Abstract
Background
To date the published data concerning the possible interplay between vitamin D (VitD) and Vit D receptor (VDR) gene polymorphism with the immune/inflammatory mediators in type 2 diabetes mellitus (DM) is insufficient. Some of the immune non-classical actions of vitamin D may point to its role in the pathogenesis of type 2 DM through down-regulation of cytokines (IL-6). Although there is evidence to support a relationship among vitamin D status, chronic inflammation and insulin resistance, the underlying mechanism requires further exploration. We aimed to investigate the role of vitamin D in chronic inflammation and insulin resistance in type 2 DM. Moreover, to examine the association of VDR gene polymorphisms [VDR 2228570 C > T (FokI); VDR 1544410 A > G (BsmI)] with the components of metabolic syndrome (MetSyn) in type 2 diabetic Egyptian patients .
Subjects and methods
A total of 190 subjects were enrolled in this study, 60 controls and 130 type 2 diabetic patients (Group II). Group II was subdivided into 63 patients without MetSyn (subgroup IIa) and 67 patients with MetSyn (subgroup IIb). Genetic analysis for VDR gene polymorphisms was done in all subjects. VitD and IL-6 plasma levels were estimated.
Results
The TT genotype for the VDR FokI was significantly more frequent in subgroup IIb than in subgroup IIa and controls (X2 = 6.83, P = 0.03 and X2 = 16.592, P = 0.000) respectively. The T allele was more frequent in the MetSyn group as compared to diabetics without MetSyn (p = 0.001), odds ratio (OR) and 95% CI for the T allele of C > T (FokI) = 2.30 (1.37–3.86). We did not detect any significant difference in VDR BsmI genotypes between patients and control groups (P = 0.947). FokI VDR was significantly associated with the lipid profile parameters, VitD and IL-6 plasma levels in subgroup IIa and associated with HOMA-IR, insulin, VitD, IL-6 levels, waist circumference (WC) and body mass index (BMI) in subgroup IIb while BsmI VDR variant was associated only with VitD values in both subgroups.
Conclusion
The present study suggests an interaction between VDR polymorphisms and important components of MetSyn, VitD and pro-inflammatory cytokines (IL-6). FokI VDR polymorphisms may be linked to mild inflammation and insulin resistance and might represent a genetic determinant for developing MetSyn in type 2 diabetic Egyptian patients. The challenge is determining the mechanisms of VitD action for recommendation of VitD supplementation that reduces the risks of MetSyn, insulin resistance and progression to type 2 diabetes.
Abbreviations: VitD, Vitamin D; DM, diabetes mellitus; VDR, Vit D receptor; MetSyn, metabolic syndrome; HOMA, Homeostasis of Metabolic Assessment; WC, waist circumference; OR, odds ratio; BMI, body mass index; IL-6, interleukin -6; SOCS, suppressors of cytokine signaling; IRS, insulin receptor substrates; CRP, C-reactive protein; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; FPI, fasting plasma insulin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HPLC, High performance liquid chromatography; SD, standard deviation; X2, Chi-square; CI, confidence intervals; PGs, pro-inflammatory prostaglandins; NHANES III, National Health and Examination Survey; PTH, parathyroid hormone
Keywords: Insulin resistance, Type 2 diabetes mellitus (DM), Metabolic syndrome, Vitamin D, Vitamin D Receptor gene, Polymorphisms, Pro-inflammatory cytokines, Interleukin-6 (IL-6)
Introduction
Type 2 diabetes is one of the main non-communicable chronic diseases and its complications have become a major cause of morbidity and mortality worldwide. It has been estimated that 285 million individuals have diabetes, most of them type 2 diabetes (International Diabetes Federation (IDF), 2011). Vitamin D deficiency is also considered a public health problem around the world. In 2008, it was estimated that 1 billion individuals presented vitamin D insufficiency or deficiency (James, 2008) and its role in abnormal glucose metabolism as well as in type 2 diabetes has been demonstrated (Melamed et al., 2008, Palomer et al., 2008). Clinical studies have demonstrated a positive correlation between circulating vitamin D (25-hydroxyvitamin D; 25(OH)D) levels and insulin sensitivity, they indicate that vitamin D deficiency may predispose to glucose intolerance, altered insulin secretion and type 2 diabetes (Pittas et al., 2010), either through a direct action via vitamin D receptor (VDR) activation or indirectly via calcemic hormones and also via inflammation (Sung et al., 2012, Thorand et al., 2011). Chronic low-grade inflammation, frequently observed in obese individuals, is involved in the development of insulin resistance, which increases the risk of type 2 diabetes (McGill et al., 2008). The first link between obesity, inflammation and insulin action came from a study developed by Hotamisligil et al. (1993). There is strong evidence that activation of inflammatory pathways interferes with normal metabolism and disrupts proper insulin signaling resulting in increased expression of pro-inflammatory cytokines (Gregor and Hotamisligil, 2011). These cytokines can target cell membrane receptors, feeding into inflammatory response and exacerbating insulin resistance (Solinas et al., 2007). Another important molecular mediators that link pro-inflammatory cytokine to inhibition of insulin signaling are suppressors of cytokine signaling (SOCS) 1 and 3, induced by IL-6, which lead to ubiquitinylation and degradation of insulin receptor substrate (IRS) proteins (Lebrun and Van Obberghen, 2008). There is increasing evidence from clinical and observational studies that a systemic, sub-clinical, low-intensity inflammatory reaction not only co-exists, but also precedes the development of T2DM (Engström et al., 2003, Flores, 2005, Mezza et al., 2012, Palomer et al., 2008).
The inflammatory markers that have shown the strongest predictive capacity in the development of T2DM are C-reactive protein (CRP) and IL-6 (Engström et al., 2003, Pickup, 2004). Epidemiological studies have shown that TNF-a, CRP and IL-6 are positively correlated with BMI and percentage body fat (Bermudez et al., 2002, Pittas et al., 2004). Several recent human studies have associated vitamin D status with type 2 diabetes development (Mezza et al., 2012). Currently, the involvement of vitamin D in the components of metabolic syndrome (MetSyn) has been suggested (Melamed et al., 2008). MetSyn is defined as a combination of medical disorders that, when occurring together, increase the risk of developing cardiovascular disease and type 2 diabetes mellitus (T2DM) (Melamed et al., 2008). Although the mechanisms underlying the role of vitamin D in MetSyn remain incompletely explained, it was showed that 25(OH)D3 could be related to the occurrence of reduced insulin secretion and sensitivity (Liu et al., 2012), obesity (Martins et al., 2007), diabetes (Martins et al., 2007), and hypertension (Forman et al., 2007). Vitamin D receptor (VDR) is widely distributed in more than 38 tissues, where it clearly controls vital genes related to bone metabolism, oxidative damage, chronic diseases and inflammation (Haussler et al., 2008). Vitamin D and its receptor complex play the role in regulating the B-cell insulin secretion (Knekt et al., 2008, Pittas et al., 2007a, Pittas et al., 2007b). Several polymorphisms, such as BsmI and FokI, have been described in the VDR genes that are able to alter the activity of VDR protein (Filus et al., 2008). All VDR polymorphisms are located between exons 8 and 9 except that FokI is in exon 2 (Naito et al., 2007). However, the genetic background of the disease still remains unclear, it has been demonstrated that some of these polymorphisms are associated with type 2 diabetes mellitus, insulin secretion (Ortlepp et al., 2001) as well as with metabolic changes related to obesity (Filus et al., 2008). Although the relationship between vitamin D insufficiency and components of MetSyn has been previously demonstrated (Ford et al., 2005, Pittas et al., 2007a, Pittas et al., 2007b), few studies have examined VDR gene polymorphisms for associations with the risk of these disorders (Filus et al., 2008, Schuch et al., 2013a). Human studies investigating the influence of VitD status with VDR genetic SNPs on inflammatory biomarkers of subjects with or at high risk of developing type 2 diabetes and its complications are scarce and have generated conflicting results. To our knowledge, no published data are available concerning the possible interplay between VitD and the immune/inflammatory mediators in type 2 diabetes mellitus in Egypt. To date, however, it remains to be elucidated whether the low-grade inflammation observed in type 2 diabetes mellitus might be influenced by the immune properties of VitD. Thus, this study was designed to examine the role of VitD status in low-intensity chronic inflammation and insulin resistance in T2DM. Moreover, we intended to investigate the association of VDR gene polymorphisms [VDR 2228570 C > T (FokI); VDR 1544410 A > G (BsmI)] with the components of MetSyn in type 2 diabetic Egyptian patients.
Subjects and methods
Subjects, blood pressure and anthropometric measurements
This case–control study was started in February 2012 to December 2013. It included 190 subjects. They were recruited from Endocrinology outpatient clinics of the Internal Medicine Department, Zagazig University hospitals. All subjects were Egyptians from Sharkia, Egypt and they belonged to the same ethnic group. A written informed consent was obtained from all patients. The study was approved by Zagazig University's ethics committee. Type 2 diabetes was defined by the 1999 criteria of the World Health Organization (fasting blood glucose level > 126 mg/dl and/or 2-h postprandial blood glucose level > 200 mg/dl) (World Health Organization, 2006). Patients who did not meet these criteria as under treatment but who gave a history of T2DM were also included in the study. Exclusion criteria were: the presence of chronic illnesses that potentially alter vitamin D metabolism, the use of medications that affect bone metabolism, pregnant or breastfeeding women, and the use of vitamin D supplements.
Subjects were classified into three main groups:
-
–
Group I “control group”: 60 normal volunteers (28 females and 32 males). Their mean ages were ranged from 37 to 61 years with a mean value ± S.D. of 47.96 ± S.D. of 5.61 years. They had been matched for BMI, sex, age and socioeconomic background, they had no evidence of DM, hypertension, obesity, hypercholesterolemia, family history or previous history of stroke or transient ischemic attacks and smoking on the basis of their clinical history and physical examination.
