Abstract
Nitric oxide plays a major role in the regulation of cerebral blood flow and loss of its function leads to alteration of the vascular relaxation given its central role in the physiology of the vascular system. G894T eNOS polymorphism could have adverse effects on the expression and activity of endothelial nitric oxide synthase, which can result in functional impairment of the endothelium and contribute to the development of ischemic stroke in the different models of transmission.
In this study, genotyping with PCR-RFLP and HRM (high resolution melting) methods were conducted on 165 ischemic stroke patients as well as 182 controls. The goal here was to compare genotyping with PCR-RLFP primer sequences of eNOS gene (size < 300 bp) to HRM.
Our data suggests a statistically significant association between G894T eNOS polymorphism and ischemic stroke in recessive, dominant and additive models with P < 0.05 and odds ratio of 2.68 (1.08–6.70), 1.78 (1.16–2.73), and 1.71 (1.21–2.43) respectively.
In sum, although the sample size is relatively small, it suggests that G894T eNOS polymorphism could be a potentially important genetic marker of ischemic stroke in the Moroccan population. Future studies should be conducted in this direction taking into consideration the functional activity of eNOS.
Abbreviations: 894T eNOS, mutant allele of endothelial nitric oxide synthase; AB, applied biosystems; bp, base pair; CI, confidence interval; DNA, deoxyribonucleic acid or deoxyribose nucleic acid; eNOS, endothelial nitric oxide synthase; Fig., figure; G894T eNOS, replacement guanine by thymine at position 894 of the endothelial nitric oxide synthase; GG eNOS, homozygous wild of endothelial nitric oxide synthase; GT eNOS, heterozygous mutant of endothelial nitric oxide synthase; HRM, high resolution melt; IS, ischemic stroke; LGPM, genetic and molecular pathology laboratory; NO, nitric oxide; OR, odds ratio; P, P value; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; Ref., reference; TOAST, Trial of Org 10172 in Acute Stroke Treatment; TT eNOS, homozygous mutant of endothelial nitric oxide synthase; vs, versus; χ2, chi square
Keyword: G894T eNOS, Ischemic stroke, Genetics models
Introduction
Ischemic stroke (IS) caused by either thrombosis or embolism is the most common disease leading to disability and/or death (Flemming et al., 2004, Saver and Tamburi, 2000). In the past decade, in addition to the known traditional risk factors such as arterial hypertension, diabetes, obesity, smoking, and dyslipidemia, other important factors including genetic variability of phenotypic profile have been identified (Domingues-Montanari et al., 2008). This genetic variability which is ethnic related is itself due to the common and uncommon risk factors for IS. However, the genes involved in the pathogenesis of this disease remain largely understudied (Hassan and Markus, 2000, Szentirmai and Carter, 2008). In the pathogenesis of stroke, according to the few clinical and experimental data available, endothelial dysfunction plays a key role in vascular alteration (Yetik-Anacak and Catravas, 2006). It has been also established that nitric oxide (NO), an endothelium-derived product of endothelial nitric oxide synthase (eNOS) is a potent vasodilator, anti-thrombotic, anti-inflammatory and anti proliferative, which all play important roles in the regulation of blood flow (Endres et al., 2004, Furchgott and Zawadszki, 1980, Loscalzo, 1995). Therefore, loss of function of NO contributes to the alteration of the vascular relaxation (Huang et al., 1995). The involvement in the physiology of the vascular system suggests that eNOS gene could be a potential susceptibility candidate of stroke. Located on chromosome 7q35–36 which contains 26 exons, the eNOS gene is crucial for the exchange rate of NO (Wang and Wang, 2000, Wattanapitayakul et al., 2001). The polymorphism of G894T eNOS gene is frequently encountered in vascular diseases such as myocardial infarction, coronary spasm and arterial hypertension (Wang et al., 2013). Several studies have been conducted around the world on the risk of stroke with this polymorphism, but the results remain controversial, and most of these studies have focused only on the susceptibility to stroke rather than studying different models of transmission. It has been shown in some studies that G894T eNOS polymorphism is associated with high risk of stroke (Wang et al., 2013) but others found in contrast a low or moderate risk (Tao and Chen, 2009). In Morocco, to study the association between eNOS polymorphism and IS first, we conducted this study to better assess the G894T eNOS variant hand in IS. We hypothesized, in this study, that this polymorphism could have an important impact on different genetic models of IS.
