Abstract
Incretin-based therapy has clearly emerged as one of the most sought out strategy in managing type 2 diabetes, primarily because they generally do not causes hypoglycemia and possess weight-neutral or weight losing properties. Efficacy-wise too, these agents, are more or less similar to commonly used drugs metformin and sulfonylureas. Interestingly, some studies recently suggested that glycemic response to these incretin-based therapies could also differ ethnicity-wise. Subsequently, meta-analysis from these studies also suggested that Asians may have better response to these incretin-based therapies. This review will be an attempt to critically analyze those studies available in literature and to address as to why East-Asians and South-Asians may have different incretin response compared to non-Asians.
Keywords: Adiponectin, DPP-4 activity, GLP-1 level in Asians, insulin resistance, incretin response, insulin secretory defect
INTRODUCTION
Current international diabetes federation (IDF) estimates indicate that 8.3% of adults (382 million people) have diabetes, and the number is set to rise beyond 592 million in less than 25 years. Close to one-fifth of all adults with diabetes in the world live in the South-East Asia region, of which 65.1 million of whom live in India, which could further increase to 109 million by 2035. India is the largest contributor to regional mortality, with 1.1 million deaths attributable to diabetes in 2013.[1] The Asian region is of prime importance because it constitutes 60% of the world's population.[2] Asian population are also racially heterogeneous and have differing demographic, cultural and socioeconomic characteristics. The increase in type 2 diabetes in Asia differs from that reported in other parts of the world, as it develops in a much shorter time, in a younger age group and in people in a much lower body mass index (BMI). South-Asian type 2 diabetes patients are typically characterized by a relatively lower BMI but with higher amounts of visceral fat at given BMI or waist circumference, while East-Asians have predominant insulin secretory defect, when compared to their white counterparts.[2,3,4] Nevertheless, the exact contribution of secretory defect and insulin resistance in the pathogenesis of Asian type 2 diabetes still remains elusive.
Reports from WHO multinational study, The Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe (the DECODE) and in Asia (the DECODA) study revealed that both insulin resistance and insulin secretion capacity are higher in Caucasians.[5,6,7] Although insulin resistance with obesity is the predominant patho-physiological defects in Europeans and Americans, impaired insulin secretion is major mechanism in East-Asians.[8,9] Insulin secretory capacity has been shown to be half in Japanese type 2 diabetes compared to Caucasian patients, a difference that is particularly pronounced for meal-related secretion.[10] The reasons for decreased insulin secretion in East-Asian patients with type 2 diabetes are yet to be determined but may be explained by lower beta cell mass, impaired beta cell function and genetic differences.[2,3,4] These findings observed primarily in East-Asians are in sharp contrast to findings which suggests, that young healthy South-Asian people are more insulin resistant and have significantly higher insulin level following a 6 hour oral glucose tolerance test (OGTT) compared to their Caucasian counterparts.[11] There are significant differences in body composition between South-Asians and Caucasians. South-Asians typically have increased central obesity with thin limbs suggestive of smaller muscle mass, as evidenced by higher waist-to-hip ratio and greater sub-scapular: Triceps skinfold ratio.[12] Interestingly, with the same BMI, visceral fat mass is higher in South-Asians compared with Caucasians.[13] Consequently, South-Asians exhibits increased insulin resistance as demonstrated by fasting hyperinsulinemia, reduced insulin sensitivity on OGTT and reduced glucose disposal during the euglycemic-hyperinsulinemic clamp study.[13,14,15,16,17,18,19] A recent meta-analysis demonstrated that, there could be a hyperbolic relationship amongst Africans, Caucasian and East-Asians when insulin sensitivity index is kept on the X-axis and acute insulin response on the Y-axis.[20] This study clearly revealed that Caucasian are located around the middle point of hyperbolic curve, while African and East-Asian are located around unstable extreme points of hyperbola and it is highly likely that a slightest change in one variable could lead to a large change in the other variables. It is also believed, that East-Asians have a limited innate capacity of insulin secretion which may decrease by progressive aging or beta cell exhaustion due to continuous ongoing insulin resistance. Therefore, it can be presumed that in East-Asians, even with slightest instability and vulnerability in canalization of beta cell, with a concomitant decrease in insulin secretion can easily promote diabetogenesis. This hypothesis may contribute to the increased prevalence of diabetes in Asia as a whole.[19,20]
Interestingly, treatment response to medication could also be heterogeneous.[4,19] Given the contribution of the insulin secretory defect and insulin resistance to the differences in the patho-physiology of type 2 diabetes between East-Asians, South-Asians and non-Asians, the response to certain anti-diabetic drugs including incretin-based therapies could also differ by ethnic groups.[20] This review will be an attempt to critically analyze those studies available in literature and to learn as to why Asians may have different response to incretin-based therapies compared to non-Asians.
Ethnic differences in secretion and metabolism of endogenous incretin
There is no substantial clarity as to what happens to GLP-1 level on varying degree of dysglycemia. Literature also varied a lot on this issue. One of the very first and largest cross-sectional studies by Toft-Nielsen et al. demonstrated that the postprandial GLP-1 levels, the area under the curve (AUC) and the GLP-1 increments following a 4-hour mixed meal tolerance tests were significantly lower in type 2 diabetes, when compared to impaired glucose tolerance (IGT) or normal glucose tolerance (NGT) groups, albeit fasting GLP-1 were normal in all groups including type 2 diabetes. This study suggested a highly significant (53%) reduction in incremental GLP-1 concentrations and overall 19% reduction in the AUC in type 2 diabetes, compared to healthy controls.[21] Subsequently, several other investigators also supported this finding and suggested a progressive decrease in GLP-1 level and GLP-1 responsiveness with worsening degree of hyperglycemia, starting from NGT to IGT to frank type 2 diabetes.[22,24,25,26,27,28] However, some recent studies challenge those findings and point no changes in GLP-1 levels in either IGT or type 2 diabetes.[23,29,30,31] Furthermore, two meta-analysis available also suggested no changes in GLP-1 level.[31,32] Nevertheless, it should be noted that all these studies measuring GLP-1 level in varying degree of dysglycemia are not concordant and therefore, any conclusion regarding GLP-1 level still remains elusive.
