Abstract
The compilation of simple sequence repeats (SSRs) in viruses and its analysis with reference to incidence, distribution and variation would be instrumental in understanding the functional and evolutionary aspects of repeat sequences. Present study encompasses the analysis of SSRs across 30 species of alphaviruses. The full length genome sequences, assessed from NCBI were used for extraction and analysis of repeat sequences using IMEx software. The repeats of different motif sizes (mono- to penta-nucleotide) observed therein exhibited variable incidence across the species. Expectedly, mononucleotide A/T was the most prevalent followed by dinucleotide AG/GA and trinucleotide AAG/GAA in these genomes. The conversion of SSRs to imperfect microsatellite or compound microsatellite (cSSR) is low. cSSR, primarily constituted by variant motifs accounted for up to 12.5% of the SSRs. Interestingly, seven species lacked cSSR in their genomes. However, the SSR and cSSR are predominantly localized to the coding region ORFs for non structural protein and structural proteins. The relative frequencies of different classes of simple and compound microsatellites within and across genomes have been highlighted.
Abbreviations: SSR, Simple sequence repeat; cSSR, Compound simple sequence repeat; IMEx, Imperfect Microsatellite Extraction; RD, Relative density; RA, Relative abundance
Keywords: Imperfect Microsatellite Extraction (IMEx), Simple sequence repeats (SSR), Compound microsatellite, Relative density, Relative abundance
Highlights
-
•
This is the first analysis of SSR and cSSR in alphaviruses.
-
•
We analysed differential frequency and distribution patterns of SSRs and cSSRs.
-
•
We studied localization of SSR and cSSR in alphaviruses proteomics
-
•
This study would help in better understanding of evolutionary biology of alphaviruses.
Introduction
According to International Committee on Taxonomy of Viruses (ICTV) report (King et al., 2012), the family Togaviridae has two genera namely Alphavirus and Rubivirus. Rubivirus genus has a solo member, Rubella virus whereas the Alphavirus includes thirty small, enveloped, plus-strand RNA viruses. These are responsible for human or animal diseases and are classified antigenically into six complexes (Garmashova et al., 2007, Powers et al., 2001). Further, the species are categorized into either the New World or Old World group based upon their prevalence.
Alphaviruses form icosahedral spherical particles of 65–70 nm in diameter and are considered a model system for structural studies of enveloped viruses. The species herein are known to cause several human diseases such as chickengunya, encephalitis and salmon pancreas diseases. Each alphavirus genome is a single-strand positive sense ~ 11.5Kb RNA molecule with terminal un-translated regions and, in between them, a single open reading frame (ORF) that is translated into a poly-protein. The poly-protein is cleaved after translation into four non-structural (virus replication and pathogenesis) and five structural proteins (virion synthesis) by proteinases that are a part of the polyprotein (Strauss and Strauss, 1994).
Repeat sequences have been established as highly divergent but ubiquitously present genome components across both prokaryotic and eukaryotic genomes. However, the viral genomes are now being actively pursued for their presence and significance. Tandem repeats of short motifs of DNA are called simple sequence repeats (SSR). On the basis of presence of interruptions in SSR; they are classified as interrupted, pure, compound, interrupted compound, complex and interrupted complex (Chambers and MacAvoy, 2000). These SSRs are termed compound SSR (cSSR) if two SSR are separated by a defined distance. dMAX is the maximum difference between the two SSRs of a cSSR as discussed later.
The repeat number, length, and motif size influence microsatellite mutability. For instance, more the number of repeats; higher is the mutability (Pearson et al., 2005). Moreover, variations in copy number due to strand slippage and unequal recombination highlight the instability of the microsatellites (Tóth et al., 2000); which in turn makes it a predominant source of genetic diversity and a crucial player in viral genome evolution (Deback et al., 2009, Kashi and King, 2006). Their role in gene regulation, transcription and protein function has also been elucidated (Kashi and King, 2006, Usdin, 2008).
The presence and possible functional significance of SSRs in viruses have been recognized only recently (Alam et al., 2013a, Alam et al., 2013b, Alam et al., 2014a, Alam et al., 2014b, Chen et al., 2009, Chen et al., 2011, Chen et al., 2012, Xiangyan et al., 2011). Concerted efforts are required to identify and confirm the presence, distribution and variations of SSRs in human infecting viruses. Here, we systematically analyzed the occurrence, size, and density of different microsatellites in different species of alphaviruses, which can serve as a model for understanding functional aspects and evolutionary relationships.
Materials and methods
Genome sequences
Complete genome sequence of 30 alphaviruses species were assessed form NCBI (http://www.ncbi.nlm.nih.gov/) and analyzed for simple and compound microsatellites. Genome size of these species ranged from 11245 nt (Acc No- HM147990) to 12052 nt (Acc No- HM147991). The accession numbers, genome size and GC content of studied alphaviruses genomes have been summarized in Table 1.
Table 1.
