to lower TSH in persons with clinical and subclinical hypothyroidism, but not in euthyroid people.[14] However, there is not enough evidence to suggest that this TSH lowering effect of metformin is mediated by lowering insulin resistance. A change in the affinity or in the number of TSH receptors; an increase in the central dopaminergic tone and direct effect of metformin on TSH regulation has been proposed as potential explanations.[15]
Figure 2.
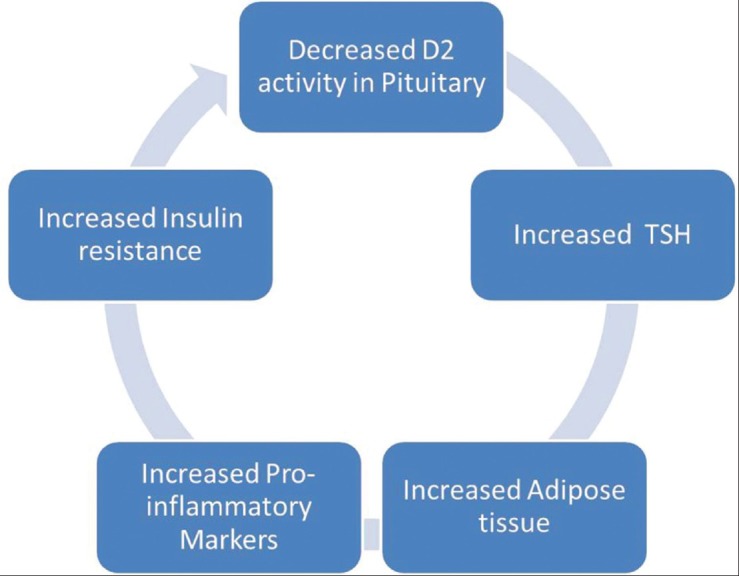
Hypothesis linking adiposity and raised thyroid stimulating hormone[10]
The pitfall of this pathway is that it fails to explain increased incidence of thyroid autoimmunity in patients with PCOS. Thyroid autoimmunity is increased in patients of PCOS. Females with PCOS have higher thyroid antibody levels, larger thyroid volumes and their thyroids are more hypoechogenic (compatible with thyroiditis) when compared to controls.[4] Thyroid peroxidase (TPO) antibodies have been shown to be present in 27% of the patients when compared to 8% in controls.[16]
Are we right in saying, therefore that women with PCOS are more predisposed to autoimmune diseases? There seem to be some theoretical basis for this statement. PCOS is known to be a hyperestrogenic state. Hyperestrogenism has been proposed as one explanation for the occurrence of increased autoimmune diseases in females when compared to males.[17] Estrogen receptors have a proliferative action on B-lymphocytes and estrogen receptors are also present on T-cell as well as macrophages.[18]
In fact, there are some reports of increased autoimmunity in PCOS patients towards organs other than thyroid as well. Positive anti-ovarian antibodies for at least one isotype (IgG - 27%, IgA - 3%, IgM - 27%) were present in 15 (44%) of 34 of the PCOS women.[19] In another study comparing 109 women with PCOS to 109 age-matched healthy controls, women with PCOS had significantly elevated serum levels of antihistone and anti-double-stranded deoxyribonucleic acid antibodies whereas serum levels of antinuclear antibodies (ANAs) and antinucleo some antibodies were similar.[20] A more recent study has reported ANA positivity in 8.6% of PCOS patients, while none in the control group was positive.[21] Raised levels of smooth muscle antibody have also been reported.[22] Keeping this data in mind and also numerous reports of increased thyroid autoimmunity, increased incidence of thyroid autoimmunity cannot be ignored and refuted anymore. As of now, the pathophysiological explanation of this phenomenon is unclear. There seem to be a complex interplay of PCOS, adiposity, thyroid dysfunction and autoimmunity, working to produce varying clinical pictures, all belongs to different parts of a wide spectrum. The relative contribution of each aspect, to the propagation of other factors, as well as the direction of causality, is far from certain. A multi-directional link seems to be the best explanation as of now.
