Abstract
Objective
To clarify the association of rs11196218 polymorphism in transcription factor 7-like 2 (TCF7L2) and type 2 diabetes mellitus (T2DM) in Asian population by a case–control study and meta-analysis.
Methods
In the case–control study, 1842 patients with T2DM and 7777 normal glucose-tolerant controls in the Henan province of China were genotyped for rs11196218 in TCF7L2 by PCR–ligase detection reaction. We used allele, co-dominant, dominant and recessive models to evaluate the risk association and performed a meta-analysis of the results of different genetic models in previous studies and the current study.
Results
The AG genotype of rs11196218 was associated with risk of T2DM in the Henan population (odds ratio 1.37, 95% confidence interval 1.06–1.78), and dominant model showed marginal significant association (1.28, 0.99–1.67). Meta-analysis of 10 studies revealed the dominant model associated with T2DM in the overall population (1.20, 1.05–1.36). When stratified by region (southern and northern China and Japan), both the AG genotype and the dominant model were associated with risk of T2DM in southern Chinese (1.31, 1.03–1.66; 1.27, 1.01–1.60, respectively).
Conclusion
The rs11196218 polymorphism in TCF7L2 is associated with risk of T2DM in Asian population.
Abbreviations: TCF7L2, factor 7-like 2 gene; T2DM, type 2 diabetes mellitus; SNP, single nucleotide polymorphism; PCR–LDR, polymerase chain reaction–ligase detection reaction; OR, odds ratio; 95% CI, 95% confidence interval; PARP, population-attributable risk proportion; MAF, minor allele frequency
Keywords: TCF7L2, rs11196218, Single nucleotide polymorphism, Type 2 diabetes mellitus
Introduction
The transcription factor 7-like 2 gene (TCF7L2) is one of the genes associated with type 2 diabetes mellitus (T2DM) (Grant et al., 2006, Guinan, 2012). In 2007, rs11196218 single nucleotide polymorphism (SNP) in TCF7L2 was first found associated with T2DM in Hong Kong Chinese (Ng et al., 2007). Following the initial report, there have been several association studies in different groups (Lin et al., 2010, Luo et al., 2009, Ma et al., 2009, Miyake et al., 2012, Qiao et al., 2012, Zhang, 2008, Zheng et al., 2012, Zhu, 2011). However, all of these replication studies were carried out in Asian populations, and most of them focused on Chinese (Lin et al., 2010, Luo et al., 2009, Ma et al., 2009, Qiao et al., 2012, Zhang, 2008, Zheng et al., 2012, Zhu, 2011), especially southern Chinese (Lin et al., 2010, Ma et al., 2009, Zhang, 2008, Zheng et al., 2012, Zhu, 2011), except for one Japanese study (Miyake et al., 2012). Although all participants were Asians, the results were confusing rather than conclusive, and showed strong regional variations. Some studies suggested that this SNP was associated with T2DM (Ng et al., 2007, Zhu, 2011) while the others reported contradictory results (Lin et al., 2010, Ma et al., 2009, Zhang, 2008, Zheng et al., 2012). This apparent difference among Asian populations may be due to regional variation or the relatively small sample sizes studied. Two previous meta-analyses had systematically reviewed the association of rs11196218 SNP and risk of T2DM, but one included only 3 studies (Luo et al., 2009) and the other focused on only Chinese studies (Wang et al., 2013); neither comprehensively reviewed the association of this SNP and T2DM in Asians.
In the present study, we evaluated the association of rs11196218 polymorphism and T2DM in the Han population in Henan Province in northern China, using a relatively large sample (1842 Chinese individuals with T2DM and 7777 normal glucose-tolerant controls). Then, we combined our data with those of all published studies for a meta-analysis to clarify the association of rs11196218 polymorphism in TCF7L2 and risk of T2DM in Asians.