Although the mechanism for these discrepancies is far from clear, several factors could be responsible for showing these diverging results. Sample size, sampling time, treatment influence, detection methods and diagnostic criteria of pre-diabetes or diabetes across the studies as well as course of disease can possibly influence the GLP-1 measurements. Age, body weight, non-esterified fatty acid (NEFA) and glucagon level can also influence the GLP-1 secretion.[31] Taken together, it is apparently clear that only studies, which longitudinally assess GLP-1 secretion in pre-diabetic cohort followed until frank diabetes and subsequent progression over some years, can probably yield any substantial conclusion in this regard.
It should be noted, that ethnic differences may also be attributed to significant difference in fasting and glucose-stimulated GLP-1 levels among different races [Figure 1].[33] From Asians perspective, data are even more conflicting as significant differences in total GLP-1, intact GLP-1 and GIP have been observed in some studies. Total GLP-1 level in Asians also varied from low to normal.[28,29,30,34] A study by Yabe et al. showed negligible GLP-1 response after meal ingestion despite robust GIP response in both healthy and diabetic Japanese subjects [Figure 2].[34] The reason for this reduced GLP-1 response is not exactly clear but may be explained by different meal size as well as meal composition (nutrient-induced) that may be sometime critical to GLP-1 response.[35,36,37,38] In contrast, young healthy South-Asian showed higher GLP-1, higher insulin level after a glucose load when compared to Caucasian counterparts.[39] It still remains to be elucidated whether this higher GLP-1 response in South-Asians are due to a compensatory increased secretion or GLP-1 resistant state. Nevertheless, this study by Sleddering et al. suggested that peak GLP-1 levels preceded the peak insulin response and paralleled with insulinogenic index, thereby suggesting a direct relation between the increased GLP-1 response and the insulin secretion by the β-cell.[39]
Figure 1.
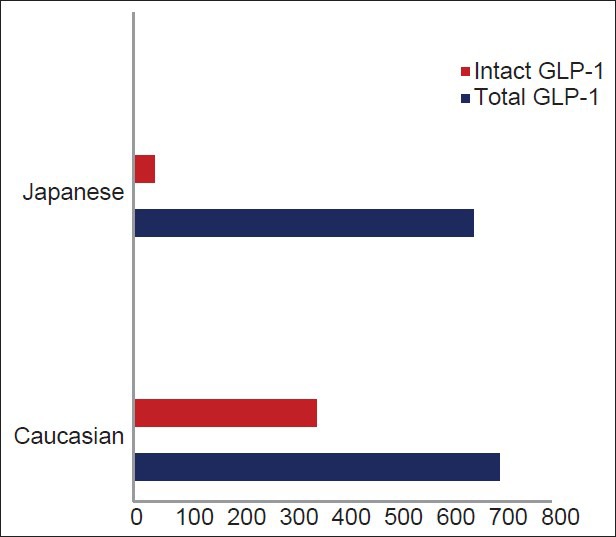
GLP-1 level in Asian versus Caucasian (adapted from Lee et al.)
Figure 2.
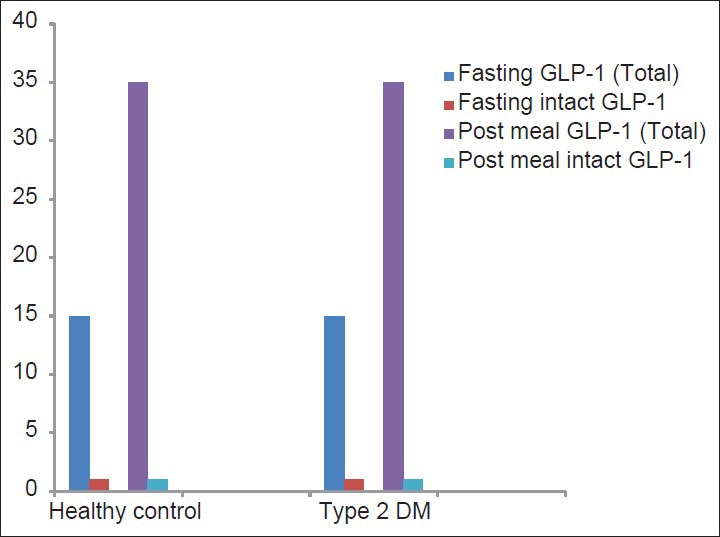
Total GLP-1 and intact GLP-1 (pmol-min/L) in Japanese healthy control versus type 2 DM (adapted from Yabe et al.)
Intact GLP-1 level were found to be considerably low in both Japanese type 2 diabetes and healthy controls, compared to Caucasians.[34,40,41] The very low levels of intact GLP-1 can occur, due to either impaired secretion from the gut or accelerated metabolism by DPP-4, or both. Logically, any finding of low intact GLP-1 despite significant peak of total GLP-1 (following a glucose load) would hint toward a possible DPP-4-enhanced GLP-1 metabolism. Intriguingly, this study by Yabe et al. also showed a higher intact GIP: total GIP ratio, thereby implying that enhanced DPP-4 activity was selective to GLP-1. Although, it appears that GLP-1 is more liable to DPP-4 processing compared to GIP, further studies are really required to understand the basis of the selective reduction of intact GLP-1 in Japanese.[42] Nevertheless, these data must be interpreted with caution in the light of different assay and methodology used to measure total GLP-1 or intact GLP-1 in these studies.[43] Interestingly, total GIP level following a glucose load or mixed meal were higher in Japanese and levels of intact GIP were similar compared to Caucasians.[22,34,40,41] This might suggests, a possible increase in processing of GIP by DPP-4 in Japanese. It should be noted that although the GIP response are enhanced in both Caucasians and Japanese type 2 diabetes (compared to healthy controls), the GLP-1 response in Japanese type 2 diabetes is significantly reduced. The reason for perceived enhanced GIP response in diabetic patients is not yet clear fully.