An overview of the alphavirus genomes used for the study.
| S. No | Species Id⁎ | Name | Acc number | Genome size | GC% |
|---|---|---|---|---|---|
| 1 | A1 | Aura virus | AF126284 | 11824 | 48.5 |
| 2 | A2 | Barmah Forest virus | U73745 | 11488 | 51.52 |
| 3 | A3 | Bebaru virus | HM147985 | 11877 | 48.5 |
| 4 | A4 | Cabassou virus | AF075259 | 11385 | 49.28 |
| 5 | A5 | Chikungunya virus | FN295484.2 | 11612 | 49.9 |
| 6 | A6 | Eastern equine encephalitis virus | AY722102 | 11680 | 48.71 |
| 7 | A7 | Everglades virus | AF075251 | 11395 | 49.8 |
| 8 | A8 | Fort Morgan virus | HM147986 | 11423 | 48.48 |
| 9 | A9 | Getah virus | EF631999 | 11689 | 52.3 |
| 10 | A10 | Highlands J virus | HM147988 | 11557 | 48.9 |
| 11 | A11 | Madariaga virus | EF151503 | 11661 | 48.5 |
| 12 | A12 | Mayaro virus | AF237947 | 11411 | 50.37 |
| 13 | A13 | Middelburg virus | EF536323 | 11674 | 52.6 |
| 14 | A14 | Mosso das Pedras virus (78 V3531) | AF075257 | 11465 | 49.64 |
| 15 | A15 | Mucambo virus | AF075253 | 11391 | 48.21 |
| 16 | A16 | Ndumu virus | HM147989 | 11688 | 50 |
| 17 | A17 | O'nyong-nyong virus | M20303 | 11835 | 48.32 |
| 18 | A18 | Pixuna virus | AF075256 | 11344 | 53.3 |
| 19 | A19 | Rio Negro virus | AF075258 | 11494 | 48.67 |
| 20 | A20 | Ross River virus | GQ433354 | 11948 | 51.03 |
| 21 | A21 | Salmon pancreas disease virus | AJ316244 | 11919 | 56.46 |
| 22 | A22 | Semliki Forest virus | X04129 | 11442 | 53.22 |
| 23 | A23 | Sindbis virus | HM147984 | 11739 | 51.55 |
| 24 | A24 | Southern elephant seal virus | HM147990 | 11245 | 48.63 |
| 25 | A25 | Tonate virus | AF075254 | 11530 | 49.07 |
| 26 | A26 | Trocara virus | HM147991 | 12052 | 47.81 |
| 27 | A27 | Una virus | HM147992 | 11964 | 50.5 |
| 28 | A28 | Venezuelan equine encephalitis virus | L01442 | 11447 | 49.8 |
| 29 | A29 | Western equine encephalitis virus | GQ287646 | 11554 | 48.28 |
| 30 | A30 | Whataroa virus | HM147993 | 11616 | 49.28 |
The species Id given here would be used for representation throughout the manuscript.
Microsatellites identification
The microsatellite search was performed using the IMEx software (Mudunuri and Nagarajaram, 2007). Earlier studies on eukaryotes and E. coli genomes have focused on microsatellites with lengths of 12 bp or more (Tóth et al., 2000) but due to smaller size of alphaviruses genome, simple and compound microsatellite search using these parameters did not yield any results. Therefore, the search for simple and compound microsatellites was accomplished using the ‘Advance- Mode’ of IMEx using the parameters as used for HIV (Chen et al., 2012). The parameters were set as follows: Type of Repeat: perfect; Repeat Size: all; Minimum Repeat Number: 6, 3, 3, 3, 3, 3; Maximum distance allowed between any two SSRs (dMAX) is 10. Other parameters were used as default. Compound microsatellites were not standardised in order to determine real composition.
Statistical analysis
The simple mathematical calculations were performed using Microsoft Office Excel 2007. However, the Pearson correlation coefficient (r) was calculated using GraphPad Prism Software, version 5 (La Jolla, CA, USA) to evaluate the influence of genome size and GC content, if any, on SSRs and cSSRs. A P-value < 0.05 was considered to be significant.
Results
Number, relative abundance and relative density
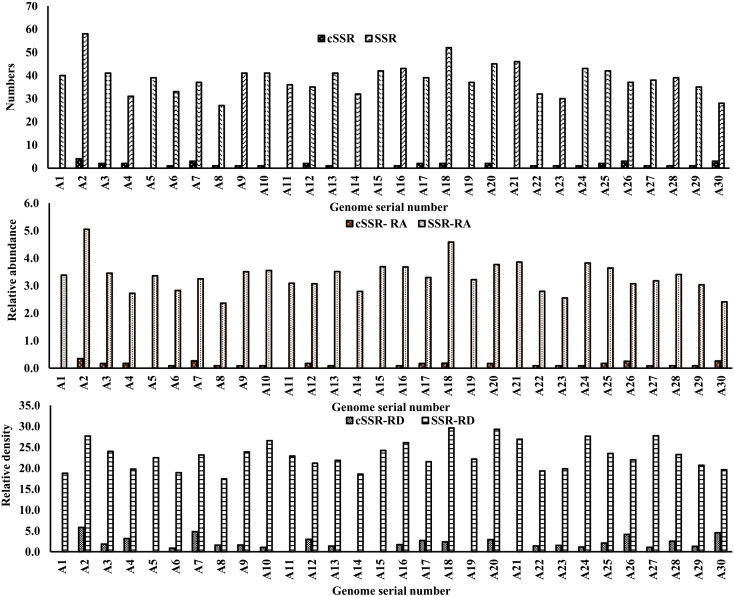
Genome-wide scan of thirty alphavirus genomes using IMEx revealed a total of 1160 SSRs distributed across all the species with varying incident frequencies ranging from 27 in A8 (Acc No- HM147986) to 58 in A2 (Acc No- U73745) (Table 2, Supplementary Table S1 and Fig. 1a). Further, the relative abundance values lied between a minimum of 2.41 for A30 to a maximum of 5.05 bp/kb for A2. (Table 2, Fig. 1b). Comparatively, relative density of SSRs varied from 18.58 bp/kb in A14 to 29.62 bp/kb for A18 (Table 2, Fig. 1c).
