Abstract
As an indispensable component of the eukaryotic ribosome, ribosomal protein L23a plays an important role in protein synthesis, folding and sorting. In this study, the cDNA fragment of ribosomal protein L23a with 471 bp in size was screened from the Small Tail Han sheep ear marginal tissue cDNA expression library, it has 157 amino acids and a molecular weight of 17.69 kDa. The nucleotide sequence of L23a shares a high homology with those of human, mouse, cattle and pig of 91.51%, 88.32%, 96.18% and 93.84%, respectively. L23a is highly basic, containing a combined 45 Arg, Lys, and His residues and only 14 Asp and Glu residues. The expression pattern and intra-cellular distribution of recombinant L23a proteins in Ujumqin sheep fibroblast cells were analyzed after transfected with the plasmid pEGFP-N3-RPL23A, there were green fluorescence signals both in the cytoplasm and nucleolus of transfected cells after 24 h, the number of positive cells was increased with time, and they reached the peak level after 48 h of transfection. The transfection efficiency was 22.8%. Expression patterns of recombinant L23a gene in Escherichia coli were different with induction temperature, inductor concentration and induction time, when the IPTG concentration was 0.1 mmol/L and induction temperature was 37°, L23a protein expression was increased with induction time.
Abbreviations: RPL23A, ribosomal protein L23a; Arg, arginine; Lys, lysine; His, histidine; Asp, aspartic acid; Glu, glutamic acid; EGFP, enhanced green fluorescence protein; IPTG, isopropyl-β-D-thiogalactopyranoside; NADH, nicerinamide adenine dinucleotide; TSK, tsukushin
Keywords: cDNA expression library, Ribosomal protein L23a, Expression pattern, Small Tail Han sheep
Introduction
Small Tail Han sheep (Ovis aries) is a meat and fur sheep breed originated from Mongolian sheep in ancient north China, In the past years, scientists have achieved tremendous success with the molecular genetics study of Small Tail Han sheep (Chu et al., 2007, Chu et al., 2009, Chu et al., 2011). Recently, functional gene study became one of the hottest issues in the current Small Tail Han sheep research (He et al., 2012, Zhang et al., 2013). Our group has engaged in molecular mechanism studies on growth and development of Small Tail Han sheep for many years, a series of genes were found, such as NADH dehydrogenase subunit 2(ND2), subunit 4 (ND4) (Guo et al., 2005), TSK21 (Wang et al., 2007), cytochrome c oxidase subunit VIa polypeptide 1-like gene (NCBI accession number: GU585577) and so on, now we are paying more attention to ribosomal protein L23a of Small Tail Han sheep because of its many biological functions especially in relation to extra-ribosomal functions.
Ribosomal proteins are composed of approximately 80 structurally distinct proteins (Wool et al., 1995). They were divided into small 40S subunit and large 60S subunit according to their subunit size, they played an important role in protein synthesis (Filip et al., 2009, Trifa et al., 1998), and there are lots of extra-ribosomal functions for ribosomal proteins (Blumenthal and Carmichael, 1979), for example, they can regulate gene transcription, mRNA translation and cell apoptosis (Das et al., 2013, Wool, 1996). Based on these extra-ribosomal functions, ribosomal protein gene mutations or disturbance in their expression levels has been found in many inherited genetic diseases, such as Diamond–Blackfan anemia, hypoplasia and cancer. Obviously, the further study on the ribosomal proteins has important significance in the mechanism of development, gene function, prevention and treatment of disease, that was also the main purpose of this study.
The ribosomal protein L23a gene is a component of the 60S subunit, which belongs to the L23P family, L23a is located in the contact area of ribosome, chaperones, signal recognition particle and Translocon Sec61. It also has a homologous protein L23 in prokaryote, there were significant differences in size and sequence between eukaryotic L23a and prokaryotic L23, so the more complex regulation mechanism of L23a in eukaryotic is a topic which is worth studying. There are multiple processed pseudogenes of ribosomal protein L23a gene dispersed through the genome (Fan et al., 1997). At present, the sequence information of ribosomal protein L23a gene for human (Fan et al., 1996, Uechi et al., 2001), mouse (Kleiter et al., 2000), cattle (Zimin et al., 2009), pig (Uenishi et al., 2004) and Giant Panda (Sun et al., 2012) has been reported. However, ribosomal protein L23a gene from the Small Tail Han sheep has not been reported, especially in aspect of in vitro expression characteristics. The present study was carried out to evaluate in vitro expression characteristics of ribosomal protein L23a from Small Tail Han sheep. This study will provide scientific data and means for postulating the mechanism of development, pharmaceutical research and the development of functional genes from the Small Tail Han sheep.