Materials and methods
Study design
A case–control study design was used to evaluate the association of rs11196218 polymorphism in TCF7L2 and risk of T2DM. The software Sample Size Calculations (Mark Woodward, The George Institute International Health; Lesley Francis, MIS Consultants) was used to calculate the sample size and power. Because the minor allele frequency of rs11196218 is 0.407 in the Chinese population (HapMap-HCB), for an OR of 1.20 for the risk allele, with ratio of controls to cases 4 and two-sided significant level 5%, a total of 8079 participants (1616 cases and 6463 cases) were needed to guarantee 80% statistical power. We conducted a free diabetic screen for the adult residents of two random sampling communities in Henan province, northern China. Those having fasting plasma glucose (FPG) ≥ 7.0 mmol/L or previous diagnosis of T2DM (who was receiving insulin treatment or an oral hypoglycemic agent or presented with a history of diabetes during the interview) were included as cases, while those having FPG < 7.0 mmol/L and no identifiable positive family history were recruited as controls. In total, 810 cases and 7777 normal glucose-tolerant controls were recruited from community populations. Additionally, we recruited 1032 cases from the outpatient clinics of 3 hospitals in Henan province in order to obtain enough cases. In hospitals, T2DM was diagnosed according to American Diabetes Association (ADA) criterion (American Diabetes Association, 2005). Participants with body mass index (BMI) ≤ 18.5 kg/m2 and who were pregnant, handicapped, mentally disturbed, obese caused by disease or taking certain drugs, and had cancer were excluded. The study was approved by the ethics committee of Zhengzhou University, and informed consent was obtained from each participant before data collection.
Data collection
Demographic characteristics were collected by interviewer-administered questionnaire. Body weight, body height, waist circumference (WC), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured. Blood samples were collected for measuring FPG and lipid profile. Total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) were measured by use of an automatic biochemical analyzer (Hitachi 7600-020, Tokyo, Japan) with reagents purchased from Ningbo Rui Bio-technology (Ningbo, China). Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula (Friedewald et al., 1972). Central obesity based on WC (WC ≥ 85 cm for men or WC ≥ 80 cm for women) was defined according to the recommended criteria for the Chinese population (Zhou and Cooperative Meta-Analysis Group of the Working Group on Obesity in China, 2002).
DNA isolation and genotyping
Genomic DNA was extracted from whole blood by use of a blood genome DNA extraction kit (Yaneng BIO, Shenzhen, China). PCR–ligase detection reaction (PCR–LDR) was used for genotyping. The PCR primers for the loci were 5′-AGC CAT TTC AAT TTC GTA CAT-3′ (forward) and 5′-AAC ATC AAA CTC CAG AGG GTC-3′ (reverse). PCR involved the LabCycler Gradient (SensoQuest GmbH, Göttingen, Germany) in a system with a total volume of 15 μl, containing 1 μl genomic DNA, 1.5 μl 10 × PCR buffer, 1.5 μl MgCl2, 0.25 μl each primer, 0.3 μl deoxyribonucleo-tide triphosphate (dNTP), 0.2 μl Taq DNA polymerase (Qiagen GmbH, Hilden, Germany) and 10 μl H2O. The cycling parameters were 94 °C for 2 min; 35 cycles at 94 °C for 15 s, 55 °C for 15 s, 72 °C for 25 s; and a final extension step at 72 °C for 3 min. Probe sequences were 5′-AAC CAA CAT GGC TTG TTT TCA TAA A-3′ (A specific), 5′-TTT AAC CAA CAT GGC TTG TTT TCA TAA G-3′ (G specific), and 5′-P-CAC CCG AGA AGG TTT AAA TAA CAT T-FAM-3′ (for LDR). For each PCR product, the ligation reaction was performed in a final volume of 10 μl, including 2 μl PCR product, 1 μl 10 × Taq DNA ligase buffer, 0.03 μl probe mixture, 0.125 μl Taq DNA ligase (New England Biolabs, Beverly, MA, USA) and 7 μl H2O. The LDR parameters were 30 cycles at 94 °C for 30 s and 56 °C for 2 min. The LDR reaction products were analyzed on the ABI 377 DNA sequencer (Applied Biosystems). Genotyping success rates in cases and controls were 97.9% and 98.6%, respectively. To confirm the accuracy of the PCR–LDR genotyping method, 5% samples were randomly selected for direct DNA sequencing. PCR–LDR genotyping showed 100% concordance with direct DNA sequencing of the randomly selected PCR products.
