Abstract
Many studies have accessed the association between eNOS-4b/a polymorphism and the risk of diabetic nephropathy (DN) among type 2 diabetic subjects. However, the results are conflicting and inconclusive. The aim of current meta-analysis was to more precisely estimate the relationship. Pubmed, Embase, the China National Knowledge Infrastructure and the Wanfang Database were searched for articles published up to May 26th, 2013 that addressed eNOS-4b/a polymorphism and the risk of DN among type 2 diabetic subjects. 18 studies were included in this meta-analysis. eNOS-4b/a polymorphisms were associated with an overall significantly increased risk of DN (allele model: OR = 1.44, 95% CI = 1.14–1.82; additive model: OR = 2.03, 95% CI = 1.14–3.62; dominant model: OR = 1.34, 95% CI = 1.07–1.68; recessive model: OR = 2.01, 95% CI = 1.12–3.61). Subgroup analysis revealed a significant association between the eNOS-4b/a polymorphism and DN in Asian population, especially in Chinese population, but not in non Asian populations. Our meta-analysis supported an association between the 4b/a polymorphism of eNOS gene and increased risk of DN in type 2 diabetes among Asians, especially in Chinese population.
Abbreviations: eNOS, endothelial nitric oxide synthase; DN, diabetic nephropathy; ESRD, end-stage renal disease; ACE, angiotensin-converting enzyme; MTHFR, methylenetetrahydrofolate reductase; CNKI, China National Knowledge Infrastructure; OR, odds ratio; CI, confidence interval; HWE, Hardy–Weinberg equilibrium; FEM, fixed-effects model; REM, random-effects model
Keywords: Diabetic nephropathy, eNOS, 4b/a, Polymorphism, Meta-analysis
Introduction
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes and is the leading cause of end-stage renal disease (ESRD) globally (Collins et al., 2012, Liao, 2012). It has become the primary diagnosis in almost 50% of patients starting renal replacement therapy in some Asian countries (Tang, 2010), in Europe and in the US. Asians, including Chinese type 2 diabetic subjects, have a higher prevalence of nephropathy, with 20% having clinical proteinuria and 40% microalbuminuria (Wu et al., 2005).
Why some type 2 diabetics develop nephropathy, whereas others do not, despite having a long-term hyperglycemia remains unknown. Accumulated evidences suggest that both environmental and genetic factors are related with the etiology of DN (Adler et al., 2003, McCarthy, 2010, Stanton and King, 2011). A number of genes have been suggested as diabetic nephropathy candidate genes, for example, angiotensin-converting enzyme (ACE) (Wang et al., 2012), eNOS, methylenetetrahydrofolate reductase (MTHFR) (Yang et al., 2013), among which eNOS may play a critical role because endothelial dysfunction is considered as an important pathophysiologic factor for DN (Futrakul et al., 2006).
Nitric oxide (NO), a vasodilator molecule, is produced through the oxidation of l-arginine by eNOS (Moncada and Higgs, 1993). NO can regulate endothelial function and is an important factor in the maintenance of homeostasis. The presence of eNOS polymorphisms might contribute to a decreased eNOS activity and a reduced NO level, and has been reported to be a potential factor in the pathogenesis and development of DN (Ahluwalia et al., 2008, Palm et al., 2009). This polymorphism consists of the two alleles of eNOS 4a with 4 tandem 27-repeats and eNOS 4b with 5 repeats (Bellini et al., 2007).
To date, many studies have been carried out to investigate the relationship between eNOS-4b/a polymorphism and the risk of DN among type 2 diabetic subjects, but results of these studies were conflicting and inconclusive. Some studies observed that there was an association between eNOS-4b/a polymorphism and the risk of DN in patients with type 2 diabetes (Ahluwalia et al., 2008); while others suggested that there was no significant association (Fujita et al., 2000, Shoukry et al., 2012). To draw a more reliable conclusion, we performed a meta-analysis of all available studies dealing with the relationship between the eNOS-4b/a polymorphism and DN among type 2 diabetic subjects. Subgroup analyses were performed according to different geographic location to investigate ethnicity-specific effects.
Materials and methods
Literature search strategy
Two investigators (ZJM and HZR) independently searched Pubmed, Embase, the China National Knowledge Infrastructure (CNKI) database and the Wanfang Database. The last updated search was performed on May 26th, 2013. We used any possible combinations of relevant keywords of “Endothelial nitric oxide synthase”, “eNOS”, “4b/a”, “polymorphism” and each term designating “T2DM-DN” (e.g., “type 2 diabetes”, “T2D”, “nephropathy”, “albuminuria”, “proteinuria”, and “ESRD”). We also reviewed all the references cited in these articles to identify additional relevant publications. There was no language limitation. For overlapping publications, only the most recent or complete study was included in this meta-analysis.
