Abstract
Aim
CHP2 (calcineurin B homologous protein 2) is identified as a tumor-associated antigen highly expressed in different malignancies. It plays a critical role in cancer cell development, proliferation, motility and survival. It is suggested that the human tumor related gene CHP2 expression in leukemia primary cells and leukemia cell lines significantly increase, which may play an important role in growth process of leukemia cells.
Methods
In this study, the expression of CHP2 gene was analyzed in 10 normal healthy controls and 40 patients with de novo acute leukemia (20 AML and 20 ALL). CHP2 expression was analyzed using a real-time quantitative reverse-transcriptase polymerase chain reaction (RTQ-PCR) to investigate a possible relation, association or correlation with the clinical features of AL (acute leukemia) at diagnosis, such as age, gender, lineage, HB, TLC, platelet count, BM blast cell infiltration and risk group.
Results
CHP2 was highly expressed in 13/40 AL studied patients (7/20 AML and 6/20 ALL) with mean expression level of 2.7 while it was not expressed in any of the controls.
Conclusions
Many studies suggest that CHP2 expression is a novel prognostic marker in AL and thus needs to be incorporated into the patient stratification and treatment protocols. In addition, a quarter of AL patients fail therapy and novel treatments that are focused on undermining specifically the leukemic process are needed urgently.
Keywords: AL, CHP2, RTQ-PCR
Introduction
Calcineurin B homologous protein 2 (CHP2), also known as hepatocellular carcinoma antigen 520 (HCA520), belongs to the super family of N-myristolated, EF-hand Ca2 +-binding protein CHPs (Pang et al., 2001, Wang et al., 2002). Three isoforms of CHPs have been identified to date. CHP1 (also called P22) is expressed ubiquitously in virtually all tissues; CHP 3 (also called tescalcin) is expressed only in a few normal tissues (Zaun et al., 2008). Unlike CHP1 and 3, the expression of CHP2 is restricted to cancer (Pang et al., 2002) and the small intestine (Inoue et al., 2003).
CHP2 was initially identified as a tumor-associated antigen highly expressed in different malignancies. Its biological function remains largely unknown except for a potential role in transmembrane Na+/H+ exchange. In many studies, it was observed that ectopic expression of CHP2 promoted the proliferation of different cell lines, whereas knockdown of endogenous CHP2 expression in cell lines inhibited cell proliferation (G. Li et al., 2008).
The Na+/H+ exchangers (NHEs) comprise a family of membrane proteins that catalyze the electroneutral exchange of Na+ and H+. Calcineurin homologous protein (CHP) acts as a crucial cofactor for NHE activity through direct interaction with the carboxyl-terminal tail region of NHEs (Inoue et al., 2003).
Many studies have characterized the function of another isoform of CHP (designated CHP2) that has a 60% amino acid identity with CHP1. CHP2, like CHP1, conferred the ability to NHEs 1–3 to express a high exchange activity by binding to the juxtamembrane region of the cytoplasmic domain of the exchanger, but it interacts more strongly (approximately 5-fold) with NHE1 than does CHP1. Although CHP1 is expressed ubiquitously at relatively high levels, CHP2 expression was extremely low in most human tissues but was higher in tumor cells. Different studies have produced stable cell clones over expressing either CHP1 or CHP2 in which one of them is predominantly bound to NHE1. Surprisingly, most (> 80%) of CHP2/NHE1 cells unlike CHP1/NHE1 cells were viable even after long serum starvation (> 7 days). Thus, the expression of CHP2 appears to protect cells from serum deprivation-induced death by increasing pH. These properties of CHP2/NHE1 cells are similar to those of malignantly transformed cells. It is proposed that serum-independent activation of NHE1 by bound CHP2 is one of the key mechanisms for the maintenance of high pH and the resistance to serum deprivation-induced cell death in malignantly transformed cells (Pang et al., 2002).