Statistical analysis
Categorical data are shown as number (percentage) and analyzed by chi-square test. Continuous data are shown as median (Q1, Q3) because data were non-normally distributed. Mann–Whitney–Wilcoxon and Kruskal–Wallis rank tests were used to assess differences in continuous data between cases and controls and among different genotypes in controls, respectively. Odds ratios (ORs), 95% confidence intervals (95% CIs) and corresponding P values for risk of T2DM were calculated by binary logistic regression analyses. Sex, age, anthropometric measurements (BMI, WC, SBP and DBP), and lipid levels (TC, TG, HDL-C and LDL-C) were covariates. All tests were two-sided and P < 0.05 was considered statistically significant. Statistical analysis involved SAS version 9.1 for Windows (SAS Institute Inc., NC, USA).
Meta-analysis
We searched MEDLINE, EMBASE, Cochrane, and Chinese databases (CNKI, CQVIP and Wan-fang Databases) for all articles published in English and Chinese between January 2007 and August 2013 using the keywords “TCF7L2”, “rs11196218”, “genetic polymorphism” and “T2D/T2DM”. The meta-analysis involved use of Review Manager version 5.0 (http://www.cc-ims.net/RevMan). Crude ORs with 95% CIs were used to assess the strength of association between rs11196218 polymorphism and T2DM risk. The allele model (G vs. A), the co-dominant model (GG vs. AA; AG vs. AA), the dominant model (GG + AG vs. AA) and the recessive model (GG vs. AG + AA) were used to evaluate the risk. Pearson's goodness-of-fit chi-square test with 1 degree of freedom was used to assess departure from the Hardy–Weinberg equilibrium (HWE) for the control group in each study. To assess heterogeneity among the studies, Q-test and I2 statistics were used. The fixed-effects model (the Mantel–Haenszel method) was used to calculate the pooled OR with I2 < 50%; otherwise, the random-effects model was used. Calculation of population-attributable risk proportion (PARP) was based on the estimated OR and risk genotype frequency of the SNP showing significant association with T2DM. Subgroup analyses were performed by region (southern and northern China and Japan).
Results
Association study of rs11196218 polymorphism and T2DM
Levels of BMI, WC, SBP, DBP, TC, TG, LDL-C were higher and level of HDL-C was lower for T2DM patients than controls (Table 1). Cases and controls differed in genotype distribution (P = 0.001) but not allelic distribution (P = 0.579). The AG genotype and dominant model of rs11196218 were associated with risk of T2DM (OR = 1.44, 95% CI = 1.14–1.80; OR = 1.32, 95% CI = 1.06–1.65, respectively) (Table 2). However, after adjusting for sex, age, anthropometric measurements, and lipid levels, only the AG genotype remained associated with risk of T2DM (OR = 1.37, 95% CI = 1.06–1.78), dominant model showed marginal significant associated with risk of T2DM (OR = 1.28, 95% CI = 0.99–1.67) (Table 2). The results changed with WC included as a covariant. When stratified by central obesity status, obese diabetics and non-obese controls differed in genotype distribution (P < 0.001) but not non-obese diabetics and non-obese controls (P = 0.637). The rs11196218 genotypes were not associated with clinical characteristics in controls. The power of this study was 100%.
Table 1.