-
–
Group II “Type 2 diabetic patients”: included 130 patients (73 males and 57 females), aged from 34 to 63 with a mean value ± S.D. of 47.79 ± 7.24 years. Body mass index [BMI = weight (kg) / height(m)2] was calculated. Waist circumference (WC) was measured while the subjects were standing up, with a tape placed at the midpoint level between the lower intercostal border and the anterior superior iliac supine while the subject was gently exhaling. The subjects were also asked about their use of sunscreen and practice of outdoor physical activities.
-Patients with T2DM were further classified according to the presence or absence of MetSyn into two subgroups. The diagnosis of MetSyn was considered when the criteria adopted by NCEP — ATP III was achieved : WC ≥ 102 cm for men and ≥ 88 cm for women; fasting blood glucose (FBG) ≥ 110 mg/dl; triglycerides (TG) ≥ 150 mg/dl; HDL-cholesterol < 40 g/dl for men and < 50 mg/dl for women; systolic blood pressure (SBP) ≥ 130 mm Hg or diastolic blood pressure (DBP) ≥ 85 mm Hg (Grundy et al., 2005).
-
–
Subgroup IIa “Type 2 diabetic patients without MetSyn”: comprised 63 patients (36 males and 27 females) with ages ranged between 35 and 59 with a mean value ± S.D. of 47.39 ± 6.01 years.
-
–
Subgroup IIb “Type 2 diabetic patients with MetSyn”: comprised 67 patients (37 males and 30 Females) with ages ranged between 34 and 63 years with a mean value ± S.D. of 48.16 ± 8.26 years.
-There was no statistical difference (P > 0.05) regarding age and sex among the groups.
Biochemical analyses of blood samples
Sample collection
Overnight fasting venous blood samples were collected from the subjects in EDTA containing tubes using standardized protocol and equipment., separated into two samples one whole blood for DNA extraction and VitD gene SNPs detection and the measurement of glycated hemoglobin (HbA1c) (Abraham et al., 1978). The other plasma specimen was used for measuring VitD and IL-6 levels. Other basic biochemical blood tests were measured by standard chemical and enzymatic commercial methods in the Medical Biochemistry department and hospital laboratories.
Laboratory investigations, including:
-
(a)
Fasting plasma glucose (FPG) levels according to Trinder (1969) using glucose enzymatic (GODPAP)-liquizyme Kits (Biotechnology, Egypt).
-
(b)
Determination of HbA1c in blood (Abraham et al., 1978).
-
(c)
Lipid profile: plasma levels of total cholesterol (TC), Triglyceride (TG), and HDL-C (Assmann et al., 1983). LDL cholesterol (LDL-C) was measured according to Friedwald et al. (1972). LDL was calculated as follows: LDL = TC − HDL − TG \ 5.
-
(d)
Fasting plasma insulin (FPI) by enzyme amplified sensitivity immunoassay according to Starr et al. (1978) using KAP1251-INSEASIA (Enzyme Amplified SensitivityImmunoassay) Kits (BioSource Europe S.A., Belgium).
-
(e)
HOMA-IR: it was assessed by homeostasis model assessment (where HOMA = fasting insulin (μU/ml) × fasting plasma glucose (mg/dl) / 405) (Matthews et al., 1985).
-
(f)
Estimation of plasma levels of IL-6 applying ELISA technique according to DeRijk (1996): ACCUCYTE® Human IL-6, U.S. Patent No. 5,587,294,8075 Green mead Drive College Park, Maryland 20740, is a competitive enzyme immunoassay (EIA), which measures the natural and recombinant forms of the cytokine Interleukin-6.
-
(g)
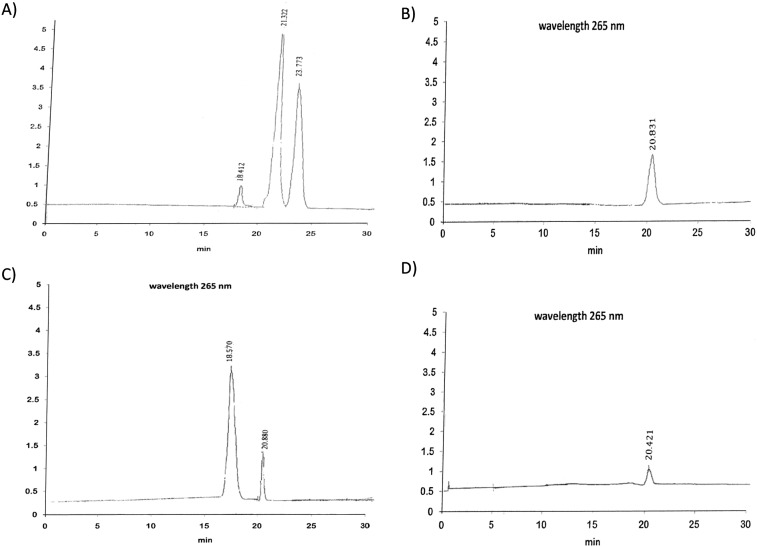
Determination of 25-Hydroxyvitamin D in Plasma by HPLC (Fig. 1): Plasma levels of 25(OH)D3 were measured by High Performance Liquid Chromatography (HPLC; Immundiagnostik AG, Germany) (Ursula et al., 2003) Vitamin D status is usually assessed by measuring the plasma concentration of 25-hydroxyvitamin D [25(OH)D]. Quantitative HPLC assays have been developed based on ultraviolet detection and normal-phase separation (Gilbertson and Stryd, 1977). Earlier HPLC methods for 25(OH)D3 in plasma were designed mainly for research purposes and were therefore too complicated for routine use. The present method was designed to be easy to use, sensitive, and rapid with simple sample preparation. To 0.5 ml of plasma, we added 350 μl of methanol-2-propanol (80:20 by volume). The tubes were mixed in a Multi tube vortex mixer for 30 s. 25(OH)D was extracted by mixing three times (60 s each time) with 2 ml of hexane. The phases were separated by centrifugation, and the upper organic phase was transferred to a tube and dried. The residue was dissolved in 100 μl of mobile phase. Calibration curves were constructed using four concentrations of 25(OH)D3 (15–120 nmol/L; cat. no. H-4014; Sigma Chemical Co.) and human plasma albumin (50 g/L). For chromatography we used an Instrument of Perkin Elmer model LC 1020 plus, carried out on a gradient pump (Binary LC pump 250) LC 18 column (particle size 5 μm, 250 mm × 4 mm inner diameter, Supelco Inc., Supelco Park, Bellefonte, PA USA). The mobile phase was 760 ml/L methanol in water, and the flow rate was 1 ml/min. Detection was at 265 nm, and the injected volume was 50 μl. The chromatographic separations obtained with calibrators and human plasma are shown in Fig. 1.
Fig. 1.
Chromatogram of vitamin D, detected by UV detector at a wavelength of 265 nm. A and C represent patient samples; B and D represent different VitD standard concentrations.
The 25(OH)D3 and 25(OH)D2 peaks are completely resolved with retention times of 20.4–21.32 min and 23.77 min, respectively. The prominent peak at 18.41–18.57 min is retinol. Our HPLC assay is based on that of Aksnes (1994). The percentage of methanol in the mobile phase is critical for the separation of these analytes. Extraction of the plasma samples with hexane before HPLC analysis was simple and fast (30 min), and it gave high and reproducible recoveries of 25(OH)D3. The peak areas of the endogenous analyte were subtracted from the supplemented plasma before comparison. 25(OH)D2 and 25(OH)D3 can be separately quantified, and there were no interfering peaks. A column 250 mm in length was necessary to clearly separate all of the peaks. The sensitivity of the ultraviolet detector is also critical. Samples containing up to 5 g/L hemoglobin or 100 μmol/L bilirubin did not interfere with the quantification of 25(OH)D3. HPLC method with ultraviolet detection enables reliable quantification of 25(OH)D3. The short and relatively simple sample preparation and ease of use make it useful for routine determinations.
Genotyping
Genomic DNA was isolated using the Wizard Genomic DNA Purification Kit purchased from Promega. After extraction, the quality of the extracted material was visualized in 1% agarose gel and the concentration was obtained by a spectrophotometer. Two VDR SNPs, namely, BsmI (VDR 1544410 A > G) and FokI (VDR 2228570 C > T), were genotyped using the polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP). Primer sequences (Iontec, Bioron). Amplification reactions were set up separately for FokI and BsmI polymorphic sites of VDR gene using primers given by Harris et al. (1997) and Morrison et al. (1994) (Table 1). The PCR was done using Taq PCR Master Mix kit (Qiagen, GmbH) as following: 25 μL of Taq PCR master mix was dispensed into each PCR tube, and then the following materials were added to each tube containing 100 ng of extracted DNA, 25 mM forward primer, and 25 mM reverse primer (Operon Biotechnologies, Inc.) and then 19 μL dd H2O was added giving a final volume of 50 μL. Following initial denaturation at 94 °C for 5 min, amplification was performed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 63 °C for 30 s for BsmI SNP and annealing of FokI at 58 °C for 30 s extension at 72 °C for 30 s. The final extension was allowed to proceed at 72 °C for 5 min; 8 μL of the PCR products was digested overnight with 10 U BsmI (at 37 °C and for 3 h with 10 U FokI at 65 °C; MBI, Fermentas, Lithuania). The digested PCR products were resolved on 3% agarose gels stained with ethidium bromide.
Table 1.
PCR-RFLP pattern of FokI (VDR 2228570 C > T) and BsmI (VDR 1544410 A > G) of VitD receptor gene.
| Polymorphisms | Primers | Annealing temp (C) | PCR-RFLP products |
|---|---|---|---|
| FokI (VDR 2228570 C > T) intron 8 and exon 2 |
Forward 5′-AGC TGG CCC TGG CAC TGA CTC TGC TCT-3′ Reverse 5′-ATG GAA ACA CCT TGC TTC TTC TCC TCC CTC-3′ |
58 °C | TT (ff):196 - 69 bp TC (fF):265,196,69 bp CC (FF):265 bp |
| BsmI (VDR 1544410 A > G) intron 8 |
Forward 5′-CAA CCA AGA CTA CAA GTA CCG CGT CAT GA-3′ Reverse 5′-AAC CAG CGG GAA GAG GTC AAG GG-3′ |
63 °C | GG (bb):650, 175 bp GA (bB):825, 650, 175 bp AA (BB):825 bp |
PCR-RFLP = Polymerase chain reaction-restriction fragment length polymorphism.