Materials and methods
Study population
This is a randomized case–control study focused on a series of 165 subjects with IS recruited at the Neurology Services of the Teaching Hospital of Casablanca and Rabat between March 2009 and February 2013, and 182 healthy control subjects recruited from volunteer blood donors with no history of stroke and cognitive disorders. Informed consent has been developed for each patient and control in our study. The distribution of our patients was performed according to the international classification of TOAST (Trial of Org 10172 in Acute Stroke Treatment) after a cardiovascular neurological examination, and biological, physiological and radiological investigations (Cerebral Scanner, Doppler echography, Electrocardiogram).
DNA extraction
Genomic DNA was extracted from whole blood samples using the standard method with phenol/chloroform extraction and ethanol precipitation. The determination of DNA concentration was made with NanoVue Plus.
Genotyping with PCR-RFLP
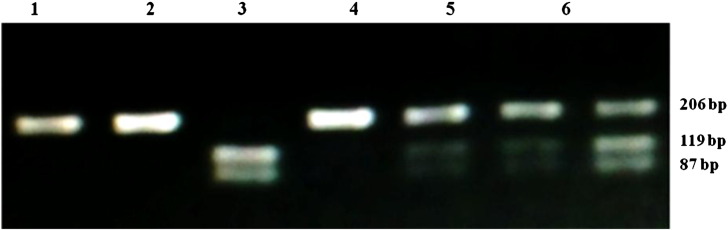
Genotypes of G894T eNOS gene was detected by PCR-RFLP as described by Hingorani et al. (1999). G to T substitution at nucleotide position 894 of eNOS gene creates a restriction site with MseI enzyme for G894T eNOS polymorphism. After amplification and digestion, PCR product showed one fragment of 206 bp for wild GG homozygous, three fragments of 206, 119 and 87 bp for GT heterozygous and two fragments of 119 and 87 bp for mutated TT homozygous (Fig. 1). The three genotypic profiles GG, GT and TT of eNOS gene obtained by PCR-RFLP were used as controls for this genotyping by high resolution melting (HRM).
Fig. 1.
PCR-RFLP analysis on agarose gel of G894T eNOS polymorphism. Lanes 1, 2, and 4 represent GG homozygous wild-type; lanes 5, 6, and 7 represent GT heterozygous and lane 3, TT homozygous mutated.
Genotyping by high resolution melting (HRM)
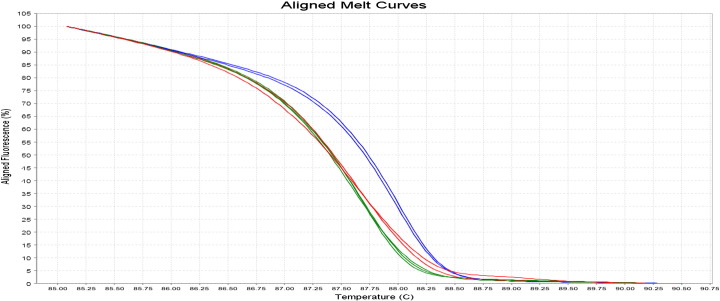
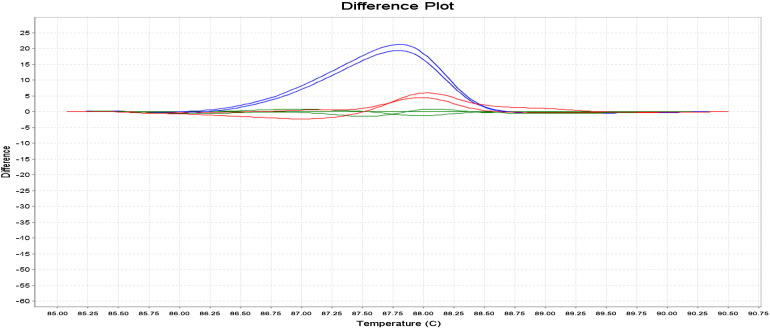
The HRM analysis on 7500 Fast Real-Time PCR (Applied Biosystems®, AB) was used to genotype G894T eNOS gene with an amplicon size of 206 bp. The protocol used for the reconstitution of HRM reaction was made from a reagent kit, MeltDoctor™ HRM. HRM assays were performed in a final volume of 20 μl including the MeltDoctor™ HRM Master Mix (Applied Biosystems®) which contains SYTO9 as the DNA intercalating dye (Giffard et al., 2007, Graham et al., 2005, Reed et al., 2007). Forward and reverse sequences of primers described by Hingorani et al. (1999) were used for amplifications. The melting curve analysis was performed with 7500 Fast System SDS Software v2.0.1. The interpretation of GG, GT and TT genotypes was performed from the temperature curves in reference to the curves of the controls (Fig. 2, Fig. 3).