Materials and methods
Animals and cDNA expression library construction
All procedures involving animals were approved by the animal care and use committee at the institution where the experiment was conducted. All procedures involving animals were approved and authorized by the Chinese Ministry of Agriculture.
Ear margin tissue samples were collected from Small Tail Han sheep at the Conservation Center of Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (Beijing, PR China). The samples were frozen in liquid nitrogen and then used for RNA isolation. cDNA expression library was constructed using SMART™ technology (SMART™ cDNA Library Construction Kit, Clontech), titer of the unamplified library, percentage of recombinant clone and titer of the amplified library were determined subsequently.
Cloning of L23a gene by screening of the cDNA expression library
Ribosomal protein L23a gene was obtained by screening the cDNA expression library using PCR method. The PCR primers were designed by Primer Premier 5.0, based on the mRNA sequence of L23a gene from Bos taurus (NM_001045958), the specific primers of cDNA sequence were as follows: RPL23A-F: 5′-ATGGCGCCGAAGGCAA-3′, RPL23A-R: 5′-TTAAATGATCCCAATTTTGTTGGC-3′. The PCR products were cloned into TA-cloning vector pGEM®-T Easy (Promega). Plasmid DNA was sequenced by Huada Zhongsheng Scientific Corporation (Beijing, China).
Over-expression of recombinant L23a gene in fibroblast cells
The DNA fragment of L23a gene was flanked with Xho I and EcoR I sites, after double digestion, the final fragment was inserted into pEGFP-N3 vector (Clontech) for transfection. Ujumqin sheep fibroblast line which has been established in our laboratory was used as target cells. Cells were seeded in 24-well plates and transfected with the plasmid DNA of L23a gene (pEGFP-N3-RPL23A) by Lipofectamine 2000 (Invitrogen). The medium was refreshed 6 h after transfection, and cells were observed 24, 48 and 72 h after transfection using Nikon TE-2000-E inverted confocal microscope with excitation wavelengths of 488 nm to determine the transfection efficiency and morphology of positive cells. For each experimental group, images were captured from 10 visual fields to determine the total and positive cell counts in each field for the calculation of transfection efficiencies.
Expression characteristics of recombinant L23a gene in Escherichia coli
The DNA fragment of L23a gene was flanked with BamH I and Xho I sites, after double digestion, the final fragment was inserted into pGEX-4T-1 vector (GE Healthcare) for protein expression. The constructed expression plasmid was transformed into BL21 (DE3) competent cells (Tiangen), and the recombinant protein production was carried out using auto-induction method. Briefly, proteins were expressed in cells by induction with isopropyl-1-thio-β-D-galactopyranoside (IPTG). Integrality of recombinant proteins was tested by western blotting, the expression condition was optimized, inducer concentration, induction time and temperature were tested respectively for high level protein expression (Hu et al., 2012). The results were confirmed using SDS-PAGE.
Data analysis
Homology research of the Small Tail Han sheep ribosomal protein L23a compared with the gene sequences of other species was performed using Blast 2.1 (http://www.ncbi. nlm.nih.gov/blast/). ORF of the DNA sequence was searched using ORF finder software (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The values of WM and pI were computed using the Compute pI/Mw tool (http://www.expasy.org/tools/pi_tool.html). Protein structure of the ribosomal protein L23a sequence cloned was analyzed using PredictProtein software (http://cubic.Bioc.columbia.edu/predictprotein/). Multiple sequence alignment was performed by software DNAstar Lasergene and DNAMAN 6.0.