Demographic characteristics of the study participants.
| Characteristics | Cases (n = 1842) | Controls (n = 7777) | P value |
|---|---|---|---|
| Male | 925 (50.2) | 3,214 (41.3) | < 0.001 |
| Age (year) | 54.00 (45.0, 61.0) | 49.00 (40.0, 58.0) | < 0.001 |
| BMI (kg/m2) | 27.38 (24.5, 30.7) | 23.83 (21.6, 26.2) | < 0.001 |
| WC (cm) | 90.50 (83.5, 101.3) | 81.35 (74.9, 88.5) | < 0.001 |
| SBP (mm Hg) | 128.33 (120.0, 140.0) | 121.33 (110.7, 134.7) | < 0.001 |
| DBP (mm Hg) | 81.33 (74.0, 90.0) | 77.00 (70.7, 85.0) | < 0.001 |
| FPG (mmol/L) | 7.62 (6.1, 9.9) | 5.19 (4.8, 5.5) | < 0.001 |
| TC (mmol/L) | 4.87 (4.2, 5.6) | 4.28 (3.7, 4.9) | < 0.001 |
| TG (mmol/L) | 1.73 (1.2, 2.6) | 1.32 (1.0, 1.9) | < 0.001 |
| HDL-C (mmol/L) | 1.10 (1.0, 1.3) | 1.14 (1.0, 1.3) | < 0.001 |
| LDL-C (mmol/L) | 2.81 (2.2, 3.4) | 2.42 (2.0, 2.9) | < 0.001 |
Data are presented as number (%) or median (Q1, Q3).
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; and LDL-C, low-density lipoprotein cholesterol.
Table 2.
Association of rs11196218 polymorphism and type 2 diabetes mellitus (T2DM) in different models.
| Genetic models | Cases | Controls | P value | OR (95% CI) | P value⁎ | OR (95% CI)⁎ |
|---|---|---|---|---|---|---|
| Allele model | ||||||
| A | 967 (26.82) | 4041 (26.37) | – | 1.00 | – | – |
| G | 2639 (73.18) | 11,287 (73.63) | 0.579 | 0.98 (0.90, 1.06) | – | – |
| Co-dominant model | ||||||
| AA | 98 (5.44) | 540 (7.05) | – | 1.00 | – | 1.00 |
| AG | 771 (42.76) | 2961 (38.64) | 0.002 | 1.44 (1.14, 1.80) | 0.018 | 1.37 (1.06, 1.78) |
| GG | 934 (51.80) | 4163 (54.31) | 0.067 | 1.24 (0.99, 1.55) | 0.173 | 1.20 (0.93, 1.55) |
| Dominant model | ||||||
| AA | 98 (5.44) | 540 (7.05) | – | 1.00 | – | 1.00 |
| AG + GG | 1705 (94.56) | 7124 (92.95) | 0.014 | 1.32 (1.06, 1.65) | 0.060 | 1.28 (0.99, 1.67) |
| Recessive model | ||||||
| AG + AA | 869 (48.20) | 3501 (45.69) | – | 1.00 | – | 1.00 |
| GG | 934 (51.80) | 4163 (54.31) | 0.054 | 0.90 (0.82, 1.00) | 0.118 | 0.91 (0.81, 1.02) |
Data are presented as number (%).
OR, odds ratio; 95% CI, 95% confidence interval.
Adjusted by sex, age, BMI, WC, SBP, DBP, TC, TG, HDL-C and LDL-C.
