Abstract
Background:
Aldose reductase (AR) is the rate-limiting enzyme in the glucose metabolism, which has been implicated in the pathogenesis of diabetic microvascular complications (MVCs). Frequent C-106T polymorphism in the promoter of the AR gene may change the expression of the gene.
Aims:
The aim of the following study is to study the association between AR C106T genotypes and diabetic MVCs in Iranian population.
Materials and Methods:
We included 206 type 2 diabetic patients categorized into two groups according to the presence or absence of diabetic microangiopathy. The cases of interest were diabetic neuropathy, retinopathy and nephropathy identified during clinical and or laboratory examination. In addition, 114 age- and sex-matched individuals were selected to serve as a control group. AR genotyping was done using an amplification gel electrophoresis.
Results:
The frequency of CC genotype was specifically higher in subjects with diabetic retinopathy as compared to those without it (53.2% vs. 38.1%, P = 0.030). Patients with diabetic microangiopathy in general; however, did not differ significantly between AR genotype groups.
Conclusion:
The C-106T polymorphism in the AR gene is likely a risk factor for development of only retinal complication of diabetes microvascular in Iranian individuals.
Keywords: Aldose reductase genotypes, C-106T polymorphisms, diabetic microvascular complications
INTRODUCTION
Diabetic microvascular disease, one of the most important complications in diabetes mellitus (DM), has been considered a major cause of health impairment world-wide.[1,2,3,4] Many biochemical mechanisms have explained the structural and functional abnormalities associated with overexposure of the vascular tissues to hyperglycemia including advanced glycation end products, increase in aldose reductase (AR) activity, free radicals accumulation and protein-kinase C activation through diacylglycerol accumulation. All these pathways, are associated with the production and signaling of vascular endothelial growth factor, contributing to the development of diabetic complications.[4] Although disease duration is the main predictive factor for the development of diabetic microvascular complications (MVCs); including nephropathy, neuropathy and retinopathy; an associated condition including glycemic control, blood pressure and genetic susceptibility specially in the polyol pathway may modify development of these complications.[5,6,7,8,9,10,11,12,13,14,15,16] AR, the first enzyme of the polyol pathway, probably plays a vital role in the pathogenesis of the long-term complications of diabetes by means of alterations that result from sorbitol increase and myo-inositol reduction due to increase enzyme activity and expression of the AR gene.[17] Persistent hyperglycemia causes increased enzyme activity and expression of AR and a reduced flux of enzyme sorbitol dehydrogenase. This imbalance results in the accumulation of sorbitol, which leads to several abnormalities in cellular metabolism and hence contributes to the development of diabetic complications.[3,18] Although, a C-106T single nucleotide polymorphism in the promoter of the AR gene (AKR1B1) is associated with AKR1B1 expression, however, whether this polymorphism is associated with the development of diabetic MVCs is still challenging.[19,20,21,22,23,24,25] The prevalence of DM in the Iranian population is estimated to be relatively high; around 8% in the population aged between 30 and 69 years.[26,27] To the best of our knowledge, there are no studies investigating the influence of AR polymorphism on this subpopulation to date. Hence, this study was undertaken to investigate whether the C-106T allele of the AR gene polymorphism is associated with MVCs in a group of Iranian participants with type 2 diabetes.
MATERIALS AND METHODS
Ethical certificate was obtained from the ethics committee of the Molecular Biology Research Center of Mazandaran University of Medical Sciences. The study was performed in Sari city where subjects in this study were Iranian people living in Mazandaran province and all provided informed consent to participate. Deoxyribonucleic acid (DNA) samples from 206 Iranian patients with type 2 diabetes were taken. A total of 114 DNA samples from normal healthy Iranian subjects who had no family history of diabetes were randomly taken to obtain control frequencies. Participants were recruited from the Eye Clinic and from the diabetic out-patient clinic of the Diabetes Research Centre Sari. The inclusion criteria were defined as all diabetic patients with at least 5 years disease duration. Patients with pregnancy, heritable diseases (such as Biedle), chronic drugs using (such as corticosteroids), uveitis and retinal dystrophy and those aged 75 years or older were excluded from study. Patients underwent a comprehensive clinical and ophthalmic evaluation. The following demographic, clinical and laboratory information was documented for each subject: Age, sex, diabetes, age of onset and duration, presence of hypertension (blood pressure was corrected according to sex and age, presence of chronic complications (neuropathy and proteinuria), funduscopy, average three last glycosylated HbA1c, serum lipids and insulin usage. Blood pressure was measured, with hypertension being defined as systolic pressure 140 mm Hg or greater or diastolic pressure 90 mm Hg or greater. In this case-control study, comparisons between the groups of patients were performed according to the presence or absence of MVCs. Patients were also compared with a control group of 114 healthy individuals who had been studied from the same geographical region. All the subjects were informed about the aim of the study and gave their written consent.