Table 2.
Summary of the SSRs and cSSRs observed in the studied alphavirus genomes.
| S. No | Species Id | SSR | RA | RD | cSSR | cRA | cRD | cSSR% |
|---|---|---|---|---|---|---|---|---|
| 1 | A1 | 40 | 3.38 | 18.78 | 0 | 0.00 | 0.00 | 0.00 |
| 2 | A2 | 58 | 5.05 | 27.68 | 4 | 0.35 | 5.83 | 6.90 |
| 3 | A3 | 41 | 3.45 | 24.00 | 2 | 0.17 | 1.85 | 4.88 |
| 4 | A4 | 31 | 2.72 | 19.76 | 2 | 0.18 | 3.16 | 6.45 |
| 5 | A5 | 39 | 3.36 | 22.48 | 0 | 0.00 | 0.00 | 0.00 |
| 6 | A6 | 33 | 2.83 | 18.92 | 1 | 0.09 | 0.86 | 3.03 |
| 7 | A7 | 37 | 3.25 | 23.17 | 3 | 0.26 | 4.83 | 8.11 |
| 8 | A8 | 27 | 2.36 | 17.42 | 1 | 0.09 | 1.58 | 3.70 |
| 9 | A9 | 41 | 3.51 | 23.87 | 1 | 0.09 | 1.63 | 2.44 |
| 10 | A10 | 41 | 3.55 | 26.56 | 1 | 0.09 | 1.04 | 2.44 |
| 11 | A11 | 36 | 3.09 | 22.90 | 0 | 0.00 | 0.00 | 0.00 |
| 12 | A12 | 35 | 3.07 | 21.21 | 2 | 0.18 | 2.98 | 5.71 |
| 13 | A13 | 41 | 3.51 | 21.84 | 1 | 0.09 | 1.37 | 2.44 |
| 14 | A14 | 32 | 2.79 | 18.58 | 0 | 0.00 | 0.00 | 0.00 |
| 15 | A15 | 42 | 3.69 | 24.23 | 0 | 0.00 | 0.00 | 0.00 |
| 16 | A16 | 43 | 3.68 | 26.10 | 1 | 0.09 | 1.71 | 2.33 |
| 17 | A17 | 39 | 3.30 | 21.55 | 2 | 0.17 | 2.70 | 5.13 |
| 18 | A18 | 52 | 4.58 | 29.62 | 2 | 0.18 | 2.38 | 3.85 |
| 19 | A19 | 37 | 3.22 | 22.19 | 0 | 0.00 | 0.00 | 0.00 |
| 20 | A20 | 45 | 3.77 | 29.29 | 2 | 0.17 | 2.93 | 4.44 |
| 21 | A21 | 46 | 3.86 | 26.93 | 0 | 0.00 | 0.00 | 0.00 |
| 22 | A22 | 32 | 2.80 | 19.31 | 1 | 0.09 | 1.40 | 3.13 |
| 23 | A23 | 30 | 2.56 | 19.85 | 1 | 0.09 | 1.53 | 3.33 |
| 24 | A24 | 43 | 3.82 | 27.66 | 1 | 0.09 | 1.16 | 2.33 |
| 25 | A25 | 42 | 3.64 | 23.50 | 2 | 0.17 | 2.08 | 4.76 |
| 26 | A26 | 37 | 3.07 | 21.99 | 3 | 0.25 | 4.15 | 8.11 |
| 27 | A27 | 38 | 3.18 | 27.75 | 1 | 0.08 | 1.09 | 2.63 |
| 28 | A28 | 39 | 3.41 | 23.24 | 1 | 0.09 | 2.53 | 2.56 |
| 29 | A29 | 35 | 3.03 | 20.69 | 1 | 0.09 | 1.30 | 2.86 |
| 30 | A30 | 28 | 2.41 | 19.63 | 3 | 0.26 | 4.56 | 10.71 |
Fig. 1.
Analysis of SSRs and cSSRs (a) Incidence (b) Relative abundance: SSRs/cSSRs present per Kb of genome (c) Relative density: Total length covered by SSR/cSSR per Kb of genome.