Results of the meta-analysis
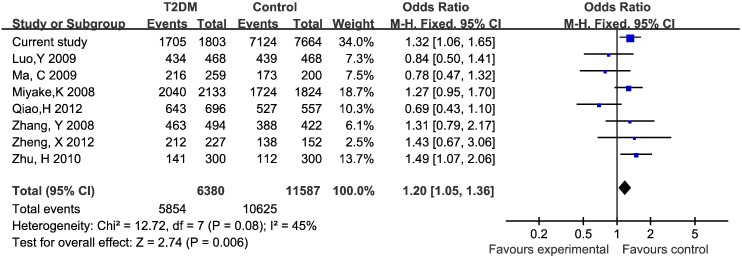
We included 9 eligible published articles (Lin et al., 2010, Luo et al., 2009, Ma et al., 2009, Miyake et al., 2012, Ng et al., 2007, Qiao et al., 2012, Zhang, 2008, Zheng et al., 2012, Zhu, 2011) and current study, for 8342 cases and 13,445 controls. Six studies were for southern China, 3 for northern China, and only one study was for Japan. The distributions of genotypes in controls for all studies were in Hardy–Weinberg equilibrium (Table 3). Because of absence of genotype data, ten studies were used for the allele model, with 8 studies for the other genetic models. We found the dominant model of rs11196218 significantly associated with risk of T2DM in the overall population (OR = 1.20, 95% CI = 1.05–1.36) (Fig. 1). The PARP for the dominant model was 15.5%. On subgroup analysis by region, the results suggested a consistent association of the dominant model and T2DM risk in southern Chinese (OR = 1.27, 95% CI = 1.01–1.60) but not northern Chinese and Japanese populations. The AG genotype was associated with T2DM risk in southern Chinese (OR = 1.31, 95% CI = 1.03–1.66) (Table 4).
Table 3.
Characteristics of the studies included in the meta-analysis.
| Authors | Year | Region | Source of controls | Genotyping method | Cases |
Controls |
P value⁎ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | AG | AA | G | A | GG | AG | AA | G | A | ||||||
| Current study | – | NC | Community | PCR–LDR | 934 | 771 | 98 | 2639 | 967 | 4163 | 2961 | 540 | 11,287 | 4041 | 0.67 |
| Ng et al. | 2007 | SC | Community | MALDI-TOF | – | – | – | 676 | 190 | – | – | – | 599 | 239 | – |
| Lin et al. | 2010 | SC | Community | ABI SNaPshot | – | – | – | 2174 | 884 | – | – | – | 2023 | 855 | – |
| Luo et al. | 2009 | NC | Hospital | ABI SNaPshot | 250 | 184 | 34 | 684 | 252 | 257 | 182 | 29 | 696 | 240 | 0.67 |
| Ma et al. | 2009 | SC | Hospital | PCR-PFLP | 93 | 123 | 43 | 309 | 209 | 67 | 106 | 31 | 240 | 168 | 0.30 |
| Qiao et al. | 2012 | NC | Hospital | PCR–LDR | 382 | 261 | 53 | 1025 | 367 | 292 | 235 | 30 | 819 | 295 | 0.05 |
| Zhang et al. | 2008 | SC | Hospital | Taqman | 253 | 210 | 31 | 716 | 272 | 235 | 153 | 34 | 623 | 221 | 0.20 |
| Zheng et al. | 2012 | SC | Hospital | MALDI-TOF | 128 | 84 | 15 | 340 | 114 | 80 | 58 | 14 | 218 | 86 | 0.46 |
| Zhu et al. | 2010 | SC | Hospital | Taqman | 14 | 127 | 159 | 155 | 445 | 10 | 102 | 188 | 122 | 478 | 0.39 |
| Miyake et al. | 2008 | Japan | Community | Taqman | 1316 | 724 | 93 | 3356 | 910 | 1112 | 612 | 100 | 2836 | 812 | 0.19 |
NC, northern China; SC, southern China.
P value for Hardy–Weinberg equilibrium.
Fig. 1.
Forest plot for the dominant model of rs11196218 polymorphism and T2DM.
Table 4.