Statistical analysis
The statistical analysis was performed using a commercially available software program (SPSS 16.0, SPSS Inc., Chicago, Illinois, USA). Descriptive data were expressed as mean and standard deviation (SD). One-way analysis of variance (ANOVA, F test) was used to examine the variation in different MetSyn variables with the genotypes. Differences between the genotypes in studied parameters were assessed by using the t-test. To determine whether any significant differences in polymorphism frequencies occurred between the case and the control populations the allele and genotype frequencies were compared, using the Chi square (X2) method. Where significant P-values were generated, the odds ratio (OR) was calculated. Associations between the disease and genotypes were assessed by calculating odds ratios and 95 confidence intervals (CI). Statistical significance was assumed when p values were < 0.05.
Results
Demographic, clinical, laboratory characteristics and VitD status of the all studied groups are summarized in Table 2, Table 3
Table 2.
Basic characteristics of all participant groups.
| Parameters | Group I (n = 60) | Subgroup IIa (n = 63) | Subgroup IIb (n = 67) |
ANOVA (F-test) |
P value |
|---|---|---|---|---|---|
| Age (years) | 47.96 ± 5.61 | 47.39 ± 6.01 | 48.16 ± 8.26 | 0.221 | P = 0.802 |
| Sex | 28 F (46.7%) 32 M (53.3%) |
27 F (42.85%) 36 M (57.15%) |
30 F (44.8%) 37 M (55.2%) |
X2 = 0.180 | P = 0.914 |
| FPI (μU/ml) | 16.83 ± 2.55 | 26.58 ± 5.84 | 31.45 ± 5.06 | 154.508 | P = 0.000 |
| FPG (mg/dl) | 100.35 ± 9.89 | 138.86 ± 7.91 | 139.15 ± 8.70 | 391.139 | P = 0.000 |
| HbA1c % | 5.62 ± 1.36 | 11.37 ± 2.46 | 12.86 ± 1.60 | 258.015 | P = 0.000 |
| HOMA-IR | 4.19 ± 0.88 | 9.18 ± 2.39 | 10.86 ± 2.20 | 193.999 | P = 0.000 |
| TC (mg/dl) | 199.57 ± 20.34 | 288.55 ± 69.04 | 357.67 ± 60.84 | 131.311 | P = 0.000 |
| TG (mg/dl) | 142.96 ± 16.38 | 177.80 ± 32.44 | 222.97 ± 19.13 | 182.063 | P = 0.000 |
| LDL-C (mg/dl) | 106.49 ± 11.45 | 228.22 ± 22.03 | 274.79 ± 40.39 | 605.298 | P = 0.000 |
| HDL-C (mg/dl) | 54.47 ± 9.86 | 43.00 ± 4.43 | 40.80 ± 6.26 | 64.635 | P = 0.000 |
| VitD (ng/ml) | 35.25 ± 6.06 | 19.21 ± 3.20 | 14.74 ± 4.01 | 349.51 | P = 0.000 |
| IL-6 (ng/ml) | 0.848 ± 0.47 | 7.58 ± 1.87 | 9.44 ± 1.88 | 510.538 | P = 0.000 |
| SBP (mmHg) | 110.95 ± 12.3 | 118.73 ± 11.46 | 138.21 ± 9.15 | 104.821 | P = 0.000 |
| DBP (mmHg) | 77.33 ± 8.04 | 81.34 ± 9.51 | 94.92 ± 9.27 | 67.849 | P = 0.000 |
| WC (cm) | 91.19 ± 8.97 | 101.68 ± 7.67 | 108.82 ± 8.81 | 68.392 | P = 0.000 |
| BMI (kg/m2) | 21.36 ± 2.60 | 30.04 ± 2.94 | 33.84 ± 3.71 | 239.114 | P = 0.000 |
FPI: fasting plasma insulin; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; VitD: Vitamin D; IL-6: Interleukin-6; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; BMI: body mass index; X2 = Chi-square for non parametric values.
Table 3.
Number and percentage of vit D status in all subjects.
| Vit D status | Group I (n = 60) |
Subgroup IIa (n = 63) |
Subgroup IIb (n = 67) |
|||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| < 20 (ng/ml) Vit D deficiency |
1 | 1.66% | 48 | 76.19% | 64 | 95.52% |
| 21–29 (ng/ml) Vit D insufficiency |
13 | 21.66% | 15 | 23.8% | 3 | 4.47% |
| > 30 (ng/ml) Vit D sufficiency |
46 | 76.66% | 0 | 0% | 0 | 0% |
| P value | 0.000 | 0.000 | 0.000 | |||
The average age of the studied population was 47.84 ± 6.75 years with mean BMI of 28.64 ± 6.07 kg/m 2. About 130 (68.42%) of our participants were complaining of T2DM. MetSyn was present in 51.5% of the diabetic patients. FPI, FPG, HbA1c, HOMA index, TC, TG, LDL-C, WC, BMI, SBP, DBP and plasma IL-6 levels were significantly higher in individuals with MetSyn when compared to diabetic patients without MetSyn and controls, except for HDL-C which showed significant decreased levels (Table 2). Mean study population vitamin D status was insufficient (22.7 ± 9.85 ng/ml), the mean of VitD was significantly lower among patients without MetSyn than patients with MetSyn and controls (t = − 7.78, t = − 22.11, P = 0.000 respectively). There is a significant negative correlation between plasma VitD and each of FPI, FPG, HOMA-IR,HbA1c,TC, IL-6, SBP, DBP, WC and BMI levels in all studied groups (r = − 0.581, r = − 0.793, r = − 0.646, r = − 0.640, r = − 0.490, r = − 0.91, r = − 0.354, r = − 0.302, r = − 0.454, r = − 0.619, P = 0.000) respectively . Otherwise, VitD had positive significant correlation with HDL-C plasma levels in all studied populations (r = 0.589, P = 0.000).
Distribution of VDR 2228570 C > T (FokI) and VDR 1544410 A > G (BsmI) gene polymorphisms
The allele and genotype frequency distribution and carriage rate of VDR (FokI and BsmI) genes among patients and controls were shown in Table 4. The results showed significant differences between diabetic patients with MetSyn and without MetSyn regarding the genotype and allele distributions of the VDR 2228570 C > T (FokI) polymorphism. The TT genotype for the VDR 2228570 C > T (FokI) was significantly more frequent in diabetics with MetSyn than in diabetics without MetSyn and controls (X2 = 6.83, P = 0.03 and X2 = 16.592, P = 0.000) respectively. Otherwise we could not find a significant association between diabetic patients without MetSyn and controls regarding the VDR 2228570 C > T (FokI) polymorphism genotype and allele distributions (X2 = 4.841, P = 0.089. X2 = 3.69,P = 0.055) respectively . The T allele was more frequent in the MetSyn group as compared to diabetics without MetSyn (p = 0.001), odds ratio (OR) and 95% CI for the T allele of C > T (FokI) = 2.30 (1.37–3.86). We did not observe any significant difference in VDR BsmI genotypes between patient and control groups (P = 0.947). In addition, we also compared the VDR (FokI and BsmI) genotypes with different clinical parameters of all studied groups.
Table 4.
Genotype and allele frequency for VDR gene polymorphisms at FokI (VDR 2228570 C > T) and BsmI (VDR 1544410 A > G) in all studied groups.
| FokI (VDR 2228570 C > T) |
BsmI (VDR 1544410 A > G) |
||||||
|---|---|---|---|---|---|---|---|
| Genotype | Groups |
Genotype | Groups |
||||
| Group I (N = 60) | Subgroup IIa (N = 63) N (%) | Subgroup IIb (N = 67) N (%) | Group I (N = 60) N (%) | Subgroup IIa (N = 63) N (%) | Subgroup IIb (N = 63) N (%) | ||
| CC | 44 (73.3%) | 39 (62%) | 27 (40.3%) | AA | 40 (66.6%) | 38 (60.3%) | 42 (62.7%) |
| CT | 11 (18.3%) | 13 (21%) | 17 (25.4%) | AG | 14 (23.4%) | 16 (25.4%) | 17 (25.3%) |
| TT | 5 (8.3%) | 11 (17%) | 23 (34.3%) | GG | 6 (10%) | 9 (14.3%) | 8 (12%) |
|
X2 P-value |
17.84 0.001 |
X2 P-value |
0.738 0.947 (NS) |
||||
| OR 95% CI |
0.448a (0.299–0.677) | 0.515b (0.37–0.717) | 0.415c (0.288–0.587) | OR 95% CI |
1.312a (0.37–4.62) | 0.11b (0.31–3.92) | 0.837c (0.26–2.7) |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| C allele | 99 (82.5%) | 91 (72.2%) | 71 (53%) | A allele | 94 (78.3%) | 92 (73%) | 101 (75.4%) |
| T allele | 21 (17.5%) | 35 (27.8%) | 63 (47%) | G allele | 26 (21.7%) | 34 (27%) | 33 (24.6%) |
|
X2 P OR 95% CI |
3.69a 0.055 1.81 (0.98–3.41) |
24.91b 0.000 4.18 (2.34–7.47) |
10.23c 0.001 2.30 (1.37–3.86) |
X2 P OR 95% CI |
0.942a 0.206a 1.336 (0.74–2.4) |
0.311b 0.341b 1.181 (0.66–2.12) |
0.032c 0.485c 1.053 (0.60–1.84) |
Comparison of group I and subgroup IIaa, group I and subgroup IIbb, and subgroup IIa and subgroup IIbc.