Acute leukemias account for approximately 2% of all cancers, but have a disproportionately large effect on cancer survival statistics. Although leukemia is 10 times more prevalent in adults than in children, it accounts for more than 30% of all childhood cancers (Bozzone, 2009).
The purpose of many studies was to investigate the expression feature of a human tumor related gene chp2 in leukemia primary cells. Real-time quantitative PCR (RQ-PCR) was performed to detect the expression level of CHP2 gene in peripheral blood mononuclear cells from healthy individuals (as control) and cases of acute and chronic, myeloid and lymphoid leukemia, to assess if it is higher than that in normal controls. It is suggested that the human tumor related gene CHP2 expression in leukemia primary cells and leukemia cell lines significantly increase, which may play an important role in growth process of leukemia cells (B. Li et al., 2008).
Materials and methods
Patients and controls series
Forty Egyptian patients with acute leukemia were included in this study. Patients were recruited from Cairo University hospital and Beni-Suef University hospital, from December 2010 to July 2011. Informed consent was taken from all contributors prior to their inclusion in the study. All work was performed in accordance with the ethical standards of the 2000 declaration of Helsinki as well as declaration of Istanbul 2009.
Patients were 20 acute myeloid leukemia (AML) and 20 acute lymphoblastic leukemia (ALL), 21 male and 19 females. Their age ranged between 1.25 and 80 years with a mean of 33.7 ± 22.8 years. All the patients were newly diagnosed and did not receive any treatment.
Ten age and sex matched healthy individuals with normal laboratory findings were included as a control group. They were 5 males and 5 females. Their age ranged between 10 and 45 years with a mean of 23.2 ± 12.4 years.
Diagnosis of acute leukemia was based on (1) morphologic findings from Giemsa stained smears of bone marrow (BM) aspirates, (2) cytochemical stains criteria such as negativity for myeloperoxidase (MPO) and Sudan Black B (SBB) in cases of acute lymphoblastic leukemia (ALL) or their positivity in cases of acute myeloid leukemia (AML) and positivity for acid phosphatase in T-cell acute lymphoblastic leukemia (T-ALL) and (3) immunophenotyping criteria as CD10 +/− CD19 +, CD20 +, CD22 + for B-ALL, CD2 +/−, CD3 +, CD5 +/−, CD7 + for T-ALL, and positivity of CD13 and CD33 for AML cases. A complete blood count and a differential count including blast cell percentage were done for all patients. Peripheral blood (PB) samples and bone marrow (BM) aspiration samples were collected at diagnosis from the 40 Egyptian acute leukemia patients, while peripheral blood samples were obtained from the control group.
CHP2 gene was analyzed in patients and controls using real-time quantitative reverse transcriptase polymerase chain reaction (RTQ-PCR) to study mRNA expression levels.
RNA isolation and real-time quantitative RT-PCR for CHP2
PB and BM mononuclear cells (MNCS) were isolated at diagnosis by Ficoll density gradient centrifugation. Total RNA was extracted from MNCs using a QIAamp RNA Blood Kit (Qiagen, Germany) according to manufacturer's instructions. Complementary DNA (cDNA) was synthesized using (dt) 15-mer primer by Superscript III Reverse Transcriptase and stored at − 20 °C till use.
The mRNA expression levels of CHP2 gene and GAPDH (endogenous control) were measured by quantitative RT-PCR using an ABI PRISM 7000 Sequence Detector System (Applied Biosystems, Foster City, CA). The quantitative RT-PCR amplification was performed using the predeveloped Assays-on-demand Gene Expression Set for the CHP2 and TaqMan GAPDH control reagents (Applied Biosystems) with the following sequence: the forward primers (GAPDH-Fw: 5′-GTCCATGCCATCACTGCCAC-3′) and the reverse (GAPDH-Rv: 5′-ATGACCTTGCCCACAGCCTT-3′) with the TaqMan Universal PCR Master Mix (Applied Biosystems).