Results
Amplification and detection of the constructed cDNA expression library
Titers of the unamplified and amplified libraries were determined by counting the number of colonies according to the protocol of cDNA Library Construction Kit, the results showed that unamplified and amplified libraries had titers of 2.76 × 106 pfu/mL and 1.5 × 1010 pfu/mL, respectively. To test for ligation efficiency, the percentage of recombinant clones was determined by screening cDNA insert using PCR method, ligation of the cDNA to the λTriplEx2 Vector was 95.8% recombinants, the average length of cDNA inserts was 910 bp (Fig. 1).
Fig. 1.
PCR products of the cDNA fragment.
M.2000 bp 1–23 and 1–22. cDNA fragment.
Analysis of the cDNA of ribosomal protein L23a gene from Small Tail Han sheep
The cDNA fragment with 471 bp in size was amplified from the cDNA expression library (Fig. 2). On the basis of the high identity, it was concluded that the cDNA isolated is the cDNA encoding the Small Tail Han sheep ribosomal protein L23a. The ribosomal protein L23a gene sequence was submitted to Genbank (accession number: GU585576.1). Alignment analysis of ribosomal protein L23a gene of the Small Tail Han sheep and those of human, mouse, cattle and pig, indicated that both the nucleotide sequence and the deduced amino acid sequence are highly conserved. There is no deletion or insertion of nucleotide and amino acid residue. As determined by BLAST analysis, the nucleotide sequence ribosomal protein L23a cloned from the Small Tail Han sheep shares a high homology with those of human, mouse, cattle and pig of 91.51%, 88.32%, 96.18% and 93.84%, respectively; the homologies for amino acid sequences are all 100% compared with the four species.
Fig. 2.

PCR products of the Small Tail Han sheep ribosomal protein L23a gene.
M: Molecular Marker DL2000; 1: amplified ribosomal protein L23a.
Primary structure analysis revealed that the molecular weight of the putative L23a protein of the Small Tail Han sheep is 17.69 kDa with a theoretical pI 10.44. Like most ribosomal proteins, ribosomal protein L23a is highly basic, containing a combined 45 Arg, Lys, and His residues and only 14 Asp and Glu residues, in which the highest content is the Lys residues (19.23%), far higher than other amino acids and without Cys and Trp residues (Figs. S1 and S2). The secondary structure analysis of L23a protein indicated that the protein was 61.4% in helix, 23.6% in sheet, 15.0% in turns and 11.4% in coil. Topology prediction shows that there is one bipartite nuclear localization signal profile, one N-glycosylation site, one Tyrosine kinase phosphorylation site, three Protein kinase C phosphorylation sites and one Ribosomal protein L23 signature in the L23a protein of the Small Tail Han sheep (Fig. 3).
Fig. 3.
Intra-domain features of the L23a amino acid sequences of Small Tail Han sheep.
 : Bipartite nuclear localization signal profile, ____: Ribosomal protein L23 signature,
: Bipartite nuclear localization signal profile, ____: Ribosomal protein L23 signature,  : Protein kinase C phosphorylation site,
: Protein kinase C phosphorylation site,  : N-glycosylation site,
: N-glycosylation site,  : Tyrosine kinase phosphorylation site.
: Tyrosine kinase phosphorylation site.
Over-expression of recombinant ribosomal protein L23a gene in fibroblast cells
The expression pattern and intra-cellular distribution of recombinant L23a proteins in Ujumqin sheep fibroblast cells were analyzed after transfected with the plasmid pEGFP-N3-RPL23a. In the initial stage of transfection, the majority of the cells had no significant change in morphology, some of the cells were shrinking and the number of live cells was decreased to some extent, there were green fluorescence signals both in the cytoplasm and nucleolus of transfected cells after 24 h (Fig. 4A), the number of positive cells was increased with time, and they reached the peak level after 48 h of transfection (Fig. 4B), the transfection efficiency was 22.8%; there was a significant decrease in fluorescence intensity after 72 h of transfection (Fig. 4C). Fluorescence intensity was stronger in the nucleolus than in the cytoplasm during the transfection (Fig. 4D, E and F).
Fig. 4.
