Abstract
Background:
The peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor subfamily of transcription factors. It has been reported that they play important roles in obesity and the development of type 2 diabetes mellitus (T2DM).
Materials and Methods:
This case-control study was carried out among 764 Qatari patients with diabetes and 764 healthy subjects above 20 years of age at Primary Healthcare Clinics (PHCs) from January 2011 to December 2012. Face-to-face interviews were based on a questionnaire that included variables such as age, sex, sociodemographic status, body mass index (BMI) and other clinical parameters. The Pro12Ala in the PPARγ2 gene was detected on the LightCycler using two specific probes. Univariate and multivariate statistical analysis were performed.
Results:
The study revealed that in the diabetes group, Pro/(10.2% vs 9.4%; P = 0.606) and Ala/Ala (1.4% vs 0.9%; P = 0.343) were higher than in controls, whereas Pro/Pro (88.4% vs 89.7%;P = 0.413) was lower in diabetes patients, but no significant difference was observed among the genotype groups. In obese patients with diabetes, Pro/Pro (89% vs 89.9%;P = 0.792) and Pro/Ala (8.9% vs 10.1%;P = 0.671) were lower than in obese healthy subjects. No homozygous Ala/Ala was found in obese healthy subjects, whereas 6 Ala/Ala homozygotes were in obese diabetes group. But in diabetes group, obese patients had higher homozygous of Pro/Pro (89.3% vs 87.8%;P = 0.523) and Ala/Ala (1.8% vs 1.2%;P = 0.771) compared to non-obese patients.
Conclusion:
The current study did not reveal an association between the Pro12Ala polymorphism of the PPAR γ2 gene and type 2 diabetes (T2D) in Qatari's population.
Keywords: Allele frequency, consanguinity, diabetes mellitus, obesity, PPARγ2 gene, Pro12Ala polymorphism
INTRODUCTION
Type 2 diabetes has a strong genetic component. There is evidence that the Pro12Ala polymorphism is linked to obesity and T2D.[1] The PPARγ is now recognized to play a main role in the formation of fat cells and insulin resistance. The PPARγ2 is a candidate gene for obesity and T2D. Obesity may be associated with elevated PPARγ2 expression.[2] The 12 Ala allele may protect from type 2 diabetes mellitus.[3] There was no association found with markers of adiposity and insulin resistance.[4] The contradictory findings observed in various studies highlighted the importance to study the impact of Pro12Ala polymorphism of the PPARγ2 gene on diabetes and obesity.[3,4,5,6]
MATERIALS AND METHODS
This is a matched case-control study carried out among patients with diabetes and healthy subjects at the Primary Healthcare Clinics (PHCs). The survey was conducted among primary health care visitors during the period from January 2011 to December 2012. All subjects were native Qataris identified by themselves as being native Qataris by at least two pedigrees (parents and grand parents). The study was approved by the Research Ethics Committee of the Hamad General Hospital, Hamad Medical Corporation. All the persons who agreed to participate in this study gave their informed consent prior to their inclusion in the study.
Sample size was calculated with 80% power, 18% exposure among controls, Odds ratio 1.50, at the 1% level of significance and 99% confidence interval which were found to be 856 subjects in each group. We have approached 856 patients with diabetes and 764 gave their verbal consent to participate in this study with a response rate of 89.3%. Similarly, 938 healthy subjects were approached and 764 controls agreed to take part in this study (81.4%).
The data was collected through a validated questionnaire with the help of pre-trained nurses. This was an originally developed questionnaire in English and translated to Arabic language in order to understand the questions mentiond in the questionnaire. A standard forward-backward procedure was applied to translate the English version to Arabic and vice versa. The translated Arabic version of the questionnaire was checked by a bilingual consultant. Internal consistency defined by Cronbach's alpha coefficient was used to assess the internal consistency of the translated instrument. The internal consistency of the designed questionnaire Cronbach's alpha coefficient was evaluated and alpha equal to or greater than 0.85 was considered satisfactory.
The designed survey instrument included information on socio-demographic charecteristics, anthropometry measures, and laboratory investigation. Qualifed nurses were instructed to interview and assist the patients to complete the questionnaire. The study included male and female Qatari nationals above 20 years of age. The study excluded patients aged below 20 years, patients with any serious physical illness and who refused to give consent to take part in the study. The reason for exclusion of individuals <20 yrs is that the study focused on adults because of its objective to find the association on diabetes mellitus and obesity in consanguineous population. The survey instrument was tested on 100 patients who visited the health centers and thus validated the questionnaire. The survey was based on standardized interviews performed by trained health professionals and nurses.