All reactions were performed in triplicate using 20 μl samples containing 50 ng cDNA. The reaction protocol used involved heating for 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of amplification (15 s at 95 °C and 1 min at 60 °C). Analysis was performed using ABI PRISM 7000 Sequence Detection Software (Applied Biosystems).
The expression levels of CHP2 gene in tested samples were expressed in the form of CT (cycle threshold) level (Fig. 1); then normalized copy number (relative quantitation) was calculated using the ∆∆CT equation as follows: ∆∆CT = ∆CT of case — ∆CT of control, then the normalized copy number (relative quantitation) = 2 − ∆∆CT. A negative control without template was included in each experiment (See Fig. 2, Fig. 3.)
Fig. 1.
Receiver operating characteristic (ROC) curve of CHP2 level.
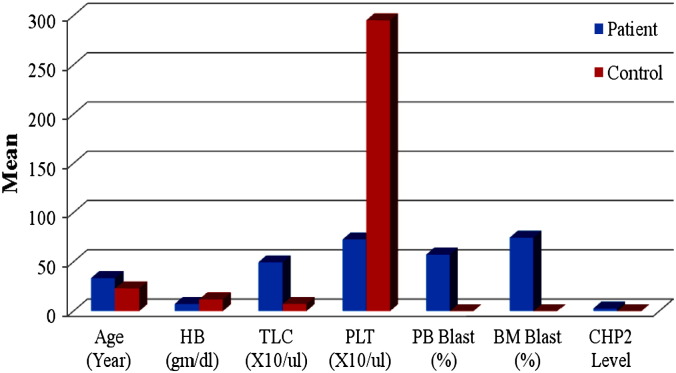
Fig. 2.
Comparison between patients and control as regards different clinical and laboratory parameters.
Fig. 3.
Comparison between AL patients and controls as regards CHP2 expression rate.
Expression level of CHP2 was correlated with the clinical and laboratory features of the studied patients at diagnosis including: age, sex, total leukocytic count (TLC), hemoglobin (Hb), platelet count, lineage and blast cell percentage.
Statistical methods
Data was analyzed using IBM SPSS advanced statistics version 17 (SPSS Inc., Chicago, IL). Numerical data of scores were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher's exact test) was used to examine the relation between qualitative variables. For not normally distributed quantitative data, comparison between two groups was done using Mann–Whitney test (non-parametric t-test). Comparison between 3 groups was done using Kruskal–Wallis test (non-parametric ANOVA) then post-hoc “Scheffe test” on rank of variables was used for pair-wise comparison. Spearman-rho method was used to test correlation between numerical variables. Survival analysis was done using Kaplan–Meier method and comparison between two survival curves was done using log-rank test. Odds ratio (OR) with 95% confidence interval (CI) were used for risk estimation. The receiver operating characteristic (ROC) curve was used for prediction of cut off values. Kappa test was used to evaluate agreement between two diagnostic methods. A p-value < 0.05 was considered significant.
Results
Clinical and laboratory data of the studied group are presented in (Table 1, Table 2, Table 3, Table 4). The study was carried out on 40 newly diagnosed acute leukemia patients. They were 21 males and 19 females; 12 patients < 18 years of age and 28 patients ≥ 18 years of age; 20 AML and 20 ALL; 25 with total leukocytic count (TLC) < 50 × 103/μl and 15 with TLC ≥ 50 × 103/μl; 19 with bone marrow (BM) blast infiltration < 90% and 21 with bone marrow blast infiltration ≥ 90%; 23 with hepatomegaly and 17 without hepatomegaly; 36 with splenomegaly and 4 without splenomegaly; 27 with lymphadenopathy and 13 without lymphadenopathy.
Table 1.