Transfection of recombinant pEGFP-N3-RPL23A in Ujumqin sheep fibroblast cells.
A, B, C. transfection after 24 h, 48 h and 72 h, respectively (100 ×).
D, E, F. transfection after 24 h, 48 h and 72 h, respectively (400 ×).
Expression of recombinant ribosomal protein L23a gene in Escherichia coli
In this study, the DNA fragment of L23a gene was inserted into pGEX-4T-1 vector for protein expression, it was concluded that the recombinant protein was ribosomal protein L23a after Western-blot analysis (Fig. 5). Protein expression was increased with temperature raising, the highest amount of protein expression appeared in the temperature of 37°, then the yield of recombinant protein decreased as the temperature raised to 40° (Fig. 6). When the IPTG concentration was 0.1–1.2 mmol/L, there was no significant change in recombinant ribosomal protein L23a expression under 37° induction (Fig. 7). When the inducer concentration was 0.1, 0.2, 0.4, 0.6, 0.8, and 1.2 mM, respectively, the expression of the recombinant proteins were initiated, but there were no significant changes in expression, when the induction of recombinant ribosomal protein L23a was performed with the IPTG at 0.1 mM for 0, 2, 4, 6 and 8 h at 37°, respectively, protein expression was increased with induction time, the first 8 h induction was shown in Fig. 8, there was a noticeable increase in expression from 2 to 8 h.
Fig. 5.

Western-blot analysis of recombinant protein.
Fig. 6.

Effect of induction temperature on recombinant protein expression.
Fig. 7.
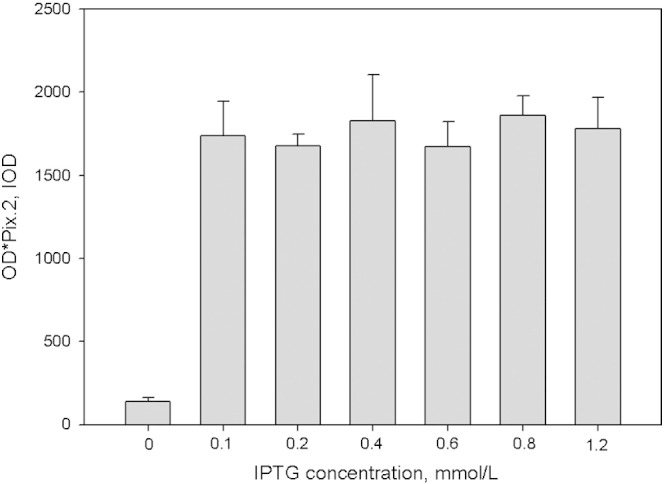
Effect of IPTG concentration on recombinant protein expression.
Fig. 8.

Effect of induction time on recombinant protein expression.
In summary, the optimal inducer concentration for the expression of recombinant ribosomal protein L23a was 0.1 mM, the optimum induction time was 8 h and the highest amount of protein expression temperature was 37°. But we did not get soluble proteins, refolding conditions of the recombinant ribosomal protein L23a required to be improved.
Discussion
The full-length cDNA library constructed from Small Tail Han sheep conformed to the requirements of a standard library (Ying, 2004). The cDNA library construction and analysis is considered to be an indispensable tool for functional genomic analysis as it provides much more detailed information on the genomic mechanisms underlying diverse processes of the organism (Shao et al., 2009). The SMART™ technique used in this study provides a method for producing high-quality and full-length cDNA libraries that preserve the complete 5′ terminal sequence of mRNA (Liu et al., 2010).
Bioinformatics analysis of ribosomal protein L23a gene was required, which could provide more valuable information in further studies, for example, basic information could be illustrated by DNA chromosome location, introns/exons and ORF analysis; gene regulation mechanism could be identified by the prediction of promoter, CpG island and transcription factor analysis; characteristics of gene encoding protein could be determined by main properties, subcellular localization and antigenic site analysis; and gene functions could be further determined by similarity search, function sites and structure analysis. It was reported that the nucleotide sequence RPL23A cloned from the Giant Panda shares a high homology with those of Homo sapiens (90.02%), Mus musculus (88.96%), Macaca mulatta (91.30%) and Rattus norvegicus (88.32%); the homologies for amino acid sequences are all 99.87% compared with the four species (Sun et al., 2012). In this study, the nucleotide sequence of Small Tail Han sheep L23a shares a high homology with those of human, mouse, cattle and pig of 91.51%, 88.32%, 96.18% and 93.84%, respectively. The homologies for amino acid sequences are all 100% compared with the four species, the striking pattern of evolutionary conservation is considered reasonable, as ribosomal protein genes are a group of highly conserved housekeeping genes.