Meta-analysis of the association between rs11196218 polymorphism and T2DM in different models.
| Study populations | G vs. A |
GG vs. AA |
AG vs. AA |
(GG + AG) vs. AA |
GG vs. (AG + AA) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value⁎ | OR (95% CI) | P value ⁎ | OR (95% CI) | P value ⁎ | OR (95% CI) | P value ⁎ | OR (95% CI) | P value⁎ | |
| Overall | 1.03 (0.99, 1.08) | 0.06 | 1.15 (1.00, 1.33) | 0.42 | 1.16 (0.94, 1.44)a | 0.04 | 1.20 (1.05, 1.36) | 0.08 | 0.97 (0.90, 1.04) | 0.40 |
| Region | ||||||||||
| Southern China | 1.13 (0.99, 1.29)a | 0.04 | 1.22 (0.89, 1.67) | 0.74 | 1.31 (1.03, 1.66) | 0.32 | 1.27 (1.01, 1.60) | 0.23 | 0.98 (0.81, 1.18) | 0.35 |
| Northern China | 0.98 (0.91, 1.05) | 0.87 | 1.08 (0.90, 1.31) | 0.09 | 0.95 (0.55, 1.64)a | 0.01 | 0.95 (0.62, 1.48)a | 0.02 | 0.94 (0.86, 1.02) | 0.28 |
| Japan | 1.06 (0.95, 1.18) | – | 1.27 (0.95, 1.71) | – | 1.27 (0.94, 1.72) | – | 1.27 (0.95, 1.70) | – | 1.03 (0.91, 1.17) | – |
P value for heterogeneity.
Estimates for random-effects model.
Discussion
T2DM is a complex disease affected by both genetic and environmental factors (Gujral et al., 2013). Many genetic association studies have suggested that several genes and specific SNPs are associated with risk of T2DM (Nair et al., 2012, Ng et al., 2008, Rai et al., 2012, Yu et al., 2013). In fact, TCF7L2 is considered the most influential gene in determining genetic susceptibility for T2DM in humans (Luo et al., 2009, Ng et al., 2008, Qiao et al., 2012, Wang et al., 2013). Mechanistic studies suggested that TCF7L2 could impair β-cell function and down-regulate the expression of glucagon-like peptide 1 receptor (GLP-1R) and glucose-dependent insulinotropic polypeptide receptor (GIP-R), thus reducing insulin level (Le-Bacquer et al., 2011, Shu et al., 2009, Vazquez-Roque et al., 2011). However, because of the low frequency of the at-risk allele of the SNP variants rs7903146, rs12255372 and rs11196205, originally reported in European populations, the association of these variants and T2DM in Asian populations varies among studies (Guinan, 2012).
In a 2008 study involving a “gene-based approach” to test all common genetic variants within TCF7L2 in a Chinese population, rs11196218 was found associated with T2DM in Hong Kong Chinese (G vs. A: OR = 1.43, 95% CI = 1.14–1.79) (Ng et al., 2007). Since then, some replication studies have been performed in Chinese and Japanese populations (Lin et al., 2010, Luo et al., 2009, Ma et al., 2009, Miyake et al., 2012, Qiao et al., 2012, Zhang, 2008, Zheng et al., 2012, Zhu, 2011); the minor allele frequency (MAF) of rs11196218 was > 20% in Asians. Even for the common SNP, discrepancies existed among studies performed in the same ethnic groups (Lin et al., 2010, Ma et al., 2009, Ng et al., 2007, Zhang, 2008, Zheng et al., 2012, Zhu, 2011). Therefore, with the existing information, evaluating the impact of this gene on the genetic susceptibility of T2DM is difficult. Studies with larger sample size and sufficient power as well as a well-designed meta-analysis are needed to clarify this issue.
With 1842 T2DM and 7777 normal controls in our study, the power to detect the association of rs11196218 polymorphism and T2DM reached 100%. Our results suggest that the GA genotype and the dominant rs11196218 model are associated with risk of T2DM. Our findings are similar to those of Anhui population (Zhu, 2011) but opposite to those of Beijing (Luo et al., 2009), Chongqing (Zheng et al., 2012) and Japan (Miyake et al., 2012) populations. This inconsistence may due to the intricate substructure of study populations, but confounding cannot be neglected. In the present study, the association of the dominant model of rs11196218 polymorphism and T2DM disappeared after adjustment for sex, age, anthropometric measurements, and lipid levels. The results of different adjustments show that WC was the confounder. For controls, we found no association of anthropometric or metabolic measurements and rs11196218 genotypes.