Inclusion and exclusion criteria
All studies included in the meta-analysis were required to meet the following criteria: (1) case–control, (2) studies investigating the association of eNOS-4b/a polymorphism with T2DM-DN as the outcome, (3) the control group with subjects who had T2DM but were free of diabetic kidney disease, (4) available data to estimate an odds ratio (OR) with 95% confidence interval (CI),and (5) genotype distribution among the control group must be in Hardy–Weinberg equilibrium (HWE).
Studies were excluded if one of the following existed: (1) review articles and editorials; (2) case reports; (3) repeat or overlapping publications; (4) no report about the genotype frequency, or insufficient information for data extraction.
Data extraction
Two investigators (ZJM and HZR) collected the data independently from all eligible publications. Disagreements about eligibility were resolved during a consensus with a third reviewer (RC) until consensus was achieved on every item. The following data were extracted from each study: name of the first author, year of publication, country of the study, ethnicity, number of cases and controls, genotype and allele frequencies, and genotyping method. Populations were categorized into Asian and non-Asian.
Statistical analysis
The strength of relationship between eNOS-4b/a polymorphism and DN risk was expressed with OR and corresponding 95% CIs. The association was evaluated with the use of the allelic model (a vs b), as well as the additive model (aa vs. bb), the dominant model (aa + ab vs bb) and the recessive model (aa vs ab + bb). Heterogeneity was assessed using the Cochran Q chi-square test and the I2 test (DerSimonian and Laird, 1986). A P value less than 0.10 indicated significant heterogeneity. We considered I2 values less than 25% to represent low heterogeneity, values between 25% and 50% to represent moderate heterogeneity, and values of 50% or greater to represent high heterogeneity. The fixed-effects model (FEM) was used when there was no heterogeneity among the included studies; otherwise, the random-effects model (REM) was used. In the subgroup analysis, statistical analysis was conducted according to different geographic location. Sensitivity analysis was performed to estimate the stability by omitting one study at a time to examine influence of one study on the overall summary estimate. Funnel plot and Egger's test were performed to assess publication bias (Begg and Mazumdar, 1994, Egger et al., 1997). An asymmetric plot or the P-value of Egger's test less than 0.1 suggested possible publication bias. Analyses were performed using the software Review Manage 5.2, publication bias was conducted using STATA software (version 12.0; Stata, College Station, TX). A two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of the studies
A total of 59 articles were identified. The flow chart of study selection is shown in Fig. 1. Of these, 41 were excluded because they did not meet the criteria or were over-lapping publications. Finally, a total of 18 studies published between 2000 and 2013 were identified according to our inclusion criteria, involving 2972 cases and 2700 controls. The sample size in these case–control studies varied considerably (range from 84 to 903 individuals). All of the included studies used polymerase chain reaction (PCR) or PCR-restriction fragment length polymorphism (PCR-RFLP) to check genotypes. The main study characteristics were listed in Table 1. The genotype and allele distributions for each study and HWE in controls were summarized in Table 2. Of the 18 studies, three were non-Asian population (Degen et al., 2001, Ezzidi et al., 2008, Shoukry et al., 2012), 15 studies were Asian population, including eight studies conducted in the Chinese population (Dong et al., 2005, Guo, 2011, Huang et al., 2003, Lin et al., 2002, Luo, 2003, Sun et al., 2004, Xing et al., 2004, Zhang et al., 2005) and seven studies conducted in the non-Chinese population (Ahluwalia et al., 2008, Cheema et al., 2013, Fujita et al., 2000, Neugebauer et al., 2000, Rahimi et al., 2013, Shimizu et al., 2002, Taniwaki et al., 2001).
Fig. 1.
Flow chart of included studies.
Table 1.
Main characteristics of included studies in the meta-analysis.
| Author | Year | Country | Ethnicity | Case/control | Genotyping methods |
|---|---|---|---|---|---|
| Ahluwalia et al. | 2008 | India | South-Asian | 195/255 | PCR-RFLP |
| Cheema et al. | 2013 | India | South-Asian | 320/490 | PCR-RFLP |
| Degen et al. | 2001 | Germany | Caucasian | 197/217 | PCR |
| Dong et al. | 2005 | China | East-Asian | 70/64 | PCR |
| Ezzidi et al. | 2008 | Tunisia | Arabs | 505/398 | PCR-RFLP |
| Fujita et al. | 2000 | Japan | East-Asian | 86/69 | PCR |
| Guo et al. | 2011 | China | East-Asian | 69/73 | PCR-RFLP |
| Huang et al. | 2003 | China | East-Asian | 141/61 | PCR |
| Lin et al. | 2002 | China | East-Asian | 79/48 | PCR |
| Luo et al. | 2003 | China | East-Asian | 49/35 | PCR |
| Neugebauer et al. | 2000 | Japan | East-Asian | 133/82 | PCR |
| Rahimi et al. | 2013 | Iran | West-Asian | 121/52 | PCR-RFLP |
| Shimizu et al. | 2002 | Japan | East-Asian | 230/203 | PCR |
| Shoukry et al. | 2012 | Egypt | Arabs | 200/200 | PCR-RFLP |
| Sun et al. | 2004 | China | East-Asian | 188/114 | PCR |
| Taniwaki et al. | 2001 | Japan | East-Asian | 79/48 | PCR |
| Xing et al. | 2004 | China | East-Asian | 130/136 | PCR |
| Zhang et al. | 2005 | China | East-Asian | 180/154 | PCR |
Table 2.
