Abstract
Background
We studied the association between erythrocyte glutathione peroxidase1 (GPx1) activity and rs1050450 (Pro198Leu) site with the stenosis of coronary arteries and, evaluated the Pro/Leu position within the predicted tertiary structure.
Methods
Subjects were recruited from who underwent coronary angiography (controls; n = 55, Stenosis < 5% and Patients; n = 95, Stenosis ≥ 50%). The GPx1 activity and rs1050450 C/T variants were determined using enzyme assay and RFLP-PCR techniques, respectively. The conserved regions and GPx1 tertiary structure were predicted using bioinformatics tools.
Results
We did not find significant association between GPx1 activity (P = 0.96), rs1050450 genotype distribution and coronary artery disease (adjusted OR = 0.79; 95%CI 0.28–2.2, P = 0.6). The polymorphic variants were not located at the predicted structural and functional domains so that it had not the significant role on the GPx1stability and function.
Conclusions
In agreement with the results predicted from bioinformatics tools, we suggested that the GPx1 activity and rs1050450 (Pro198Leu) site are not involved in the development of stenosis of coronary arteries in the study population.
Keywords: Glutathione peroxidase1 (GPx1), rs1050450 (Pro198Leu), Coronary artery disease, Bioinformatics
Highlights
-
•
GPx1 Pro198Leu genotype distribution was evaluated in the stenosis of coronary arteries.
-
•
Serum GPx1 activity was measured in the stenosis of coronary arteries.
-
•
GPx1 tertiary structure was predicted using bioinformatics tools.
Introduction
The vascular injury occurs usually due to infection, excessive blood pressure, glycation and inflammatory reactions (Basta et al., 2002, Gattone et al., 2001). Although it proposed to be involved in atherosclerosis process but its role remains unclear so that, other concepts such as molecular failures in vessel remodeling process are also suggested in the recent studies (Nissen et al., 2004). Some studies have reported that the oxidative modification hypothesis, suggested by Steinberg, may explain biological events related to atherosclerosis process. Based on the above hypothesis, the oxidant/antioxidant imbalance causes to increase reactive molecular species in body's fluids and triggers molecular events in vessel subendothelial space (Najafi et al., 2011). However, some interventional studies have not confirmed the retardation of atherosclerosis process using antioxidant supplements.
The protective role of glutathione peroxidase isoenzymes in the oxidant/antioxidant balance is reported in many studies. Glutathione peroxidase1 (GPx1) is an intracellular soluble selenoprotein which converts peroxides such as H2O2 and ROOH into water and alcohol (Arthur, 2000). A fragment 184 aa (12–196) containing conserved sequence of GPx family is reported in PDB database (2F8A). Based on the dbSNP records (www.ncbi.nlm.nih.gov/snp), the polymorphic sites are widely distributed within the introns, exons and UTR regions of GPx1 gene so that they may affect the GPx1 function (Hamanishi et al., 2004). The rs1050450 (Pro198Leu) polymorphism is a site located within the GPx1 C-terminal region. Several studies have reported that the proline (CCC Codon) to leucine (CTC Codon) substitution is related to the risk of breast cancer and coronary artery disease (Ravn-Haren et al., 2006, Tang et al., 2008). Proline is an imino acid that often found at the end of alpha-helix or turns and, can cause a kink in the protein chain. Thus, its substitution with other amino acids may change structural conformation of the active site region and modify the enzyme activity resulting in oxidant/antioxidant imbalance (Ratnasinghe et al., 2000).
With the consideration of these reports, we studied the genotype and phenotype relationship of rs1050450 polymorphism with the stenosis of coronary arteries and evaluated the polymorphic variants within the GPx1 tertiary structure based on the prediction tools.