In our meta-analysis, the dominant model of rs11196218 was associated with risk of T2DM in the overall population. However, when stratified by region, this association was found in only the southern Chinese population, not northern Chinese or Japanese populations. In the southern Chinese population, the AG genotype was associated with increased risk of T2DM. The main reason might due to the complex composition of the Chinese and Japanese populations and the great differences among ethnic groups in genetics and living environment. As well, studies of rs11196218 polymorphism and T2DM have focused on southern Chinese populations. Except for our study, with only 2 studies of northern Chinese (Luo et al., 2009, Qiao et al., 2012) and 1 study of Japanese (Miyake et al., 2012), the number of studies is relatively less. More independent replication studies with a large sample size in these regions are needed to verify the results.
Although our study combined data from all qualified studies and had a large sample size with sufficient power to assess the rs11196218 polymorphism in TCF7L2 in the Asian population, the possible heterogeneity among subjects recruited from different studies might be a limitation. Moreover, we lacked detailed information on sex, age, anthropometric measurements and metabolic measurements for the meta-analysis, so the effects of these factors could not be adequately addressed.
In conclusion, both our case–control study and meta-analysis suggest an association of rs11196218 polymorphism in TCF7L2 and risk of T2DM in Chinese population.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant no. 81072359), the Natural Science Foundation of Guangdong Province (grant no. S2013010016791), the Science and Technology Development Foundation of Shenzhen (grant nos. JCYJ20120613112221107 and JCYJ20130326110246234), and the Natural Science Foundation of Shenzhen University (grant no. 801-00035911).
Contributor Information
Yujia Zhai, Email: zhaiyujiamodi@163.com.
Jingzhi Zhao, Email: 15137165136@163.com.
Haifei You, Email: youhaifei1987@163.com.
Chao Pang, Email: smartduoduo1@163.com.
Lei Yin, Email: yinlei3008@163.com.
Tan Guo, Email: 1328618233@qq.com.
Tianping Feng, Email: besdwujing1@163.com.
Chongjian Wang, Email: tjwcj2005@126.com.
Kaiping Gao, Email: gao_kaiping@hotmail.com.
Xinping Luo, Email: lxp2005@yahoo.cn.
Ming Zhang, Email: caroline.zhang@hotmail.com.
Jinjin Wang, Email: wangjinjin510@163.com.
Linlin Li, Email: lilinlin@zzu.edu.cn.
Bingyuan Wang, Email: wangby95@163.com.
Yongcheng Ren, Email: ryc12@sina.com.
Yanxia Shen, Email: 690455673@qq.com.
Tian Zhu, Email: 1466284965@qq.com.
Dongsheng Hu, Email: dongsheng_hu@hotmail.com.