The distribution of the 4b/a genotype and allele frequency for cases and controls.
| Distribution of 4b/a eNOS genotype |
Allele frequency |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Cases |
Controls |
Cases |
Controls |
HWE |
||||||
| aa | ab | bb | aa | ab | bb | a | b | a | b | ||
| Ahluwalia et al. | 21 | 28 | 146 | 5 | 61 | 189 | 70 | 320 | 71 | 439 | Yes |
| Cheema et al. | 51 | 61 | 208 | 10 | 118 | 363 | 163 | 477 | 138 | 844 | Yes |
| Degen et al. | 2 | 53 | 142 | 6 | 50 | 161 | 57 | 337 | 62 | 372 | Yes |
| Dong et al. | 0 | 27 | 43 | 0 | 11 | 53 | 27 | 113 | 11 | 117 | Yes |
| Ezzidi et al. | 29 | 162 | 314 | 21 | 143 | 234 | 220 | 790 | 185 | 611 | Yes |
| Fujita et al. | 2 | 21 | 63 | 0 | 19 | 50 | 25 | 147 | 19 | 119 | Yes |
| Guo et al. | 6 | 7 | 56 | 3 | 0 | 70 | 19 | 119 | 6 | 140 | Yes |
| Huang et al. | 2 | 31 | 108 | 0 | 8 | 53 | 35 | 247 | 8 | 114 | Yes |
| Lin et al. | 0 | 8 | 71 | 1 | 6 | 41 | 8 | 150 | 8 | 88 | Yes |
| Luo et al. | 27 | 2 | 20 | 8 | 4 | 23 | 56 | 42 | 20 | 50 | Yes |
| Neugebauer et al. | 6 | 26 | 101 | 1 | 10 | 71 | 38 | 228 | 12 | 152 | Yes |
| Rahimi et al. | 3 | 32 | 86 | 0 | 14 | 38 | 38 | 204 | 14 | 90 | Yes |
| Shimizu et al. | 6 | 44 | 180 | 3 | 44 | 156 | 56 | 404 | 50 | 356 | Yes |
| Shoukry et al. | 12 | 64 | 124 | 9 | 60 | 131 | 88 | 312 | 78 | 322 | Yes |
| Sun et al. | 4 | 48 | 136 | 2 | 11 | 101 | 56 | 320 | 15 | 213 | Yes |
| Taniwaki et al. | 0 | 8 | 71 | 1 | 6 | 41 | 8 | 150 | 8 | 88 | Yes |
| Xing et al. | 4 | 16 | 110 | 0 | 8 | 128 | 24 | 236 | 8 | 264 | Yes |
| Zhang et al. | 0 | 21 | 159 | 2 | 21 | 131 | 21 | 339 | 25 | 283 | Yes |
HWE: Hardy–Weinberg equilibrium.
Overall meta-analysis and subgroup analyses
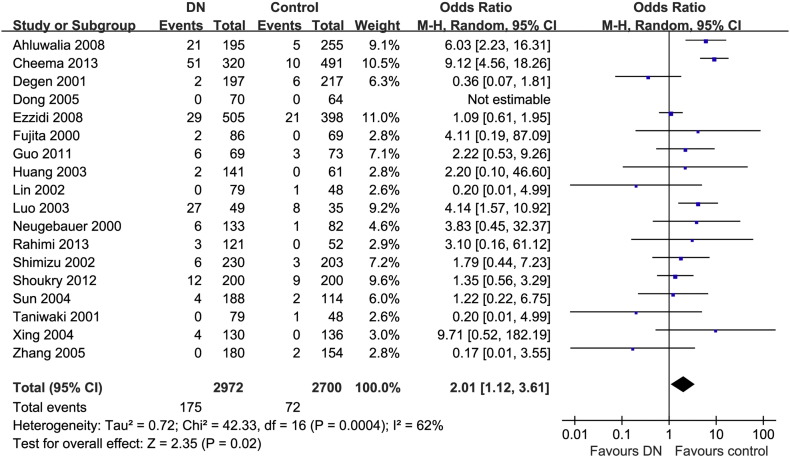
The summary of the meta-analysis for eNOS-4b/a gene polymorphisms and DN was shown in Table 3. Significant association was found between the 4a allele and increased risk of DN when compared with the 4b allele in overall populations (REM OR = 1.44, 95% CI = 1.14–1.82) (Fig. 2). Significant associations were also found under additive model (REM OR = 2.03, 95% CI = 1.14–3.62) (Fig. 3), dominant model (REM OR = 1.34, 95% CI = 1.07–1.68) (Fig. 4) and recessive model (REM OR = 2.01, 95% CI = 1.12–3.61) (Fig. 5).