Materials and methods
Subjects
Among subjects who underwent angiography, one hundred fifty subjects (patients and controls) were recruited between February 2010 and March 2011. All subjects with myocardial infarction occurred at the last three months, diabetes and these with systemic diseases were excluded from the study. The stenosis of coronary arteries was studied in proximal, medial and distal portions of three vessels; right coronary artery, left coronary artery and left anterior descending artery. Patients (n = 95) had at least one stenosed coronary artery (≥ 50% stenosis) (Herzog et al., 2007) and, controls (n = 55) had normal coronary arteries (< 5% stenosis). The clinical data was obtained through cardiologist records. University ethics committee wasapproved the study and informed consent was obtained from all subjects.
Sample preparation
Peripheral venous blood samples were collected in EDTA-containing tubes. An aliquot was used for preparation of erythrocyte hemolysate and was diluted with distilled water (1:1). Furthermore, genomic DNA was extracted from white blood cells using salting out technique (Miller et al., 1988). All samples were rapidly stored at − 80 °С.
Lipid profile
Total cholesterol, TG and HDL levels and other biochemical parameters were measured with routine laboratory methods. LDL was calculated by Friedewald formula.
GPx1 activity
The erythrocyte hemolysate GPx1 activity was measured in a reaction coupled with glutathione reductase (GR). It was normalized with the hemoglobin level as described by Paglia and Valentine (Paglia and Valentine, 1967). An enzyme unit was defined as NADPH amount (micromole) converted to NADP+ per minute. 20 μl of the diluted hemolysate was added to 400 μl reactive solution (8 ml phosphate buffer (100 mM, PH 7.4), 4 ml GR (5000 u/l), 2 ml GSH (2.5 mM), and 2 ml NADPH (2.5 mM)). Then, TBHP (25 mM, 20 μl) was added to the previous solution and the absorbance changes (ΔOD) were kinetically calculated after an incubation period (3 min). GPx1 activity on the basis of hemolysate Hb was calculated by the following formula (Najafi et al., 2012):
Genotyping
For genotyping rs1050450 (Pro(C)/Leu(T)) site, a fragment 1195 bp was amplified by two primers;
5′-AGACAGCAGCACTGCAACTGCCAA-3′ (1 μM) and
5′-AGACCAGACATGCCTGCTGCTCCTT-3′ (1 μM).
The cycling condition was followed after initial incubation at 94 °C for 1 min by 60 cycles (94 °C for 30 s, 59 °C for 30 s, and 72 °C for 60 s) and a final elongation at 72 °C for 5 min. The PCR product (1195 bp) was digested with ApaI (NEB) when the C allele was within rs1050450 position so that; we observed two fragments 1131 bp and 64 bp on agarose gel (3 %). CC and TT homozygotes showed two fragments (1131 bp and 64 bp) and a fragment (1195 bp) on the gel (3 %), respectively. Also, TC heterozygote showed three fragments 1195 bp, 1131 bp and 64 bp on the gel (Fig. 1).