Enhanced DPP-4 activities in type 2 diabetes have been observed by many researchers; nevertheless, these findings are discordant among individual studies. Some studies suggested increased DPP-4 activity, some unchanged and some decreased DPP-4 activity.[44,45,46,47,48,49,50,51,52,53,54] However, a recent meta-analysis by Fadini et al. suggested a 33% enhanced DPP4 activity in type 2 diabetes.[54] Therefore, it can be assumed that the reduction of postprandial active GLP-1 in type 2 diabetes could possibly be attributable to either impairment in GLP-1 secretion or an increase of its degradation (because of enhanced DPP-4 activity) or both. If the latter mechanism is found to be predominant in Asians as suggested by Lee and Yabe et al. and substantiated further, than it can be speculated that DPP-4 inhibitors might do a trick in East-Asians by suppressing enhanced DPP-4 activity.[29,34]
In summary, as there is no significant difference in either GLP-1 or GIP levels between T2DM and healthy control, incretin deficiency does not seems to be accountable for the reduced insulin response in Japanese. Nevertheless, findings of low intact GLP-1 levels and low GLP-1 response after meals might have some correlation to reduced insulin secretory capacity and hence there could be exaggerated response to incretin-based therapies in the Japanese diabetic patients. Whether this finding from Japanese study can be extrapolated to South-Asians seems debatable. Also, very limited numbers of literatures are currently available on GLP-1 response in South-Asians. One study by Ahluwalia et al. suggested no significant differences in the gastro-intestinally mediated glucose disposal (GIGD %) between South-Asians and Caucasians.[55] Therefore, we clearly need further studies to learn whether GLP-1 secretion could be similar or different in South-Asians compared to East-Asians or Caucasians.
Role of adiponectin and its relation to incretin response in Asians
Adiponectin is an adipokine, secreted exclusively by adipose tissue and has putative insulin-sensitizing, anti-atherogenic and anti-inflammatory properties.[56] Total serum adiponectin concentration (all multi-meric isoforms) is inversely proportional to both visceral fat and insulin resistance. Low adiponectin has been found to predict the future development of incident type 2 diabetes in various populations including Pima Indian, Caucasian, Japanese, and native South-Asian people.[57,58] Few studies consistently suggests that South-Asian have significantly lower adiponectin compared to Caucasian [Table 1].[59,60,61,62,63,64]
Table 1.
Adiponectin level in South-Asian versus Caucasians
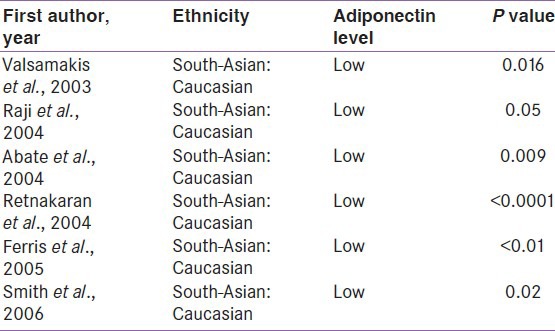
Study from India, showed a typical association of low adiponectin in both type 2 diabetes and the metabolic syndrome.[65] Another prospective Indian study also suggested that baseline hypoadiponectinemia independently predicted the future development of type 2 diabetes, after adjustment for covariates in IGT patients.[66] All these data confirm that hypoadiponectinemia in South-Asians is clinically significant and plays a crucial role in the pathophysiology of insulin resistance and type 2 diabetes.
It is believed that the intervention to increase adiponectin level could provide benefit against atherosclerosis and diabetes. Activation of PPAR-γ by thiazolidinediones increases adiponectin at the transcriptional level and pioglitazone is a significant enhancer of adiponectin.[67,68,69] Exendin-4 a GLP-1 receptor agonist, has shown to promote adiponectin secretion via the protein kinase-A pathway in high fat-fed rats. Few other experimental studies also showed that both GLP-1 agonist or DPP4 inhibitors increased serum adiponectin levels.[70,71,72,73] Unfortunately, GLP-1 agonist-mediated increase of adiponectin still remains unknown in human.[74] Interestingly, some data exist with DPP-4 inhibitors. An Italian multicenter, randomized, double-blind, placebo controlled trial, demonstrated that sitagliptin and metformin combination resulted in the significant increase of adiponectin, without change of body weight at 9 months.[75] In contrast, other Italian multicenter, randomized, double-blind clinical trial assessing the effect of sitagliptin or metformin added to pioglitazone monotherapy in T2DM patients indicated, no change of adiponectin level by sitagliptin at 12 months.[76] It is argued that sitagliptin-induced increase of adiponectin might have been masked by pioglitazone in this Italian study. A very recent randomized control trial in Japanese type 2 diabetes also showed sitagliptin increasing adiponectin without change of BMI.[77] Another observational study also showed an increase in plasma adiponectin level after 12 weeks of sitagliptin treatment in Japanese T2DM.[78] Recently, the 6 months treatment with vildagliptin has been found to increase adiponectin level.[79]
Taken together, it can be presumed that the long term DPP-4 inhibitor treatment will likely elevate serum adiponectin levels and in turn reduce insulin resistance in South-Asians with predominant hypoadiponectinemia. This could possibly be another mechanism by which these incretin-based drugs might modulate different response in Asians with hypoadiponectinemia.