Table 3.
Meta analysis of the association of eNOS-4b/a gene polymorphism with DN in type 2 diabetes.
| Genetic model |
Populations |
Studies |
Number of |
Heterogeneity |
I2 (%) |
OR (95% CI) |
P value |
|---|---|---|---|---|---|---|---|
| (n) | cases/controls | Q test P-value | |||||
| a versus b | All | 18 | 2972/2700 | < 0.00001 | 74 | 1.44 (1.14–1.82) (REM) | 0.0002 |
| Non-Asian | 3 | 902/815 | 0.52 | 0 | 0.99 (0.84–1.17) (FEM) | 0.92 | |
| Asian | 15 | 2070/1885 | 0 < 0.0001 | 67 | 1.59 (1.22–2.09) (REM) | 0.0007 | |
| China | 8 | 906/685 | 0.001 | 70 | 2.02 (1.31–3.11) (REM) | 0.006 | |
| Non-China | 7 | 1164/1200 | 0.01 | 64 | 1.34 (0.98–1.83) (REM) | 0.07 | |
| aa versus bb | All | 18 | 2972/2700 | 0.0006 | 61 | 2.03 (1.14–3.62) (REM) | 0.02 |
| Non-Asian | 3 | 902/815 | 0.38 | 0 | 1.02 (0.64–1.61) (FEM) | 0.95 | |
| Asian | 15 | 2070/1885 | 0.10 | 35 | 3.94 (2.72–5.71) (FEM) | < 0.00001 | |
| China | 8 | 906/685 | 0.28 | 20 | 2.25 (1.22–4.13) (FEM) | 0.009 | |
| Non-China | 7 | 1164/1200 | 0.18 | 32 | 5.31 (3.29–8.56) (FEM) | < 0.00001 | |
| aa + ab versus bb | All | 18 | 2972/2700 | 0.0004 | 61 | 1.34 (1.07–1.68) (REM) | 0.010 |
| Non-Asian | 3 | 902/815 | 0.41 | 0 | 0.98 (0.80–1.20) (FEM) | 0.85 | |
| Asian | 15 | 2070/1885 | 0.002 | 60 | 1.48 (1.12–1.95) (REM) | 0.006 | |
| China | 8 | 906/685 | 0.008 | 63 | 2.05 (1.25–3.36) (REM) | 0.005 | |
| Non-China | 7 | 1164/1200 | 0.22 | 27 | 1.20 (0.99–1.45) (FEM) | 0.06 | |
| aa versus bb + ab | All | 18 | 2972/2700 | 0.0004 | 62 | 2.01 (1.12–3.61) (REM) | 0.02 |
| Non-Asian | 3 | 902/815 | 0.36 | 1 | 1.04 (0.66–1.64) (FEM) | 0.87 | |
| Asian | 15 | 2070/1885 | 0.07 | 39 | 4.01 (2.78–5.80) (FEM) | < 0.00001 | |
| China | 8 | 906/685 | 0.24 | 24 | 2.21 (1.21–4.05) (FEM) | 0.010 | |
| Non-China | 7 | 1164/1200 | 0.18 | 33 | 5.52 (3.43–8.89) (FEM) | < 0.00001 |
OR, odds ratio; CI, confidence interval.
Fig. 2.
The forest plot of a vs. b of eNOS polymorphism and overall DN risk.
Fig. 3.
The forest plot of aa vs. bb of eNOS polymorphism and overall DN risk.
Fig. 4.
The forest plot of aa + ab vs. bb of eNOS polymorphism and overall DN risk.
Fig. 5.
The forest plot of aa vs bb + ab of eNOS polymorphism and overall DN risk.