Fig. 1.
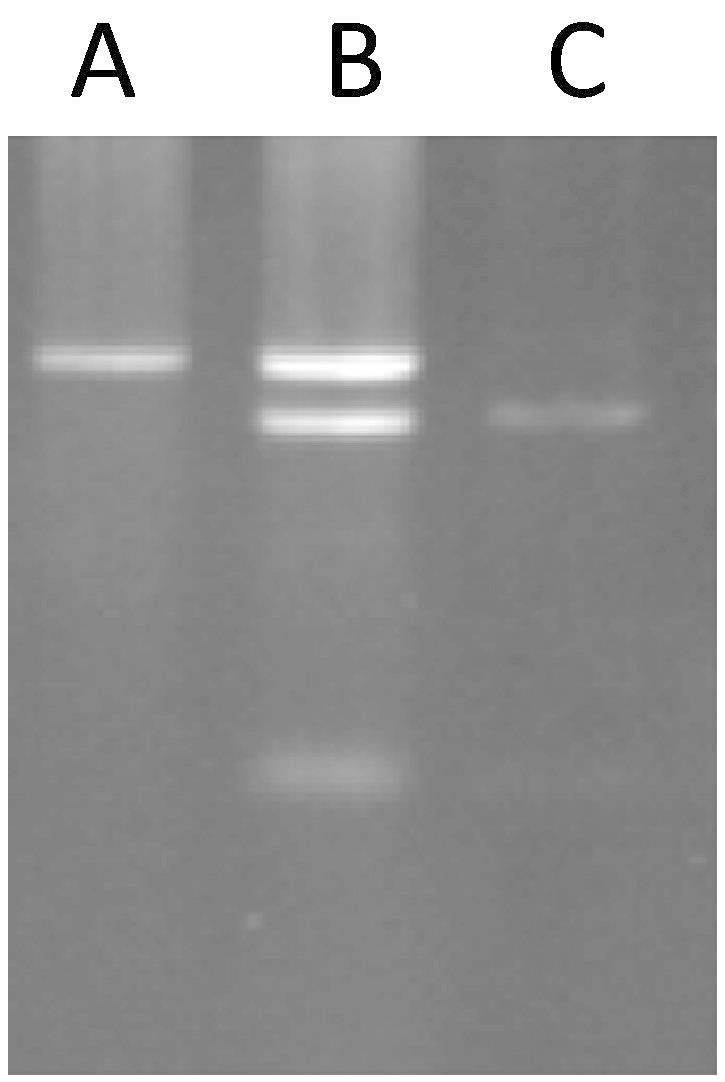
Digestion of PCR product. The PCR products were digested with ApaI and were run on gel (3%). A; TT Homozygote (undigested fragment 1195 bp). B; CT Heterozygote (undigested and digested fragments 1195 bp, 1131 bp and 64 bp). C; CC Homozygote (digested fragments 1131 bp and 64 bp).
Bioinformatic tools
The conserved and functional regions were predicted within Gpx1 protein sequence (ENSP00000407375) using PROSITE, SMART, PRINTS, Pfam A and InterPro servers. Then, the sequences were aligned with T-coffee server. In addition, we predicted the tertiary structure for the complete protein sequence (203 aa) with HMM-based tool (SAM-T08) and PDB template (2F8A). The effect of polymorphic variants on the stability and function of the predicted structure was also evaluated using MUpro, PANTHER and SNAP servers.
Statistical analysis
Data was analyzed using statistical software package (ver. 18.0. Chicago: SPSS Inc.). Kolmogorov–Smirnov test was applied to evaluate the normal distribution of variables. The statistical differences between groups were evaluated by Student-t and Chi squaretests. The Chi square goodness-of-fit test was used to identity significant departures from the Hardy–Weinberg equilibrium. Odds ratio (OR) and 95% confidence interval (CI) were calculated for genotype distribution and were adjusted with age, sex, smoking and BMI parameters. The linear and binary logistic regression analyses were performed to evaluate the effect of variables on GPx1 activity and their relationships with the stenosis of coronary arteries. Wald chi-square test was calculated by dividing the predictor's estimate square by its standard error and, the P value was the probability that the Wald test presents difference than null hypothesis when the regression coefficient for the predictor is estimated to be zero. P value less than 0.05 was considered to be significant.
Results
Population characteristics
Demographic information, clinical records and biochemical parameters of the study population (95 patients and 55 controls) who underwent coronary angiography are summarized in Table 1. The coronary left coronary artery (LCA) was the most important vessel stenosed in patients. The serum LDL (P < 0.014) level was significantly higher among patients. Other parameters such as blood pressure, total cholesterol, BMI, serum HDL and TG had no significant differences between the groups (P > 0.2).
Table 1.
Characteristics of the study population.