Response to incretin based therapy in Asians versus non-Asians
Ethnic differences in response to drug treatment, have been identified in the literature. Few individual studies [Table 2] and a meta-analysis primarily conducted in Asian subjects, hinted at better HbA1c reduction with incretin-based therapies, when indirectly compared with the results from phase 3 global trials, done primarily in Caucasian, African-American and Hispanic populations.[80]
Table 2.
Effectiveness of incretin-based therapies in asians
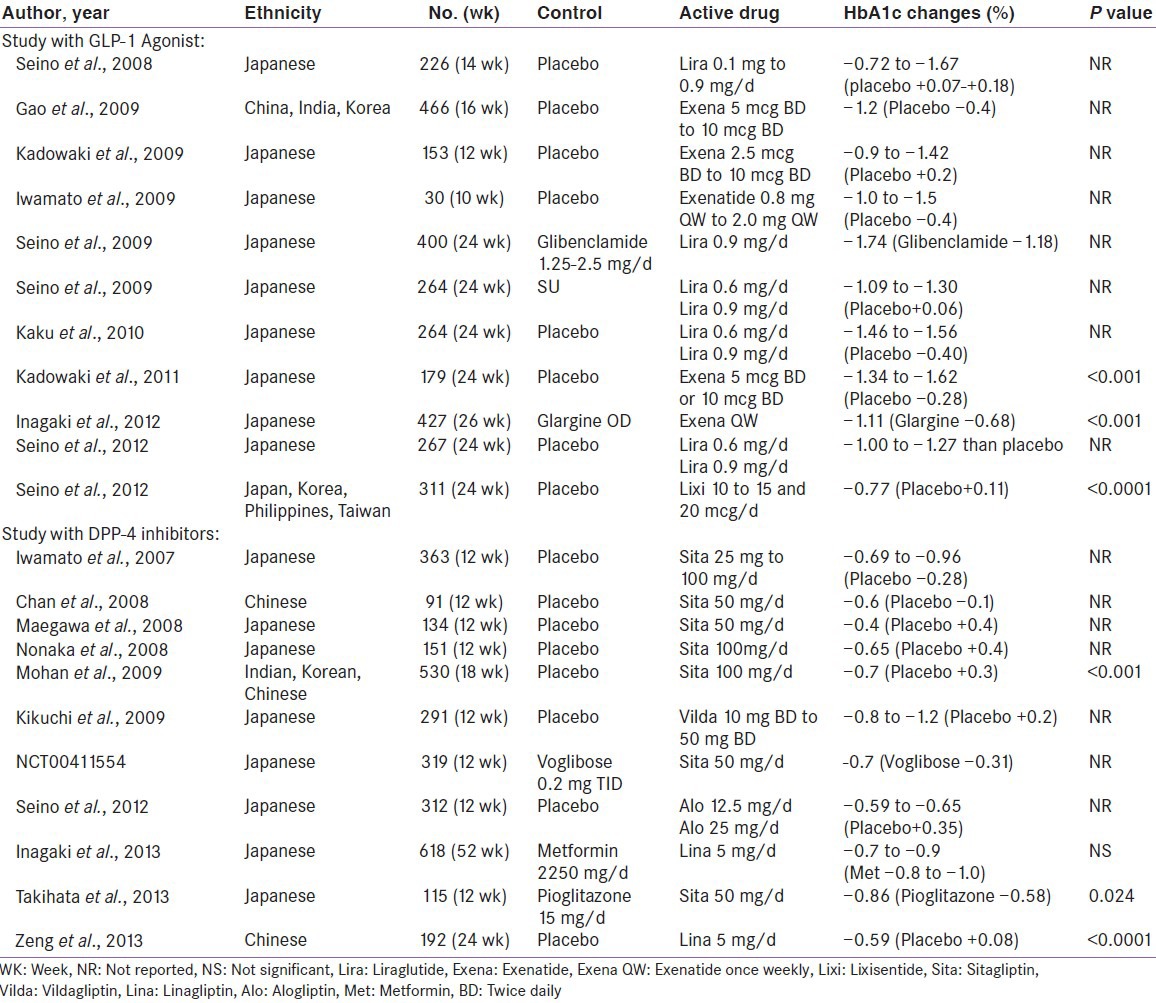
A randomized, double-blind, placebo-controlled, 18-week trial (n = 530) conducted by Mohan et al., evaluating efficacy and safety of Sitagliptin amongst Asian population (Korea, China and India) revealed significant glucose lowering (placebo-subtracted, -1.0%; P < 0.001) with Sitagliptin. Although, similar HbA1c reduction were noted in all three subpopulation relative to baseline, Indians and Koreans exhibited better HbA1c lowering (-1.4% each) compared to Chinese (-0.7%), against placebo. However, this appears to have occurred due to increase HbA1c in placebo arm in Indians (+0.7%) and Koreans (+0.6%) but decrease HbA1c in placebo arm of Chinese (-0.2%) patients.[81] A 24-week, real-life observational study (n = 14) conducted by Kesavadev et al. evaluating efficacy and safety of Liraglutide in Indian patients showed remarkable lowering of HbA1c (-2.26%, P < 0.001), which appears to be quite higher reduction compared to what had been observed in six phase 3 global randomized Liraglutide effect and Action in Diabetes study (maximum HbA1c reduction of -1.5% in LEAD-4 study).[82] Interestingly, a 16-week double blind randomized study (n = 929) by Yang et al. suggested a similar glucose lowering with liraglutide among all Asians (Chinese, Koreans and Indians).[83]
Subsequently, a meta-analysis done from 62 randomized controlled trial by Park et al. suggested a better glucose-lowering effect of DPP-4 inhibitor in Asian compared to non-Asians (Asians: −1.67%; 95%CI, −1.89 to −1.44 versus non-Asians: −0.65%; 95% CI, −0.71 to −0.60; P < 0.05; Figure 3).[84] Another recent meta-analysis done by Kim et al. also reported that both DPP-4 inhibitors (Asians: −0.92%; 95% CI, −1.03 to −0.82 versus non-Asians: −0.65%; 95%CI, −0.69 to −0.60; P < 0.001) and GLP-1 agonists (Asians: −1.16%; 95%CI, −1.48 to −0.85 versus non-Asians: −0.83%; 95%CI, −0.97 to −0.70; P = 0.044) were more effective in Asians compared to non-Asians when used in oral combination therapy [Table 3 and Figure 4]. Asian-dominant studies (studies with ≥50% Asians participants) clearly showed a greater HbA1c lowering effect than non-Asian-dominant studies (between-group difference for DPP-4 inhibitors: −0.18%, P = 0.006; between-group difference for GLP-1 agonist: −0.32%, P = 0.04).[85,86] Asian-dominant studies also suggested better fasting glucose reduction with DPP-4 inhibitor compared to non-Asian-dominant studies.[85] Univariate meta-regression analysis revealed a significant correlation of BMI with A1c reduction and Asian with lower BMI had better response with DPP-4 inhibitors. This significant correlation with BMI was also reported in other Japanese studies.[87] Ironically, a recent two-nation wise audit database from UK suggested lower incretin response and trends of lesser weight reduction with GLP-1 agonist in South-Asians compared to Caucasian.[88]
Figure 3.