Table 5 presents the distribution of all clinical variables according to the VDR genotypes observed in controls. No association between VDR FokI and VDR BsmI polymorphisms was observed with the components of MetSyn, VitD, IL-6 plasma levels or blood pressure. Lipid profile exhibited non-significant associations with both VDR polymorphism as shown in Table 5.
Table 5.
Different studied parameters and different VDR gene polymorphisms at FokI (VDR 2228570 C > T) and BsmI (VDR 1544410 A > G) genotypes in control group.
| Parameters |
FokI (VDR 2228570 C > T) genotypes |
BsmI (VDR 1544410 A > G) genotypes |
||||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 44) | CT (n = 11) | TT (n = 5) | ANOVA P-value | AA (n = 40) | AG (N = 14) | GG (n = 6) | ANOVA P-value | |
| FPI (μU/ml) | 16.84 ± 2.6 | 16.6 ± 2.5 | 17.28 ± 2.9 | 0.119 0.888 |
16.80 ± 2.57 | 16.87 ± 2.60 | 17.0 ± 2.71 | 0.018 0.983 |
| FPG (mg/dl) | 99.61 ± 10.2 | 104.4 ± 7.8 | 98.0 ± 10.4 | 1.174 0.316 |
100.70 ± 9.82 | 99.85 ± 10.82 | 9.70 ± 9.70 | 0.083 0.921 |
| HbA1c % | 5.5 ± 1.27 | 6.19 ± 1.6 | 5.48 ± 1.54 | 1.175 0.316 |
5.53 ± 1.28 | 6.08 ± 1.46 | 5.15 ± 1.59 | 1.241 0.297 |
| HOMA-IR | 4.25 ± 0.85 | 4.29 ± 1.03 | 3.46 ± 0.55 | 1.937 0.153 |
4.25 ± .86 | 4.32 ± .95 | 3.45 ± .49 | 2.502 0.091 |
| TC (mg/dl) | 196.9 ± 21.9 | 208.4 ± 15.8 | 203.4 ± 4.3 | 1.517 0.228 |
195.94 ± 22.02 | 210.39 ± 13.9 | 198.50 ± 12.6 | 2.784 0.070 |
| TG(mg/dl) | 143.6 ± 16.9 | 138.9 ± 15.6 | 146.0 ± 13.4 | 0.456 0.636 |
143.94 ± 17.4 | 138.21 ± 14.2 | 147.55 ± 12.6 | 0.892 0.415 |
| LDL-C(mg/dl) | 107.9 ± 11.8 | 105.0 ± 9.2 | 97.4 ± 9.6 | 2.070 0.136 |
107.98 ± 12.32 | 104.64 ± 7.69 | 100.83 ± 12.0 | 1.265 0.290 |
| HDL-C(mg/dl) | 54.79 ± 10.85 | 55.62 ± 6.29 | 49.12 ± 5.4 | 0.830 0.441 |
55.32 ± 10.8 | 52.9 ± 6.76 | 52.45 ± 10.06 | 0.444 0.644 |
| VitD (ng/ml) | 35.28 ± 5.36 | 36.54 ± 8.86 | 32.22 ± 4.59 | 0.872 0.424 |
35.46 ± 5.53 | 34.78 ± 7.11 | 34.96 ± 7.88 | 0.071 0.932 |
| IL-6(ng/ml) | 0.851 ± 0.44 | 0.866 ± 0.63 | 0.784 ± 0.4 | 0.053 0.949 |
0.837 ± 0.46 | 0.94 ± 0.55 | 0.85 ± 0.47 | 0.524 0.595 |
| SBP (mmHg) | 112.77 ± 12.3 | 105.45 ± 12.3 | 107.0 ± 9.08 | 1.893 0.160 |
112.4 ± 12.42 | 106.07 ± 11.5 | 112.67 ± 12.35 | 1.459 0.241 |
| DBP (mmHg) |
78.40 ± 7.4 | 75.45 ± 9.6 | 72.0 ± 9.1 | 1.841 0.168 |
78.75 ± 7.22 | 75.35 ± 9.49 | 72.50 ± 8.21 | 2.21 0.119 |
| WC(cm) | 92.16 ± 8.3 | 90.62 ± 10.33 | 86.4 ± 12.6 | 0.959 0.390 |
91.81 ± 8.49 | 91.10 ± 9.11 | 89.33 ± 13.41 | 0.201 0.819 |
| BMI (kg/m2) |
21.2 ± 2.69 | 22.24 ± 2.45 | 20.98 ± 1.8 | 0.777 0.465 |
21.11 ± 2.74 | 22.36 ± 2.30 | 20.73 ± 1.74 | 1.419 0.250 |
FPI: fasting plasma insulin; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; VitD: Vitamin D; IL-6: Interleukin-6; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; BMI: body mass index; X2 = Chi-square for non parametric values.
Then, the same analyses were performed for the subgroup IIa patients without MetSyn (Table 6). Higher plasma IL-6, TC, TG, and LDL-C with lower HDL-C and VitD levels were seen in TT genotype than CC genotype carriers of the VDR 2228570 C > T polymorphism (t = 6.703, P = 0.00, t = 9.08, t = 13.45,t = 11.43, t = − 3.141, P = 0.010, t = − 9.63, P = 0.000) respectively. While GG homozygous for the BsmI VDR variant was associated with lower VitD values than seen in others. Additionally, the values of FPI, FPG, HbA1c, DBP, SBP, BMI and WC were similar among all participants, independently of presence or absence of both VDR BsmI and FokI polymorphisms.
Table 6.
Different studied parameters and different VDR gene polymorphisms at FokI (VDR 2228570 C > T) and BsmI (VDR 1544410 A > G) genotypes in subgroup IIa.
| Parameters |
FokI (VDR 2228570 C > T) genotypes |
BsmI (VDR 1544410 A > G) genotypes |
||||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 39) | CT (n = 13) | TT (n = 11) | ANOVA P-value | AA (n = 38) | AG (N = 16) | GG (n = 9) | ANOVA P-value | |
| FPI (μU/ml) | 25.58 ± 6.2 | 26.54 ± 5.7 | 29.27 ± 3.12 | 2.032 0.119 |
25.82 ± 6.42 | 26.76 ± 5.15 | 29.47 ± 3.43 | 1.448 0.243 |
| FPG (mg/dl) | 139.12 ± 8.8 | 139.58 ± 7.38 | 137.37 ± 5.77 | 0.214 0.887 |
139.25 ± 8.7 | 138.92 ± 7.12 | 137.12 ± 6.02 | 0.256 0.775 |
| HbA1c % | 10.9 ± 2.47 | 12.2 ± 2.6 | 11.51 ± 2.1 | 1.557 0.209 |
11.10 ± 2.53 | 11.79 ± 2.48 | 11.78 ± 2.24 | 0.577 0.565 |
| HOMA | 9.3 ± 2.56 | 9.7 ± 2.26 | 8.43 ± 1.63 | 1.335 0.272 |
9.13 ± 2.58 | 9.93 ± 2.13 | 8.05 ± 1.55 | 1.833 0.169 |
| TC (mg/dl) | 250.15 ± 46.8 | 353.32 ± 55.9 | 351.15 ± 42.6 | 22.836 0.000 |
289.59 ± 70.53 | 275.68 ± 67.4 | 309.97 ± 62.08 | 0.722 0.490 |
| TG (mg/dl) | 169.27 ± 28.0 | 170.77 ± 28.6 | 219.57 ± 13.6 | 11.841 0.000 |
181.55 ± 30.5 | 169.86 ± 29.6 | 176.10 ± 44.96 | 0.739 0.482 |
| LDL-C (mg/dl) | 216.16 ± 14.5 | 239.96 ± 11.04 | 259.75 ± 14.98 | 32.620 0.000 |
225.68 ± 24.45 | 234.02 ± 14.95 | 230.07 ± 23.04 | 0.819 0.446 |
| HDL-C (mg/dl) | 42.23 ± 4.26 | 40.39 ± 2.52 | 36.23 ± 3.79 | 10.265 0.000 |
41.27 ± 4.73 | 41.11 ± 3.77 | 38.27 ± 3.73 | 1.752 0.182 |
| VitD (ng/ml) | 20.37 ± 1.67 | 19.95 ± 1.73 | 14.22 ± 4.0 | 33.054 0.000 |
20.70 ± 1.48 | 18.29 ± 2.79 | 14.60 ± 4.2 | 24.85 0.000 |
| IL-6 (ng/ml) | 6.70 ± 1.64 | 8.38 ± 0.75 | 9.72 ± 1.41 | 20.523 0.000 |
7.242 ± 1.87 | 8.52 ± 1.10 | 7.32 ± 2.49 | 2.90 0.062 |
| SBP (mmHg) | 118.68 ± 11.72 | 117.31 ± 12.84 | 120.91 ± 9.95 | 0.226 0.878 |
118.42 ± 11.68 | 118.44 ± 11.8 | 120.56 ± 11.02 | 0.129 0.879 |
| DBP (mmHg) | 80.78 ± 9.41 | 82.69 ± 9.26 | 82.27 ± 11.03 | 0.303 0.823 |
80.78 ± 9.41 | 82.5 ± 8.56 | 81.66 ± 12.24 | 0.183 0.833 |
| WC (cm) | 100.96 ± 7.16 | 100.98 ± 9.19 | 104.22 ± 7.57 | 0.937 0.428 |
101.21 ± 7.31 | 100.45 ± 8.35 | 105.82 ± 7.43 | 1.619 0.207 |
| BMI (kg/m2) | 29.63 ± 3.36 | 30.76 ± 2.37 | 30.65 ± 1.83 | 0.661 0.579 |
29.74 ± 3.29 | 30.62 ± 2.63 | 30.27 ± 1.73 | 0.533 0.590 |
FPI: fasting plasma insulin; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; VitD: Vitamin D; IL-6: Interleukin-6; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; BMI: body mass index; X2 = Chi-square for non parametric values.