Sub-group analysis was performed according to different geographic location (Asian and non-Asian) in this meta-analysis. For Asian population, the 4a allele was found contributing significantly to increased DN risk in allelic model (REM: OR = 1.59, 95% CI = 1.22–2.09), additive model (FEM: OR = 3.94, 95% CI = 2.72–5.71), dominant model (REM: OR = 1.48, 95% CI = 1.12–1.95), and recessive model (FEM: OR = 4.01, 95% CI = 2.78–5.80), respectively. No significant association was found in any of the above mentioned models for non-Asian population (data were shown in Table 3).
Sub-group analysis according to different country (Chinese population and non Chinese population) in Asian populations was also performed in this meta-analysis. Of all, eight case–control studies with 906 cases and 685 controls were conducted in the Chinese population, seven case–control studies with 1164 cases and 1200 controls were examined in the non-Chinese population. Significant associations were found under allelic model (REM: OR = 2.02, 95% CI = 1.31–3.11), recessive (FEM: OR = 2.25, 95% CI = 1.22–4.13), dominant (REM: OR = 2.05, 95% CI = 1.25–3.36) and additive (FEM: OR = 2.25, 95% CI = 1.22–4.13) models among the Chinese population. For non-Chinese population, significant associations were found under recessive (FEM: OR = 5.52, 95% CI = 3.43–8.89) and additive (FEM: OR = 5.31, 95% CI = 3.29–8.56) models, but the association was not found in allelic model (REM: OR = 1.34, 95% CI = 0.98–1.83) and dominant model (FEM: OR = 1.20, 95% CI = 0.99–1.45).
Publication bias diagnostics
Begg's funnel plot and Egger's test were performed to evaluate the publication bias in this meta-analysis. The shapes of the funnel plots in all genetic models did not reveal any sign of obvious asymmetry (Figure not shown). The Egger's test showed that there was no significant publication bias for any of the four genetic models (for a versus b: P = 0.837; for aa versus bb: P = 0.387; for aa + ab versus bb: P = 0.902; for aa versus ab + bb: P = 0.434).
Sensitivity analysis
Sensitivity analysis was performed by sequentially excluding one study at a time. The results indicated that fixed-effects estimates and/or random-effects estimates before and after sequential omission of each study were similar at large (data not shown), which suggested that the results of this meta-analysis were reliable and robust.
Discussion
DN is the major cause of end-stage renal disease globally and is the second cause of blood dialysis in China (13.5%) (Lu et al., 2010). The etiology of DN is multi-factorial and involves both environmental and genetic factors. It is still a major challenge for clinicians and researchers to elucidate the pathogenesis of DN and identify patients at DN risk. Genetic studies may provide valuable information with regard to the pathobiology of DN, and in the recent years, interest in the genetic susceptibility to DN has evoked a growing attention to the investigation of susceptibility polymorphisms of DN (Hellemons et al., 2012, Thomas et al., 2012).
Vascular endothelial dysfunction is prevalent in diabetes and has been shown to be an important pathophysiologic factor for DN (Cheng et al., 2012). eNOS plays a critical role in the regulation of endothelial function through production of NO (Moncada and Higgs, 2006). The mechanism responsible for the potential association between eNOS polymorphisms and risk of DN is still unclear. However, eNOS activity and NO levels have been suggested as one of the critical factor for whether patients with diabetes are at risk for developing DN. Increased availability of eNOS may be an effective strategy in restoring endothelial function in patients with diabetic nephropathy (Badal and Danesh, 2012). Polymorphisms in the eNOS gene may lead to defective NO synthesis and decreased NO levels, contribute to the development and progression of DN (Ahluwalia et al., 2008, Palm et al., 2009). Therefore, this metabolic pathway of diabetes may be associated with renal complications of diabetes.
In the past two decades, a number of case–control studies were performed to evaluate the association between eNOS-4b/a polymorphism and risk of DN in type 2 diabetes patients, but because of relatively small sample size and the low statistical power of individual studies, results remained inconclusive. A meta-analysis was published in 2009 to assess the association between eNOS-4b/a polymorphisms and risk of DN in type 2 diabetes patients (Zintzaras et al., 2009). However, the meta-analysis didn't report an obvious association between eNOS-4b/a polymorphisms and risk of DN in type 2 diabetes patients. From 2009, several new studies have been performed to further investigate this association, but the association between the eNOS-4b/a polymorphisms and risk of DN is still unclear. To obtain a more reliable conclusion, we conducted the present meta-analysis of all available studies based on 18 studies published from 2000 to 2013, involving 2972 cases and 2700 controls. Significant association was found between the 4a allele and increased risk of DN when compared with the 4b allele in all populations from the present meta-analysis. According to those results, we conclude that eNOS-4b/a would be considered as an effective genetic factor, which contributed to the pathology of DN in type 2 diabetes patients.