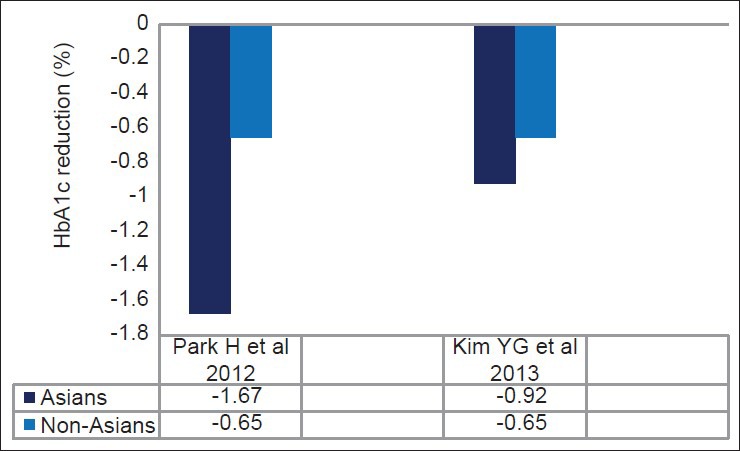
Effect of DPP-4 inhibitors in Asians versus non-Asians: Meta-analysis
Table 3.
Effectiveness of incretin based therapies in Asian versus non-Asians: Meta-analysis
Figure 4.
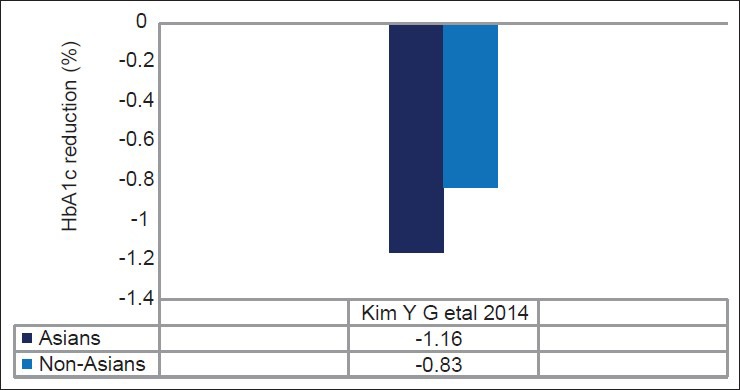
Effect of GLP-1 agonist in Asians versus non-Asians: Meta-analysis
It is worth mentioning that this meta-analysis of Asian-dominant studies by Kim et al. mainly represents East-Asians and cannot be extrapolated to South-Asians, where insulin resistance contributes majorly. Heterogeneity in the studies included in these studies may also contribute to possible bias which is inherent to any meta-analysis and therefore these results should be interpreted cautiously.
CONCLUSION
It is apparently clear that pathogenesis of type 2 diabetes may have different underlying mechanism between Asians and non-Asians. Even among Asians, etio-pathogenesis could be different between East-Asians and South-Asians. While insulin resistance could be major mechanism for Caucasian, Europeans and South-Asians; insulin secretory defects seem to be underlying predominant mechanism in East-Asians. Hypoadiponectinemia could be another emerging mechanism for type 2 diabetes in South-Asians.
Although literature intriguingly varied about GLP-1 secretion with progressive dysglycemia, two meta-analyses from these studies suggest no significant deterioration in GLP-1 secretion. Ethnic differences in GLP-1 secretion are another important factor. Amplitude, responsiveness and pattern of GLP-1 secretion following a meal may also differ in different ethnicity. Difference in meal size and composition can also influence GLP-1 enhancement. Lesser intact: total GLP-1 in East-Asian may suggest enhanced DPP-4 activity. Taken together, these theories may suggest a differential impact of incretin-based therapies in East-Asian. On the other hand, South-Asians doesn’t show similar characteristics secretory defect seen with East-Asians, however, further studies are clearly required to understand differential GLP-1 response among all Asians.
Hypoadiponectinemia have been demonstrated unequivocally in South-Asians and suggested to be critically responsible for ensuing insulin resistance. Long-term uses of DPP-4 inhibitors have been found to be associated with an enhancement in adiponectin levels in some of Italian and Japanese studies, however, no studies have been conducted so far in South-Asian, so as to suggest that enhancement of adiponectin by DPP-4 inhibitors could make any differential impact on them.