This relationship has been further complicated by report of linkage between autoimmunity and adiposity. In UK Medical Research Council 1946 British Birth Cohort study, positive anti-TPO antibodies among women at age 60-64 years were positively associated with childhood body weight, childhood overweight, adult BMI as well as childhood weight gain between 0 and 14 years.[23] An interesting explanation has been proposed via leptin pathway but needs further elucidation [Figure 3].[24] Increased leptin, as a result of increased adiposity, increases TRH secretion from the hypothalamus via Janus activating kinase-2/signal transducer and activator of transcription 3 factor. Increased TSH again induces proliferation of adipocytes via TSH receptors on adipocytes. Leptin also mediates autoimmunity by preferentially inducing effector T-cells and down-regulating regulator T-cells.[25]
Figure 3.
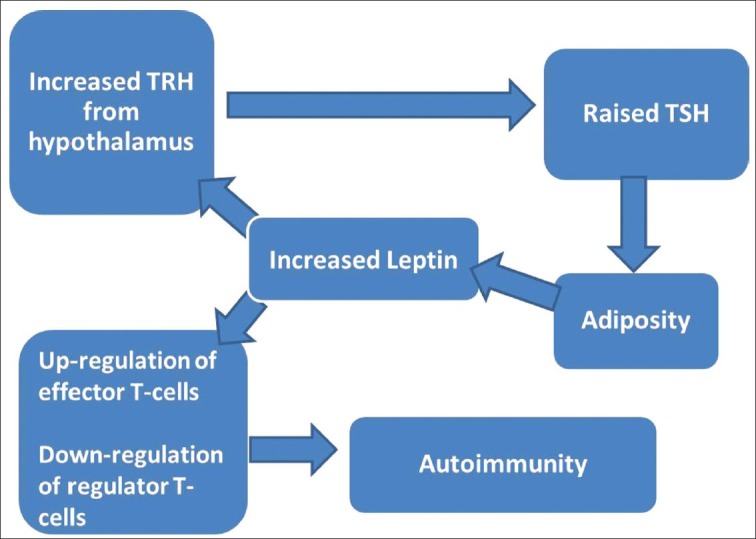
Complex pathway linking adiposity with raised thyroid stimulating hormone as well autoimmunity, via leptin[18,25]
Implications of subclinical hypothyroidism or thyroid autoimmunity in patients with PCOS are still a work in progress. Studies have reported deranged lipid parameters like rise in LDL and triglycerides in PCOS patients with subclinical hypothyroidism, but long term follow-up is lacking.[2,26] There are contradictory reports on worsening of insulin resistance in these patients.[19,27]
CONCLUSION
There is enough literature support to argue that prevalence of subclinical hypothyroidism/thyroid autoimmunity is increased in women with PCOS patients. The pathophysiological pathway and contributing factors behind this association are yet to be elucidated. Long-term studies are required to assess the significance of thyroid dysfunction in patients with PCOS, especially on fertility.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sinha U, Sinharay K, Saha S, Longkumer TA, Baul SN, Pal SK. Thyroid disorders in polycystic ovarian syndrome subjects: A tertiary hospital based cross-sectional study from Eastern India. Indian J Endocrinol Metab. 2013;17:304–9. doi: 10.4103/2230-8210.109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benetti-Pinto CL, Berini Piccolo VR, Garmes HM, Teatin Juliato CR. Subclinical hypothyroidism in young women with polycystic ovary syndrome: An analysis of clinical, hormonal, and metabolic parameters. Fertil Steril. 2013;99:588–92. doi: 10.1016/j.fertnstert.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ramanand SJ, Ghongane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013;17:138–45. doi: 10.4103/2230-8210.107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gärtner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150:363–9. doi: 10.1530/eje.0.1500363. [DOI] [PubMed] [Google Scholar]
- 5.Van Wyk JJ, Grumbach MM. Syndrome of precocious menstruation and galactorrhea in juvenile hypothyroidism. An example of hormonal overlap in pituitary feedback. J Pediatr. 1960;57:416–35. [Google Scholar]
- 6.Muderris II, Boztosun A, Oner G, Bayram F. Effect of thyroid hormone replacement therapy on ovarian volume and androgen hormones in patients with untreated primary hypothyroidism. Ann Saudi Med. 2011;31:145–51. doi: 10.4103/0256-4947.77500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganie MA, Marwaha RK, Aggarwal R, Singh S. High prevalence of polycystic ovary syndrome characteristics in girls with euthyroid chronic lymphocytic thyroiditis: A case-control study. Eur J Endocrinol. 2010;162:1117–22. doi: 10.1530/EJE-09-1012. [DOI] [PubMed] [Google Scholar]
- 8.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 9.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 10.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–37. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 11.Asvold BO, Bjøro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94:5023–7. doi: 10.1210/jc.2009-1180. [DOI] [PubMed] [Google Scholar]