Previous studies have demonstrated that the recombinant protein RPL23A had a time- and dose-dependency on the cell growth inhibition rate. The data also indicated that the effect at low concentrations was better than at high concentrations on Hep-2 cells, and that the concentration of 0.185 μg/mL had the best rate of growth inhibition of 36.31% (Sun et al., 2012). Another study showed that the over expression of 60sRL23a in SAG sensitive parasite decreases the sensitivity of the parasite towards SAG, miltefosine and paramomycin (Das et al., 2013). While in our study, it was found that there were no significant effects of recombinant proteins on cell growth and proliferation, a possible explanation is that the mechanism of RPL23A was different in some cell types. Meanwhile, the expression pattern and intra-cellular distribution of recombinant L23a proteins in Ujumqin sheep fibroblast cells were analyzed after transfected with the plasmid pEGFP-N3-RPL23A, there were green fluorescence signals both in the cytoplasm and nucleolus of transfected cells after 24 h, the number of positive cells was increased with time, and they reached the peak level after 48 h of transfection. pEGFP-N3 encodes a red-shifted variant of wild-type GFP which has been optimized for brighter fluorescence and higher expression in somatic cells of livestock and poultry, which had been demonstrated in our early studies (Li et al., 2009, Na et al., 2010, Wu et al., 2008), the high level of recombinant ribosomal protein L23a gene expression in fibroblast cells could last for 1 week in this study, indicating that gene duplication, transcription, protein synthesis and modification were highly effective in transfected cells.
In the present study 60sRL23a has been characterized for its probable association with Sodium antimony gluconate (SAG) resistance mechanism. Das S has cloned L.donovani 60sRL23a, and it was expressed in E. coli system and purified for raising antibody in swiss mice and was observed to have cytosolic localization in L. donovani. 60sRL23a was further over-expressed in sensitive strain of L. donovani to check its sensitivity profile against SAG (Sb V and III) and was found to be altered towards the resistant mode (Das et al., 2013). In this study, the DNA fragment of RPL23A gene was inserted into pGEX-4T-1 vector for protein expression, the pGEX-4T-1 vector is designed for inducible, high level intracellular expression of genes or gene fragments as fusions with Schistosoma japonicum GST (Smith and Johnson, 1988). There was target protein expression after 6 h induction by 1 mmol/L IPTG, molecular weight of target protein was 43.69 kDa, GST occurs naturally as a 26 kDa protein that can be expressed in E. coli with full enzymatic activity, while ribosomal protein L23a has a molecular weight of 17.69 kDa, fusion proteins that possess the complete amino acid sequence of GST also demonstrate GST enzymatic activity and can undergo dimerization similar to that observed in nature. Yield of fusion protein is highly variable and is affected by the nature of the fusion protein, the host cell, and the culture conditions used, during the optimization of expression conditions. Samples from evaluations of media, growth temperature, culture density, induction conditions and other variables were processed successively in this study. It was concluded that recombinant proteins were completely expressed by induction with IPTG. Without inducer, there was little recombinant protein expression, and a small quantity of inducer was needed when the protein expression was in control of the tac promoter.
Conclusion
Consequently, analysis of the expression of ribosomal protein L23a combined with the knowledge of their functions can facilitate the understanding and allows us to take a glimpse of the overall picture of ribosomal protein L23a in Small Tail Han sheep. The findings will give scientific support and orientation for postulating the mechanism of development, pharmaceutical research and the development of functional genes from the Small Tail Han sheep.