Fig. 2.
Aligned melting curve HRM analysis of G894T eNOS polymorphism. Blue—GG homozygous wild type, red—GT heterozygous mutant and green—TT homozygous mutant.
Fig. 3.
Difference plots of HRM analysis of G894T eNOS polymorphism. Blue—GG homozygous wild type, red—GT heterozygous mutant and green reference—TT homozygous mutant.
Statistical analysis
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The law of the genotype distribution for G894T eNOS polymorphism among disease cases and controls were performed with Hardy–Weinberg equilibrium test. Categories of IS subtype relations to different genotypes were performed using χ2 test. A P value < 0.05 was considered as significant. The association between the polymorphism of G894T eNOS gene and risk of IS in transmission models was evaluated using odds ratio (OR) of 95% confidence interval (CI).
Results
By comparing HRM analysis (Fig. 2, Fig. 3) and PCR-RFLP technique (Fig. 1) for genotyping of G894T eNOS gene, no discordant results were observed.
Test cases and controls were in Hardy–Weinberg equilibrium for the distribution of G894T eNOS polymorphism (χ2 = 0.30, P = 0.86; χ2 = 0.63, P = 0.98). The average age of patients and controls was 56.58 ± 2 and 54.15 ± 2 respectively. Older age patients (age > 50 years) were overrepresented in our study sample compared to young subjects (age ≤ 50 years). The proportion of males was more important than that of the females. No positive correlation was seen between G894T eNOS polymorphism and age (P = 0.11) or sex (0.51) (Table 1).
Table 1.
Frequencies of G894T eNOS polymorphism according to age, gender and subtypes in ischemic stroke subjects.
| IS characteristic | N |
G894T eNOS polymorphism |
P value (P < 0.05) |
||
|---|---|---|---|---|---|
| GG% | GT% | TT% | |||
| Age | 165 | 0.11 | |||
| ≤ 50 years | 47 | 21 (44.7) | 24 (51.1) | 2 (4.3) | |
| > 50 years | 118 | 62 (52.5) | 42 (35.6) | 14 (11.9) | |
| Gender | 165 | 0.51 | |||
| Female | 74 | 38 (51.4) | 31 (41.9) | 5 (6.8) | |
| Male | 91 | 45 (49.5) | 35 (38.5) | 11 (12.1) | |
N: total number, GG eNOS wild genotypes, GT eNOS variant heterozygous, TT eNOS variant homozygous.
Among the IS subtypes included in our studies, atherosclerosis (93 cases) and cardioembolic (53 cases) forms were more frequent than lacunars (8 patients) and other (11 cases) forms. Statistically, no significant difference was observed between G894T eNOS gene and IS subtypes (P = 0.89) (Table 2).
Table 2.