The MVCs of interest were diabetic neuropathy, retinopathy and nephropathy, as previously described.[28] These are summarized as follows.
Retinal evaluation was performed by trained ophthalmologist and in case of abnormalities, documented by fundus fluorescein angiography. In the presence of diabetic retinopathy, it was classified as non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR).[29]
All patients provided two separate 24 h urine collection samples. Nephropathy was classified as an albumin excretion rate 30-300 mg/l/24 h as microalbuminuria and >300 mg/l/24 h as gross albuminuria in a timed urine collection after excluding urinary tract infection.[30]
Clinical evaluation for peripheral neuropathy was performed according to neurophysiologic studies with nerve conduction velocity (NCV). The clinical staging of peripheral neuropathy was defined according to involvement of the 14 nerves (both median, ulnar, peroneal, posterior tibial motor nerves and both median, ulnar, sural sensory nerves) as follows: Stage 0 (no neuropathy); Stage 1 (mild), one or two nerves affected; Stage 2 (moderate), three or four nerves affected; Stage 3 (severe), five or more nerves affected.[31]
The tests described in autonomic dysfunction are based on the responses of the heart rate and blood pressure to a variety of stimuli. A test reflecting cardiac parasympathetic damage was immediate heart rate response to standing, (30th beat: 15th beat ratios) <1.00 was abnormal. Sympathetic neuropathy occurred if the postural fall in blood pressure to standing was >30 mmHg.[32]
The blood samples (5 ml) were drawn from the antecubital vein from all subjects into ethylenediaminetetraacetic acid and processed for AR genotyping. A fasting blood sample for determining glycosylated hemoglobin (HbA1c) was also taken.[33]
The genomic DNA was extracted from the remaining white blood cells using a Macrogen DNA purification kit (Macrogen Inc., South Korea). The DNA samples were stored and shipped at 4°C. All kits and reagents were used according to their manufacturer's instructions. The C-106T polymorphism in the promoter of the AR gene was determined using conventional polymerase chain reaction (PCR).[17] The fragment containing the C-106T polymorphism was amplified using the following primers PRAF1 5’-CAG ATA CAG CAG CTG AGG AAC-3’ and PRAR1 5’-GCC TTC TGA TTG GTT GC A CT-3’. In the reverse primer the mismatch was introduced to give a restriction site for the BseN I restriction enzyme in the presence of allele C-106. The 159-bp PCR product was digested with the BseN I restriction enzyme into two fragments, 138 and 21 bp, in the presence of allele C-106 and remained undigested when the T-106 allele was present.
Statistical analysis
Data is expressed as mean ± standard deviation, or proportions for categorical variables. AR genotype variation was calculated using the Chi-square test. Categorical valuables were compared using Fisher's exact test. Non-parametric data was analyzed by the odds ratio test. Associations between the AR genotypes and DM, blood pressure, serum lipid values and diabetic MVCs were evaluated. P < 0.05 was considered to be statistically significant.
RESULTS
Age, sex, duration of type 2 diabetes, hypertension, hypercholesterolemia and HbA1c level of the subjects and the proportion of patients with diabetic MVCs in our study group are presented in Table 1. After adjusting for age and sex, patients with diabetic MVCs had higher HbA1c and longer diabetes duration than those without MVCs with statistical significance of P < 0.017 and <0.001 respectively.
Table 1.