| Parameter | Control (n = 55) |
Patient (n = 95) |
P-value |
|---|---|---|---|
| Sex (male/female) | 15/40 | 62/33 | 0.0001 |
| Age (year) | 56.11 ± 13.51 | 61.62 ± 10.49 | 0.01 |
| Body mass index (kg/m2) | 25.92 ± 6.09 | 25.25 ± 3.77 | 0.67 |
| Systolic blood pressure (mm Hg) | 125.15 ± 23.36 | 132.13 ± 24.92 | 0.92 |
| Diastolic blood pressure (mm Hg) | 75.50 ± 13.61 | 78.54 ± 19.45 | 0.31 |
| Smoking (yes/no) | 16/39 | 33/62 | 0.19 |
| LDL (mg/dl) | 86.13 ± 38.79 | 104.71 ± 46.40 | 0.014 |
| HDL (mg/dl) | 40.15 ± 14.44 | 36.76 ± 17.17 | 0.22 |
| Triglyceride (TG) (mg/dl) | 153.11 ± 75.47 | 171.10 ± 90.51 | 0.22 |
| Total cholesterol (mg/dl) | 123.52 ± 74.88 | 132.13 ± 24.92 | 0.19 |
| GPx1 activity (U/gr Hb) | 53.08 ± 21.40 | 52.92 ± 19.29 | 0.95 |
Biochemical parameters
GPx1 activity had no significant difference (P > 0.9) between the patient and control groups (Table 1). The binary regression analysis showed that the coronary artery stenosis mostly occurred in old men with high serum LDL level (Table 2). In addition, the linear regression analysis showed that the GPx1 activity is not related to sex, BMI, age and polymorphic site (Table 3).
Table 2.
Effect of study parameters on stenosis of coronary artery.
| Parameter | Betaa | Standard error | Wald | P value | Odds ratio (OR) |
|---|---|---|---|---|---|
| Sex (female 1, male 2) | 1.406 | 0.622 | 5.1 | 0.025 | 4.08 |
| Age (year) | 0.05 | 0.026 | 3.6 | 0.05 | 1.05 |
| BMIb | − 0.033 | 0.058 | 0.32 | 0.56 | 0.96 |
| LDL (mg/dl) | 0.034 | 0.014 | 6.01 | 0.014 | 1.03 |
| TG (mg/dl) | − 0.001 | 0.005 | 0.056 | 0.81 | 0.99 |
| Cholesterol (mg/dl) | 0.005 | 0.005 | 1.039 | 0.3 | 1.01 |
| GPX1 activity (U/gr Hb) | − 0.023 | 0.017 | 1.73 | 0.18 | 0.97 |
Based on the P and OR values, the sex, age and LDL changes related directly (Beta > 0) and significantly (P value < 0.05) to the stenosis of coronary arteries.
Beta, regression model coefficient.
BMI (body mass index): 1, under-nutrition; 2, normal; 3, overweight; and 4, obese.
Table 3.
Effect of age, sex, BMI and rs1050450 genotype on glutathione peroxidase1 (GPx1) activity.
| Parameter | Betaa | t | P value |
|---|---|---|---|
| Age (year) | − 0.039 | − 0.37 | 0.71 |
| BMIb | − 0.062 | − 0.57 | 0.56 |
| Sex (male 1, female 2) | − 0.013 | − 0.11 | 0.9 |
| CC/CT + TT | 0.057 | 0.54 | 0.58 |
Based on P values, demographic parameters and genotype distribution can not affect erythrocyte GPX1 activity.
Beta, regression model coefficient.
BMI (body mass index): 1, under-nutrition; 2, normal; 3, overweight; and 4, obese.
Pro198 Leu genotype
The allele frequency was not significant between control (C/T = 61/49) and patient (C/T = 100/90) groups (P = 0.6).The risk estimates for rs1050450 polymorphism had no significant differences between patients and controls (Table 4). The results persisted after adjustment of age, sex, smoking and BMI (adjusted OR = 0.79; 95%CI 0.28–2.2, P = 0.6). Moreover, the genotype effects on the risk of coronary artery stenosis in the age (< 55 y and ≥ 55 y) and sex (male and female) subgroups were not significant even after adjustment of sex, age, smoking and BMI (Table 5).
Table 4.
Risk estimates for rs1050450.
| Genotype | Control//patient | Unadjusted model OR (95%CI) | P-value | Adjusted model OR (95%CI) | P-value |
|---|---|---|---|---|---|
| CC/CT + TT | 20/35//30/65 | 1.23 (0.61–2.5) | 0.54 | 0.79 (0.28–2.2) | 0.6 |
Adjusted for age, sex, smoking and BMI.