Table 7 presents the distribution of all clinical variables according to the VDR genotypes observed in individuals with MetSyn. No association between VDR BsmI polymorphism was observed with the components of MetSyn, or other biochemical parameters except for VitD plasma levels which showed lower significant values in GG carriers. However, individuals carrying the mutant recessive homozygous TT genotype of the VDR 2228570 C > T polymorphism presented significantly higher insulin levels, insulin resistance index (HOMA-IR), IL-6 plasma levels, WC and BMI than individuals with the homozygous CC genotype (t = 3.42, P = 0.002, t = 4.51, P = 0.000, t = 10.482, P = 0.000, t = 3.35, P = 0.003, t = 13.58, P = 0.000) respectively . Additionally, the presence of TT genotype of VDR FokI polymorphism was associated with decreased VitD concentrations compared to TC and CC genotypes (t = − 3.58, P = 0.003, t = − 9.63, P = 0.000) respectively.
Table 7.
Different studied parameters and different VDR gene polymorphisms at FokI (VDR 2228570 C > T) and BsmI (VDR 1544410
A > G) genotypes subgroup IIb.
| Parameters |
C/T genotypes |
A/G genotypes |
||||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 27) | CT (n = 17) | TT (n = 23) | ANOVA P-value | AA (n = 42) | AG (N = 17) | GG (n = 8) | ANOVA P-value | |
| FPI (μU/ml) | 29.15 ± 5.07 | 31.38 ± 4.25 | 34.18 ± 4.36 | 7.290 0.001 |
30.63 ± 4.99 | 32.77 ± 4.74 | 32.91 ± 5.78 | 1.481 0.235 |
| FPG (mg/dl) | 137.96 ± 8.01 | 141.48 ± 9.18 | 138.82 ± 9.15 | 0.875 0.422 |
139.31 ± 8.75 | 140.11 ± 9.15 | 136.24 ± 7.81 | 0.551 0.579 |
| HbA1c % | 12.97 ± 1.73 | 13.04 ± 1.62 | 12.59 ± 1.45 | 0.501 0.608 |
13.00 ± 1.71 | 12.81 ± 1.44 | 12.20 ± 1.32 | 0.852 0.431 |
| HOMA | 9.71 ± 2.05 | 11.12 ± 1.94 | 12.03 ± 1.94 | 8.638 0.000 |
10.5 ± 2.21 | 11.7 ± 2.14 | 11.01 ± 2.02 | 1.856 0.165 |
| TC (mg/dl) | 356.49 ± 69.29 | 356.68 ± 59.63 | 359.78 ± 53.28 | 0.020 0.980 |
355.76 ± 66.1 | 351.80 ± 57.8 | 380.14 ± 31.67 | 0.638 0.532 |
| TG (mg/dl) | 219.44 ± 18.48 | 226.33 ± 21.46 | 224.64 ± 18.23 | 0.804 0.452 |
223.39 ± 19.2 | 220.35 ± 21.58 | 226.36 ± 13.93 | 0.289 0.750 |
| LDL-C (mg/dl) | 278.59 ± 39.42 | 273.28 ± 50.56 | 271.43 ± 34.17 | 0.206 0.815 |
275.66 ± 44.4 | 274.16 ± 34.98 | 271.50 ± 32.01 | 0.037 0.963 |
| HDL-C (mg/dl) | 41.51 ± 5.94 | 45.40 ± 6.28 | 42.98 ± 6.32 | 2.074 0.134 |
43.06 ± 6.23 | 44.59 ± 6.57 | 39.33 ± 4.66 | 1.976 0.147 |
| VitD (ng/ml) | 17.03 ± 2.49 | 16.33 ± 4.02 | 10.88 ± 2.28 | 31.503 0.000 |
16.58 ± 3.17 | 12.67 ± 3.55 | 9.49 ± 1.35 | 22.165 0.000 |
| IL-6 (ng/ml) | 7.83 ± 1.30 | 9.55 ± 1.02 | 11.24 ± 1.13 | 51.965 0.000 |
9.11 ± 2.0 | 9.97 ± 1.61 | 10.03 ± 1.54 | 1.758 0.180 |
| SBP (mmHg) | 140.93 ± 9.51 | 135.88 ± 7.75 | 136.74 ± 9.24 | 2.101 0.131 |
138.57 ± 9.12 | 139.41 ± 10.13 | 133.75 ± 6.4 | 1.132 0.329 |
| DBP (mmHg) |
96.48 ± 8.96 | 95.00 ± 9.84 | 93.04 ± 9.26 | 0.851 0.432 |
96.42 ± 9.05 | 93.82 ± 9.76 | 89.37 ± 7.76 | 2.180 0.121 |
| WC (cm) | 104.0 ± 9.34 | 109.86 ± 7.97 | 113.64 ± 5.53 | 9.386 0.000 |
110.41 ± 7.57 | 105.52 ± 10.44 | 107.50 ± 10.16 | 2.023 0.141 |
| BMI (kg/m2) | 30.61 ± 2.43 | 34.94 ± 3.15 | 36.64 ± 2.39 | 35.17 0.000 |
34.39 ± 3.69 | 32.53 ± 3.97 | 33.71 ± 2.85 | 1.547 0.221 |
FPI: fasting plasma insulin; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; VitD: Vitamin D; IL-6: Interleukin-6; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; BMI: body mass index; X2 = Chi-square for non parametric values.
Discussion
In contrast to type 1 diabetes, which is related to autoimmune destruction of pancreatic β cells, leading to absolute insulin deficiency, type 2 diabetes development involves impaired pancreatic β cell function, insulin resistance and inflammation (Takiishi et al., 2010). Insulin resistance is defined as systemic impairment of insulin function which precedes the development of hyperglycemia (Mirzaei Mirzaei et al., 2009). Although mechanistically unclear, it has been suggested that both environmental and genetic factors seem to be involved in type 2 diabetes development (Pittas et al., 2010). The relationship between vitamin D and low-intensity chronic inflammation with insulin resistance in type 2 diabetes can be mediated in part by the immune-modulating properties of the 1,25(OH)2D3, which is able to downregulate the production of pro-inflammatory cytokines (Wolden-Kirk et al., 2011). Some cross-sectional studies indicate that hypovitaminosis D is associated with higher serum levels of inflammatory biomarkers, such as IL-6 (Jablonski et al., 2011, Ngo et al., 2010, Peterson and Heffernan, 2008) and in obese subjects (Bellia et al., 2011), while others could not confirm these findings (Hyppoenen et al., 2010, Jorde et al., 2007, Vilarrasa et al., 2010).
To our knowledge, there are few studies which discussed the influence of VitD levels with VDR genetic SNPs on the risk of pathogenesis of type 2 DM and MetSyn in diabetic Egyptian patients. Thus, our study was designed to examine the role of VitD status in low-intensity chronic inflammation and insulin resistance in type 2 diabetes mellitus (T2DM). Moreover, we intended to investigate the association of VDR gene polymorphisms [VDR 2228570 C > T (FokI); VDR 1544410 A > G (BsmI)] with the components of MetSyn in type 2 diabetic Egyptian patients.
About 68.42% of our participants were suffering from T2DM. MetSyn was present in 51.5% of diabetic patients. Patients with MetSyn exhibited significant higher levels of FPI, FPG, HbA1c, and HOMA index than those of diabetic patients without metabolic syndrome and controls. We revealed a significant increase of lipid profile parameters (TC, TG, LDL-C) with significant decreased levels of HDL-C in diabetic patients with MetSyn when compared to two other groups. Additionally, in the present study VitD levels were negatively correlated with TC and positively correlated with HDL-C plasma levels in all studied groups.
The association of MetSyn with increased cardiovascular disease may be modulated by non-bony vitamin D effects on lipid profiles (Wang et al., 2009). Different results were observed in humans in the NHANES 2000–2004 survey, which demonstrated that vitamin D deficiency (< 15 ng/mL) was associated with lower levels of HDL-C in adults (Kim et al., 2008).
In the present study, the anthropometric measurements (WC and BMI), the blood pressure (SBP and DBP) and plasma IL-6 levels were significantly higher among patients with MetSyn when compared to diabetic patients without MetSyn and controls, and they also showed significant negative correlations with plasma VitD levels. Mean study population vitamin D status was insufficient (22.7 ng/ml), and VitD plasma levels were significantly lower among diabetic patients than controls with more decrease in diabetic patients with MetSyn.
We detected a significant negative correlation between plasma VitD and each of insulin levels, FPG, HOMA-IR and HbA1c levels in all studied groups. Our results were in harmony with Schuch et al. (2013b), Mukhopadhyaya et al. (2010) and Bid et al. (2009). A multi-ethnic sample of 6000 adults who participated in the Third National Health and Examination Survey (NHANES III) in the USA reported an inverse association between vitamin D status, diabetes and insulin resistance (Scragg et al., 2004). Clinical studies have demonstrated a positive correlation between circulating vitamin D levels and insulin sensitivity (Chiu et al., 2004, Pittas et al., 2007a, Pittas et al., 2007b). Shorter-term intervention studies also demonstrate a positive impact of vitamin D or its active metabolites on insulin resistance (Borissova et al., 2003).
To evaluate the role of vitamin D in low-intensity chronic inflammation and insulin resistance in T2DM we should take into consideration that vitamin D status is related to adiposity (Arunabh et al., 2003, Parikh et al., 2004), and that adiposity is in turn related to both chronic inflammation and insulin resistance (Bays et al., 2004). The strong positive correlation between adiposity and pro-inflammatory markers may reflect in part the fact that adipocytes are the source of a large proportion of baseline IL-6 (Kershaw and Flier, 2004, Pittas et al., 2004).
It has also been observed that macrophages infiltrate adipose tissue in obese individuals, contributing to the release of pro-inflammatory cytokines which are able to cause insulin resistance in adipocytes (Xu et al. 2003).
The present study exhibited a negative significant correlation between the VitD and the IL-6 plasma levels in all studied groups. Several studies support a role for vitamin D and 1,25(OH)2D, as anti-inflammatory agents (Cohen-Lahav et al., 2007, Zhang et al., 2007). In addition, 1,25(OH)2D down-regulates the increased levels of inflammatory markers in monocytes from type 2 diabetic patients compared with monocytes from healthy controls (Giulietti et al., 2007).