Statistical comparison between patients group and control group as regard their clinical data.
| Parameter | AL group (n = 40) Freq (%) | Control group (n = 10) Freq (%) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 21/40 (52.5%) | 5/10 (50%) | 0.914 |
| Female | 19/40 (47.5%) | 5/10 (50%) | |
| Hepatomegaly | |||
| Absent | 17/40 (42.5%) | 10/10 (100%) | 0.004a |
| Present | 23/40 (57.5%) | 0/10 (0%) | |
| Splenomegaly | |||
| Absent | 4/40 (10%) | 10/10 (100%) | 0.001a |
| Present | 36/40 (90%) | 0/10 (0%) | |
| Lymphadenopathy | |||
| Absent | 13/40 (32.5%) | 10/10 (100%) | 0.001a |
| Present | 27/40 (67.5%) | 0/10 (0%) |
p-Value is significant ≤ 0.05.
Table 2.
Statistical comparison between patients group and control group as regard their age and their laboratory data.
| Variable | AL group mean ± SD | Control group mean ± SD | p-Value |
|---|---|---|---|
| Age (years) | 33.7 ± 22.8 | 32.2 ± 12.4 | 0.167 |
| HB (g/dl) | 7.4 ± 2.2 | 11.96 ± 0.3 | 0.001a |
| TLC (× 103/μl) | 49.6 ± 49.3 | 7.4 ± 2.0 | 0.010a |
| PLT (× 103/μl) | 72.9 ± 64.0 | 295.3 ± 106.7 | 0.001a |
| BM blast (%) | 74.7 ± 30.0 | 0 ± 0 | 0.001a |
p-Value is significant ≤ 0.05.
Table 3.
Statistical comparison between patients group and control group as regard CHP2 gene level.
| Variable | AL group mean ± SD | Control group mean ± SD | p-Value |
|---|---|---|---|
| CHP2 level | 2.7 ± 4.1 | 0.1 ± 0.3 | 0.042a |
p-Value is significant ≤ 0.05.
Table 4.
Patients characteristics and the CHP2 expression rates and levels in different clinical groups.
| Group | No. of patients | CHP2 rates | CHP2 level (mean) | Range | p-Value |
|---|---|---|---|---|---|
| Total patient | 40 | 13/40 (32.5%) | 2.7 | 0–10.5 | |
| Control | 10 | 1/10 (10%) | 0.1 | 0–1.0 | P1: 0.042a |
| Sex | |||||
| Male | 21 | 7/21 (33.3%) | 2.7 | 0–10.5 | P1: 0.042a |
| Female | 19 | 6/19 (31.5%) | 2.7 | 0–10.5 | P2: 1.0 |
| Age | |||||
| Less than18 | 12 | 3/12 (25%) | 1.9 | 0–9.5 | P1: 0.042a |
| At least18 | 28 | 10/28 (35.7%) | 3.0 | 0–10.5 | P2: 0.715 |
| Type | |||||
| AML | 20 | 7/20 (35%) | 3.1 | 0–10.5 | P1: 0.042a |
| ALL | 20 | 6/20 (30%) | 2.3 | 0–10.5 | P2: 0.740 |
| TLC | |||||
| Less than 50 × 103/μl | 25 | 8/25 (32%) | 2.4 | 0–10.5 | P1: 0.042a |
| At least 50 × 103/μl | 15 | 5/15 (33.3%) | 3.1 | 0–10.5 | P2: 1.0 |
| BM blast infiltration | |||||
| Less than 90% | 19 | 7/19 (36.8%) | 2.7 | 0–9.6 | P1: 0.042a |
| Total infiltration | 21 | 6/21 (28.5%) | 2.7 | 0–10.5 | P2: 0.737 |
| Hepatomegaly | |||||
| Absent | 17 | 4/17 (23.5%) | 2.1 | 0–10.5 | P1: 0.042a |
| Present | 23 | 9/23 (39.1%) | 3.1 | 0–10.5 | P2: 0.332 |
| Splenomegaly | |||||
| Absent | 4 | 0/4 (0%) | 0.0 | 0 | P1: 0.042a |
| Present | 36 | 13/36 (36.1%) | 3.0 | 0–10.5 | P2: 0.284 |
| Lymphadenopathy | |||||
| Absent | 13 | 3/13 (23%) | 1.6 | 0–8.4 | P1: 0.042a |
| Present | 27 | 10/27 (37%) | 3.2 | 0–10.5 | P2: 0.484 |
P1:relation of total 40 AL patients to 10 control calibrators.