Table 5.
Genotype analysis for sex and age subgroups in controls and patients.
| Parameter | Control//patient (CC/CT + TT) | Unadjusted model OR (95%CI) | P-value | Adjusted model OR (95%CI) | P-value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 6/9//22/40 | 1.21 (0.38–3.8) | 0.74 | 1.5 (0.33–6.84) | 0.79 |
| Female | 15/25//14/19 | 0.81 (0.37–2.1) | 0.66 | 0.79 (0.28–2.24) | 0.29 |
| Age | |||||
| < 55 y | 13/18//12/13 | 0.78 (0.27–2.25) | 0.65 | 0.3 (0.05–1.93) | 0.2 |
| ≥ 55 y | 8/16//20/50 | 1.25 (0.46–3.38) | 0.66 | 1.07 (0.24–4.60) | 0.92 |
Adjusted for age, sex, smoking and BMI.
Prediction
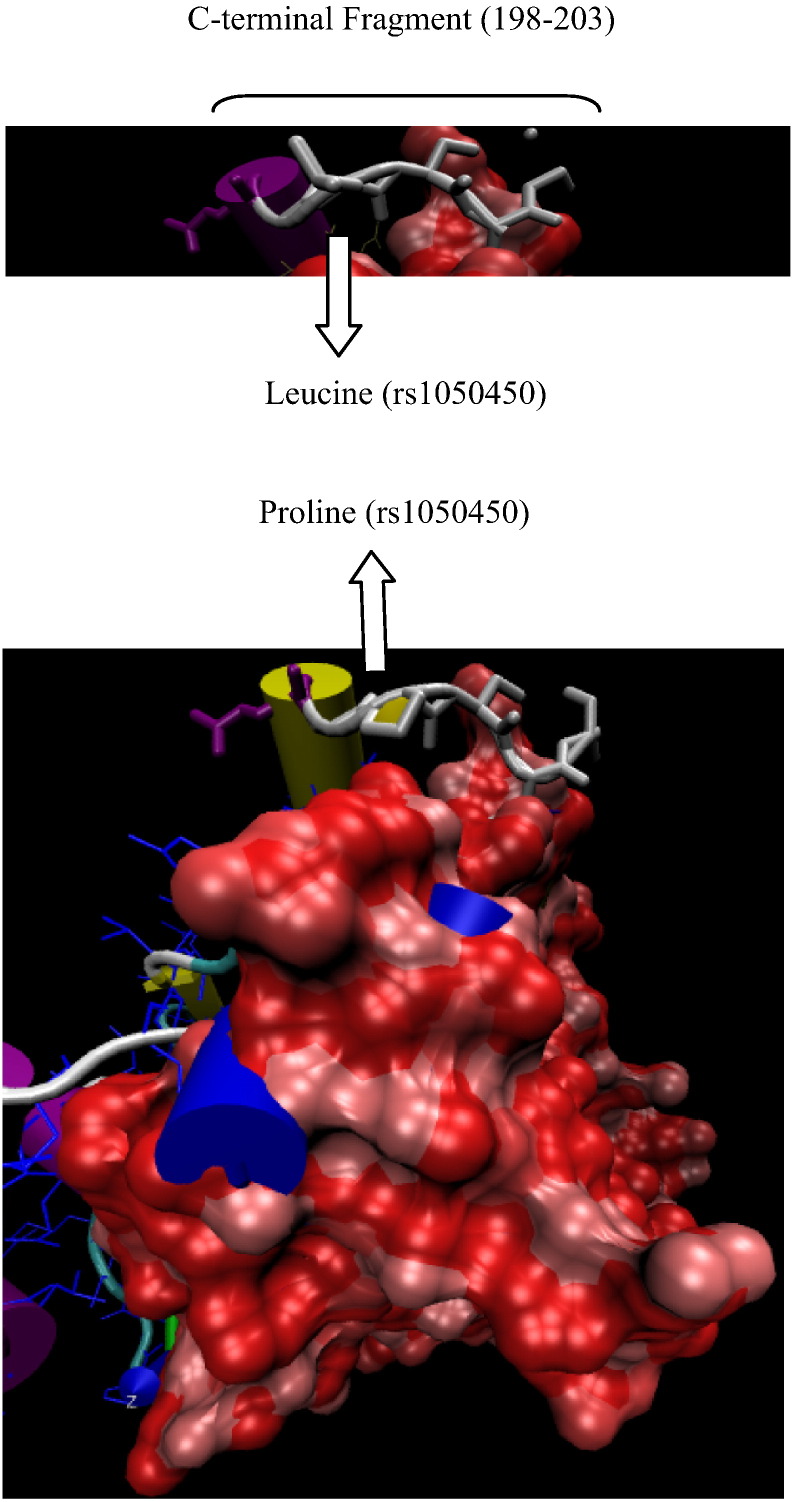
Protein secondary databases showed that the polymorphic site is not located within the Gpx1 conserved regions (Fig. 2). Furthermore, the predicted tertiary structure showed that C-terminal fragment containing polymorphic site is located on the protein surface and within non-functional fragment (determined using Pfam A database) (Fig. 3). The secondary predictors also confirmed that the Proline-to-Leucine substitution does not affect the protein stability (MUpro; Score 0.26), structural damage (PANTHER; subPSEC−-2.25, Pdeleterious 0.32) and disease probability (SNAP; neutral).
Fig. 2.
Conserved sequence alignment of Gpx1. Wild is complete sequence (www.ensembl.org) and, Prosite (http://prosite.expasy.org), Pfam (http://pfam.sanger.ac.uk), PRINTS (www.bioinf.manchester.ac.uk/dbbrowser/PRINTS) and SMART (http://smart.embl-heidelberg.de/) are conserved fragments prepared from secondary protein databases.
Fig. 3.
Predicted Gpx1 structure. (A) C-terminal fragment containing Leucine variant. (B) Complete tertiary structure containing conserved region (space-filling model, fragment 16 –130aa) and the C-terminal fragment containing Proline variant (fragment 198–203 aa).
Discussion