The geographic and ethnic differences might be an important factor to effect the association of gene polymorphism with the susceptibility of DN in type 2 diabetes patients. The results of subgroup analyses indicated that significant association of eNOS-4b/a polymorphism with DN only existed in Asian population, but not in non-Asian population. Furthermore, such association appeared to be enhanced when subgroup studies were performed among Chinese population. The differences in ethnic backgrounds, lifestyle or public sanitation may partly explain this discrepancy (Hossain et al., 2007, Lee, 2003). Further ethnic-specific association studies should be performed to investigate the population discrepancy of DN susceptibility.
Heterogeneity is one of the important issues when interpreting the results of meta-analysis (Nakaoka and Inoue, 2009). Our results indicated that heterogeneity existed in the overall analysis. After subgroup analysis by ethnicity and country, the heterogeneity was decreased or removed, suggesting that certain effects of genetic variants are ethnic specific. Publication bias is an important factor to affect us to get a reliable conclusion for meta-analysis. In this meta-analysis, no significant publication bias for 4b/a polymorphism in any of the above-mentioned inherited models was found, suggesting the associations observed should be stable. Sensitivity analyses did not significantly alter the results, also suggesting that the association was reliable and stable.
Despite our efforts in performing a comprehensive analysis, several limitations exist in this meta-analysis. First, only published studies in English or Chinese were included for data analysis, some potential studies with other languages or unpublished could be missed. Second, the majority of studies involved Asian populations, whereas non-Asian populations were only seen in three studies, more studies needed to be performed to investigate the association in non-Asian populations. Third, the ORs obtained by all comparison models were unadjusted, and a more precise analysis should be conducted if all individual raw data were available. This would allow for adjustment by other covariates such as age, gender, glycemic control, hypertension, smoking status, family history, lifestyle, environmental factors, and so on. Fourth, genotyping methods were different among the selected studies, which might affect the results. Finally, DN is a complex disease, and many genes are related to DN, however, we could not address gene–gene interactions in our study due to the lack of the related information. Despite these limitations, our study provided a better understanding of the association between eNOS-4b/a gene polymorphisms and risk of DN in type 2 diabetes.
In conclusion, our meta-analysis supported an association between the 4b/a polymorphism of eNOS gene and increased risk of DN in type 2 diabetes. However, significant association only existed in Asian populations, especially in Chinese population. As for non-Asian populations, no significant association was found under all the genetic models. Taking into account the limitations of this meta-analysis, further larger well-designed studies involving different ethnic populations, particularly referring to gene–gene and gene–environment interactions are required to warrant and validate our findings.
Author contributions
ZJM and LMC conceived and designed the experiments. ZJM, XG and RC carried out the literature searching and data extraction. ZJM, HZR, JG and LMC analyzed the data. ZJM, XG, RC and JG contributed reagents/materials/analysis tools. ZJM, XG and LMC wrote the paper.
Conflict of interests
All authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81072922 and No. 81273915).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Adler A.I., Stevens R.J., Manley S.E., Bilous R.W., Cull C.A., Holman R.R. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- Ahluwalia T.S., Ahuja M., Rai T.S., Kohli H.S., Sud K., Bhansali A., Khullar M. Endothelial nitric oxide synthase gene haplotypes and diabetic nephropathy among Asian Indians. Mol. Cell. Biochem. 2008;314:9–17. doi: 10.1007/s11010-008-9759-8. [DOI] [PubMed] [Google Scholar]