1,25(OH)2D inhibits the synthesis and actions of pro-inflammatory prostaglandins (PGs) by inhibiting cyclooxygenase-2 expression. 1,25(OH)2D influences several pathways known to regulate inflammatory responses, including increasing mitogen-activated protein kinase phosphatase-5 which down-regulates p38 mitogen-activated protein kinase activity (Krishnan et al., 2007). Therefore, vitamin D may also function to reduce the risk of diabetes by acting to reduce inflammatory responses.
Environmental and genetic factors play important roles in the mechanisms involved in the development of T2DM (Bid et al., 2009). Studies on genes inducing susceptibility to T2DM have been carried out by various groups in different populations (Almawi et al., 2006, Gragnoli et al., 2005, Grarup et al., 2007, Herder et al., 2008, Leak et al., 2008, Tong et al., 2009).
An important mediator of VitD action is the VDR gene which functions as a transcription factor when bound to 1,25(OH)2D (Palomer et al., 2008). There is evidence that VDR genotype may affect insulin resistance, both in regard to insulin secretion and insulin resistance (Oh and Barrett-Connor, 2002). Recent evidence demonstrated that the interaction of vitamin D and its receptor (VDR) has a supportive and regulatory impact on the immune system (Cantorna, 2010).
A linkage has been shown between VDR and the immune system, with the presence of VDR in several immune cells, such as monocytes, macrophages, and activated T and B lymphocytes (Matheu et al., 2003, O'Kelly et al., 2002, Pichler et al., 2002). Interference with cytokine production of monocytes and lymphocytes seems to be a key mechanism by which VitD interacts with the immune system (Mahon et al., 2003, Pichler et al., 2002). Importantly, VDR is present in the adipose tissue (Kershaw and Flier, 2004).
In the present study, we examined the associations of two potentially functional SNPs, 2228570 C > T (FokI) and 1544410 A > G (BsmI) of the VDR gene in a case–control study including controls and diabetic patients with and without MetSyn . Our results revealed that the TT genotype and the T allele of VDR 2228570 C > T (FokI) polymorphism were significantly more frequent in diabetics with MetSyn than in diabetics without MetSyn and controls. Otherwise we could not detect any significant differences regarding VDR 1544410 A > G (BsmI) alleles among patient and controls.
These results were supported by Cantorna (2010) and Schuch et al. (2013b). Otherwise, Bid et al. (2009) showed non-significant association between VDR (FokI, BsmI and TaqI) genotypes and diabetes risk in the north Indian population. A study was done by Malecki et al. (2003) on a Polish population, which showed no association between VDR FokI, ApaI, BsmI and TaqI polymorphisms and diabetes risk. The reason for the discrepancy between our results and these studies could be explained by the genetic differences in populations studied or their exposure to environmental factors.
In a trial to investigate the functional aspects of the two VDR polymorphisms, we measured several clinical and metabolic parameters of the patients and controls included the study. The findings of the present study revealed that VDR gene polymorphisms may influence the severity of MetSyn component disorder. Otherwise, we could not find significant association between BsmI VDR and the components of MetSyn in type 2 diabetic Egyptian patients except for VitD plasma levels.
In the present study, lipid profile parameters showed significant association with VDR 2228570 C > T (FokI) polymorphism in diabetic patients without MetSyn. We reported higher TC, TG , LDL-C and lower HDL levels in CT and TT genotype carriers as well lower VitD levels in homozygous recessive VDR 2228570 C > T (FokI) and VDR 1544410 A > G (BsmI). In diabetic patients with MetSyn we noticed significant associations between VDR 2228570 C > T (FokI) polymorphism and each of insulin levels, insulin resistance (HOMA-IR), IL-6 levels, WC and BMI. VitD levels were lower in homozygous recessive VDR 2228570 C > T (FokI) and VDR 1544410 A > G (BsmI).
Moreover, though glycemia is a major characteristic of diabetes mellitus type 2, no association was found between fasting plasma glucose levels and VDR gene polymorphisms evaluated in this study. Similarly, Malecki et al. (2003) and coworkers (2003) have investigated BsmI, TaqI, and FokI VDR gene polymorphisms in 548 Polish individuals with and without T2DM and did not find any association of glycemia with any of the VDR gene polymorphisms either.
On the other hand, in another study of Ortlepp et al. (2003), the BsmI VDR genotype had significantly associated with fasting glucose. Oh and Barrett-Connor (2002) investigated VDR gene polymorphisms in individuals with type 2 diabetes in the Rancho Bernando Cohort, they observed a significant association of the VDR 1544410 A > G (BsmI) polymorphism and levels of HOMA-IR.
Several mechanisms have been proposed to explain the impact of vitamin D on insulin sensitivity and glucose homeostasis. The regulation of serum Ca via VitD has been proposed to mediate the effects of vitamin D, at least in part, on insulin resistance. Vitamin D has been shown to increase levels of intracellular Ca and other rapid signaling pathways in a variety of tissues including adipocytes and muscle cells (Zemel et al., 2000). In addition to its rapid actions in cells, VitD also mediates genomic regulation through the VDR (Christakos et al., 2003). There is also evidence to support an effect of VitD at multiple levels of insulin release and action. Insulin release is low in vitamin D-deficient rats (Kadowaki and Norman, 1984) and is enhanced by treatment with 1,25(OH)2D (Bourlon et al., 1996), potentially via synthesis of proteins and increased conversion of pro-insulin to insulin (Bourlon et al., 1999). Results of studies suggest that the increase in insulin release mediated by 1,25(OH)2D may involve increases in intracellular Ca through the phosphoinositide/protein kinase C pathway and facilitating Ca entry by Ca channels (Billaudel et al., 1995).
Further, the 1α-hydroxylase is expressed in pancreatic islet Cells (Bland et al., 2004) consistent with the ability of vitamin D to enhance insulin secretion concentrations, but not in normal subjects (Li et al., 2008).
An increase in 25(OH)D could promote an increased insulin receptor expression with a net effect to induce insulin sensitivity in adipocytes. However, the effect of improved vitamin D status alone on insulin receptor expression has not been explored (Dorothy and Shawn, 2009).
Results of this study corroborate with the study of Schuch et al. (2013b), in suggesting that VDR gene polymorphism associated with lower serum VitD, may be linked to lower HDL-C and higher other lipid profile parameters in diabetic patients. There are three possible mechanisms by which vitamin D-VDR axis could affect lipid profiles. First: Vitamin D induced suppression of PTH secretion, and it has been reported that PTH could reduce lipolysis (Zemel et al., 2000). Second: Vitamin D increases intestinal calcium absorption and this can trigger a decrease in serum triglycerides levels by reducing the hepatic triglyceride formation and secretion (Cho et al., 2005) and Third: Vitamin D might improve insulin secretion and insulin sensitivity, thereby indirectly influencing lipid metabolism (Kamycheva et al., 2007).
In summary, genetic changes or alterations in the VDR might contribute to the pathogenesis of type 2 diabetes mellitus by at least four different pathways: alteration in calcium metabolism, modulation of adipocyte function, modulation of insulin secretion and modification of cytokine expression (Christakos et al., 2003). Vitamin D has been suggested to inhibit the expression of interleukins, which is produced by activated T lymphocytes (Mahon et al., 2003). Thus, the inhibition of vitamin D binding to its receptor and subsequent signaling might alter the cytokine secretion profile. Further examinations in larger cohorts with data permitting genome wide association studies are required.
Conclusion
Recent studies support a role for vitamin D as a vascular protective agent against the effects of advanced glycation end products, which are proposed to mediate the devastating consequences of diabetes on cardiac complications (Wang, 2013). The present study suggests an interaction between FokI VDR and Bsm VDR gene polymorphisms with type 2 DM pathogenesis and MetSyn. The former through its association with important components of MetSyn, VitD levels and pro-inflammatory cytokines (IL-6), linking the VDR 2228570 C > T to mild inflammation and insulin resistance. While the Bsm VDR through its association only to plasma levels of VitD. The positive effects of vitamin D seem to be primarily related to its action on enhancement of insulin synthesis and release, increasing the insulin receptor expression, and suppression of the inflammation, all three mechanisms acting to reduce the potential risks for insulin resistance. Additionally the association of VDR 2228570 C > T polymorphism with the lipid profile parameters, BMI and WC provides an explanation of the role of vitamin D to reduce adiposity. Therefore, the role of vitamin D in potentially reducing adiposity, improving insulin sensitivity and the reduction in vitamin D status with increased adiposity are factors that need to be carefully considered when examining the relationship of vitamin D with diabetes and its complications or insulin sensitivity.
Recommendations and future notes
The effects of vitamin D, either acting in concert or alone, all serve to improve insulin sensitivity. The challenge that lies ahead is in determining the mechanisms and relative strengths of these pleiotropic actions of vitamin D in order to make informed recommendations for vitamin D intakes that promote health and reduce the risks of onset of insulin resistance and progression to type 2 diabetes (Teegarden and Donkin, 2009). Future studies specifically designed to investigate the role of vitamin D on type 2 diabetes using inflammation as the main outcome are urgently needed in order to provide a more robust link between vitamin D, inflammation and type 2 diabetes. Furthermore, it is important to examine more genetic polymorphisms on a larger sample size in order to identify individuals that are more susceptible to vitamin D deficiency, type 2 diabetes and to developing MetSyn in the population.