In this study, we investigated the GPx1 activity and rs1050450 (Pro198Leu) polymorphism in patients with the coronary artery stenosis. The arteries were evaluated using 64-section multi-detector row CT coronary angiography with accuracy values for LCX = 89%, RCA = 94.5% and LCA = 90.9% (Herzog et al., 2007). Moreover, we evaluated the effect of polymorphic variants on the conserved protein sequence and tertiary structure.
In agreement with the Hoffmann et al. report (2011), GPx1 activity was not significant between patient and control groups. We showed that the age, sex and BMI parameters are not related to GPx1 activity. Moreover, our results were in agreement with clinical trials on antioxidant supplements, anti-inflammatory and anti-bacterial agents that had no benefits in CAD patients (Williams and Tabas, 2005). In contrast with our results, some studies suggested an association between the GPx1 activity and cardiovascular events (Loeper et al., 1991, Blankenberg et al., 2003, Goldschmidt-Clermont et al., 2005). Our results also showed that LDL level was high in patients. The LDL-cholesterol level is particularly known as a strong risk factor for atherogenic lesions (Boullier et al., 2001, Shi et al., 2000, Reddy, 2004).
Although some studies showed the association between coronary artery disease (Nemoto et al., 2007), cancer (Raaschou-Nielsen et al., 2007) and rs1050450 polymorphism a meta-analysis study, in agreement with our study results, did not confirm the role of rs1050450 site in development of breast cancer (Hu et al., 2010).
In agreement with laboratory results, the prediction studies confirmed that the rs1050450 site is not located within the conserved regions. Although Prosite server showed the location of polymorphism at the end of pattern it was obtained from homology data while the results of other protein secondary databases were annotated to the function. In addition, the predicted tertiary structures showed that the polymorphic variants have no essential role on protein stability and function. It may be due to the surface location of polymorphism that can not affect the functional regions.