References
- American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2005;28:S4–S36. [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Grant S.F. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Guinan K.J. Worldwide distribution of type II diabetes-associated TCF7L2 SNPs: evidence for stratification in Europe. Biochem. Genet. 2012;50:159–179. doi: 10.1007/s10528-011-9456-2. [DOI] [PubMed] [Google Scholar]
- Gujral U.P., Pradeepa R., Weber M.B., Narayan K.M., Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci. 2013;1281:51–63. doi: 10.1111/j.1749-6632.2012.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Bacquer O. TCF7L2 splice variants have distinct effects on beta-cell turnover and function. Hum. Mol. Genet. 2011;20:1906–1915. doi: 10.1093/hmg/ddr072. [DOI] [PubMed] [Google Scholar]
- Lin Y. Association study of genetic variants in eight genes/loci with type 2 diabetes in a Han Chinese population. BMC Med. Genet. 2010;11 doi: 10.1186/1471-2350-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. Meta-analysis of the association between SNPs in TCF7L2 and type 2 diabetes in East Asian population. Diabetes Res. Clin. Pract. 2009;85:139–146. doi: 10.1016/j.diabres.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Ma C., Sheng H., Ma J. Relationship of rs13266634 and rs11196218 polymorphisms in solute carrier family 30, member 8 and transcription factor 7 like-2 genes with type 2 diabetes in Han population in Southern China. Shanghai Med. J. 2009;32:1086–1091. [in Chinese] [Google Scholar]
- Miyake K. Association of TCF7L2 polymorphisms with susceptibility to type 2 diabetes in 4,087 Japanese subjects. J. Hum. Genet. 2012;53:174–180. doi: 10.1007/s10038-007-0231-5. [DOI] [PubMed] [Google Scholar]
- Nair S., Muller Y.L., Ortega E., Kobes S., Bogardus C., Baier L.J. Association analyses of variants in the DIO2 gene with early-onset type 2 diabetes mellitus in Pima Indians. Thyroid. 2012;22:80–87. doi: 10.1089/thy.2010.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M.C., Tam C.H., Lam V.K., So W.Y., Ma R.C., Chan J.C. Replication and identification of novel variants at TCF7L2 associated with type 2 diabetes in Hong Kong Chinese. J. Clin. Endocrinol. Metab. 2007;92:3733–3737. doi: 10.1210/jc.2007-0849. [DOI] [PubMed] [Google Scholar]
- Ng M.C. Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes. 2008;57:2226–2233. doi: 10.2337/db07-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H. Genetic variants of TCF7L2 are associated with type 2 diabetes in a northeastern Chinese population. Gene. 2012;495:115–119. doi: 10.1016/j.gene.2011.12.055. [DOI] [PubMed] [Google Scholar]
- Rai E. The interactive effect of SIRT1 promoter region polymorphism on type 2 diabetes susceptibility in the North Indian population. PLoS ONE. 2012;7:e48621. doi: 10.1371/journal.pone.0048621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L., Matveyenko A.V., Kerr-Conte J., Cho J.H., McIntosh C.H., Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum. Mol. Genet. 2009;18:2388–2399. doi: 10.1093/hmg/ddp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Roque M.I., Camilleri M., Vella A., Carlson P., Laugen J., Zinsmeister A.R. Association of TCF7L2 allelic variations with gastric function, satiation, and GLP-1 levels. Clin. Transl. Sci. 2011;4:183–187. doi: 10.1111/j.1752-8062.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Meta-analysis of associations between TCF7L2 polymorphisms and risk of type 2 diabetes mellitus in the Chinese population. BMC Med. Genet. 2013;14:8. doi: 10.1186/1471-2350-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.Y. Association between the TRAIL single nucleotide polymorphism rs1131580 and type 2 diabetes mellitus in a Han Chinese population. Genet. Mol. Res. 2013;12:3455–3464. doi: 10.4238/2013.September.10.2. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Central South University; 2008. Association Study of TCF7L2 Gene Polymorphisms with Susceptibility to Type 2 Diabetes in Han Chinese Population. Master. (in Chinese) [Google Scholar]
- Zheng X. Association of type 2 diabetes susceptibility genes (TCF7L2, SLC30A8, PCSK1 and PCSK2) and proinsulin conversion in a Chinese population. Mol. Biol. Rep. 2012;39:17–23. doi: 10.1007/s11033-011-0705-6. [DOI] [PubMed] [Google Scholar]
- Zhou B.F., Cooperative Meta-Analysis Group of the Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- Zhu H. Anhui Medical University; 2011. Common variants of the TCF7L2 gene with type 2 diabetes and impaired glucose regulation in a Chinese Han population. Master. (in Chinese) [Google Scholar]