P2: relation of each category to the other of the same group.
p ≤ 0.05: Significant; p > 0.05: nonsignificant.
The mean age of patients at diagnosis was 33.7 years (range, 1.25–80 years), mean HB at diagnosis was 7.4 g/dl (range 3.5–13.9 g/dl), mean TLC at diagnosis was 49.6 × 103/μl (range 3.9–254 × 103/μl), mean platelets count at diagnosis was 72.9 × 103/μl (range 10–320 × 103/μl) and mean percentage of BM blast infiltration at diagnosis was 74.7% (range 15–100%).
Ten age and sex matched healthy volunteers were included as a control group.
Both expression rates and expression levels of CHP2 gene were higher in AL patients than control
The expression rates and levels of CHP2 mRNA in normal controls and AL patients with respect to the common prognostic factors are shown in Table 3, Table 4. CHP2 mRNA was expressed in 13/40 AL patients (32.5%) with mean expression level of 2.7 (range, 0–10.5) and also in 1/10 control samples used as calibrators, with mean expression level of 0.1 (range, 0–1.0). There was statistically significant difference between rates and levels of CHP2 gene in cases when compared to normal controls (p < 0.05), while there was no statistically significant differences (p > 0.05) in the CHP2 expression rates and levels as regards clinical and laboratory findings, i.e., age, gender, type of AL, TLC, BM infiltration, presence of hepatomegaly, splenomegaly and lymphadenopathy. Also, there was no positive correlation between CHP2 expression and any of the clinical and laboratory findings (Table 5).
Table 5.
Correlation between CHP2 expression rate and different clinical and laboratory parameters.
| Parameter | r Value | p-Value |
|---|---|---|
| Sex | 0.02 | 0.906 |
| Age | 0.1 | 0.507 |
| Type | 0.05 | 0.736 |
| TLC | 0.01 | 0.931 |
| BM blast infiltration | 0.1 | 0.577 |
| Hepatomegaly | 0.1 | 0.298 |
| Splenomegaly | 0.2 | 0.144 |
| Lymphadenopathy | 0.1 | 0.377 |
p ≤ 0.05: Significant.
p > 0.05: Nonsignificant.
Discussion
Cancer is recognized as a heterogeneous collection of diseases whose initiation and progression are promoted by the aberrant function of genes that regulate DNA repair, genome stability, cell proliferation, cell death, adhesion, angiogenesis, invasion, and metastasis in complex cell and tissue microenvironments (Ushijima, 2005).
The finding that tumors are capable of shedding nucleic acids (DNA or RNA) into the blood stream, which can be recovered from both serum and plasma and used as a source of tumor DNA, has opened new areas in diagnosis and prognosis (Pathak et al., 2006).
Several reports have described the detection of circulating, cancer-related RNA molecules in serum or plasma from cancer patients (El-Hefnawy et al., 2004). Despite substantial advances in cancer diagnostics, the search persists for simple and cost-effective diagnostic tests. Many studies have explored the possibility of using circulating tumor cells for detection or monitoring of cancer (Lo, 2001, Mehes et al., 2001, Molnar et al., 2001, Taback et al., 2001, Wong and Lo, 2002). Although circulating tumor cells are frequently detectable in blood from patients with advanced stages of the disease, these researches have been unable to demonstrate reliable utility for early cancer detection or recurrence monitoring.
Calcineurin B homologous protein isoform 2 (CHP2) was identified to be expressed in various malignant cell lines, but not in the normal tissue counterpart. The biological function of CHP2 related to cancer progression is still unknown. In many studies, cell proliferation, adhesion, motility, and invasion capacities were assessed in transfected cell lines to explore the possible functions of CHP2 in cancer progression (Jin et al., 2007).