Nevertheless, clinical trials comparing effect of incretin-based therapies indirectly hint a significant and exaggerated response to incretin-based therapies in Asians (mainly comprising East-Asians) compared to non-Asians. Although the exact reasons for better response to incretin-based therapies are not yet fully known but this might be explained by the fact that East-Asians are less obese and possess “thrifty” genotype, which probably ensues more insulin secretory defects compared to non-Asians and hence these agents could be more effective. However, whether these results can be extrapolated to South-Asians or Indians is not yet known and neither has been demonstrated through any dedicated studies so far. A prospective study involving different ethnic groups may enlighten these possible differences in incretin response if at all it exists.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1. [Last accessed on 2014 Jul 7]. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf .
- 2.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–18. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 5.Chturvedi N, Stevens LK, Fuller JH, Lee ET, Lu M. The WHO Multinational Study Group, Risk factors, ethnic differences and mortality associated with lower extremity gangrene and amputation in diabetes. The WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44:S65–71. doi: 10.1007/pl00002941. [DOI] [PubMed] [Google Scholar]
- 6.Lee ET, Keen H, Bennett PH, Fuller JH, Lu M. The WHO Multinational Study Group, WHO Multinational Study Group: Follow-up of the WHO Multinational study of vascular disease in diabetes: General description and morbidity. Diabetologia. 2001;44:S3–13. doi: 10.1007/pl00002936. [DOI] [PubMed] [Google Scholar]
- 7.The DECODE-DECODA Study Group. Age, body mass index and type 2 diabetes: Associations modified by ethnicity. Diabetologia. 2003;46:1063–70. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 8.Seino Y. Relevance of incretins in the treatment of Asian patients with Type 2 diabetes. Diabetes Res Clin Pract. 2008;79:S4. [Google Scholar]
- 9.Takeuchi M, Okamoto K, Takagi T, Ishii H. Ethnic difference in patients with type 2 diabetes mellitus in inter-East Asian populations: A systematic review and metaanalysis focusing on fasting serum insulin. Diabetes Res Clin Pract. 2008;81:370–6. doi: 10.1016/j.diabres.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66S:S37–43. doi: 10.1016/j.diabres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Sleddering MA, Bakker LE, Janssen LG, Meinders AE, Jazet IM. Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism. 2014;63:226–32. doi: 10.1016/j.metabol.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Yajnik CS. The insulin resistance epidemic in India: Fetal origins, later lifestyle, or both? Nutr Rev. 2001;59:1–9. doi: 10.1111/j.1753-4887.2001.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 13.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–71. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 14.Retnakaran R, Hanley AJ, Connelly PW, Sermer M, Zinman B. Ethnicity modifies the effect of obesity on insulin resistance in pregnancy: A comparison of Asian, South Asian, and Caucasian women. J Clin Endocrinol Metab. 2006;91:93–7. doi: 10.1210/jc.2005-1253. [DOI] [PubMed] [Google Scholar]
- 15.Raji A, Gerhard-Herman MD, Warren M, Silverman SG, Raptopoulos V, Mantzoros CS, et al. Insulin resistance and vascular dysfunction in nondiabetic Asian Indians. J Clin Endocrinol Metab. 2004;89:3965–72. doi: 10.1210/jc.2004-0087. [DOI] [PubMed] [Google Scholar]
- 16.Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:2329–35. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 17.Liew CF, Seah ES, Yeo KP, Lee KO, Wise SD. Lean, nondiabetic Asian Indians have decreased insulin sensitivity and insulin clearance, and raised leptin compared to Caucasians and Chinese subjects. Int J Obes Relat Metab Disord. 2003;27:784–9. doi: 10.1038/sj.ijo.0802307. [DOI] [PubMed] [Google Scholar]
- 18.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–44. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 19.Cockram CS. Diabetes mellitus: Perspective from the Asia-Pacific region. Diabetes Res Clin Pract. 2000;50:S3–7. doi: 10.1016/s0168-8227(00)00202-3. [DOI] [PubMed] [Google Scholar]
- 20.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, et al. Determinants of the impaired secretion of glucagon-like peptide- 1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 22.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 23.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–87. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 24.Lugari R, Dei CA, Ugolotti D, Finardi L, Barilli AL, Ognibene C, et al. Evidence for early impairment of glucagon-like peptide 1-induced insulin secretion in human type 2 (Non Insulin-Dependent) Diabetes. Horm Metab Res. 2002;34:150–4. doi: 10.1055/s-2002-23199. [DOI] [PubMed] [Google Scholar]
- 25.Rask E, Olsson T, Soderberg S, Holst JJ, Tura A, Pacini G, et al. Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism. 2004;53:624–31. doi: 10.1016/j.metabol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vanttinen M, Stancakova A, et al. Insulin sensitivity, insulin release and glucagon like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;1:502–11. doi: 10.1007/s00125-007-0899-2. [DOI] [PubMed] [Google Scholar]
- 27.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–8. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 28.Fang Z, Tang X, Cao H, Lu Q, Li N, Liu Y, et al. Impaired secretion of total glucagon-like peptide-1 in people with impaired fasting glucose combined impaired glucose tolerance. Int J Med Sci. 2012;9:574–81. doi: 10.7150/ijms.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Yabe D, Nohtomi K, Takada M, Morita R, Seino Y, et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J. 2010;7:119–26. doi: 10.1507/endocrj.k09e-269. [DOI] [PubMed] [Google Scholar]
- 30.Kozawa J, Okita K, Imagawa A, Iwahashi H, Holst JJ, Yamagata K, et al. Similar incretin secretion in obese and non-obese Japanese subjects with type 2 diabetes. Biochem Biophys Res Commun. 2010;393:410–3. doi: 10.1016/j.bbrc.2010.01.134. [DOI] [PubMed] [Google Scholar]