Genotype frequencies of G894T eNOS gene according to ischemic stroke subtype.
| Genotypes | TOAST classification (%) |
|||
|---|---|---|---|---|
| Atherosclerosis N = 93 |
Cardioembolic N = 53 |
Lacunar N = 8 |
Others N = 11 |
|
| GG | 45 (48.4) | 29 (54.7) | 4 (50.0) | 5 (45.5) |
| GT | 38 (40.9) | 21 (39.6) | 3 (37.5) | 4 (36.4) |
| TT | 10 (10.8) | 3 (5.7) | 1 (12.5) | 2 (18.2) |
Chi-square test of all IS subtypes (χ2 = 2.27, P = 0.89).
GG eNOS wild genotypes, GT eNOS variant heterozygous, TT eNOS variant homozygous, TOAST (Trial of Org 10172 in Acute Stroke Treatment).
Table 3 shows the genotypic and allelic distribution and transmission models of G894T eNOS polymorphism among cases compared to controls. Genotypic frequencies found among cases and controls were 50.3% GG, 40.0% GT, and 8.0% TT and 61.1% GG, 34.6% GT, and 3.3% TT respectively. Allele frequencies of G and T of eNOS gene were respectively 70.3%, 29.7% and 79.4% for G, and 20.6% for T in the IS group and control group. In our study, a significant increase of risk of IS was observed in both TT (OR: 3.22, 95% CI = 1.27–8.18; P = 0.014) and GT (OR: 1.60, 95% CI = 1.02–2.52; P = 0.040) genotypes.
Table 3.
Genotype and allele frequencies of G894T eNOS polymorphism in patients with ischemic stroke and controls.
| Genotypes/alleles | Cases (%) N = 165 |
Control (%) N = 182 |
OR (95% CI) | P value |
|---|---|---|---|---|
| GG | 83 (50.3) | 117 (64.3) | 1 | |
| GT | 66 (40.0) | 58 (31.9) | 1.60 (1.02–2.52) | 0.040a |
| TT | 16 (8.0) | 7 (3.8) | 3.22 (1.27–8.18) | 0.014a |
| GG + GTb | 149 (90.3) | 175 (96.2) | 1 | |
| TT | 16 (9.7) | 7 (3.8) | 2.68 (1.08–6.70) | 0.034a |
| GGc | 83 (50.3) | 117 (64.3) | 1 | |
| GT + TT | 82 (46.7) | 65 (35.7) | 1.78 (1.16–2.73) | 0.009a |
| Gd | 232 (70.3) | 292 (80.2) | 1 | |
| T | 98 (29.7) | 72 (19.8) | 1.71 (1.21–2.43) | 0.003a |
N: Number, OR: odds ratio, CI: confidence interval, P < 0.05, vs.: versus, Ref.: reference, %: percentage.
Significant.
TT vs. GG + GT (recessive model) for G894T eNOS.
GT + TT vs. GG (dominant model) for G894T eNOS.
T vs. G (additive model) for G894T eNOS.
There was also a positive correlation between G894T eNOS polymorphism and IS in additive (T vs. G, OR: 1.71, 95% CI = 1.21–2.43; P = 0.003), recessive (TT vs. GG + GT, OR: 2.68, 95% CI = 1.08–6.70, P = 0.034) and dominant (GT + TT vs. GG: OR: 1.78, 95% CI = 1.16–2.73, P = 0.009) models. However, the risk was considerably higher in the recessive model than that of the other two models. So G894T eNOS polymorphism could be a potential genetic marker for ischemic stroke in the Moroccan population.
Discussions
Concerning the comparison of genotyping PCR-RFLP and HRM from the same primers (size < 300 bp), the results of G894T eNOS genotyping by both techniques were similar. Once genotypic profiles are known, the HRM method can be used in the rapid detection of single base sequences. This will save time with less work relative to PCR-RFLP.