Selection of diabetic subjects
We studied Qatari diabetic subjects diagnosed for T2D in accordance with the established diagnostic criteria. A total number of 764 diabetic Qatari patients aged above 20 years were studied by a simple random process from the Primary Health Care Centers. These patients were classified as diabetes if their venous blood glucose values were higher than or equal to 7 mmol/l or if they were taking medication for diabetes at the time of the study.
Selection of control subjects
Control subjects were self-reported, aged above 20 years of age with random glucose value of <6.1 mmol/L, and if had never taken any diabetic medication. Since T2D is a late onset disorder, the control subjects selected were above the age of 35. This group consisted of a random sample of 764 healthy Qatari subjects who visited the PHC Centers for any reason other than acute or chronic disease.
Height and weight were measured using standardized methods and all the participants wore light clothes and no shoes for this part of the examination. Body mass index (BMI) was calculated as weight (kilograms)/height (meters).[1] Subjects were classified into three categories: acceptable weight, BMI <25; overweight, BMI 25-30; and obese, BMI >30.[2]
Blood pressure measurement was carried out by trained practical nurses according to World Health Organization (WHO) standardized criteria.[7] The mean value obtained from three readings was used in the analysis. Hypertension was defined according to WHO criteria as SBP >140 mmHg and/or DBP >90 mmHg and/or the use of antihypertensive medication. Mean blood pressure was defined as 1/3 systolic pressure +2/3 diastolic pressure. The pulse pressure was calculated as the difference between systolic and diastolic pressures.
Procedures
Blood samples were collected by venipuncture in vacutainer EDTA tubes and used for manual DNA purification using the QiaAmp mini DNA kits. Random glucose values were determined by using the One Touch Sure Step Kit.
The single nucleotide polymorphism (SNP) detection protocol was based on a modified procedure of Ghoussaini et al., and consisted of an amplification step by polymerase chain reaction (PCR) followed by mutation analysis of the PCR product by real time PCR.[4] The PCR primer set consisted of: PGE × 1F 5′-AAT AGG ACA GTG CCA GCC-3′ and PGE × 1R 5′-TAC ATA AAT GCC CCC ACG-3′. The reaction was performed in a final volume of 25 μl and contained: 1 × GeneAmp® PCR Gold buffer; 2.5 mM MgCl2, 1% DMSO, 0.25 mM each dNTP, two primers (PGE × 1F: 4.3 nM and PGE × 1R 3.58 nM), 0.75U AmpliTaq Gold® DNA Polymerase, and 100-500 ng template DNA. The PCR cycles consisted of an initial denaturation at 95°C for 12 minute followed by 35 cycles, which consisted of denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds, elongation at 72°C for 30 seconds.
The Pro12Ala in the PPAR γ2 gene was detected on the LightCycler using two specific probes: (Sensor [G] 5′-CTC CTA TTG ACG CAG AAA GCG-FL and PPAR Anchor 5′ LC Red 640- TCC TTC ACT GAT ACA CTG TCT GCA AAC ATA TC-PH). The melting temperature profiles were interpreted as follows; wild type (CC): 54°C, mutant (GG): 60°C and heterozygous (CG): 54°C and 60°C.
Statistical analysis
Student-t test was used to ascertain the significance of differences between two means of a continuous variable and confirmed by non-parametric Mann-Whitney test. Chi-square analysis was performed to test for differences in proportions of categorical variables between two or more groups. In 2 × 2 tables, the Fisher's exact test (two-tailed) replaced the Chi-square test if the assumptions underlying Chi-square violated, namely in case of small sample size and where the expected frequency is less than 5 in any of the cells. Odds ratio (OR) and their 95% confidence intervals (CI) was calculated by using Mantel-Haenszel test. One Way Analysis of variance (ANOVA) was employed for comparison of several group means and to determine the presence of significant differences between group means. The level P ≤ 0.05 was considered as the cut-off value for significance.