The calcineurin homologous protein (CHP) belongs to an evolutionary conserved Ca2 + binding protein sub-family. The CHP sub-family is comprised of CHP1, CHP2 and CHP3, which in vertebrates share significant homology at protein level amongst each other and between other Ca+ 2-binding proteins (Di Sole et al., 2011).
CHP1 is expressed ubiquitously and is an essential cofactor for NHE1 to NHE3. CHP3 is restricted to the heart, brain, stomach and testes. CHP2 is restricted to cancer tissues or cells, such as hepatic carcinoma, colon adenocarcinoma, cervical carcinoma, leukemia, ovarian cancer cell line but not their normal tissue counterpart. These results implied that CHP2 expression may represent a useful biomarker for the detection and diagnosis of certain types of cancer (Wang et al., 2002).
In the present study the expression of CHP2 mRNA was measured by QRT-PCR in 40 de novo AL patients in comparison with 10 healthy control subjects, to find a possible relation or correlation with the clinical and laboratory features of AL patients at diagnosis, such as age, gender, lineage (AML or ALL), HB, TLC, platelets count and bone marrow blast cell infiltration.
In this study, CHP2 mRNA was expressed in 13/40 AL patients (32.5%) with mean expression level of 2.7 (range, 0–10.5) and was expressed in 1/10 (10%) control samples used as calibrators with mean expression level of 0.1 (range 0–1.0). The CHP2 expression rate in AL patients (32.5%) was statistically significant higher than those of controls (p < 0.05), and the analysis of CHP2 expression showed that all the AL patients who expressed CHP2 mRNA exhibited CHP2 expression above the calibrators. There was no statistically significant differences (p > 0.05) in the CHP2 expression rates and levels as regards clinical and laboratory findings, i.e., age, gender, type of AL, TLC, BM infiltration, presence of hepatomegaly, splenomegaly and lymphadenopathy. Also, there was no positive correlation between CHP2 expression and any of the clinical and laboratory findings.
This was in accordance with B. Li et al. (2008) in which real-time quantitative PCR (RQ-PCR) was performed to detect the expression level of CHP2 gene in peripheral blood mononuclear cells from 10 healthy individuals (as control) and 24 cases of leukemia, and in 4 kinds of leukemia cell lines. The results showed that the detection rate of CHP2 gene in 10 normal controls was 80%, with mean expression level (0.744 ± 0.682). Difference in expression rates and levels may be due to different sample size. The expression levels of CHP2 mRNA leukemia primary cells and leukemia cell lines were significantly higher than that in the normal control (p < 0.05). They concluded that the human tumor related gene CHP2 expression was significantly increased in leukemia primary cells and leukemia cell lines, which may play an important role in growth process of leukemia cells.
Li et al. (2011) also reported that nuclear accumulation of calcineurin B homologous protein 2 (CHP2) results in enhanced proliferation of tumor cells. The C-terminal region of CHP2 contains a nuclear export sequence (NES). When the six leucines of NES were mutated to alanines, the resulting CHP2 protein was predominantly localized to the nucleus. Furthermore, mutation of the NES resulted in enhanced proliferation and oncogenic potential of HeLa cells. These results provided new insight into understanding the role of CHP2 in tumor cell growth.
Also, Jin et al. (2007) reported that with RT-PCR analysis, CHP2-transfected OVCAR3/CHP2 cancer cells showed high CHP2 gene expression, whereas non-transfected clones did not produce detectable CHP2 mRNA. CHP2-transfected OVCAR3/CHP2 cells showed increased proliferation rates and exhibited increased activities of cell adhesion, migration and invasion. This study provided the first evidence that over expression of the CHP2 gene affects the biological behavior of ovarian cancer cell line OVCAR3 and is one of key mechanisms for ovarian carcinoma progression, suggesting that CHP2 may be an attractive target for biological anticancer therapy.