- Badal S.S., Danesh F.R. Strategies to reverse endothelial dysfunction in diabetic nephropathy. Kidney Int. 2012;82:1151–1154. doi: 10.1038/ki.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Bellini M.H., Figueira M.N., Piccoli M.F., Marumo J.T., Cendoroglo M.S., Neto M.C., Dalboni M.A., Batista M.C., Goes M.A., Schor N. Association of endothelial nitric oxide synthase gene intron 4 polymorphism with end-stage renal disease. Nephrol. (Carlton) 2007;12:289–293. doi: 10.1111/j.1440-1797.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- Cheema B.S., Kohli H.S., Sharma R., Bhansali A., Khullar M. Endothelial nitric oxide synthase gene polymorphisms and renal responsiveness to RAS inhibition therapy in type 2 diabetic Asian Indians. Diabetes Res. Clin. Pract. 2013;99:335–342. doi: 10.1016/j.diabres.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Cheng H., Wang H., Fan X., Paueksakon P., Harris R.C. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int. 2012;82:1176–1183. doi: 10.1038/ki.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.J., Foley R.N., Chavers B., Gilbertson D., Herzog C., Johansen K., Kasiske B., Kutner N., Liu J., St Peter W., Guo H., Gustafson S., Heubner B., Lamb K., Li S., Li S., Peng Y., Qiu Y., Roberts T., Skeans M., Snyder J., Solid C., Thompson B., Wang C., Weinhandl E., Zaun D., Arko C., Chen S.C., Daniels F., Ebben J., Frazier E., Hanzlik C., Johnson R., Sheets D., Wang X., Forrest B., Constantini E., Everson S., Eggers P., Agodoa L. 'United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am. J. Kidney Dis. 2012;59(A7):e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Degen B., Schmidt S., Ritz E. A polymorphism in the gene for the endothelial nitric oxide synthase and diabetic nephropathy. Nephrol. Dial. Transplant. 2001;16:185. doi: 10.1093/ndt/16.1.185. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dong Y.H., Qu S.P., Lu W.S., Ding M., Dong C., Jiang H.W. Gene po lymorphism in chromosome 7q35 and susceptibility to diabetic nephropathy. Chin. J. Endocrinol. Metab. 2005;21:4750-50. [Google Scholar]
- Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzidi I., Mtiraoui N., Mohamed M.B., Mahjoub T., Kacem M., Almawi W.Y. Association of endothelial nitric oxide synthase Glu298Asp, 4b/a, and -786T>C gene variants with diabetic nephropathy. J. Diabetes Complicat. 2008;22:331–338. doi: 10.1016/j.jdiacomp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Fujita H., Narita T., Meguro H., Ishii T., Hanyu O., Suzuki K., Kamoi K., Ito S. Lack of association between an ecNOS gene polymorphism and diabetic nephropathy in type 2 diabetic patients with proliferative diabetic retinopathy. Horm. Metab. Res. 2000;32:80–83. doi: 10.1055/s-2007-978594. [DOI] [PubMed] [Google Scholar]
- Futrakul N., Butthep P., Vongthavarawat V., Futrakul P., Sirisalipoch S., Chaivatanarat T., Suwanwalaikorn S. Early detection of endothelial injury and dysfunction in conjunction with correction of hemodynamic maladjustment can effectively restore renal function in type 2 diabetic nephropathy. Clin. Hemorheol. Microcirc. 2006;34:373–381. [PubMed] [Google Scholar]
- Guo X.J.a.L., S.J. The association between endothelial nitric oxide synthase gene polymorphism and type 2 diabetic nephropathy. Natl Med. Front. China. 2011;6:3–5. [Google Scholar]
- Hellemons M.E., Kerschbaum J., Bakker S.J., Neuwirt H., Mayer B., Mayer G., de Zeeuw D., Lambers Heerspink H.J., Rudnicki M. Validity of biomarkers predicting onset or progression of nephropathy in patients with Type 2 diabetes: a systematic review. Diabet. Med. 2012;29:567–577. doi: 10.1111/j.1464-5491.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- Hossain P., Kawar B., El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N. Engl. J. Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Huang H.B., Ling L.X., Chen M.Q. Relationship between eNOS gene polymophism and diabetic nephropathy. Chin. J. Nephrol. 2003;19:181–182. [Google Scholar]
- Lee G. End-stage renal disease in the Asian-Pacific region. Semin. Nephrol. 2003;23:107–114. doi: 10.1053/snep.2003.50009. [DOI] [PubMed] [Google Scholar]
- Liao E.P. Management of type 2 diabetes: new and future developments in treatment. Am. J. Med. 2012;125:S2–S3. doi: 10.1016/j.amjmed.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Lin S., Qu H., Qiu M. Allele A in intron 4 of ecNOS gene will not increase the risk of diabetic nephropathy in type 2 diabetes of Chinese population. Nephron. 2002;91:768. doi: 10.1159/000065048. [DOI] [PubMed] [Google Scholar]
- Lu B., Yang Z., Wang M., Yang Z., Gong W., Yang Y., Wen J., Zhang Z., Zhao N., Zhu X., Hu R. High prevalence of diabetic neuropathy in population-based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res. Clin. Pract. 2010;88:289–294. doi: 10.1016/j.diabres.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Luo H.a.N., Y.Y. Association of polymorphism of endothelial nitric oxide synthase gene with diabetic nephropathy. Chin. J. Diabetes. 2003;11:317–320. [Google Scholar]