Acknowledgments
This research was funded by the Transgenic Research Program of China (2013ZX08009-003-006, 2013ZX08012-002-06), National Nature Science Foundation of China (31201765, 31272403), the Agricultural Science and Technology Innovation Program (cxgc-ias-01) and the earmarked fund for Modern Agro-industry Technology Research System (nycytx-40-01).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.mgene.2014.06.005.
Appendix A. Supplementary data
Figure S1. Nucleotide and deduced amino acid sequences of cDNA encoding ribosomal protein L23a from Small Tail Han sheep.
The asterisk (***) represents stop codon.
Figure S2. Amino acid composition of ribosomal protein L23a from Small Tail Han sheep.
References
- Blumenthal T., Carmichael G.G. RNA replication: function and structure of Qbeta-replicase. Annu. Rev. Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Chu M.X., Liu Z.H., Jiao C.L., He Y.Q. Mutations in BMPR-IB and BMP-15 genes are associated with litter size in Small Tailed Han sheep (Ovis aries) J. Anim. Sci. 2007;85(3):598–603. doi: 10.2527/jas.2006-324. [DOI] [PubMed] [Google Scholar]
- Chu M.X., Wang X.C., Jin M., Di R. DNA polymorphism of 5′ flanking region of prolactin gene and its association with litter size in sheep. J. Anim. Breed. Genet. 2009;126(1):63–68. doi: 10.1111/j.1439-0388.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- Chu M.X., Yang J., Feng T., Cao G.L. GDF9 as a candidate gene for prolificacy of Small Tail Han sheep. Mol. Biol. Rep. 2011;38(8):5199–5204. doi: 10.1007/s11033-010-0670-5. [DOI] [PubMed] [Google Scholar]
- Das S., Shah P., Baharia R.K., Tandon R. Over-expression of 60s ribosomal L23a is associated with cellular proliferation in SAG resistant clinical isolates of Leishmania donovani. PLoS Negl. Trop. Dis. 2013;7(12):e2527. doi: 10.1371/journal.pntd.0002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Cai W., Parimoo S., Schwarz D.C. Identification of seven new human MHC class I region genes around the HLA-F locus. Immunogenetics. 1996;44(2):97–103. doi: 10.1007/BF02660056. [DOI] [PubMed] [Google Scholar]
- Fan W., Christensen M., Eichler E., Zhang X. Cloning, sequencing, gene organization, and localization of the human ribosomal protein RPL23A gene. Genomics. 1997;46(2):234–239. doi: 10.1006/geno.1997.5038. [DOI] [PubMed] [Google Scholar]
- Filip A.M., Klug J., Cayli S., Fröhlich S. Ribosomal protein S19 interacts with macrophage migration inhibitory factor and attenuates its pro-inflammatory function. J. Biol. Chem. 2009;284(12):7977–7985. doi: 10.1074/jbc.M808620200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Du L.X., Ma Y.H., Guan W.J. A novel maternal lineage revealed in sheep (Ovis aries) Anim. Genet. 2005;36(4):331–336. doi: 10.1111/j.1365-2052.2005.01310.x. [DOI] [PubMed] [Google Scholar]
- He J.N., Zhang B.Y., Chu M.X., Wang P.Q. Polymorphism of insulin-like growth factor 1 gene and its association with litter size in Small Tail Han sheep. Mol. Biol. Rep. 2012;39(10):9801–9807. doi: 10.1007/s11033-012-1846-y. [DOI] [PubMed] [Google Scholar]
- Hu P.F., Guan W.J., Li X.C., Ma Y.H. Construction of recombinant proteins for reprogramming of endangered Luxi cattle fibroblast cells. Mol. Biol. Rep. 2012;39(6):7175–7182. doi: 10.1007/s11033-012-1549-4. [DOI] [PubMed] [Google Scholar]
- Kleiter N., Artner I., Copeland N.G., Gilbert D.J. Genomic organization and chromosome location of the murine Rpl23 gene. Cytogenet. Cell Genet. 2000;90(3–4):227–230. doi: 10.1159/000056774. [DOI] [PubMed] [Google Scholar]
- Li X.C., Yue H., Li C.Y., He X.H., Zhao Q.J., Ma Y.H., Guan W.J., Ma J.Z. Establishment and characterization of a fibroblast cell line derived from Jining Black Grey goat for genetic conservation. Small Ruminant Res. 2009;87:17–26. [Google Scholar]