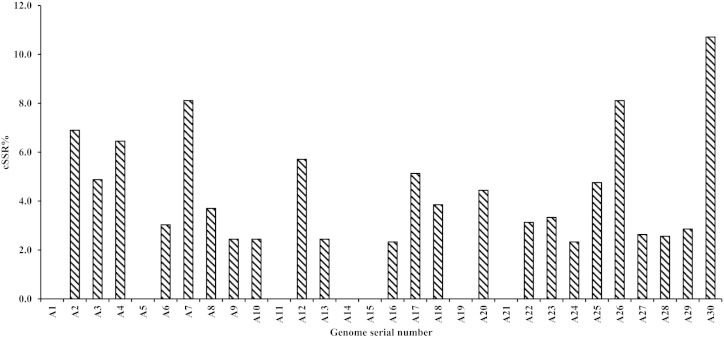
IMEx scan for alphavirus genomes revealed a total of 39 cSSRs (Table 2 and Supplementary Table S2). In contrast to the ubiquitous presence of SSRs, seven alphavirus genomes lacked any cSSR. Further, relative abundance varies from 0.00 bp/kb in 7 alphavirus sequence (A1, A5, A11, A14, A15, A19, A21) to 0.35 bp/kb in A2(Acc No- U73745) (Table 2, Fig. 1b) whereas relative density varies from 0.00 bp/kb in 7 alphavirus sequence to 5.83 bp/kb in A2 (Acc No- U73745) (Table 2, Fig. 1c). The correlation of cSSR incidence in a genome to SSR abundance led to few interesting observations. cSSR% is the percentage of individual microsatellites being part of a compound microsatellite and it has been summarized in Fig. 2. It was ‘nil’ in C8 (Acc No- AJ620300) which was harbouring 46 SSRs as compared to 10.71% in A30 (Acc No- HM147993) with just 28 SSRs.
Fig. 2.
Analysis of cSSR-% (Number of cSSR/Total number of SSR*100) across different alphavirus genomes.
Varying dMAX and incidence of cSSR
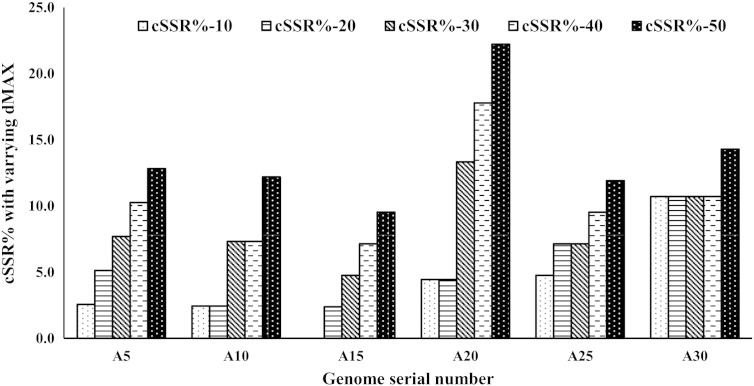
dMAX is the maximum distance between any two adjacent microsatellites and if the distance separating two microsatellites is less than or equivalent to dMAX, these microsatellites are classified as cSSR (Kofler et al., 2008). To determine the impact of dMAX, compound microsatellites from A5, A10, A15, A20, A25 and A30 were chosen at random to determine the variability of compound microsatellite with increasing dMAX by analyzing cSSRs%. It is noteworthy that the dMAX value can only be set between 0 and 50 for IMEx (Mudunuri and Nagarajaram, 2007). Our analysis revealed an overall increase in cSSRs% with increasing dMAX in all the six alphavirus genomes analysed (Fig. 3).
Fig. 3.
Frequency of cSSR-% (Percentage of individual microsatellites being part of a compound microsatellite) in relation to varying dMAX (10 to 50) across six randomly selected alphavirus species.
Genome parameters and SSR/cSSR distribution
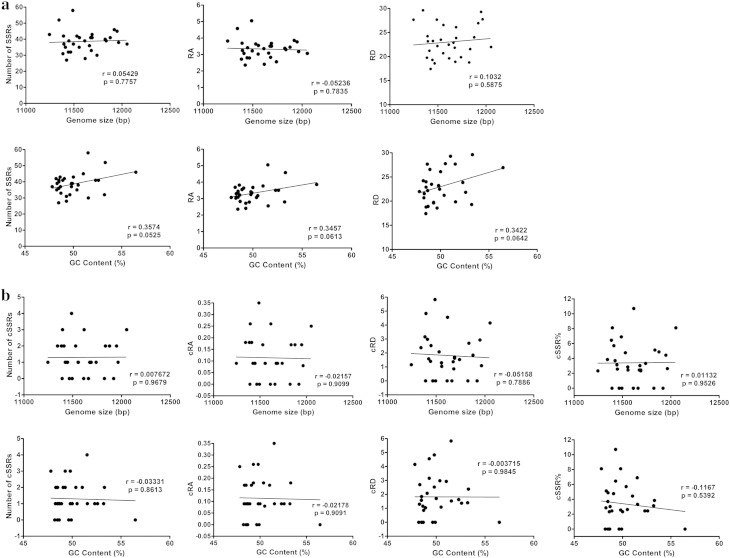
We assessed the possible influence of genome size and GC content on number/RA/RD of SSRs and cSSRs (Fig. 4; Supplementary Table S3). Genome size of assessed alphavirus genomes had a non significant influence on number of SSRs (r = 0.05429; P > 0.05), RA (r = − 0.05236; P > 0.05) and RD (r = 0.1032; P > 0.05). Genome size showed similar influence on number of cSSRs (r = 0.007672; P > 0.05), and their RA (r = − 0.02157; P > 0.05) and RD (r = − 0.05158; P > 0.05) and cSSR% (r = 0.001132; P > 0.05). Similarly, GC content in assessed alphavirus genomes also showed non-significant correlations with number of SSRs (r = 0.3574; P > 0.05), RA (r = 0.3457; P > 0.05) and RD (r = 0.3422; P > 0.05), and also with number of cSSRs (r = − 0.03331; P > 0.05), and their RA (r = − 0.02178; P > 0.05) and RD (r = − 0.00371; P > 0.05) and cSSR%(r = − 0.1167; P > 0.05).