Clinical and biochemical data of type 2 diabetic patients with and without DR
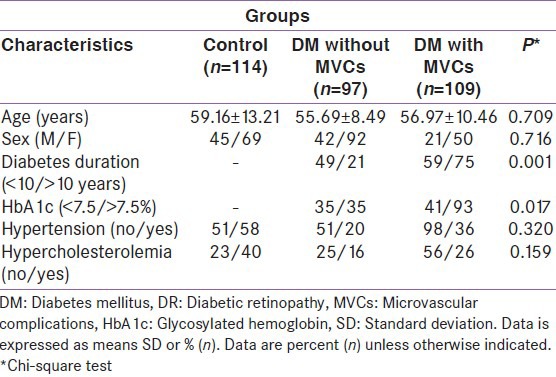
Among the diabetic participants; retinopathy, nephropathy and neuropathy were found in 109 (52.9%), 57 (28.8%) and 103 (58.5%) individuals, respectively. Diabetic nephropathy was found in 18.7% with microalbuminuria and10.1% with gross albuminuria. The patients with peripheral diabetic neuropathy was in 103 (33.5% mild, 17% moderate and 8% severe neurophysiologic change) individuals and autonomic dysfunction was in 61 (6.3% sympathetic, 16.5% parasympathetic or 11.9% both) respectively. In diabetic patients, 65.4% had at least one form of MVCs. With special emphasis on retinopathy, it was found that, 72 had NPDR, 37 had PDR and 35 had diabetic macular edema.
Table 2 shows the distribution of C-106T genotypes and alleles in the control and study group. When the whole study population was analyzed, no distortion in the genotype and alleles frequency among the study groups was observed. For the C-106T polymorphism, allele C was present in 67% of the subjects and allele T in 33%. The most common genotype of the C-106T polymorphism was CC corresponding to 46.1% of the cases, followed by CT and TT corresponding to 41.7% and 12.1% of the analyzed cases respectively. The frequencies of these polymorphisms were found to be in Hardy–Weinberg equilibrium both in diabetic and non-diabetic subjects.
Table 2.
Analysis of the frequency of C-106T allele polymorphism in the promoter of the AR gene in control and subgroups of type 2 diabetic patients
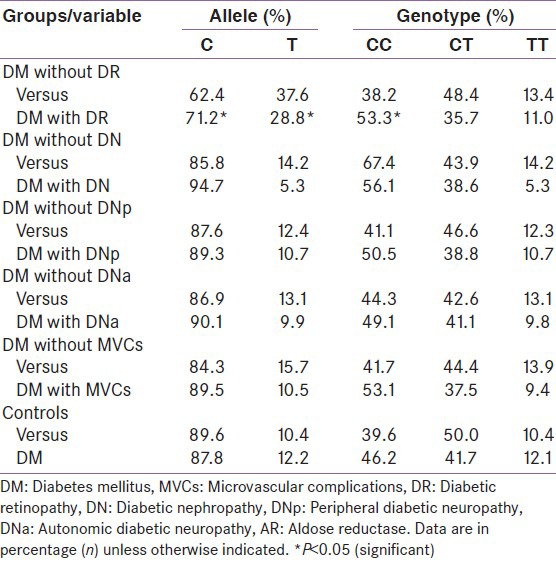
There were no significant differences in diabetic nephropathy, diabetic neuropathies and MVCs except retinopathy between patients with different ALR genotypes.
The frequency of CC genotype was significantly higher in subjects with DR as compared to those without it (53.3% vs. 38.1%, P = 0.030). The prevalence of DR tended to be higher as the number of C alleles increased (CC [53.3%], CT [35.7%], TT [11%], P = 0.0036). Furthermore, severity of DR was higher in subjects with CC genotype compared to those with CT or TT genotype (22.1% vs. 14.4%, P = 0.031). Among type 2 diabetic patients, the carriers of the T allele of the ALR had a significantly lower DR rate compared with those without the T allele (28.8% vs. 37.6%, P = 0.036).
There were no significant differences in HbA1c levels, hypertension, hypercholesterolemia or duration of diabetes between patients with different ALR genotypes.