- 12.Muscogiuri G, Sorice GP, Mezza T, Prioletta A, Lassandro AP, Pirronti T, et al. High-normal TSH values in obesity: Is it insulin resistance or adipose tissue's guilt? Obesity (Silver Spring) 2013;21:101–6. doi: 10.1002/oby.20240. [DOI] [PubMed] [Google Scholar]
- 13.Duntas LH, Biondi B. The interconnections between obesity, thyroid function, and autoimmunity: The multifold role of leptin. Thyroid. 2013;23:646–53. doi: 10.1089/thy.2011.0499. [DOI] [PubMed] [Google Scholar]
- 14.Lupoli R, Di Minno A, Tortora A, Ambrosino P, Lupoli GA, Di Minno MN. Effects of treatment with metformin on TSH levels: A meta-analysis of literature studies. J Clin Endocrinol Metab. 2014;99:E143–8. doi: 10.1210/jc.2013-2965. [DOI] [PubMed] [Google Scholar]
- 15.Rotondi M, Cappelli C, Magri F, Botta R, Dionisio R, Iacobello C, et al. Thyroidal effect of metformin treatment in patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2011;75:378–81. doi: 10.1111/j.1365-2265.2011.04042.x. [DOI] [PubMed] [Google Scholar]
- 16.Garelli S, Masiero S, Plebani M, Chen S, Furmaniak J, Armanini D, et al. High prevalence of chronic thyroiditis in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;169:248–51. doi: 10.1016/j.ejogrb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10:2005–11. doi: 10.3201/eid1011.040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutolo M, Sulli A, Straub RH. Estrogen metabolism and autoimmunity. Autoimmun Rev. 2012;11:A460–4. doi: 10.1016/j.autrev.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Fénichel P, Gobert B, Carré Y, Barbarino-Monnier P, Hiéronimus S. Polycystic ovary syndrome in autoimmune disease. Lancet. 1999;353:2210. doi: 10.1016/S0140-6736(99)00256-1. [DOI] [PubMed] [Google Scholar]
- 20.Hefler-Frischmuth K, Walch K, Huebl W, Baumuehlner K, Tempfer C, Hefler L. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil Steril. 2010;93:2291–4. doi: 10.1016/j.fertnstert.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 21.Samsami Dehaghani A, Karimaghaei N, Parsanezhad ME, Malekzadeh M, Mehrazmay M, Erfani N. Anti-Nuclear Antibodies in Patients with Polycystic Ovary Syndrome before and after Laparoscopic Electrocauterization. Iran J Med Sci. 2013;38:187–90. [PMC free article] [PubMed] [Google Scholar]
- 22.Reimand K, Talja I, Metsküla K, Kadastik U, Matt K, Uibo R. Autoantibody studies of female patients with reproductive failure. J Reprod Immunol. 2001;51:167–76. doi: 10.1016/s0165-0378(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 23.Ong KK, Kuh D, Pierce M, Franklyn JA. Childhood weight gain and thyroid autoimmunity at age 60-64 years: The 1946 British birth cohort study. J Clin Endocrinol Metab. 2013;98:1435–42. doi: 10.1210/jc.2012-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duntas LH, Biondi B. The interconnections between obesity, thyroid function, and autoimmunity: The multifold role of leptin. Thyroid. 2013;23:646–53. doi: 10.1089/thy.2011.0499. [DOI] [PubMed] [Google Scholar]
- 25.Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: Many questions, some answers. Tissue Antigens. 2007;70:87–95. doi: 10.1111/j.1399-0039.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 26.Ganie MA, Laway BA, Wani TA, Zargar MA, Nisar S, Ahamed F, et al. Association of subclinical hypothyroidism and phenotype, insulin resistance, and lipid parameters in youngwomen with polycystic ovary syndrome. Fertil Steril. 2011;95:2039–43. doi: 10.1016/j.fertnstert.2011.01.149. [DOI] [PubMed] [Google Scholar]
- 27.Mueller A, Schöfl C, Dittrich R, Cupisti S, Oppelt PG, Schild RL, et al. Thyroid-stimulating hormone is associated with insulin resistance independently of body mass index and age in women with polycystic ovary syndrome. Hum Reprod. 2009;24:2924–30. doi: 10.1093/humrep/dep285. [DOI] [PubMed] [Google Scholar]


