Abstract
In Genetics Out-patient Department of Shanghai Children's Medical Center, we consulted a 3-year-old boy with multiple anomaly syndrome (congenital heart disease, cryptorchidism, congenital deafness, mental retardation, exophthalmos, laryngeal cartilage dysplasia and high arched palate). We ruled out the possibility of multiple deformities caused by genomic imbalances. The patient was then clinically considered to have CHARGE syndrome, an autosomal dominant multi-system disorder involving defects in multiple organs, and CHD7 is the only known gene associated with the syndrome. Sequencing analysis of CHD7 of the proband identified a de novo heterogeneous mutation (c.2916_2917del, p.Gln972HisfsX22), a two-nucleotide deletion causing reading frame shift and resulting in a truncated CHD7 protein. Computational structure analysis suggests that the truncated protein only contains the chromodomains of CHD7, but lacks the SWI2/SNF2-like ATPase/helicase domain and the DNA binding domain, which are indispensable for the proper function of the protein, especially on chromatin remodeling. The patient then received follow up treatment in different clinical departments in a long period. To our best knowledge, this is the first CHARGE syndrome in Chinese patients diagnosed by gene analysis. In summary, the clinical symptoms and the description of treatment in the present case, combined with genetic test and functional prediction of CHD7, are helpful for further understanding and genetic counseling of the CHARGE syndrome.
Keywords: CHARGE syndrome, CHD7, Gene mutation
Highlights
-
•
CHD7 is a member of the chromodomain family.
-
•
CHD7 gene in a Chinese boy clinically diagnosed with CHARGE syndrome was sequenced.
-
•
A new heterozygous, two-base deletion of CHD7 located in exon11 (c.2916_2917del) was identified.
-
•
The truncated CHD7 (Q972X) only maintains the chromodomains but lacks all the other functional domains.
-
•
This is the first CHRAGE syndrome in Chinese patients diagnosed by gene analysis.
Introduction
CHARGE syndrome is an autosomal dominant multi-system disorder involving coloboma, heart defects, choanal atresia, retarded growth and development, genital hypoplasia, ear anomalies and/or deafness (Jongmans et al., 2006). In 1998, Blake et al. established reformative diagnostic criteria for CHARGE syndrome comprising of major and minor criteria. Major criteria include coloboma, choanal atresia, ear anomalies/deafness and cranial nerve dysfunction; while minor criteria include heart defects, genital hypoplasia, growth deficiency, developmental delay, tracheoesophageal fistula, orofacial cleft, and distinctive facial appearance (Blake and Prasad, 2006).
CHD7 gene, located at chromosome 8 (8q12) and starting at 61.59 Mb from the p-arm telomere, has a genomic size of 188 kb and consists of 38 exons. It encodes for the chromodomain helicase DNA binding protein, a member of the chromodomain family. In human neural crest cells, CHD7 forms a protein complex with PBAF (polybromo- and BRG1-associated factor-containing complex) that regulates chromatin structure, gene expression and embryonic development (Bajpai et al., 2010, Hargreaves and Crabtree, 2011). Currently, CHD7 is the only gene known to be associated with the CHARGE syndrome (Lalani et al., 2006); both heterozygous mutations and deletions of CHD7 could result in CHARGE syndrome. Up to date, several types of mutations within the CHD7 coding region have been identified, including nonsense mutations (44%), frame shift causing deletions or insertions (34%), splice sites (11%), missense mutations (8%), larger deletions and duplication (2%), translocations (< 1%) and small in-frame deletions (< 1%) (Janssen et al., 2012).
In the present study, we consulted and followed up a Chinese boy with multiple deformities and clinically diagnosed with CHARGE syndrome. Molecular genetic analysis identified a new frame-shift-causing deletion of CHD7, resulting in a truncated form of CHD7 protein. The clinical symptoms and the description of treatment in the present case, combined with genetic test and functional prediction of CHD7, are helpful for further understanding and genetic counseling of the CHARGE syndrome.
Materials and methods
Clinical information and follow-up
A 3-year-old little boy was the first child born by non-consanguineous Chinese healthy parents with an unremarkable family history, after uncomplicated pregnancy and delivery. After birth, the infant had been suffering from recurrent respiratory tract infections and thus admitted to hospital frequently. Subsequent examinations revealed multiple malformations such as unique facial features, atrial septal defect (ASD), bilateral ear anomalies, and micropenis. Based on the above observations, he was suspected to have CHARGE syndrome. The clinical features, laboratory results and follow-up treatment of the patient were investigated and recorded in detail.