DISCUSSION
Previous studies concerning the C-106T polymorphism of the AR gene in relation to MVCs have been conflicting. Diabetic patients carrying the T allele of the C-106T polymorphism of the AR gene had a significantly higher urinary albumin excretion rate and frequencies of microalbuminuria at the time of diagnosis than subjects with the C-106C genotype. However, the association between the T allele of the C-106T polymorphism and the prevalence of gross albuminuria was not observed in many studies.[25,34,35] In some studies, no association between the polymorphisms and the risk of retinopathy were observed,[36,37] which is in contrast with other reports from type 2 diabetic patients.[22,38,39,40] Diabetic patients with the T allele of the C-106T polymorphism of the AR gene had lower sensory response amplitudes and in cases with longer duration, a greater decrease in NCV of the motor nerve than the subjects with the C-106C genotype. This AR gene polymorphism may contribute to an early development of neurophysiologic deterioration in type 2 diabetic patients. However, no associations were found between polymorphisms and clinical polyneuropathy.[13,41]
It is interesting that in this case-control of subjects with type 2 diabetes, we found no association between the AR genotype and MVCs in general but found an association between diabetic retinopathy and the C-106T genotype in the population of type 2 diabetes patients. According to present study diabetic patients with the T allele of the AR gene had lower rate of DR than the subjects with the C-106C genotype.
A possible explanation is that discrepancy in AR genotype and MVCs with other population could be attributed to ethnic difference. Although the increased risk of DR associated with the presence of the C allele observed in the present study is relevant with the functional studies showing that the C allele is associated with an increased expression of AKR1B1 gene.[19,20]
CONCLUSION
No association was found between AR C106T polymorphism and other MVC except retinopathy.
ACKNOWLEDGMENT
This study was supported by grants-in-aid for scientific search from the Mazandaran University of Medical Sciences, Iran. We would like to thank Dr. Leila Sarparast and Mrs. Soheila Shahmohammadi, CRNA, Research Fellow, of the Bu Ali Sina Clinical Research Development Unit of the Mazandaran University of Medical Sciences, Sari, Iran for statistical analyses and technical assistance. The authors wish to sincerely thank Mr. Nakhai Head of Laboratory and Mrs. Mosavi staff head nursery of eye department, Bu Ali-Sina Hospital for support during sample collection. Appreciation also goes to the Molecular Biology and Biotechnology Department, Sari Medical School for his constructive criticism after reading the manuscript.
Footnotes
Source of Support: Financial support was provided by Mazandaran University of Medical Sciences, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Rosenblatt BJ, Benson WE. retinopathy. In: Yanoff M, Duken JS, editors. Ophtalmology. 2nd ed. St. Louis: Mosby; 2004. pp. 877–86. [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Ng MC, Lee SC, So WY, Tong PC, Cockram CS, et al. Phenotypic heterogeneity and associations of two aldose reductase gene polymorphisms with nephropathy and retinopathy in type 2 diabetes. Diabetes Care. 2003;26:2410–5. doi: 10.2337/diacare.26.8.2410. [DOI] [PubMed] [Google Scholar]
- 4.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diab Eye. 2004;27:5–10. [Google Scholar]
- 6.Fioretto P, Dodson PM, Ziegler D, Rosenson RS. Residual microvascular risk in diabetes: Unmet needs and future directions. Nat Rev Endocrinol. 2010;6:19–25. doi: 10.1038/nrendo.2009.213. [DOI] [PubMed] [Google Scholar]