NO synthesized by eNOS, being a potent vasodilator, anti-thrombotic, anti-inflammatory and anti-proliferative plays an important role in the regulation of blood flow. eNOS polymorphism plays a major role in endothelial dysfunction (Leeson et al., 2002). G894T eNOS variant has been implicated in the pathogenesis of several diseases such as cardiovascular disease and erectile dysfunction (Charalambos et al., 2006, Lee et al., 2009, Tang et al., 2008). However, the results of various studies on this variant as a factor in genetic predisposition to IS stay contradictory (Tao and Chen, 2009). We carried out this work in order to better assess the interaction between G894T eNOS polymorphism and IS in the Moroccan population. The large number of IS subjects aged ≥ 50 years and male in our study sample was in conformity with what has been reported in the literature that IS is a pathology that affects both sexes at any time of life but the elderly (50 years or older) are more affected than younger subjects with a male predominance (Reeves et al., 2008). Our data showed no positive association between G894T polymorphism of eNOS gene and demographic characteristics (age and gender). This result is consistent with those that have been found by Berger et al. (2007) and Saidi et al. (2010). They respectively found in German and Tunisian populations that association of this polymorphism with age and gender was independent.
We observed no significant difference in the distribution of G894T eNOS polymorphism and IS subtypes. This result was similar to what has been obtained by Grewal et al. (2007), Markus et al. (1998), Hassan et al. (2004) and MacLeod et al. (1999). The first reported that G894T eNOS gene is not associated with an increased risk of IS subtypes in Caucasian and African American populations. Markus et al. found in a British population that G894T eNOS variant was not associated with ischemic stroke secondary to atherosclerosis forms. In the study by Hassan, this polymorphism was associated with a reduction of ischemic stroke risk secondary to lacunar infarction forms. Moreover, MacLeod et al. reported in a French population that the effect of this polymorphism has no impact on IS subtypes. By contrast, Elbaz et al. found a statistically significant association between G894T eNOS gene and IS lacunar forms (Elbaz et al., 2000). These discrepancies concerning the relation between G894T eNOS variant and the risk of ischemic stroke subtypes could be linked to several factors, including methodological differences between studies, the sample size, the frequency of subtypes in different populations and early diagnosis of radiological IS.
Regarding the association between G894T eNOS polymorphism and the risk of ischemic stroke, the results of our study suggest that GT and TT genotypes were significantly associated with ischemic stroke. This data was similar to that observed by Saidi et al. who reported on a Bahrain population increased risk of ischemic stroke (P < 0001 for the two genotypes) (Saidi et al., 2010). Berger et al. found the same result in a German population where the risk of CT and TT genotypes was 1.16 (P = 0.03) and 0.01 (P = 0.01) respectively (Berger et al., 2007).
Concerning the relation between G894T eNOS polymorphism and IS in different models, the present study also revealed that the G894T eNOS gene was significantly associated with an increased risk of ischemic stroke in recessive, dominant and additive models, where a strong association was observed with the first model (OR = 2.68) compared to the other two models. These are consistent with those reported by Saidi et al. on a Bahrain population, in which the three models seem to be involved in the pathogenesis of IS (Saidi et al., 2010). Similarly, Wang et al., in a meta-analysis of several populations (Asia, Turkey) and Berger et al. (German population) concluded that recessive and dominant genetic models were associated with a high risk of IS for G894T eNOS but not with the recessive model (Berger et al., 2007, Wang et al., 2013). However, in a specific subgroup analysis of Chinese population, the results were similar (Wang et al., 2013) (Table 4). Interestingly, as the conclusion of another meta-analysis in a well-diversified population, Tao and Chen (2009) reported that three genetic models were moderately associated with risk of ischemic stroke (Table 4).
Table 4.