RESULTS
The socio-demographic data of the studied diabetic and healthy subjects showed a higher proportion of patients with diabetes in the age group 50-59 years (33%), followed by 40-49 years (32.6%). Most of the patients with diabetes were married (84.6%), had secondary education (26.6%), not working (33.4%) and low economic status (QRs. 10,000-15,000) (38.4%). There was a significant association between diabetic and non-diabetic subjects in terms of age group (P < 0.001), marital status (P < 0.001) and occupation (P = 0.007). Consanguinity was significantly more prevalent in patients with diabetes (39.9%), than in non-diabetic subjects (24.9%) (P < 0.001) [Table 1].
Table 1.
Socio-demographic characteristics of the studied patients with diabetes and healthy subjects
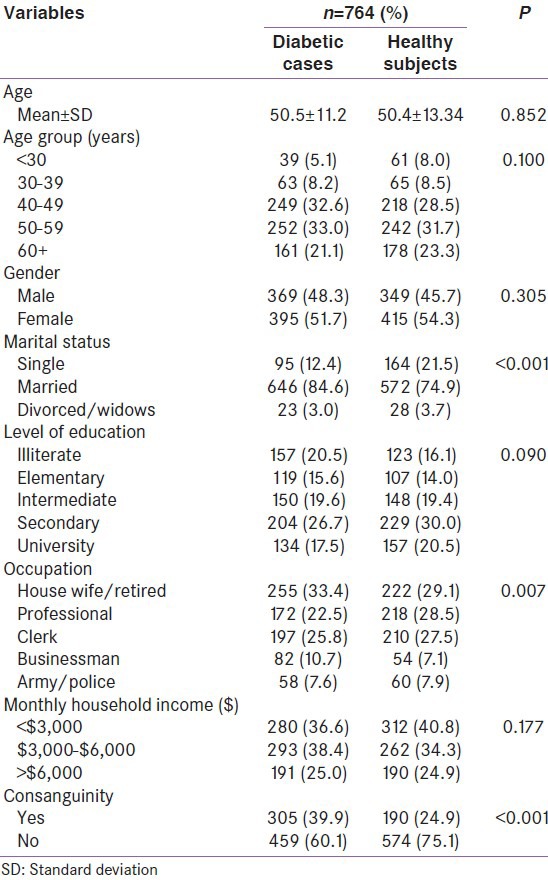
The PPARγ2 genotype distribution in patients with diabetes vs. non-diabetes and obese patients with diabetes vs. non-diabetes were examined. Genotype distribution in patients with diabetes was 88.4% homozygous for the Pro/Pro, 10.2% hetrerozygous for the Pro/Ala and 1.4% Ala/Ala/homozygous. In the non-diabetic group, Pro/Pro was slightly higher (89.7%), but Pro/Ala (9.4%) and Ala/Ala (0.9%) were lower than in the diabetic group. There was no significant difference observed in the proportions of the different genotypes between the diabeteic patients and the control group [Table 2].
Table 2.
The PPARγ2 genotype distribution in diabetic vs. non-diabetic subjects and obese diabetic vs. non-diabetic subjects
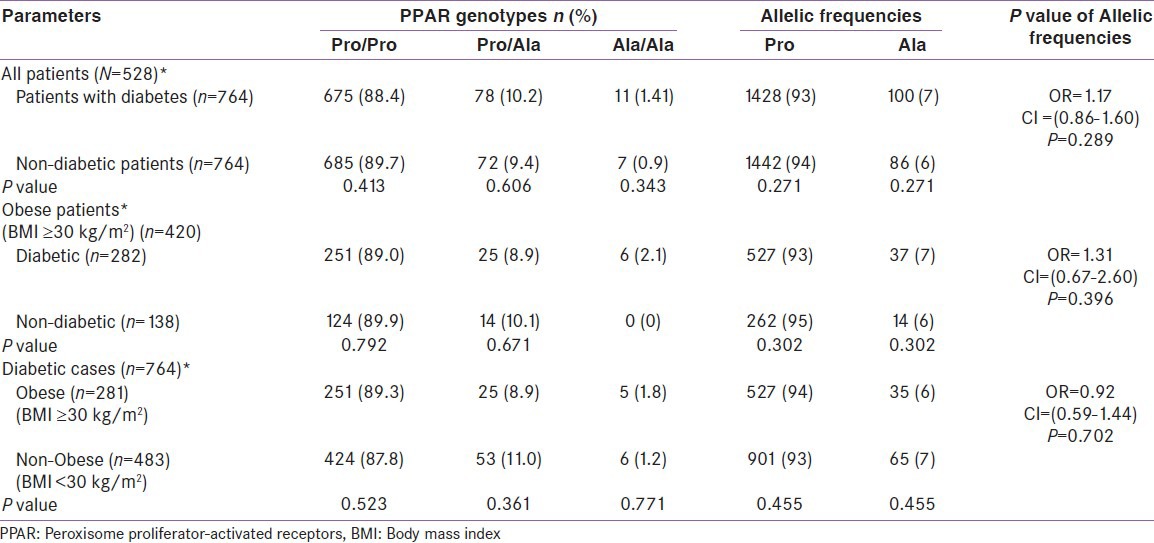