- 7.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 2003;26(Suppl 1):S99–S102. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 8.Kim HW, Ko GJ, Kang YS, Lee MH, Song HK, Kim HK, et al. Role of the VEGF 936 C/T polymorphism in diabetic microvascular complications in type 2 diabetic patients. Nephrology (Carlton) 2009;14:681–8. doi: 10.1111/j.1440-1797.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 9.Ko BC, Lam KS, Wat NM, Chung SS. An (A-C) n dinucleotide repeat polymorphic marker at the 5’ end of the aldose reductase gene is associated with early-onset diabetic retinopathy in NIDDM patients. Diabetes. 1995;44:727–32. doi: 10.2337/diabetes.44.7.727. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury TA, Dyer PH, Kumar S, Barnett AH, Bain SC. Genetic determinants of diabetic nephropathy. Clin Sci (Lond) 1999;96:221–30. [PubMed] [Google Scholar]
- 11.Parving HH, Tarnow L, Rossing P. Genetics of diabetic nephropathy. J Am Soc Nephrol. 1996;7:2509–17. doi: 10.1681/ASN.V7122509. [DOI] [PubMed] [Google Scholar]
- 12.Marre M. Genetics and the prediction of complications in type 1 diabetes. Diabetes Care. 1999;22(Suppl 2):B53–8. [PubMed] [Google Scholar]
- 13.Sivenius K, Pihlajamäki J, Partanen J, Niskanen L, Laakso M, Uusitupa M. Aldose reductase gene polymorphisms and peripheral nerve function in patients with type 2 diabetes. Diabetes Care. 2004;27:2021–6. doi: 10.2337/diacare.27.8.2021. [DOI] [PubMed] [Google Scholar]
- 14.Hanis CL, Hallman D. Genetics of diabetic retinopathy. Curr Diab Rep. 2006;6:155–61. doi: 10.1007/s11892-006-0027-6. [DOI] [PubMed] [Google Scholar]
- 15.Petrovic MG, Peterlin B, Hawlina M, Petrovic D. Aldose reductase (AC) n gene polymorphism and susceptibility to diabetic retinopathy in Type 2 diabetes in Caucasians. J Diabetes Complications. 2005;19:70–3. doi: 10.1016/j.jdiacomp.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Fanelli A, Hadjadj S, Gallois Y, Fumeron F, Betoule D, Grandchamp B, et al. Polymorphism of aldose reductase gene and susceptibility to retinopathy and nephropathy in Caucasians with type 1 diabetes. Arch Mal Coeur Vaiss. 2002;95:701–8. [PubMed] [Google Scholar]
- 17.Kao YL, Donaghue K, Chan A, Knight J, Silink M. A novel polymorphism in the aldose reductase gene promoter region is strongly associated with diabetic retinopathy in adolescents with type 1 diabetes. Diabetes. 1999;48:1338–40. doi: 10.2337/diabetes.48.6.1338. [DOI] [PubMed] [Google Scholar]
- 18.Demaine A, Cross D, Millward A. Polymorphisms of the aldose reductase gene and susceptibility to retinopathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:4064–8. [PubMed] [Google Scholar]
- 19.Stevens MJ, Killen P, Wang P, Larkin DD, Greene DA. Overexpression of aldose reductase (AR) in human retinal pigment epithelial (RPE) cell lines is associated with a polymorphism in the AR basal promoter region. Diabetes. 2000;49(Suppl 1):A167–8. [Google Scholar]
- 20.Yang B, Millward A, Demaine A. Functional differences between the susceptibility Z-2/C-106 and protective Z+2/T-106 promoter region polymorphisms of the aldose reductase gene may account for the association with diabetic microvascular complications. Biochim Biophys Acta. 2003;1639:1–7. doi: 10.1016/s0925-4439(03)00095-4. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Xie P, Huang J, Gu Y, Zeng W, Song H. Polymorphisms and functions of the aldose reductase gene 5’ regulatory region in Chinese patients with type 2 diabetes mellitus. Chin Med J (Engl) 2002;115:209–13. [PubMed] [Google Scholar]
- 22.Richeti F, Noronha RM, Waetge RT, de Vasconcellos JP, de Souza OF, Kneipp B, et al. Evaluation of AC(n) and C(-106) T polymorphisms of the aldose reductase gene in Brazilian patients with DM1 and susceptibility to diabetic retinopathy. Mol Vis. 2007;13:740–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Olmos P, Futers S, Acosta AM, Siegel S, Maiz A, Schiaffino R, et al. (AC) 23 [Z-2] polymorphism of the aldose reductase gene and fast progression of retinopathy in Chilean type 2 diabetics. Diabetes Res Clin Pract. 2000;47:169–76. doi: 10.1016/s0168-8227(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Santos KG, Tschiedel B, Schneider J, Souto K, Roisenberg I. Diabetic retinopathy in Euro-Brazilian type 2 diabetic patients: Relationship with polymorphisms in the aldose reductase, the plasminogen activator inhibitor-1 and the methylenetetrahydrofolate reductase genes. Diabetes Res Clin Pract. 