In conclusion, the GPx1 activity and rs1050450 site were not involved in the stenosis of coronary arteries based on the prediction results. We suggested that the response-to-injury events may be responsible of atherogenic lesions since high LDL level in patients provoke a series of responses in the arterial wall that contribute in vascular injuries (Libby, 2006).
References
- Arthur J.R. The glutathione peroxidase. Cell. Mol. Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta G., Lazzerini G., Massaro M. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- Blankenberg S., Rupprecht H.J., Bickel C. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003;349:1603–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- Boullier A., Bird D.A., Chang M.K. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann. N. Y. Acad. Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Gattone M., Iacoviello L., Colombo M. Chlamydia pneumoniae and cytomegalovirus seropositivity, inflammatory markers, and the risk of myocardial infarction at a young age. Am. Heart J. 2001;142:633–640. doi: 10.1067/mhj.2001.118118. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P.J., Creager M.A., Losordo D.W. Atherosclerosis 2005: recent discoveries and novel hypotheses. Circulation. 2005;22:3348–3353. doi: 10.1161/CIRCULATIONAHA.105.577460. [DOI] [PubMed] [Google Scholar]
- Hamanishi T., Furuta H., Kato H. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes. 2004;53:2455–2460. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- Herzog C., Zwerner P.L., Doll J.R. Significant coronary artery stenosis: comparison on per-patient and per-vessel or per-segment basis at 64-section CT angiography. Radiology. 2007;244:112–120. doi: 10.1148/radiol.2441060332. [DOI] [PubMed] [Google Scholar]
- Hoffmann F.W., Hashimoto A.S., Lee B.C. Specific antioxidant selenoproteins are induced in the heart during hypertrophy. Arch. Biochem. Biophys. 2011;512:38–44. doi: 10.1016/j.abb.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Zhou G.W., Wang N. GPX1 Pro198Leu polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res. Treat. 2010;124:425–431. doi: 10.1007/s10549-010-0841-z. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Loeper J., Goy J., Rozensztajn L. Lipid peroxidation and protective enzymes during myocardial infarction. Clin. Chim. Acta. 1991;196:119–125. doi: 10.1016/0009-8981(91)90064-j. [DOI] [PubMed] [Google Scholar]
- Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M., Roustazadeh A., Alipoor B. Ox-LDL particles: modified components, cellular uptake, biological roles and clinical assessments. Cardiovasc. Hematol. Disord. Drug Targets. 2011;10:119–128. doi: 10.2174/187152911798346990. [DOI] [PubMed] [Google Scholar]
- Najafi M., Ghasemi H., Roustazadeh A., Alipoor B. Phenotype and genotype relationship of glutathione peroxidase1 (GPx1) and rs 1800668 variant: the homozygote effect on kinetic parameters. Gene. 2012;505(1):19–22. doi: 10.1016/j.gene.2012.05.057. [DOI] [PubMed] [Google Scholar]
- Nemoto M., Nishimura R., Sasaki T. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc. Diabetol. 2007;6:23. doi: 10.1186/1475-2840-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen S.E., Tuzcu E.M., Schoenhagen P. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Raaschou-Nielsen O., Sørensen M., Hansen R.D. GPX1 Pro198Leu polymorphism, interactions with smoking and alcohol consumption, and risk for lung cancer. Cancer Lett. 2007;247:293–300. doi: 10.1016/j.canlet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Ratnasinghe D., Tangrea J.A., Andersen M.R. Glutathione peroxidase codon198 polymorphism variant increases lung cancer risk. Cancer Res. 2000;60:6381–6383. [PubMed] [Google Scholar]
- Ravn-Haren G., Olsen A., Tjønneland A. Associations between GPX1Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27:820–825. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- Reddy K.S. Cardiovascular disease in non-Western countries. N. Engl. J. Med. 2004;350:2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- Shi W., Haberland M.E., Jien M.L. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- Tang N.P., Wang L.S., Yang L. Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin. Chim. Acta. 2008;395:89–93. doi: 10.1016/j.cca.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Williams K.J., Tabas I. Lipoprotein retention and clues for atheroma regression. Arterioscler. Thromb. Vasc. Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]