Summary of studies of G894T eNOS polymorphism and ischemic stroke in genetic models.
| Additive model T vs. G |
Dominant model GT + TT vs. GG |
Recessive model TT vs. GG + GT |
Authors | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P < 0.05 | OR (95% CI) | P < 0.05 | OR (95% CI) | P < 0.05 | |
| 1.16 (1.05–1.13) | 0.003a | 2.26 (1.2–4.66) | 0.014a | 2.58 (0.05–13.62) | 0.26 | Berger et al. (2007) |
| 1.06 (0.99–1.13) | 0.06 | 1.07 (0.99–1.55) | 0.11 | 1.11 (0.97–1.28) | 0.12 | Tao et al. (2009) |
| 1.97 (1.59–2.43) | < 0.0001a | 2.51 (1.86–3.39) | < 0.0001a | 2.24 (1.40–3.42) | < 0.0001a | Saidi et al. (2010) |
| 1.27 (1.13–1.42) | 0.000a | 1.30 (1.15–1.49) | 0.000a | 1.273 (0.85–1.91) | 0.25 | Wang et al. (2013) |
| 1.34 (1.11–1.60) | 0.000a | 1.34 (1.10–1.62) | 0.000a | 1.27 (0.85–1.91) | 0.074 | Wang et al. (2013) |
| 1.71 (1.21–2.43) | 0.003 | 1.78 (1.16–2.73) | 0.009a | 2.68 (1.08–6.70) | 0.034a | Our study 2014 |
OR: odds ratio, CI: confidence interval, P < 0.05.
Significant.
This contradiction about G894T eNOS polymorphism and IS in a recessive model could be explained by the difference in sample sizes and genetic variability of ethnicities (Table 3). The most common problem in association studies is the inconsistency of data due to different models of inheritance (Salanti and Higgins, 2008). Thus, deep study of the genetic predisposition behind these different models of transmission is required to properly elucidate the hereditary nature of the polymorphisms. When the inheritance is unknown with two alleles of single genetic variant, dominant, recessive, and additive models may have complex characters (Salanti and Higgins, 2008, Salanti et al., 2009). To avoid one genetic model, we presented here an analysis of three different models. If the risk is the same for G894T heterozygous carrying one copy of 894T mutated allele, as for homozygosity, the underlying model is dominant. For the recessive model, two copies of 894T eNOS gene are necessary so that risk is different from the basis risk. The risk in subjects carrying two copies of 894T is double the risk in heterozygous cases, which is the additive genetic model (Attia et al., 2003, Salanti and Higgins, 2008). In addition, the maximum power is obtained, when the inherited model is unknown with codominant model and one or all three genetic models are tested together.
Conclusion
Although the PCR-RFLP method remains laborious and requires plenty of time, our results suggested that HRM is a simple, fast, reliable and profitable test, which allows genotyping of G894T eNOS gene. In this study, we showed that G894T eNOS polymorphism could play its part by endothelial dysfunction process in the onset of IS in a Moroccan population. It would be important in future studies to increase the sample size on the one hand and on the other hand to measure the impact of this polymorphism at the clinical level. In addition, it will be important to add biochemical tests of NO in order to better understand this polymorphism's direct relation with ischemic stroke.
Funding
This work was financed by Hassan II Academy of Science and Technology of Rabat and the Laboratory of Genetics and Molecular Pathology (LGPM) at the Faculty of Medicine and Pharmacy, University Hassan II, Casablanca, Morocco.
Acknowledgment
We thank all the staff of Neurology Services of Teaching Hospital of Rabat and Casablanca for their collaborations in the collection of samples and clinical data for this study. We also thank MD-PhD Mamoudou Maiga, all the staff and PhD students of LGPM for their critical contributions to this work.