- Liu C.Q., Lu T.F., Feng B.G., Liu D. Construction of cDNA library and preliminary analysis of expressed sequence tags from Siberian tiger. Int. J. Biol. Sci. 2010;6(6):584–589. doi: 10.7150/ijbs.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na R.S., Zhao Q.J., da Jin P., Su X.H. Establishment and biological characteristics of Ujumqin sheep fibroblast line. Cytotechnology. 2010;62(1):43–52. doi: 10.1007/s10616-010-9260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z.T., Cong X., Yuan J.D., Yang G.W. Construction and characterization of a cDNA library from head kidney of Japanese sea bass (Lateolabrax japonicus) Mol. Biol. Rep. 2009;36(7):2031–2037. doi: 10.1007/s11033-009-9536-0. [DOI] [PubMed] [Google Scholar]
- Smith D.B., Johnson K.S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sun B., Hou Y.L., Hou W.R., Zhang S.N. CDNA cloning, overexpression, purification and pharmacologic evaluation for anticancer activity of ribosomal protein L23A gene (RPL23A) from the giant panda. Int. J. Mol. Sci. 2012;13(2):2133–2147. doi: 10.3390/ijms13022133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifa Y., Privat I., Gagnon J., Baeza L. The nuclear RPL4 gene encodes a chloroplast protein that co-purifies with the T7-like transcription complex as well as plastid ribosomes. J. Biol. Chem. 1998;273(7):3980–3985. doi: 10.1074/jbc.273.7.3980. [DOI] [PubMed] [Google Scholar]
- Uechi T., Tanaka T., Kenmochi N. A complete map of the human ribosomal protein genes: assignment of 80 genes to the cytogenetic map and implications for human disorders. Genomics. 2001;72(3):223–230. doi: 10.1006/geno.2000.6470. [DOI] [PubMed] [Google Scholar]
- Uenishi H., Eguchi T., Suzuki K., Sawazaki T. PEDE (Pig EST Data Explorer): construction of a database for ESTs derived from porcine full-length cDNA libraries. Nucleic Acids Res. 2004;32(Database issue):D484–D488. doi: 10.1093/nar/gkh037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ma Y.H., Chen H., Guan W.J. Genetic and phylogenetic studies of Chinese native sheep breeds (Ovis aries) based on mtDNA D-loop sequences. Small Ruminant Res. 2007;72(2–3):232–236. [Google Scholar]
- Wool I.G. Extraribosomal functions of ribosomal proteins. Trends Biochem. Sci. 1996;21(5):164–165. [PubMed] [Google Scholar]
- Wool I.G., Chan Y.L., Glück A. Structure and evolution of mammalian ribosomal proteins. Biochem. Cell Biol. 1995;73(11–12):933–947. doi: 10.1139/o95-101. [DOI] [PubMed] [Google Scholar]
- Wu H., Guan W., Li H., Ma Y. Establishment and characteristics of white ear lobe chicken embryo fibroblast line and expression of six fluorescent proteins in the cells. Cell Biol. Int. 2008;32(12):1478–1485. doi: 10.1016/j.cellbi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Ying S.Y. Complementary DNA libraries: an overview. Mol. Biotechnol. 2004;27(3):245–252. doi: 10.1385/MB:27:3:245. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang G., Wang J., Ji Z. Characterization and comparative analyses of muscle transcriptomes in Dorper and Small-Tailed Han sheep using RNA-Seq technique. PLoS One. 2013;8(8):e72686. doi: 10.1371/journal.pone.0072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin A.V., Delcher A.L., Florea L., Kelley D.R. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10(4):R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Nucleotide and deduced amino acid sequences of cDNA encoding ribosomal protein L23a from Small Tail Han sheep.
The asterisk (***) represents stop codon.
Figure S2. Amino acid composition of ribosomal protein L23a from Small Tail Han sheep.