In addition, Pang et al. (2002) stated that CHP2 expression was extremely low in most human tissues but was higher in tumor cells as liver carcinoma cells and colon tumour metastatic cells. They produced stable cell clones over expressing either CHP1 or CHP2 in which one of them is predominantly bound to NHE1. Serum (10%) induced a significant cytoplasmic alkalinization (0.1–0.2 pH unit) in cells co-expressing CHP1 and NHE1 but not in cells co-expressing CHP2 and NHE1. In the latter, pH was high (7.4–7.5) even in the absence of serum, suggesting that NHE1 was already activated. Surprisingly, most (> 80%) of CHP2/NHE1 cells unlike CHP1/NHE1 cells were viable even after long serum starvation (> 7 days). Thus, the expression of CHP2 appears to protect cells from serum deprivation-induced death by increasing pH. These properties of CHP2/NHE1 cells are similar to those of malignantly transformed cells. They proposed that serum-independent activation of NHE1 by bound CHP2 is one of the key mechanisms for the maintenance of high pH and the resistance to serum deprivation-induced cell death in malignantly transformed cells.
In contrast, Inoue et al. (2003) had clearly shown that CHP2 is expressed in normal intestinal epithelia and also epithelium-like cell line. Thus they believed that CHP2 has an important role in normal intestinal epithelia not only in transformed cells, possibly with the antiporter activity of NHEs.
It has also been reported by G. Li et al. (2008) that ectopic expression of CHP2 promoted the proliferation of HEK293 (human embryonic kidney cell line 293) cells, whereas knockdown of endogenous CHP2 expression in HepG2 inhibited cell proliferation. When inoculated into nude mice, CHP2 transfected HEK293 cells displayed markedly increased oncogenic potential. In the analysis of the underlying molecular mechanisms, they found that like calcineurin B, CHP2 was able to bind to and stimulate the phosphatase activity of calcineurin A. In accordance with this, CHP2-transfected cells showed increased nuclear presence of NFATc3 (nuclear factor of activated T cells) and enhanced NFAT activity. Finally, both accelerated cell proliferation and NFAT activation following CHP2 transfection could be suppressed by the calcineurin inhibitor cyclosporine A, suggesting an intrinsic connection between these events. So these results highlighted a potential role of CHP2 in tumorigenesis and revealed a novel function of CHP2 as an activator of the calcineurin/NFAT signaling pathway. This may give an impression that CHP2 is a general cancer marker.
Finally, we conclude that the human tumor related gene chp2 expression in leukemia primary cells significantly increase, which may play an important role in growth process of leukemia cells.
Also the results showed that this procedure was highly discriminating between healthy subjects and AL patients and strongly support the idea that a valuable diagnostic test for cancer might be developed using this genetic marker in plasma.
Future studies involving large number of cases, serial blood analysis, correlation with different immunophenotypical types and monitoring response to treatment may allow a more detailed assessment of the predictive ability of this Q-RT assay. Long-term follow-up of CHP2 mRNA elevation to monitor patients or assess treatment efficacy after chemoradiotherapy of patients receiving therapy is highly recommended.
Acknowledgment
Authors would like to thank Dr. Seham Omar, professor and head of Department of Clinical Pathology, Beni Suef University, for her endless support throughout this study.