References
- Attia J., Thakkinstian A., D'Este C. Meta-analysis of molecular association studies: methodological lessons for genetic epidemiology. J. Clin. Epidemiol. 2003;56:297–303. doi: 10.1016/s0895-4356(03)00011-8. [DOI] [PubMed] [Google Scholar]
- Berger K., Stogbauer F., Stoll M., Wellmann J., Huge A., Cheng S., Kessler C., John U., Assmann G., Ringelstein E.B., Funke H. The glu298asp polymorphism in the nitric oxide synthase 3 gene is associated with the risk of ischemic stroke in two large independent case–control studies. Hum. Genet. 2007;121:169–178. doi: 10.1007/s00439-006-0302-2. [DOI] [PubMed] [Google Scholar]
- Charalambos A., Dimitris T., Carmen V., Christos P., Marina T., Costas T., Kyriakoula M., Christodoulos S. Genetic polymorphisms G894T on the eNOS gene is associated with endothelial function and vWF levels in premature myocardial infarction survivors. Int. J. Cardiol. 2006;107:95–100. doi: 10.1016/j.ijcard.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Domingues-Montanari S., Mendioroz M., delRio-Espinola A., Fernández-Cadenas I., Montaner J. Genetics of stroke: a review of recent advances. Expert. Rev. Mol. Diagn. 2008;8:495–513. doi: 10.1586/14737159.8.4.495. [DOI] [PubMed] [Google Scholar]
- Elbaz A., Poirier O., Moulin T., Chédru F., Cambien F., Amarenco P. Association between the Glu298Asp polymorphism in the endothelial constitutive nitric oxide synthase gene and brain infarction. The genic investigators. Stroke. 2000;31:1634–1639. doi: 10.1161/01.str.31.7.1634. [DOI] [PubMed] [Google Scholar]
- Endres M., Laufs U., Liao J.K., Moskowitz M.A. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Flemming K.D., Brown R.D.J., Petty G.W., Huston J., Kallmes D.F., Piepgras D.G. Evaluation and management of transient ischemic attack and minor cerebral infarction. Mayo Clin. Proc. 2004;79:1071–1086. doi: 10.4065/79.8.1071. [DOI] [PubMed] [Google Scholar]
- Furchgott R.F., Zawadszki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Giffard P.M., Huygens F., Price E.P., Smith H. High-resolution DNA melt curve analysis of the clustered, regularly interspaced short-palindromic-repeat locus of Campylobacter jejuni. Appl. Environ. Microbiol. 2007;73:3431–3436. doi: 10.1128/AEM.02702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R., Liew M., Lyon E., Meadows C., Wittwer C.T. Distinguishing different DNA heterozygotes by high-resolution melting. Clin. Chem. 2005;51:1295–1298. doi: 10.1373/clinchem.2005.051516. [DOI] [PubMed] [Google Scholar]
- Grewal R.P., Dutra A.V.C., Liao Yi C., Juo S.S.H., Papamitsakis N.I.H. The intron 4c allele of the NOS3 gene is associated with ischemic stroke in African Americans. BMC Med. Genet. 2007;8:76. doi: 10.1186/1471-2350-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A., Markus H.S. Genetics and ischemic stroke. Brain. 2000;123:1784–1812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- Hassan A., Gormley K., O'Sullivan M., Knight J., Sham P., Vallance P., Bamford J., Markus H. Endothelial nitric oxide gene haplotypes and risk of cerebral small vessel disease. Stroke. 2004;35:654–659. doi: 10.1161/01.STR.0000117238.75736.53. [DOI] [PubMed] [Google Scholar]
- Hingorani A.D., Liang C.F., Fatibene J., Lyon A., Monteith S., Parsons A., Haydock S., Hopper R.V., Stephens N.G., O'Shaughnessy K.M., Brown M.J. A common variant of the endothelial nitric oxide synthase (Glu298Asp) is a major risk factor for coronary disease in the UK. Circulation. 1999;100:1515–1520. doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- Huang P.L., Huang Z., Mashimo H., Bloch K.D., Moskowitz M.A., Bevan J.A., Fishman M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Lee Y.C., Wu W.J., Liu C.C., Wang C.J., Li W.M., Huang C.H., Yeh H.C., Ke H.L., Huang S.P. The associations among eNOS G894T gene polymorphism, erectile dysfunction, and benign prostate hyperplasia-related lower urinary tract symptoms. J. Sex. Med. 2009;6:3158–3165. doi: 10.1111/j.1743-6109.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Leeson C.P., Hingorani A.D., Mullen M.J., Jeerooburkhan N., Kattenhorn M., Cole T.J., Muller D.P., Lucas A. Glu298Asp endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ. Res. 2002;90:1153–1158. doi: 10.1161/01.res.0000020562.07492.d4. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Nitric oxide and vascular disease. N. Engl. J. Med. 1995;333:251–253. doi: 10.1056/NEJM199507273330410. [DOI] [PubMed] [Google Scholar]