References
- Abraham E.C.K., Huff T.A., Cope N.E. Determination of glycosylated hemoglobins with a new micro-column procedure. Diabetes. 1978;27:931–937. doi: 10.2337/diab.27.9.931. [DOI] [PubMed] [Google Scholar]; and insulin resistance in type 2 diabetes mellitus? Nutr. Res. Rev. 2005;18:175–182. doi: 10.1079/NRR2005104. [DOI] [PubMed] [Google Scholar]
- Aksnes L. Simultaneous determination of retinol, α-tocopherol, and 25-hydroxyvitamin D in human plasma by high-performance liquid chromatography. J. Pediatr. Gastroenterol. Nutr. 1994;18:339–343. doi: 10.1097/00005176-199404000-00015. (Medline Order article via Infotrieve Web of Science) [DOI] [PubMed] [Google Scholar]
- Almawi W.Y., Wakim-Ghorayeb S.F., Arekat M.R., Najm P., Keleshian S.H., Al-Sayed N. Association of selective HLA class II susceptibility-conferring and protective haplotypes with type 2 diabetes in patients from Bahrain and Lebanon. Clin. Vaccine Immunol. 2006;13:1296–1298. doi: 10.1128/CVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunabh S., Pollack S., Yeh J., Aloia J.F. Body fat content and 25-hydroxyvitamin D levels in healthy women. J. Clin. Endocrinol. Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- Assmann H., Schriewer G., Schmitz E.O. Hagele, Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin Chem. 1983;29:2026–2030. [PubMed] [Google Scholar]
- Bays H., Mandarino L., DeFronzo R.A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator−activated receptor agonists provide a rational therapeutic approach. Journal of Clinical Endocrinology and Metabolism. 2004;89(2):463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Bellia A., Garcovich C., D'Adamo M., Lombardo M., Tesauro M., Donadel G., Gentileschi P., Lauro D., Federici M., Lauro R. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2011 doi: 10.1007/s11739-011-0559-x. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Bermudez E.A., Rifai N., Buring J., Manson J.A., Ridker P.M. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- Bid H.K., Konwasr R., Aggarwal C.G., Gautam S., Saxena M., Nayak V.L., Banerjee M. Vitamin D receptor (FolkI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J. Med. Sci. 2009;63:187–194. ([PubMed] [Cross Ref]) [PubMed] [Google Scholar]
- Billaudel B.J., Bourlon P.M., Sutter B.C. Regulatory effect of 1,25-dihydroxyvitamin D3 on insulin release and calcium handling via the phospholipid pathway in islets from vitamin D-deficient rats. J. Endocrinol. Invest. 1995;18:673–682. doi: 10.1007/BF03349788. [DOI] [PubMed] [Google Scholar]
- Bland R., Markovic D., Hills C.E. Expression of 25-hydroxyvitamin D3-1a-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2004;89(90):121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- Borissova A.M., Tankova T., Kirilov G. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pract. 2003;57:258–261. [PubMed] [Google Scholar]
- Bourlon P.M., Faure-Dussert A., Billaudel B. Relationship between calbindin-D28K levels in the A and B cells of the rat endocrine pancreas and the secretion of insulin and glucagon: influence of vitamin D3 deficiency and 1,25-dihydroxyvitamin D3. J. Endocrinol. 1996;148:223–232. doi: 10.1677/joe.0.1480223. [DOI] [PubMed] [Google Scholar]
- Bourlon P.M., Billaudel B., Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J. Endocrinol. 1999;160:87–95. doi: 10.1677/joe.0.1600087. [DOI] [PubMed] [Google Scholar]
- Cantorna M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc. Nutr. Soc. 2010;69:286–289. doi: 10.1017/S0029665110001722. (Cross Ref Medline) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu K.C., Chu A., Go V.L., Saad M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. (PubMed) [DOI] [PubMed] [Google Scholar]
- Cho H.J., Kang H.C., Choi S.A., Ju Y.C., Lee H.S., Park H.J. The possible role of Ca2 + on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol. Pharm. Bull. 2005;28(8):1418–1423. doi: 10.1248/bpb.28.1418. (PubMed Abstract) [DOI] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Liu Y. New insights into the mechanisms of vitamin D action. J. Cell. Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- Cohen-Lahav M., Douvdevani A., Chaimovitz C. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J. Steroid Biochem. Mol. Biol. 2007;103:558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- DeRijk R.H. Changes in corticosteroid sensitivity of peripheral blood lymphocytes after strenuous exercise in humans. J. Clin. Endocrinol. Metab. 1996;81:228–235. doi: 10.1210/jcem.81.1.8550757. [DOI] [PubMed] [Google Scholar]
- Dorothy D.S., Shawn Tand. Vitamin D: emerging new roles in insulin sensitivity. Nutr. Res. Rev. 2009;22:82–92. doi: 10.1017/S0954422409389301. [DOI] [PubMed] [Google Scholar]
- Engström G., Stavenow L., Hedblad B., Lind P., Eriksson K.F., Janzon L., Lindgarde F. Inflammation-sensitive plasma proteins, diabetes, and mortality of myocardial infarction and stroke: a population-based study. Diabetes. 2003;52:442–447. doi: 10.2337/diabetes.52.2.442. [DOI] [PubMed] [Google Scholar]
- Filus A., Trzmiel A., Kuliczkowska-Plaksej J., Tworowska U., Jedrzejuk D., Milewicz A., Medras M. Relationship between vitamin D receptor BsmI and FokI polymorphisms and anthropometric and biochemical parameters describing metabolic syndrome. Aging Male. 2008;11:134–139. doi: 10.1080/13685530802273426. (PubMed Abstract | Publisher Full Text) [DOI] [PubMed] [Google Scholar]
- Flores M. A role of vitamin D in low-intensity chronic inflammation and insulin resistance in type 2 diabetes mellitus? Nutr. Res. Rev. 2005;18:175–182. doi: 10.1079/NRR2005104. [DOI] [PubMed] [Google Scholar]
- Ford E.S., Ajani U.A., McGuire L.C., Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. (PubMed Abstract) [DOI] [PubMed] [Google Scholar]
- Forman J.P., Giovannucci E., Holmes M.D., Bischoff-Ferrari H.A., Tworoger S.S., Willett W.C., Curhan G.C. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. (PubMed Abstract) [DOI] [PubMed] [Google Scholar]
- Friedwald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gilbertson T.J., Stryd R.P. High-performance liquid chromatographic assay for 25-hydroxyvitamin D3 in plasma. Clin. Chem. 1977;23:1700–1704. (Abstract/FREE Full Text) [PubMed] [Google Scholar]
- Giulietti A., van Etten E., Overbergh L. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res. Clin. Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Gragnoli C., Milord E., Habener J.F. Linkage study of the glucagon receptor gene with type 2 diabetes mellitus in Italians. Metabolism. 2005;54:786–787. doi: 10.1016/j.metabol.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Grarup N., Rose C.S., Andersson E.A., Andersen G., Nielsen A.L., Albrechtsen A. Studies of association of variants near the HHEX, CDKN2A/B, and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects: validation and extension of genome-wide association studies. Diabetes. 2007;56:3105–3111. doi: 10.2337/db07-0856. [DOI] [PubMed] [Google Scholar]
- Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. (PubMed) [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. (PubMed) [DOI] [PubMed] [Google Scholar]
- Harris S.S., Eccleshall T.R., Gross C., Dawson-Hughes B., Feldman D. The VDR start codon polymorphism (Fok-I) and bone mineral density in premenopausal American Black and White women. J. Bone Miner. Res. 1997;12:1043–1048. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- Haussler M.R., Haussler C.A., Bartik L., Whitfield G.K., Hsieh J.C., Slater S., Jurutka P.W. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008;66:S98–S112. doi: 10.1111/j.1753-4887.2008.00093.x. (PubMed) [DOI] [PubMed] [Google Scholar]
- Herder C., Rathmann W., Strassburger K., Finner H., Grallert H., Huth C. Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 genes confer risk of type 2 diabetes independently of BMI in the German KORA studies. Horm. Metab. Res. 2008;40:722–726. doi: 10.1055/s-2008-1078730. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor-necrosis-factor-alpha—direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. (PubMed) [DOI] [PubMed] [Google Scholar]
- Hyppoenen E., Berry D., Cortina-Borja M., Power C. 25-Hydroxyvitamin D and pre-clinical alterations in inflammatory and hemostatic markers: a cross sectional analysis in the 1958 British Birth Cohort. PLoS One. 2010:5:e10801. doi: 10.1371/journal.pone.0010801. ([PMC free article] [PubMed]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation (IDF) Diabetes atlas global burden, epidemiology and morbidity. Diabetes and impaired glucose tolerance. 2011. http://www.diabetesaltas.org/content/diabetes-and-impaired-glucose-tolerance [(accessed on 19 October 2011)]. Available online:
- Jablonski K.L., Chonchol M., Pierce G.L., Walker A.E., Seals D.R. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. ([PMC free article] [PubMed] [Cross Ref]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W.P.T. 22nd Marabou Symposium: the changing faces of vitamin D. Nutr. Rev. 2008;66:286–290. doi: 10.1111/j.1753-4887.2008.00034.x. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Jorde R., Haug E., Figenschau Y., Hansen J.B. Serum levels of vitamin D and haemostatic factors in healthy subjects: the Tromso study. Acta Haematol. 2007;117:91–97. doi: 10.1159/000097383. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Kadowaki S., Norman A.W. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J. Clin. Invest. 1984;73:759–766. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamycheva E., Jorde R., Figenschau Y., Haug E. Insulin sensitivity in subjects with secondary hyperparathyroidism and the effect of a low serum 25-hydroxyvitamin D level on insulin sensitivity. J. Endocrinol. Invest. 2007;30:126–132. doi: 10.1007/BF03347410. (PubMed Abstract) [DOI] [PubMed] [Google Scholar]
- Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Sabour S., Sagar U.N., Adams S., Whellan D.J. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am. J. Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. (PubMed Abstract) [DOI] [PubMed] [Google Scholar]
- Knekt P., Laaksonen M., Mattila C., Hδrkδnen T., Marniemi J., Heliφvaara M. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- Krishnan A.V., Moreno J., Nonn L. Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: role of anti-inflammatory activity. J. Bone Miner. Res. 2007;22(2):74–80. doi: 10.1359/jbmr.07s213. [DOI] [PubMed] [Google Scholar]