Frequency of the Pro/Pro (89.0% vs. 89.9%; P = 0.792) and Pro/Ala (8.9% vs. 10.1%; P = 0.671) carriers tends to be slightly lower in the obese patients with diabetes compared to controls. The frequency of Ala/Ala was zero in healthy subjects, while 6 homozygous carriers of Ala/Ala in diabetic paitents. No significant difference was found in the genotype distribution between the obese diabetic and non-diabetic subjects. In diabetes group, obese patients had higher Pro/Pro (89.3% vs. 87.8%; P = 0.523) and Ala/Ala (1.8% vs. 1.2%; P = 0.774) homozygous compared to non-obese patients, where Pro/Ala heterozygous was higher in non-obese patients with diabetes (11% vs. 8.9%; P = 0.361) compared to their counterparts.
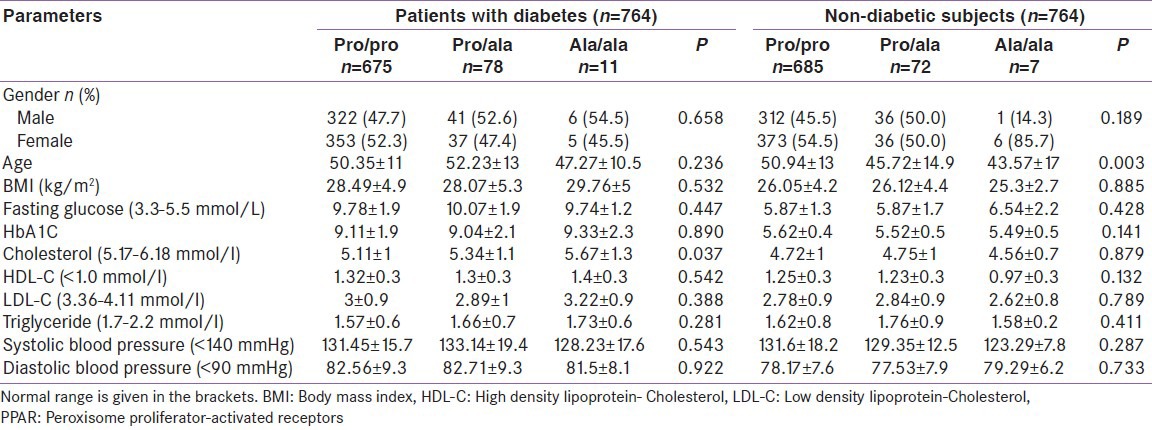
The baseline characteristics of the patients with diabetes and non-diabetes by PPARγ2 genotype revealed that the fasting insulin levels in the Pro/Ala carriers (10.07 ± 1.9) was higher within the group of diabetes (P = 0.447), while fasting insulin in the Ala/Ala carriers (6.54 ± 2.2) was higher in the non-diabetic subjects (P = 0.428). In a group of diabetes, no significant difference was observed among the genotype groups for the individual study parameters, except for serum cholesterol (5.67 ± 1.3; P = 0.037). Interms of blood pressure, higher reading was found in Pro/Ala carriers; SBP (133.14 ± 19.4; P = 0.543) and DBP (82.71 ± 9.3; P = 0.922) compared to other genotype groups Pro/Pro and Ala/Ala. In non-diabetes group, there was no significant difference in any of the parameters studied among the genotype groups; BMI (26.12 ± 4.4; P = 0.885), Cholesterol (4.75 ± 1; P = 0.879), LDL (2.84 ± 0.9; P = 0.789), and Triglyceride (1.76 ± 0.9;P = 0.411) were higher in Pro/Ala carriers [Table 3].
Table 3.