- 31.Nauck AM, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia. 2011;54:10–8. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 32.Calanna S, Christensen M, Holst JJ, Laferrere B, Gluud LL, Vilsboll T, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: Systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–72. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velasquez-Mieyer PA, Umpierrez GE, Lustig RH, Cashion AK, Cowan PA, Christensen M, et al. Race affects insulin and GLP-1 secretion and response to a long-acting somatostatin analogue in obese adults. Int J Obes Relat Metab Disord. 2004;28:330–3. doi: 10.1038/sj.ijo.0802561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yabe D, Kuroe A, Lee S, Watanabe K, Hyo T, Hishizawa M, et al. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: Comparison of type 2 diabetes patients and healthy controls. J Diabetes Investig. 2010;1:56–9. doi: 10.1111/j.2040-1124.2010.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, et al. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E779–84. doi: 10.1152/ajpendo.90233.2008. [DOI] [PubMed] [Google Scholar]
- 36.Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkanen HM. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr. 2002;75:254–62. doi: 10.1093/ajcn/75.2.254. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, et al. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69:1135–43. doi: 10.1093/ajcn/69.6.1135. [DOI] [PubMed] [Google Scholar]
- 38.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sleddering MA, Bakker LE, Janssen LG, Meinders AE, Jazet IM. Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism. 2014;63:226–32. doi: 10.1016/j.metabol.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 41.Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, et al. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes. 2004;53:654–62. doi: 10.2337/diabetes.53.3.654. [DOI] [PubMed] [Google Scholar]
- 42.Deacon CF, Nauck MA, Meier J, Hucking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–81. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 43.Deacon CF, Holst JJ. Immunoassays for the incretin hormones GIP and GLP-1. Best Pract Res Clin Endocrinol Metab. 2009;23:425–32. doi: 10.1016/j.beem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Toft-Nielsen M, Damholt M, Hilsted J, Hughes TE, Krarup T, Madsbad S, et al. GLP-1 secretion is decreased in NIDDM patients compared to matched control subjects with normal glucose tolerance. Diabetologia. 1999;A40:143. [Google Scholar]
- 45.Meneilly GS, Demuth HU, Mcintosh CH, Pederson RA. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet Med. 2000;17:346–50. doi: 10.1046/j.1464-5491.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- 46.Korosi J, Mcintosh CH, Pederson RA, Demuth HU, Habener JF, Gingerich R, et al. Effect of aging and diabetes on the enteroinsular axis. J Gerontol A Biol Sci Med Sci. 2001;56:M575–9. doi: 10.1093/gerona/56.9.m575. [DOI] [PubMed] [Google Scholar]
- 47.Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–72. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 48.Ryskjaer J, Deacon CF, Carr RD, Krarup T, Madsbad S, Holst JJ, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006;155:485–93. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 49.McKillop AM, Duffy NA, Lindsay JR, O’Harte FP, Bell PM, Flatt PR. Decreased dipeptidyl peptidase-IV activity and glucagon-like peptide-1 (7-36) amide degradation in type 2 diabetic subjects. Diabetes Res Clin Pract. 2008;79:79–85. doi: 10.1016/j.diabres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Han SJ, Kim HJ, Choi SE, Kang Y, Lee KW, Kim DJ. Incretin secretion and serum DPP-IV activity in Korean patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89:e49–52. doi: 10.1016/j.diabres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Firneisz G, Varga T, Lengyel G, Fehr J, Ghyczy D, Wichmann B, et al. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: A novel liver disease biomarker. PLoS One. 2010;5:e12226. doi: 10.1371/journal.pone.0012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pala L, Ciani S, Dicembrini I, Bardini G, Cresci B, Pezzatini A, et al. Relationship between GLP-1 levels and dipeptidyl peptidase-4 activity in different glucose tolerance conditions. Diabet Med. 2010;27:691–5. doi: 10.1111/j.1464-5491.2010.03010.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee S, Yabe D, Nohtomi K, Takada M, Morita R, Seino Y, et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J. 2010;7:119–26. doi: 10.1507/endocrj.k09e-269. [DOI] [PubMed] [Google Scholar]
- 54.Fadini GP, Albiero M, Menegazzo L, de Kreutzenberg SV, Avogaro A. The increased dipeptidyl peptidase-4 activity is not counteracted by optimized glucose control in type 2 diabetes, but is lower in metformin-treated patients. Diabetes Obes Metab. 2012;14:518–22. doi: 10.1111/j.1463-1326.2011.01550.x. [DOI] [PubMed] [Google Scholar]
- 55.Ahluwalia R, Knop FK, Vilsbøll T, Ranganath I, Meier JJ, Vora J. Chicago: ADA; 2013. 173-LB. [Google Scholar]
- 56.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–8. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 57.Cnop C, Havel PJ, Utzschneider K, Carr D, Sinha M, Boyko E, et al. Relationship of Adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 58.Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese population: The Funagata study. Diabetes Care. 2003;26:2015–20. doi: 10.2337/diacare.26.7.2015. [DOI] [PubMed] [Google Scholar]
- 59.Valsamakis G, Chetty R, McTernan PG, Al-Daghri NM, Barnett AH, Kumar S. Fasting serum adiponectin concentration is reduced in Indo-Asian subjects and is related to HDL cholesterol. Diabetes Obes Metab. 2003;5:131–5. doi: 10.1046/j.1463-1326.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- 60.Raji A, Gerhard-Herman MD, Warren M, Silverman SG, Raptopoulos V, Mantzoros CS, et al. Insulin resistance and vascular dysfunction in non diabetic Asian Indians. J Clin Endocrinol Metab. 2004;89:3965–972. doi: 10.1210/jc.2004-0087. [DOI] [PubMed] [Google Scholar]
- 61.Abate N, Chandalia M, Snell P, Grundy SM. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab. 2004;89:2750–5. doi: 10.1210/jc.2003-031843. [DOI] [PubMed] [Google Scholar]