- Leak T.S., Mychaleckyj J.C., Smith S.G., Keene K.L., Gordon C.J., Hicks P.J. Evaluation of a SNP map of 6q24-27 confirms diabetic nephropathy loci and identifies novel associations in type 2 diabetes patients with nephropathy from an African-American population. Hum. Genet. 2008;124:63–71. doi: 10.1007/s00439-008-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun P., Van Obberghen E. SOCS proteins causing trouble in insulin action. Acta Physiol. 2008;192:29–36. doi: 10.1111/j.1748-1716.2007.01782.x. (PubMed) [DOI] [PubMed] [Google Scholar]
- Li J., Byrne M.E., Chang E. 1α-25-Dihydroxyvitamin D hydroxylase in adipocytes. J. Steroid Biochem. 2008;112:122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., McKeown N.M., Pittas A.G., Meigs J.B., Economos C.D., Booth S.L., Jacques P.F. Predicted 25-hydroxyvitamin D score and change in fasting plasma glucose in the Framingham Offspring Study. Eur. J. Clin. Nutr. 2012;66:139–141. doi: 10.1038/ejcn.2011.181. (PubMed Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon B.D., Wittke A., Weaver V., Cantorna M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- Malecki M.T., Frey J., Moczulski D., Klupa T., Kozek E., Sieradzki J. VDR gene polymorphisms and association with type 2 diabetes mellitus in a Polish population. Exp. Clin. Endocrinol. Diabetes. 2003;111:505–509. doi: 10.1055/s-2003-44711. [DOI] [PubMed] [Google Scholar]
- Martins D., Wolf M., Pan D., Zadshir A., Tareen N., Thadhani R., Felsenfeld A., Levine B., Mehrotra R., Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. (PubMed/Abstrac) [DOI] [PubMed] [Google Scholar]
- Matheu V., Back O., Mondoc E., Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of Th1/Th2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003;112:585–592. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting serum glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McGill A.T., Stewart J.M., Lithander F.E. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed M.L., Michos E.D., Post W., Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch. Intern. Med. 2008;168:629–637. doi: 10.1001/archinte.168.15.1629. ([PMC free article] [PubMed]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezza T., Muscogiuri G., Sorice G.P., Prioletta A., Salomone E., Pontecorvi A., Giaccari A. Vitamin D deficiency: a new risk factor for type 2 diabetes. Ann. Nutr. Metab. 2012;61:337–348. doi: 10.1159/000342771. [DOI] [PubMed] [Google Scholar]
- Mirzaei k, Hossein-nezhad A., Hosseinzadeh-Attar M., Najmafshar A., Jafari N., Rahmani M., Larijani B. Relationship between genotype and serum levels of adipokines and bone mineral density in type 2 diabetes mellitus patients. Iran. J. Diabetes Lipid Disord. 2009:77–86. (electronic) [Google Scholar]
- Morrison N.A., Qi J.C., Tokita A., Kelly P.J., Crofts L., Nguyen T.V. Prediction of bone density from Vitamin D receptor. Nature. 1994;367:284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyaya P.N., Acharya A., Chavan Y., Purohit S.S., Mutha A. Metagenomic study of single-nucleotide polymorphisms within candidate genes associated with type 2 diabetes in an Indian population. Genet. Mol. Res. 2010;9:2060–2068. doi: 10.4238/vol9-4gmr883. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Naito M., Miyaki K., Naito T., Zhang L., Hoshi K., Hara A. Association between vitamin D receptor gene haplotypes and chronic periodontitis among Japanese men. Int. J. Med. Sci. 2007;4:216–222. doi: 10.7150/ijms.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo D.T., Sverdlov A.L., McNeil J.J., Horowitz J.D. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am. J. Med. 2010;123:335–341. doi: 10.1016/j.amjmed.2009.09.024. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- O'Kelly J., Hisatake J., Hisatake Y., Bishop J., Norman A., Koeffler H.P. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J. Clin. Investig. 2002;109:1091–1099. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.Y., Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults. Metabolism. 2002;51:356–359. doi: 10.1053/meta.2002.29969. (PubMed Abstract) [DOI] [PubMed] [Google Scholar]
- Ortlepp J.R., Lauscher J., Hoffmann R., Hanrath P., Joost H.G. The VDR gene variant is associated with the prevalence of type 2 diabetes mellitus and coronary artery disease. Diabet. Med. 2001;18:842–845. doi: 10.1046/j.1464-5491.2001.00585.x. [DOI] [PubMed] [Google Scholar]
- Ortlepp J.R., Metrikat J., Albrecht M. The vitamin D receptor gene variant and physical activity predicts fasting glucose levels in healthy young men. Diabet. Med. 2003;20:451–454. doi: 10.1046/j.1464-5491.2003.00971.x. [DOI] [PubMed] [Google Scholar]
- Palomer X., Gonzalez-Clemente J.M., Blanco-Vaca F., Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 2008;10:185–197. doi: 10.1111/j.1463-1326.2007.00710.x. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Parikh S.J., Edelman M., Uwaifo G.I., Freedman R.J., Semega-Janneh M., Reynolds J., Yanovski J.A. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J. Clin. Endocrinol. Metab. 2004;89:1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- Peterson C.A., Heffernan M.E. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J. Inflamm. 2008;5:10. doi: 10.1186/1476-9255-5-10. ([PMC free article] [PubMed] [Cross Ref]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler J., Gerstmayr M., Szepfalusi Z., Urbanek R., Peterlik M., Willheim M. 1a,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr. Res. 2002;52:12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Pickup J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes mellitus. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Pittas A., Joseph N.A., Greenberg A. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- Pittas A.G., Harris S.S., Stark P.C., Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. (PubMed Abstract | Publisher Full Text) [DOI] [PubMed] [Google Scholar]
- Pittas A.G., Lau J., Hu F., Dawson-Hughes B. The role of vitamin d and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas A.G., Sun Q., Manson J.E., Dawson-Hughes B., Hu F.B. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. (PMC free article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch Natielen Jacques, Garcia Vivian Cristina, Gouvea Ferreira Vivolo Sandra Roberta, Martini Lígia Araújo. Relationship between vitamin D receptor gene polymorphisms and the components of metabolic syndrome. Nutr. J. 2013;12:96. doi: 10.1186/1475-2891-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch N.J., Garcia V.C., Sandra R.G., Vívolo F., Martini L.R. Relationship between vitamin D receptor gene polymorphisms and the components of metabolic syndrome. Nutr. J. 2013;12:96. doi: 10.1186/1475-2891-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scragg R., Sowers M., Bell C. Serum 25-hydroxivitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- Solinas G., Vilcu C., Neels J.G., Bandyopadhyay G.K., Luo J.L., Naugler W., Grivennikov S., Wynshaw-Boris A., Scadeng M., Olefsky J.M., Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Starr J.I., Mako M.E., Juhn D., Rubenstein A.H. Measurement of serum pro-insulin–like material: cross reactivity of porcine and human proinsulin. J. Lab. Clin. Med. 1978;91:691–692. [PubMed] [Google Scholar]
- Sung C.C., Liao M.T., Lu K.C., Wu C.C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012;2012:634195. doi: 10.1155/2012/634195. (Published online 2012 Sep 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiishi T., Gysemans C., Bouillon R., Mathieu C. Vitamin D and diabetes. Endocrinol. Metab. Clin. N. Am. 2010;39:419–446. doi: 10.1016/j.ecl.2010.02.013. (PubMed) [DOI] [PubMed] [Google Scholar]
- Teegarden D., Donkin S.S. Vitamin D emerging new roles in insulin sensitivity. Nutr Res. Rev. 2009;22:82–92. doi: 10.1017/S0954422409389301. [DOI] [PubMed] [Google Scholar]
- Thorand B., Zierer A., Huth C., Linseisen J., Roden M., Peters A., Koenig Wand Herder C. Effect of serum 25-hydroxivitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/RORA Augsburg study. Diabetes Care. 2011;34:2320–2322. doi: 10.2337/dc11-0775. ([PMC free article] [PubMed]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y., Lin Y., Zhang Y., Yang J., Zhang Y., Liu H. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med. Genet. 2009;10:15. doi: 10.1186/1471-2350-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder P. Enzymatic determination of glucose. Ann. Clin. Biochem. 1969;6:24–27. [Google Scholar]
- Ursula T., Ulla H., Ulf-Håkan S. Determination of 25-hydroxyvitamin D in serum by HPLC and immunoassay. Clin. Chem. 2003;49(9):1521–1524. doi: 10.1373/49.9.1521. [DOI] [PubMed] [Google Scholar]
- Vilarrasa N., Vendrell J., Maravall J., Elio I., Solano E., San Jose P., García I., Virgili N., Soler J., Gómez J.M. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine. 2010;38:235–242. doi: 10.1007/s12020-010-9379-4. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- Wang C. Role of vitamin D in cardiometabolic diseases. J. Diabetes Res. 2013 doi: 10.1155/2013/243934. (Article ID 243934, 10 pp.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.H., Keisala T., Solakivi T., Minasyan A., Kalueff A.V., Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J. Steroid Biochem. Mol. Biol. 2009;113:222–226. doi: 10.1016/j.jsbmb.2009.01.003. (PubMed/Abstract) [DOI] [PubMed] [Google Scholar]
- Wolden-Kirk H., Overbergh L., Christesen H.T., Brusgaard K., Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Mol. Cell. Endocrinol. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. ([PubMed] [Cross Ref]) [DOI] [PubMed] [Google Scholar]
- World Health Organization Definition and Diagnosis of Diabetes Mellitus and Intermediate. Hyperglycemia. 2006;30(2):263–269. [Google Scholar]
- Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel M.B., Shi H., Greer B., Dirienzo D., Zemel P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–1138. (PubMed Abstract | Publisher Full Text) [PubMed] [Google Scholar]
- Zhang Z., Yuan W., Sun L. 1,25-Dihydroxyvitamin D3 targeting of NF-kB suppresses high glucose induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]