Fig. 4.
Correlation analysis of (a) SSRs and (b) cSSR with genome size and GC content across alphavirus genomes.
Motifs types in analysed genomes
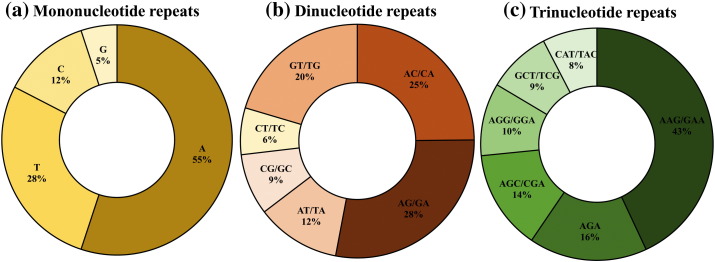
Mononucleotide repeats were observed in all the analyzed alphavirus genomes. Poly (A/T) repeats were significantly more prevalent (82.62%) than poly (G/C) repeats in each of the studied genomes (Supplementary Table S1) which may be attributed to the high (A/T) content of the genomes (Karaoglu et al., 2005). However, the (A/T) content being only slightly higher than G/C content in each of the analyzed sequences suggests a weak influence on the occurrence of poly (G/C) repeats. Similar to eukaryotic and prokaryotic genomes with reportedly more abundant poly (A/T) tracts (Gur-Arie et al., 2000, Karaoglu et al., 2005, Tóth et al., 2000) in the analyzed sequences poly A or poly T mononucleotide repeats prevailed over poly G or poly C (Fig. 5a).
Fig. 5.
Differential composition of (a) Mono-nucleotide repeats (b) Di-nucleotide repeat motifs (c) Tri-nucleotide repeat motifs.
A maximum of 64 mononucleotide A repeats was observed in genome of Una virus (A27). Further, the genomes had di-nucleotide repeats of six types: AG/GA, GT/TG, AC/CA, CT/TC, AT/TA, and CG/GC with variations in occurrence across genomes. AG/GA repeats were the most prevalent whereas, CT/TC was least represented (Fig. 5b). The average occurrence of GT/TG repeats was ~ 4 times more than CG/GC. The distribution of di-nucleotide repeats in the studied genomes has been summarized in Supplementary Table S1.
Tri-nucleotide repeats are the third most abundant SSRs within the alphavirus genomes. Of the 64 triplet repeat types, the density of AAG/GAA coding for lysine/glutamic acid was the most abundant followed by AGA coding for arginine respectively. Though differences exist in the abundance of trinucleotide repeats amongst alphavirus species but AAG/GAA showed highest prevalence in most species (Fig. 5c; Supplementary Table S1). Ten different types of tetra-nucleotide repeat motifs were distributed in 14 alphaviruses species while pentanucleotide repeat motif ATTTT was present in two genomes A7 and A27 only. There were no hexanucleotide repeats in the analysed genomes.
Comparative distribution across coding and non-coding regions
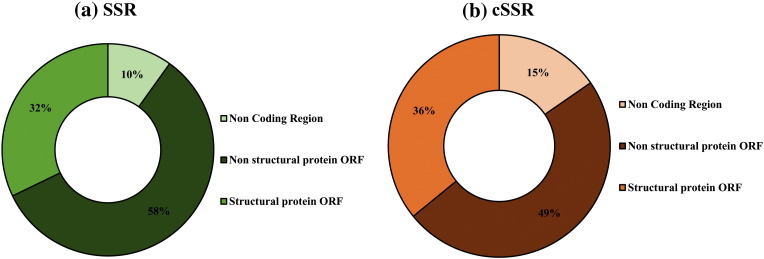
The distribution of SSRs and cSSR in coding/non-coding region revealed a clear bias of incidence in the coding regions. The coding regions accounted for 90% of the SSRs (Non-structural proteins ORF: 58%; Structural Proteins ORF: 32%) whereas residual 10% contribution was from the non-coding region of the genomes (Fig. 6a). The cSSR distribution also exhibited similar results with only 15% incidence being attributed to the non-coding regions. Their distribution across structural and non structural ORFs has been summarized in Fig. 6b. Also, the actual distribution of SSRs and cSSRs across coding and non-coding regions has been summarized in Table 3 for representative genomes.
Fig. 6.
Comparative distribution of (a) SSRs and (b) cSSR across coding and non-coding regions of alphavirus genomes.
Table 3.