- 62.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Hypoadiponectinemia in South Asian women during pregnancy: Evidence of ethnic variation in adiponectin concentration. Diabet Med. 2004;21:388–92. doi: 10.1111/j.1464-5491.2004.1151.x. [DOI] [PubMed] [Google Scholar]
- 63.Ferris WF, Naran NH, Crowther NJ, Rheeder P, Van der Merwe L, Chetty N. The relationship between insulin sensitivityand serum adiponectin levels in three population groups. Horm Metab Res. 2005;37:695–701. doi: 10.1055/s-2005-870580. [DOI] [PubMed] [Google Scholar]
- 64.Smith JD, Al-Amri M, Sniderman AD, Cianflone K. Leptin and adiponectin in relation to body fat percentage, waist to hip ratio and the apoB/apoA1 ratio in Asian Indian and Caucasian men and women. Nutr Metab. 2006;3:18. doi: 10.1186/1743-7075-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snehalatha C, Mukesh B, Simon M, Vishwanathan V, Haffner SM, Ramachandran Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26:3226–9. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 66.Mohan V, Deepa R, Pradeepa R, Vimaleswaran KS, Mohan A, Velmurugan K, et al. Association of low Adiponectin levels with the metabolic syndrome: The Chennai Urban Rural Epidemiology Study (CURES-4) Metabolism. 2005;54:476–81. doi: 10.1016/j.metabol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPAR gamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 68.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–63. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 69.Nakatsuji H, Kishida K, Funahashi T, Shimomura I. Senri Study II Group. Three-month treatment with pioglitazone reduces circulating levels of thiobarbituric acid-reacting substances, a marker of reactive oxidative stress, without change in body mass index, in Japanese patients with type 2 diabetes. Atherosclerosis. 2010;212:243–5. doi: 10.1016/j.atherosclerosis.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 70.Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, et al. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun. 2009;390:613–8. doi: 10.1016/j.bbrc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Yang G, Li Q, Tan X, Liu H, Tang Y, et al. Exenatide prevents fat-induced insulin resistance and raises adiponectin expression and plasma levels. Diabetes Obes Metab. 2008;10:921–30. doi: 10.1111/j.1463-1326.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 72.Miyagawa K, Kondo T, Goto R, Matsuyama R, Ono K, Kitano S, et al. Effects of combination therapy with vildagliptin and valsartan in a mouse model of type 2 diabetes. Cardiovasc Diabetol. 2013;12:160. doi: 10.1186/1475-2840-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakr HF. Effect of sitagliptin on the working memory and reference memory in type 2 diabetic Sprague-Dawley rats: Possible role of adiponectin receptors 1. J Physiol Pharmacol. 2013;64:613–23. [PubMed] [Google Scholar]
- 74.Diaz-Soto G, De Luis DA, Conde-Vicente R, Izaola-Jauregui O, Ramos C, Romero E. Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters and cardiovascular risk biomarkers in patients with Type 2 diabetes: A prospective study. Diabetes Res Clin Pract. 2014;104:92–6. doi: 10.1016/j.diabres.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 75.Derosa G, Carbone A, Franzetti I, Querci F, Fogari E, Bianchi L, et al. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2012;98:51–60. doi: 10.1016/j.diabres.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 76.Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Ragonesi PD, Querci F, et al. Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metabolism. 2010;59:887–95. doi: 10.1016/j.metabol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Hibuse T, Maeda N, Kishida K, Kimura T, Minami T, Takeshita E, et al. A pilot three-month sitagliptin treatment increases serum adiponectin level in Japanese patients with type 2 diabetes mellitus- a randomized controlled trial START-J study. Cardiovasc Diabetol. 2014;13:96. doi: 10.1186/1475-2840-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kubota Y, Miyamoto M, Takagi G, Ikeda T, Kirinoki-Ichikawa S, Tanaka K, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J Korean Med Sci. 2012;27:1364–70. doi: 10.3346/jkms.2012.27.11.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shestakova MV, Suhareva OI, Chernova TO, Shmushkovich IA, Aleksandrov AA, Ilin AV, et al. A combination of dipeptidyl peptidase-4 inhibitor and metformin in the treatment of patients with type 2 diabetes mellitus: Effective control of glycemia, weight, and quantitative body composition. Ter Arkh. 2013;85:49–55. [PubMed] [Google Scholar]
- 80.Louisa M, Takeuchi M, Takeuchi M, Nafrialdi, Setiabudy R. Incretin-based therapies for type 2 diabetes mellitus in Asian patients: Analysis of clinical trials. Med J Indones. 2010;19:205–12. [Google Scholar]
- 81.Mohan V, Yang W, Sone HY, Xu L, Noble L, Langdon RB, et al. Safety and efficacy of sitagliptin in the treatment of patient with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83:106–16. doi: 10.1016/j.diabres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Kesavadev J, Shankar A, Krishnan G, Jothydev S. Liraglutide therapy beyond glycemic control: An observational study in Indian patients with type 2 diabetes in real world setting. Int J Gen Med. 2012;5:317–22. doi: 10.2147/IJGM.S27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: A 16-week, randomized, double-blind, active control trial. Diabetes Obes Metab. 2011;13:81–8. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 84.Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: Meta-analysis. Ann Pharmacother. 2012;46:1453–69. doi: 10.1345/aph.1R041. [DOI] [PubMed] [Google Scholar]
- 85.Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: A systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 86.Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: A systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–9. doi: 10.1111/dom.12293. [DOI] [PubMed] [Google Scholar]
- 87.Aso Y, Ozeki N, Terasawa T, Naruse R, Hara K, Suetsugu M, et al. Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res. 2012;159:25–31. doi: 10.1016/j.trsl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Melbourne: International Diabetes Federation; 2013. The efficacy of exenatide and liraglutide among South Asians in the Association of British Clinical Diabetologists nationwide audits; p. 1349. [Google Scholar]