Baseline characteristics of the patients with diabetes and non-diabetic subjects by PPARγ2 genotype
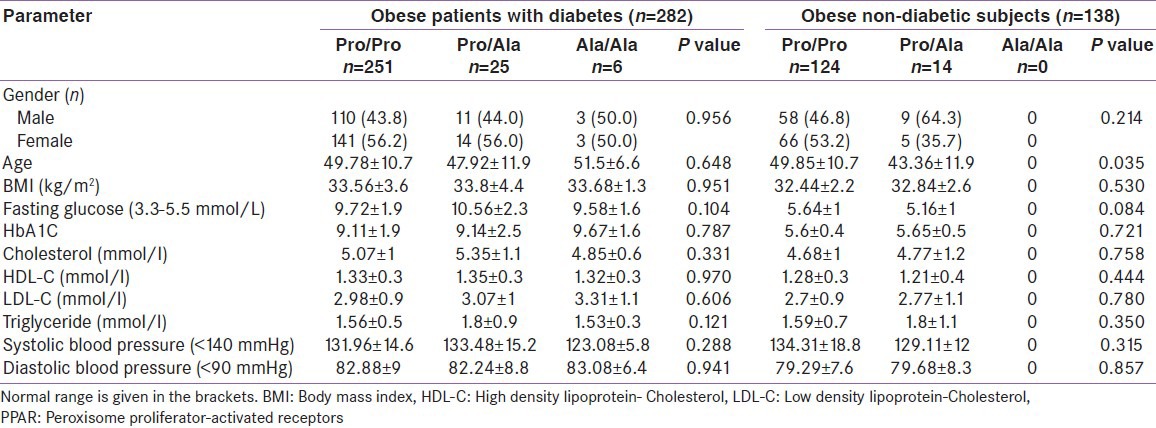
The baseline charecteristics of the obese patients with diabetes and non-diabetic subjects by PPARγ2 genotype showed that. Pro Ala had a higher fasting glucose (10.56 ± 2.3; P = 0.104), cholesterol (5.35 ± 1.1; P = 0.331), triglyceride (1.8 ± 0.9; P = 0.121) and SBP (133.48 ± 15.2;P = 0.288) than those with common allele (PP) and Ala/Ala carriers in obese patients with diabetes, but no significant difference was observed. BMI was similar in three genotypes. In the obese non-diabetic group, there were no Ala/Ala carriers. Subjects with Pro/Pro had a higher fasting glucose level compared to the Pro/Ala type (5.64 ± 1 vs. 5.16 ± 1; P = 0.084) and also a higher HDL (1.28 ± 0.3 vs. 1.21 ± 0.4; P = 0.444). Pro/Ala carriers in the obese non-diabetic subjects had higher total cholesterol (4.77 ± 1.2 vs. 4.68 ± 1.2; P = 0.758) and triglyceride (1.8 ± 1.1 vs. 1.59 ± 0.7; P = 0.350). No significant difference was detected between genotypes in both obese diabetic and non-diabetes groups [Table 4].
Table 4.
Baseline characteristics of the obese patients with diabetes and non-diabetic subjects by PPARγ2 genotype
DISCUSSION
The present study determined the frequency of the proline to alanine susbstitution in the human PPAR γ2 gene in Qatari population and investigated its role on diabetes mellitus and obesity in a highly consanguineous population. The allelic frequency of Pro12Ala, a polymorphic variant of the PPAR γ2 gene in diabetic subjects or nondiabetic subjects vary among different ethnic communities. In the studied Qatari population, the frequency of Pro12Ala allele was greater (10.2%) in diabetic population than in non-diabetic group (9.4%), but no significant difference was observed between the groups. The similar study finding that higher frequency of Pro12Ala in diabetic population, was found in other studies of Caucasians population in different countries which showed that the frequency of Pro12Ala allele was greater than 10%.[4,5] This shows that the polymorphism Pro12Ala plays some role in T2DM in the Qatari population. It was reported that the Pro12Ala allele is associated with protection against diabetes in both Caucasians and Asians.[1,8] However, no significant association was detected in the study sample between the the Pro12Ala polymorphism and type 2 diabtes. In Qatari patients with diabetes, Pro12Ala allele was found being causative rather than a protective allele. A similar result was observed in German and Italian populations.[5,9]
Also, Ala/Ala (1.41) was quite higher in the studied diabetic population compared to healthy subjects (0.9%), but not statistically significant which is similar to the result found in Czech diabetic population (1.09%).[10] On the contrast, Japanese and Caucasian studeis reported that the allelic frequency of Ala 12 was significantly lower in type 2 diabetic group than in non-diabetic group which suggests that the Ala 12 allele of the PPAR γ2 gene may protect subjects with this polymorphism from type 2 diabetes.[3,4] This is not the case in our study that the frequency of Ala 12 allele was higher in the diabetic group and the difference between the group of diabetic and non-diabetic subjects did not reach statistical significance. Hence, no significant association was detected between the Ala 12 allele and and type 2 diabetes in Qatari population. Study results of Mori et al., and Hara et al., showed that the frequency of the Ala 12 variant of the PPAR γ2 gene is higher in the control group than in the group of patients explaining the fact that PPAR γ2 is associated with a decreased risk of the type 2 diabetes.[3,8]