Distribution of SSRs and cSSRs across coding and non-coding regions for representative Alphavirus genomes.
| Regions in genome |
Number of SSRs (region) |
Number of cSSRs (region) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| S. No | Genome size (bp) | Coding (bp) | Non coding (bp) | Total | Coding | Non-coding | Total | Coding | Non-coding |
| A5 | 11612 | 26-7429, 7495-11241 | 1-25, 7430-7494, 11242-11612 | 39 | 37 | 2 | 0 | 0 | 0 |
| A10 | 11557 | 1-5553, 7440-11150 | 5554-7439, 11151-11557 | 41 | 31 | 10 | 1 | 1 | 0 |
| A15 | 11391 | 43-7410, 7446-11210 | 1-42, 7411-7445, 11211-11391 | 42 | 41 | 1 | 0 | 0 | 0 |
| A20 | 11948 | 80-7561, 7606-11370 | 1-79, 7562–7605, 11371-11948 | 45 | 41 | 4 | 2 | 1 | 1 |
| A25 | 11530 | 44-7549, 7585-11349 | 1-43, 7550–7584, 11350-11530 | 42 | 39 | 3 | 2 | 2 | 0 |
| A30 | 11616 | 61-5625, 7526-11266 | 1-60, 5626-7525, 11267-11616 | 28 | 22 | 6 | 3 | 2 | 1 |
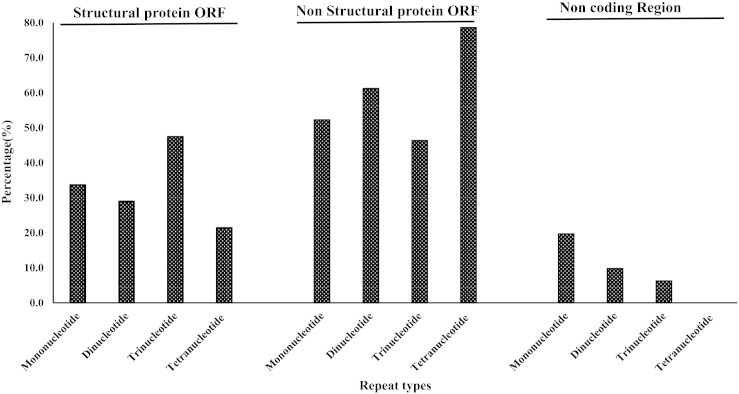
Further, we assessed the relative composition of repeat motifs. The structural proteins ORF had predominantly trinucleotide repeats (~ 50%) whereas tetranucleotide motif constituted almost eight out of every ten repeats present in the non structural proteins ORF. Contrastingly, the non coding region had mononucleotide motifs as the most prevalent one (Fig. 7).
Fig. 7.
Differential contribution of mono-, di- and tri-nucleotide SSR motifs across Structural proteins ORF, Non-structural proteins ORF and Non-coding regions of alphavirus genomes.
Discussion
In this study, we screened 30 alphavirus genomes for the presence, abundance, and composition of SSR tracts. A total of 1160 SSRs were revealed ranging from mono- to penta- nucleotide motifs. A higher prevalence of di-nucleotide repeats over tri-nucleotide repeats may be attributed to instability of the former because of higher slippage rate (Katti et al., 2001) and suggests a possible role of host in the evolution of di-nucleotide repeats within alphavirus (Alam et al., 2013a, Alam et al., 2013b, Alam et al., 2014a, Alam et al., 2014b). The repetitive sequences are hypothesized to provide for a molecular device for faster adaptation to environmental stresses (Kashi et al., 1997, Li et al., 2004) and hence, diverse repeats observed in alphavirus genomes might accelerate their evolution.
The compound microsatellites are reportedly involved in regulation of gene expression and at functional level of proteins in several species (Chen et al., 2011, Kashi and King, 2006). Though their significance in alphavirus is not clear, it suggests presence of a possibly complex regulation at the functional level. Further, varying dMAX (10 to 50) in 6 analyzed species lead to a higher cSSR% with increasing dMAX though, not in a linear way. This further adds to the significance of differential cSSRs distribution across genomes. In alphaviruses compound microsatellites were composed of a maximum of two SSRs whereas, in prokaryotes it is four and more than eight in eukaryotic species. Interestingly, cSSRs% varied between 0–11.42% in alphavirus genome; 0 to 15.5% in Human Papilloma Viruses; 0–15.15% in potyvirus genome; 0–11.43% in tobamovirus genome; 0–12.5% in carlavirus genome; 0–11.76% in potexvirus genome; (Alam et al., 2013a, Alam et al., 2013b, Alam et al., 2014a, Alam et al., 2014b, Singh et al., 2014), 0–24.24% in HIV-1 genomes (Chen et al., 2012); 4–25% in eight eukaryotic genomes (Kofler et al., 2008) and 1.75–2.85% in E. coli genomes (Kruglyak et al., 2000).
Distribution of microsatellite in the viral genome is organism specific rather than host specific. This is supported by the fact that the taxonomy of alphavirus shows no comparable congruence with host taxonomy, and species from the same lineage may have quite unrelated hosts (Gibbs et al., 2008). Accordingly, we observed that cSSR from viruses infecting common host does not possess similar number and type of cSSR motifs in their genome (data not shown). Surprisingly, seven of the alphavirus species did not possess even a single compound microsatellite this may be due to less number of strains present in these species wherein non availability of cSSR which might be restricting their variation and evolution.