References
- Bozzone D.M. Chelsea House; 2009. The Biology of Cancer: Leukemia. (An imprint of info Base Publishing, New York) [Google Scholar]
- Di Sole F., Vadnagara K., Moe O.W., Babich Calcineurin homologous protein: a multi-functional Ca2 +-binding protein family. Am. J. Physiol. Renal. 2011;10:1–7. doi: 10.1152/ajprenal.00628.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hefnawy T., Raja S., Kelly L. Characterization of amplifiable, circulating RNA in plasma and its potential as a tool for cancer diagnostics. Clin. Chem. 2004;50(3):564–573. doi: 10.1373/clinchem.2003.028506. [DOI] [PubMed] [Google Scholar]
- Inoue H., Nakamura Y., Nagita M. Calcineurin homologous protein isoform 2 (CHP2), Na +/H + exchangers-binding protein, is expressed in intestinal epithelium. Biol. Pharm. Bull. 2003;26(2):148–155. doi: 10.1248/bpb.26.148. [DOI] [PubMed] [Google Scholar]
- Jin Q., Kong B., Yang X. Overexpression of CHP2 enhances tumor cell growth, invasion and metastasis in ovarian cancer. In Vivo. 2007;21(4):593–598. [PubMed] [Google Scholar]
- Li G., Zhang X., Li R. CHP2 activates the calcineurin/nuclear factor of activated T cells signaling pathway and enhances the oncogenic potential of HEK293 cells. J. Biol. Chem. 2008;283(47):32660–32668. doi: 10.1074/jbc.M806684200. [DOI] [PubMed] [Google Scholar]
- Li B., Li H., Ma L. Expression of a tumor related gene chp2 in leukemia cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16(4):734–737. [PubMed] [Google Scholar]
- Li Q.H., Wang L.H., Lin Y.N. 2011. Nuclear accumulation of calcineurin B homologous protein 2 (CHP2) results in enhanced proliferation of tumor cells. [DOI] [PubMed] [Google Scholar]
- Lo Y.M.D. Circulating nucleic acids in plasma and serum: an overview. Ann. N. Y. Acad. Sci. 2001;945:1–7. doi: 10.1111/j.1749-6632.2001.tb03858.x. [DOI] [PubMed] [Google Scholar]
- Mehes G., Ambros P.F., Gadner H. Detecting disseminated solid tumor cells in hematopoietic samples: methodological aspects. Haematologia (Budap) 2001;31:97–109. doi: 10.1163/15685590152492918. [DOI] [PubMed] [Google Scholar]
- Molnar B., Ladanyi A., Tanko L. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin. Cancer Res. 2001;7:4080–4085. [PubMed] [Google Scholar]
- Pang T., Su X., Wakabayashi S., Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J. Biol. Chem. 2001;276:17367–17372. doi: 10.1074/jbc.M100296200. [DOI] [PubMed] [Google Scholar]
- Pang T., Wakabayashi S., Su X. Expression of calcineurin B homologous protein 2 protects serum deprivation-induced cell death by serum-independent activation of Na +/H + exchanger. J. Biol. Chem. 2002;277(46):43771–43777. doi: 10.1074/jbc.M208313200. [DOI] [PubMed] [Google Scholar]
- Pathak A.K., Bhutani M., Kumar S. Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin. Chem. 2006;52:1833–1842. doi: 10.1373/clinchem.2005.062893. [DOI] [PubMed] [Google Scholar]
- Taback B., Chan A.D., Kuo C.T. Detection of occult metastatic breast cancer cells in blood by a multimolecular marker assay: correlation with clinical stage of disease. Cancer Res. 2001;61:8845–8850. [PubMed] [Google Scholar]
- Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat. Rev. Cancer. 2005;5:223–231. doi: 10.1038/nrc1571. [DOI] [PubMed] [Google Scholar]
- Wang Y., Han K.J., Pang X.W. Large scale identification of human hepatocellular carcinoma-associated antigens by autoantibodies. J. Immunol. 2002;169:1102–1109. doi: 10.4049/jimmunol.169.2.1102. [DOI] [PubMed] [Google Scholar]
- Wong I.H., Lo Y.M.D. New markers for cancer detection. Curr. Oncol. Rep. 2002;4:471–477. doi: 10.1007/s11912-002-0058-3. [DOI] [PubMed] [Google Scholar]
- Zaun H.C., Shrier A., Orlowski J. Calcineurin B homologous protein 3 promotes the biosynthetic maturation, cell surface stability, and optimal transport of the Na+/H+ exchanger NHE1 isoform. J. Biol. Chem. 2008;283:12456–12467. doi: 10.1074/jbc.M800267200. [DOI] [PMC free article] [PubMed] [Google Scholar]