- McCarthy M.I. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The l-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006;147(Suppl. 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka H., Inoue I. Meta-analysis of genetic association studies: methodologies, between-study heterogeneity and winner's curse. J. Hum. Genet. 2009;54:615–623. doi: 10.1038/jhg.2009.95. [DOI] [PubMed] [Google Scholar]
- Neugebauer S., Baba T., Watanabe T. Association of the nitric oxide synthase gene polymorphism with an increased risk for progression to diabetic nephropathy in type 2 diabetes. Diabetes. 2000;49:500–503. doi: 10.2337/diabetes.49.3.500. [DOI] [PubMed] [Google Scholar]
- Palm F., Teerlink T., Hansell P. Nitric oxide and kidney oxygenation. Curr. Opin. Nephrol. Hypertens. 2009;18:68–73. doi: 10.1097/MNH.0b013e32831c4cdf. [DOI] [PubMed] [Google Scholar]
- Rahimi Z., Rahimi Z., Shahvaisi-Zadeh F., Sadeghei S., Vessal M., Yavari N. eNOS 4a/b polymorphism and its interaction with eNOS G894T variants in type 2 diabetes mellitus: modifying the risk of diabetic nephropathy. Dis. Markers. 2013;34:437–443. doi: 10.3233/DMA-130988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Onuma T., Kawamori R., Makita Y., Tomino Y. Endothelial nitric oxide synthase gene and the development of diabetic nephropathy. Diabetes Res. Clin. Pract. 2002;58:179–185. doi: 10.1016/s0168-8227(02)00156-0. [DOI] [PubMed] [Google Scholar]
- Shoukry A., Shalaby S.M., Abdelazim S., Abdelazim M., Ramadan A., Ismail M.I., Fouad M. Endothelial nitric oxide synthase gene polymorphisms and the risk of diabetic nephropathy in type 2 diabetes mellitus. Genet. Test. Mol. Biomark. 2012;16:574–579. doi: 10.1089/gtmb.2011.0218. [DOI] [PubMed] [Google Scholar]
- Stanton R.C., King G.L. A complex interplay of factors causes diabetic nephropathy. Metabolism. 2011;60:591–593. doi: 10.1016/j.metabol.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Sun H.Y., Yang M.G., Liu S.Q., Wang C.J., Zhang Q., Chen M.W. Study on the correlation of the polymorphisms of endothelial nitric oxide synthase gene with type 2 diabetes mellitus and diabetic nephropathy. Chin. J. Prev. Control. Chron. Non-commun. Dis. 2004;12:101–103. [Google Scholar]
- Tang S.C. Diabetic nephropathy: a global and growing threat. Hong Kong Med. J. 2010;16:244–245. [PubMed] [Google Scholar]
- Taniwaki H., Ishimura E., Matsumoto N., Emoto M., Inaba M., Nishizawa Y. Relations between ACE gene and ecNOS gene polymorphisms and resistive index in type 2 diabetic patients with nephropathy. Diabetes Care. 2001;24:1653–1660. doi: 10.2337/diacare.24.9.1653. [DOI] [PubMed] [Google Scholar]
- Thomas M.C., Groop P.H., Tryggvason K. Towards understanding the inherited susceptibility for nephropathy in diabetes. Curr. Opin. Nephrol. Hypertens. 2012;21:195–202. doi: 10.1097/MNH.0b013e328350313e. [DOI] [PubMed] [Google Scholar]
- Wang F., Fang Q., Yu N., Zhao D., Zhang Y., Wang J., Wang Q., Zhou X., Cao X., Fan X. Association between genetic polymorphism of the angiotensin-converting enzyme and diabetic nephropathy: a meta-analysis comprising 26,580 subjects. J. Renin Angiotensin Aldosterone Syst. 2012;13:161–174. doi: 10.1177/1470320311417655. [DOI] [PubMed] [Google Scholar]
- Wu A.Y., Kong N.C., de Leon F.A., Pan C.Y., Tai T.Y., Yeung V.T., Yoo S.J., Rouillon A., Weir M.R. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia. 2005;48:17–26. doi: 10.1007/s00125-004-1599-9. [DOI] [PubMed] [Google Scholar]
- Xing Q., Su B.L., Li C.C., Du J.L., Bai R., Zhang J.N. Association between edothelial nitric oxide synthase gene polymeophism and type 2 diabetic nephropathy. Chin. J. Endocrinol. Metab. 2004;20:435–437. [Google Scholar]
- Yang S., Zhang J., Feng C., Huang G. MTHFR 677 T variant contributes to diabetic nephropathy risk in Caucasian individuals with type 2 diabetes: a meta-analysis. Metabolism. 2013;62:586–594. doi: 10.1016/j.metabol.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu H., Yang H.Y., Wang Y.M., Song D.P., Li H. Relationship of endothelial nitric oxide synthase (eNOS) 4a/bgene polymorphism with diabetic nephropathyin T2DM. Chin. J. Diabetes. 2005;13:284–285. [Google Scholar]
- Zintzaras E., Papathanasiou A.A., Stefanidis I. Endothelial nitric oxide synthase gene polymorphisms and diabetic nephropathy: a HuGE review and meta-analysis. Genet. Med. 2009;11:695–706. doi: 10.1097/GIM.0b013e3181b2046b. [DOI] [PubMed] [Google Scholar]