It has been widely accepted that genetic RNA recombination is crucial for the emergence of new viral strains or species (Tan et al., 2005) and genomes are known to have recombination hot spots for the same (Jeffreys et al., 1998). The repeat sequences are possibly facilitating these recombination events (Nagy and Burjarski, 1996). Moreover, the unique composition of repeat motifs across structural/non structural proteins ORF and non coding regions as illustrated in Fig. 7 suggests that different parts of the genome might be evolving independent of one another in a dynalic manner.
A complete understanding of the functional and evolutionary role of tandem repeat sequences in viruses is still elusive. However, their ubiquitous presence though with varying frequency and complexity across species as well as coding and non-coding regions is suggestive of them being involved in the already established roles of gene regulation recombination hot spots. But owing to the smaller genome size of viruses as compared to prokaryotes and eukaryotes these tandem repeats are probably more influential in guiding the evolution of viruses. The diversity of microsatellites in alphavirus genomes may be useful for better understanding of viral genetic diversity, evolutionary biology, and strain/species demarcation
Conclusion
Genome-wide scan of thirty alphavirus genomes using IMEx revealed a total of 1160 SSRs and 39 cSSRs. The SSRs showed ubiquitous presence across the species albeit with varying incidence rates while seven alphavirus genomes lacked any cSSR. There was a bias towards occurrence of SSRs and cSSRs in the coding region as compared to the non-coding region which accounted for 10% and 15% contribution respectively. We postulate that such repeats in viral genomes could be involved in recombination, leading to sequence diversity that drives host adaptation. More detailed study of compound microsatellites in alphavirus genomes may be useful for understanding complex biological features such as changes in virulence and their emergence as new epidemics.
The following are the supplementary data related to this article.
SSRs in Alphavirus genome.
Compound SSR of Alphavirus genomes.
Raw data of correlation analysis for SSR and cSSR with genome size and GC content across Alphavirus genomes.
Acknowledgements
We thank Department of Biomedical Sciences, Shaheed Rajguru College of Applied Sciences for Women, University of Delhi, New Delhi, India and Department of Botany, Patna University, Bihar, India for all the financial and infrastructural support provided for the study.
References
- Alam C.M., Singh A.K., Sharfuddin C., Ali S. Incidence, complexity and diversity of simple sequence repeats across potexvirus genomes. Gene. 2014;537:189–196. doi: 10.1016/j.gene.2014.01.007. (ISSN: 0378–1119) [DOI] [PubMed] [Google Scholar]
- Alam C.M., Singh A.K., Sharfuddin C., Ali S. Genome-wide scan for extraction and analysis of simple and imperfect microsatellites in diverse carlaviruses. Infect. Genet. Evol. 2014;21:287–294. doi: 10.1016/j.meegid.2013.11.018. (ISSN: 1567–1348) [DOI] [PubMed] [Google Scholar]
- Alam C.M., Singh A.K., Sharfuddin C., Ali S. In silico analysis of simple and imperfect microsatellites in diverse tobamovirus genomes. Gene. 2013;530:193–200. doi: 10.1016/j.gene.2013.08.046. (ISSN: 0378–1119) [DOI] [PubMed] [Google Scholar]
- Alam C.M., George B., Sharfuddin C., Jain S.K., Chakraborty S. Occurrence andanalysis of imperfect microsatellites in diverse potyvirus genomes. Gene. 2013;521(2):238–244. doi: 10.1016/j.gene.2013.02.045. (1) [DOI] [PubMed] [Google Scholar]
- Chambers G.K., MacAvoy E.S. Microsatellites: consensus and controversy. Comp. Biochem. Physiol. B. 2000;126:455–476. doi: 10.1016/s0305-0491(00)00233-9. [DOI] [PubMed] [Google Scholar]
- Chen M., Tan Z., Jiang J., Li M., Chen H., Shen G., Yu R. Similar distribution of simple sequence repeats in diverse completed human immunodeficiency virus type 1 genomes. FEBS Lett. 2009;583:2959–2963. doi: 10.1016/j.febslet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Chen M., Zeng G., Tan Z., Jiang M., Zhang J., Zhang C., Lu L., Lin Y., Peng J. Compound microsatellites in complete Escherichia coli genomes. FEBS Lett. 2011;585:1072–1076. doi: 10.1016/j.febslet.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Chen M., Tan Z., Zeng G., Zhuotong Z. Differential distribution of compound microsatellites in various human immunodeficiency virus type 1 complete genomes. Infect. Genet. Evol. 2012;12:1452–1457. doi: 10.1016/j.meegid.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Deback C., Boutolleau D., Depienne C., Luyt C.E., Bonnafous P., Gautheret-Dejean A., Garrigue I., Agut H. Utilization of microsatellite polymorphism for differentiating herpes simplex virus type 1 strains. J. Clin. Microbiol. 2009;47:533–540. doi: 10.1128/JCM.01565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmashova N., Gorchakov R., Volkova E., Paessler S., Frolova E., Frolov I. The old world and new world alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J. Virol. 2007;81(5):2472–2484. doi: 10.1128/JVI.02073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur-Arie R., Cohen C.J., Eitan Y. Simple sequence repeats in Escherichia coli: abundance, distribution, composition, and polymorphism. Genome Res. 2000;10:62–71. [PMC free article] [PubMed] [Google Scholar]