Among Iranian population, obese healthy subjects with Pro/Ala and Ala/Ala had a higher BMI in comparison to individuals with the common allele.[11] On the contrary, the studied obese healthy subjects had higher Pro/Pro (89.9% vs. 89%) and Pro/Ala (10.1% vs. 8.9%) frequency compared to patients with diabetes, whereas Ala/Ala (2.1%) was higher in obese patients with diabetes, but no significant difference was found between the groups. This shows that the healthy subjects with Pro12Ala allele may have higher insulin sensitivity and gain weight without developing diabetes.
The Iranian non-obese diabetic group with Pro/Ala and Ala/Ala had higher BMI. In the present study, non-obese diabetic cases had higher Pro/Ala (11% vs. 8.9%, but lower Ala/Ala (1.2% vs. 1.8%) compared to diabetic obese cases. In the study population, we could not detect a significant difference in Ala allele frequency between obese diabetic and non-dioabetic groups as well as between diabetic obese and non-obese groups. In the Iranian population, a significant association between obesity and Ala allele frequency was observed.[11] In contrast, no association was reported between PA/AA polymorphism and obesity in our diabetic group which is consistent with several other studies too.
The correlation between the PPAR γ2 genotype and clinical parameters of the T2DM patients vs. non-diabetic subjects were investigated and found that fasting blood glucose (10.07 ± 1.9) was higher in diabetic subjects with the Pro12Ala compared to homozygous of Pro/Pro (9.78 ± 1.9) and Ala/Ala (9.74 ± 1.2). A study of Mori et al., also reported that 12 Ala variant is associated with a lower level of insulin secretarion in patients with diabetes.[8] Along with the fasting blood glucose, systolic blood pressure (133.14 ± 19.4) and diastolic blood pressure (82.71 ± 9.3) were higher in Diabetic group. Majority of the biochemical values were higher in patients with diabetes with Ala/Ala carriers HBA1C (9.33 ± 2.3), cholesterol (5.67 ± 1.3), HDL (1.4 ± 0.3), LDL (3.22 ± 0.9) and Triglyceride (1.73 ± 0.6) compared to PP/AA allelies. Another finding was that BMI was found higher in studied patients with diabetes with Ala/Ala carriers compared to PP/PA allelies. Although there is no significant association between the three groups of patients with diabetes, the observed phenomenon might be related to the notion that once diabetes is developed, the protective effect of Ala12 allele may be lost as reported in a study of Stumvoll et al.[12]
In obese patients with diabetes of the present study, subjects with PA/AA had higher BMI (33.8 ± 4.4 and 33.68 ± 1.3), than those with the common allele (PP) (33.56 ± 3.6) which is in agreement with the results found in the Iranian population.[11] In Iranian population, there was a significant association between obesity with Ala allele frequency, but the present study could not find a significant difference. The study found many various associations of the Pro12Ala polymorphism with BMI, insulin sensitivity, total cholesterol, HDL, LDL, Triglyceride and Blood pressure in obese patients with diabetes and controls. Studies conducted in German and Arab polulation reported no association between Pro12Ala polymorphism and obesity in DM subjects which supports our study results.[13,14]
CONCLUSION
The study findings revealed that the there is no association between Pro12Ala polymorphic variant and type 2 diabetes. The results did not support the role of the Pro12Ala polymorphism as the genetic risk factor for obesity in the Qatari diabetic population. The results reflected the actual genotype metabolic interactions in fasting and OGTT stimulated conditions.