2003;61:133–6. doi: 10.1016/s0168-8227(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 25.Sivenius K, Niskanen L, Voutilainen-Kaunisto R, Laakso M, Uusitupa M. Aldose reductase gene polymorphisms and susceptibility to microvascular complications in Type 2 diabetes. Diabet Med. 2004;21:1325–33. doi: 10.1111/j.1464-5491.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 26.Golozar A, Khademi H, Kamangar F, Poutschi H, Islami F, Abnet CC, et al. Diabetes mellitus and its correlates in an Iranian adult population. PLoS One. 2011;6:e26725. doi: 10.1371/journal.pone.0026725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Amiri AA, Hashemi-Soteh MB, Haghshenas MR, Daneshvar F, Rastegar A, Farazmand T. Haptoglobin polymorphism in individuals with type 2 diabetic microangiopathy. N Am J Med Sci. 2013;5:529–35. doi: 10.4103/1947-2714.118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CK, Swaminathan R. Rapid, economical immunoturbidimetric method for microalbuminuria. Clin Chem. 1987;33:204. [PubMed] [Google Scholar]
- 31.Bao XH, Wong V, Wang Q, Low LC. Prevalence of peripheral neuropathy with insulin-dependent diabetes mellitus. Pediatr Neurol. 1999;20:204–9. doi: 10.1016/s0887-8994(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 32.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–8. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maquart FX, Gillery P, Bernard JF, Mante JP, Borel JP. A method for specifically measuring haemoglobin AIC with a disposable commercial ion-exchange column. Clin Chim Acta. 1980;108:329–32. doi: 10.1016/0009-8981(80)90021-2. [DOI] [PubMed] [Google Scholar]
- 34.Gosek K, Moczulski D, Zukowska-Szczechowska E, Grzeszczak W. C-106T polymorphism in promoter of aldose reductase gene is a risk factor for diabetic nephropathy in type 2 diabetes patients with poor glycaemic control. Nephron Exp Nephrol. 2005;99:e63–7. doi: 10.1159/000083209. [DOI] [PubMed] [Google Scholar]
- 35.Makiishi T, Araki S, Koya D, Maeda S, Kashiwagi A, Haneda M. C-106T polymorphism of AKR1B1 is associated with diabetic nephropathy and erythrocyte aldose reductase content in Japanese subjects with type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:943–51. doi: 10.1016/j.ajkd.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Park HK, Ahn CW, Lee GT, Kim SJ, Song YD, Lim SK, et al. (AC)(n) polymorphism of aldose reductase gene and diabetic microvascular complications in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2002;55:151–7. doi: 10.1016/s0168-8227(01)00299-6. [DOI] [PubMed] [Google Scholar]
- 37.Isermann B, Schmidt S, Bierhaus A, Schiekofer S, Borcea V, Ziegler R, et al. (CA)(n) dinucleotide repeat polymorphism at the 5’- end of the aldose reductase gene is not associated with microangiopathy in Caucasians with long-term diabetes mellitus 1. Nephrol Dial Transplant. 2000;15:918–20. doi: 10.1093/ndt/15.6.918. [DOI] [PubMed] [Google Scholar]
- 38.Katakami N, Kaneto H, Takahara M, Matsuoka TA, Imamura K, Ishibashi F, et al. Aldose reductase C-106T gene polymorphism is associated with diabetic retinopathy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;92:e57–60. doi: 10.1016/j.diabres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Abhary S, Burdon KP, Laurie KJ, Thorpe S, Landers J, Goold L, et al. Aldose reductase gene polymorphisms and diabetic retinopathy susceptibility. Diabetes Care. 2010;33:1834–6. doi: 10.2337/dc09-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olmos P, Bastías MJ, Vollrath V, Toro L, Trincado A, Salinas P, et al. C(-106) T polymorphism of the aldose reductase gene and the progression rate of diabetic retinopathy. Diabetes Res Clin Pract. 2006;74:175–82. doi: 10.1016/j.diabres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Thamotharampillai K, Chan AK, Bennetts B, Craig ME, Cusumano J, Silink M, et al. Decline in neurophysiological function after 7 years in an adolescent diabetic cohort and the role of aldose reductase gene polymorphisms. Diabetes Care. 2006;29:2053–7. doi: 10.2337/dc06-0678. [DOI] [PubMed] [Google Scholar]