- Gibbs A.J., Ohshima K., Phillips M.J., Gibbs M.J. The prehistory of potyviruses: their initial radiation was during the dawn of agriculture. PLoS One. 2008;3:e2523. doi: 10.1371/journal.pone.0002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys J., Murray J., Neumann R. High-resolution mapping of crossovers in human sperm defines a minisatellite-associated recombination hotspot. Mol. Cell. 1998;2:267–273. doi: 10.1016/s1097-2765(00)80138-0. [DOI] [PubMed] [Google Scholar]
- Karaoglu H., Lee C.M., Meyer W. Survey of simple sequence repeats in completed fungal genomes. Mol. Biol. Evol. 2005;22:639–649. doi: 10.1093/molbev/msi057. [DOI] [PubMed] [Google Scholar]
- King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. Ninth Report of the International Committee on Taxonomy of Viruses, San Diego. 2012. Virus taxonomy: classification and nomenclature of viruses. [Google Scholar]
- Kashi Y., King D., Soller M. Simple sequence repeats as a source of quantitative genetic variation. Trends Genet. 1997;13:74–78. doi: 10.1016/s0168-9525(97)01008-1. [DOI] [PubMed] [Google Scholar]
- Kashi Y., King D.G. Simple sequence repeats as advantageous mutators in evolution. Trends Genet. 2006;22:253–259. doi: 10.1016/j.tig.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Katti M.V., Ranjekar P.K., Gupta V.S. Differential distribution of simple sequence repeats in eukaryotic genome sequences. Mol. Biol. Evol. 2001;18:1161–1167. doi: 10.1093/oxfordjournals.molbev.a003903. [DOI] [PubMed] [Google Scholar]
- Kofler R., Schlotterer C., Luschutzky E., Lelley T. Survey of microsatellite clustering in eight fully sequenced species sheds light on the origin of compound microsatellites. BMC Genomics. 2008;9:612. doi: 10.1186/1471-2164-9-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglyak S., Durrett R., Schug M.D., Aquadro C.F. Distribution and abundance of microsatellites in the Yeast genome can be explained by a balance between slippage events and point mutations. Mol. Biol. Evol. 2000;17:1210–1219. doi: 10.1093/oxfordjournals.molbev.a026404. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Korol A.B., Fahima T., Nevo E. Microsatellites within genes: structure, function, and evolution. Mol. Biol. Evol. 2004;21:991–1007. doi: 10.1093/molbev/msh073. [DOI] [PubMed] [Google Scholar]
- Mudunuri S.B., Nagarajaram H.A. IMEx: imperfect microsatellite extractor. Bioinformatics. 2007;23:1181–1187. doi: 10.1093/bioinformatics/btm097. [DOI] [PubMed] [Google Scholar]
- Nagy P.D., Burjarski J.J. Homologous RNA recombination in brome mosaic virus: AU-rich sequences decrease the accuracy of crossovers. J. Virol. 1996;70:415–426. doi: 10.1128/jvi.70.1.415-426.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C.E., Nichol Edamura K., Cleary J.D. Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 2005;6:729–742. doi: 10.1038/nrg1689. [DOI] [PubMed] [Google Scholar]
- Powers A.M., Brault A.C., Shirako Y., Strauss E.G., Kang W., Strauss J.H., Weaver S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001;75(21):10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Alam C.M., Sharfuddin C., Ali S. Frequency and distribution of simple and compound microsatellites in forty-eight Human Papillomavirus (HPV) genomes. Infect. Genet. Evol. 2014;24:92–98. doi: 10.1016/j.meegid.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Strauss J.H., Strauss E.G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. (Review. Erratum in: Microbiol Rev 58(4),806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z., Gibbs A.J., Tomitaka Y., Sanchez F., Ponz F., Ohshima K. Mutations in Turnip mosaic virus genomes that have adapted to Raphanus sativus. J. Gen. Virol. 2005;86:501–510. doi: 10.1099/vir.0.80540-0. [DOI] [PubMed] [Google Scholar]
- Tóth G., Gáspári Z., Jurka J. Microsatellites in different eukaryotic genomes: survey and analysis. Genome Res. 2000;10:967–981. doi: 10.1101/gr.10.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin K. The biological effects of simple tandem repeats: lessons from the repeat expansion diseases. Genome Res. 2008;18:1011–1019. doi: 10.1101/gr.070409.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiangyan Z., Zhongyang T., Haiping F., Ronghua Y., Mingfu Li, Jianhui J., Guoli S., Ruqin Y. Microsatellites in different Potyvirus genomes: survey and analysis. Gene. 2011;488:52–56. doi: 10.1016/j.gene.2011.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SSRs in Alphavirus genome.
Compound SSR of Alphavirus genomes.
Raw data of correlation analysis for SSR and cSSR with genome size and GC content across Alphavirus genomes.