ACKNOWLEDGMENT
The project was supported and funded by the Diabetic Association and Qatar Foundation for generous support and help while this project conducted. We also, would like to thank Hamad Medical Corporation for their approval this study (HMC Research Protocol # 10262/10, April 2011).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Danawati CW, Nagata M, Moriyama H, Hara K, Yasuda H, Nakayama M, et al. A possible association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma2 gene with obesity in native Javanese in Indonesia. Diabetes Metab Res Rev. 2005;21:465–9. doi: 10.1002/dmrr.543. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM. PPAR-y: Adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 3.Hara K, Okada T, Tobe K, Yasuda K, Mori Y, Kadowaki H, et al. The Pro12Ala polymorphism in PPARγ-2 may confer resistance to type 2 diabetes. Biochem Biophys Res Commun. 2000;271:212–6. doi: 10.1006/bbrc.2000.2605. [DOI] [PubMed] [Google Scholar]
- 4.Ghoussaini M, Meyre D, Lobbens S, Charpentier G, Clement K, Charles MA, et al. Implication of the Pro12Ala polymorphism of the PPAR-gamma 2 gene in type 2 diabetes and obesity in the French population. BMC Med Genet. 2005;22:6–11. doi: 10.1186/1471-2350-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altshuler D, Hirschhorn JN, Klannenmark M, Lindgren CM, Vohl MC, Nemesh J, et al. The common Pro12Ala PPAR polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 6.Badii R, Bener A, Zirie M, Al-Rikabi A, Simsek M, Al-Hammaq AO, et al. Lack of association between the Pro12Ala polymorphism of the PPAR-y2 gene and type 2 diabetes mellitus in the Qatari consanguineous population. Acta Diabetol. 2008;45:15–21. doi: 10.1007/s00592-007-0013-8. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. International Society of Hypertension Writing Group: 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Mori H, Kegami H, Kawaguchi Y, Seino S, Yokoi N, Takeda J, et al. The Pro12Ala substitution is associated with resistance to development of diabetes in the general population. Diabetes. 2001;5:891–4. doi: 10.2337/diabetes.50.4.891. [DOI] [PubMed] [Google Scholar]
- 9.Ringel J, Engeli S, Distler A, Sharma AM. Pro12Ala missense mutation of the peroxisome proliferator activated receptor γ2 and DM. Biochem Biophys Res Commun. 1999;254:450–3. doi: 10.1006/bbrc.1998.9962. [DOI] [PubMed] [Google Scholar]
- 10.Sramkova D, Kunesova M, Hainer V, Hill M, Vcelak J, Bendlova B. Is a Pro12Ala polymorphism of the PPAR γ2 gene related to obesity and type 2 Diabetes Mellitus in the Czech population. Ann N Y Acad Sci. 2002;967:265–73. doi: 10.1111/j.1749-6632.2002.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 11.Mirzaei H, Akrami SM, Golmohammadi T, Doosti M, Heshmat R, Nakhjavani M, et al. Polymorphism of Pro12Ala in the Peroxisome Proliferator-activated Receptor γ2 gene in Iranian diabetic and obese subjects. Metab Syndr Relat Disord. 2009;7:453–8. doi: 10.1089/met.2008.0099. [DOI] [PubMed] [Google Scholar]
- 12.Stumvoll M, Haring H. Perspectives in Diabetes: The Peroxisome Proliferator–Activated Receptor-γ 2 Pro12Ala Polymorphism. Diabetes. 2002;51:2341–7. doi: 10.2337/diabetes.51.8.2341. [DOI] [PubMed] [Google Scholar]
- 13.Zietz B, Barth N, Spiegel D, Schmitz G, Scholmerich J, Schazer A. Pro12Ala polymorphism in the Peroxisome Proliferator activated receptor gamma 2 (PPAR γ2) is associated with high level of total cholesterol and LDL cholesterol in male Caucasian type 2 diabetes patients. Exp Clin Endocrinol Diabetes. 2002;110:60–6. doi: 10.1055/s-2002-23487. [DOI] [PubMed] [Google Scholar]
- 14.Wakil SM, Al-rubean K, Alsmadi O, Imtiaz F, Carroll P, Rajab M, et al. The peroxisome and type 2 diabetes in an Arab population. Diabetes Care. 2006;29:171–2. doi: 10.2337/diacare.29.1.171. [DOI] [PubMed] [Google Scholar]