- MacLeod M.J., Dahiyat M.T., Cumming A., Meiklejohn D., Shaw D., St Clair D. No association between Glu/Asp polymorphism of NOS3 gene and ischemic stroke. Neurology. 1999;53:418–420. doi: 10.1212/wnl.53.2.418. [DOI] [PubMed] [Google Scholar]
- Markus H.S., Ruigrok Y., Ali N., Powell J.F. Endothelial nitric oxide synthase exon 7 polymorphism, ischemic cerebrovascular disease, and carotid atheroma. Stroke. 1998;29:1908–1911. doi: 10.1161/01.str.29.9.1908. [DOI] [PubMed] [Google Scholar]
- Reed G.H., Kent J.O., Wittwer C.T. Review: high-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8:597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- Reeves M.J., Bushnell C.D., Howard G., Gargano J.W., Duncan P.W., Lynch G., Khatiwoda A., Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi S., Mallat S.G., Almawi W.Y., Mahjoub T. Endothelial nitric oxide synthase Glu298Asp, 4b/a, and − 786T > C gene polymorphisms and the risk of ischemic stroke. Acta Neurol. Scand. 2010;121:114–119. doi: 10.1111/j.1600-0404.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- Salanti G., Higgins J.P. Meta-analysis of genetic association studies under different inheritance models using data reported as merged genotypes. Stat. Med. 2008;27:764–777. doi: 10.1002/sim.2919. [DOI] [PubMed] [Google Scholar]
- Salanti G., Southam L., Altshuler D., Ardlie K., Barroso I., Boehnke M., Cornelis M.C., Frayling T.M., Grallert H., Grarup N. Underlying genetic models of inheritance in established type 2 diabetes associations. Am. J. Epidemiol. 2009;170:537–545. doi: 10.1093/aje/kwp145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver J., Tamburi T. Genetics of cerebrovascular disease. In: Stefan M.P., editor. Neurogenetics. Oxford University Press; Oxford: 2000. pp. 403–431. [Google Scholar]
- Szentirmai O., Carter B.S. Genetic and cellular therapies for cerebral infarction. Neurosurgery. 2008;62:1465–1479. doi: 10.1227/01.neu.0000333811.56907.13. [DOI] [PubMed] [Google Scholar]
- Tang W., Yang Y., Wang B., Xiao C. Association between a G894T polymorphism of eNOS gene and essential hypertension in Hani and Yi minority groups of China. Arch. Med. Res. 2008;39:222–225. doi: 10.1016/j.arcmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Tao H.M., Chen G.Z. Endothelial NO synthase gene polymorphisms and risk of ischemic stroke: a meta-analysis. Neurosci. Res. 2009;64:311–316. doi: 10.1016/j.neures.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Wang X.L., Wang J. Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol. Genet. Metab. 2000;70:241–251. doi: 10.1006/mgme.2000.3033. [DOI] [PubMed] [Google Scholar]
- Wang M., Jiang X., Wu W., Zhang D. Endothelial NO synthase gene polymorphisms and risk of ischemic stroke in Asian population: a meta-analysis. PLoS One. 2013;8:e60472. doi: 10.1371/journal.pone.0060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanapitayakul S.K., Mihm M.J., Young A.P., Bauer J.A. Therapeutic implications of human endothelial nitric oxide synthase gene polymorphism. Trends Pharmacol. Sci. 2001;22:361–368. doi: 10.1016/s0165-6147(00)01692-8. [DOI] [PubMed] [Google Scholar]
- Yetik-Anacak G., Catravas J.D. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vasc. Pharmacol. 2006;45:268–276. doi: 10.1016/j.vph.2006.08.002. [DOI] [PubMed] [Google Scholar]