The study was approved by the Ethnics Committee of Shanghai Children's Medical Center, Shanghai Jiao Tong University School of Medicine. Informed consents were obtained from the parents.
Genetic analysis
Genomic DNA was extracted from peripheral blood of the patient and his parents using QIAamp DNA Blood Mini Kit (Qiagen GMBH, Hilden, Germany). Chromosomal Microarray Analysis was applied to detect large structural variants (SVs) using CytoScanHD chip (Affymetrix, Santa Clara, CA).
Then all of the 38 exons and exon-intron boundaries of the CHD7 (GenBank accession number NM_017780.2) were amplified by polymerase chain reaction (PCR) using 76 primers. The primer sequences, PCR mixture components and cycling conditions were available upon request. The amplified products were purified by QIAquick Gel Extraction Kit (Qiagen GMBH, Hilden, Germany) and then sequenced via ABI3730XL sequencer (Applied Biosystems, Foster City, CA).
Structure modeling
The structures of wild type human CHD7 and truncated proteins were simulated by SWISS-MODEL (http://swissmodel.expasy.org/), and the figure for modeled structure was prepared by PyMOL (Hhttp://www.pymol.org/H).
Results
Clinical information and follow-up
Upon the first admission to the hospital, the patient presented unique facial features, congenital heart disease history, severe dysaudia, genital hypoplasia and retarded growth/development. Typical and atypical symptoms were shown in Table 1.
Table 1.
Typical and atypical symptoms of the patient in this case.
| Diagnostic criteria of CHARGE | Symptoms of the patient in this case | |
|---|---|---|
| Major | Ocular coloboma or microphthalmia | + |
| Choanal atresia or stenosis | + | |
| Characteristic external ear anomaly, or middle ear malformations or mixed deafness | + | |
| Cranial nerve dysfunction | + | |
| Minor | Congenital cardiovascular malformations | + |
| Tracheoesophageal defect | + | |
| Genital hypoplasia/delayed pubertal development | + | |
| Cleft lip and/or palate | − | |
| Developmental delay | + | |
| Growth retardation | + | |
| Characteristic face | + | |
| Atypical symptoms | Laryngeal cartilage dysplasia; exophthalmos |
Coloboma
Upon admission, the patient presented with exophthalmos. After a specialist consultation and funduscopic examination, a right-sided iris coloboma was identified, while both visual acuity and visual field were not affected.
Heart defects
Congenital heart disease was diagnosed and treated at the Department of Cardiology in our hospital about 6 months before admission this time. Ultrasound cardiogram showed ASD, bicuspid aortic valve, right aortic arch, right atrial and ventricular enlargement. ASD was closed by an occlusion surgery at twenty months of age.
Choanal atresia
Nasopharyngoscopy showed choanal atresia caused by abnormal soft tissue, with half of the choanae being obstructed by nasopharyngeal adenoids hypertrophy. It is noteworthy that laryngoscope demonstrated tracheal stenosis and laryngeal cartilage dysplasia in this case.
Genital hypoplasia
External genital examination showed micropenis (about 2–3 cm), and ultrasound revealed bilateral inguinal cryptorchidism with right spermatic cord hydrocele.
Ear anomalies
Auditory brainstem response test showed bilateral hearing loss with ABR thresholds > 100 dB. Brainstem auditory evoked potential described bilateral severe BAEP abnormalities, suggesting distal acoustic nerve or cochlear damage. Temporal computed tomography (CT) showed bilateral inner ear dysplasia, including cochlear and vestibular dysplasia, horizontal and superior semicircular canal aplasia, short posterior semicircular canal, and internal auditory canal stenosis. MRI for inner ear further indicated bilateral vestibular cochlear nerve hypoplasia. Hearing loss was significantly improved after a cochlear implant surgery.
Retarded growth and development
The patient cannot sit stably yet when admitted at 3 year old. Language delay and cerebral dysplasia were also observed in this patient. Cranial MRI showed bilateral lateral ventricle and subarachnoid space enlargement, and myelination of the brain white matter was slower than full-term normal infants.
Others
A series of biochemical analysis also helped to exclude metabolic diseases. All of the biochemical results were normal. GS/MS metabolic disorder test was also negative, with no evidence of metabolic diseases.
According to the above clinical observations and laboratorial results, the patient was finally diagnosed as CHARGE syndrome.
Genetic analysis
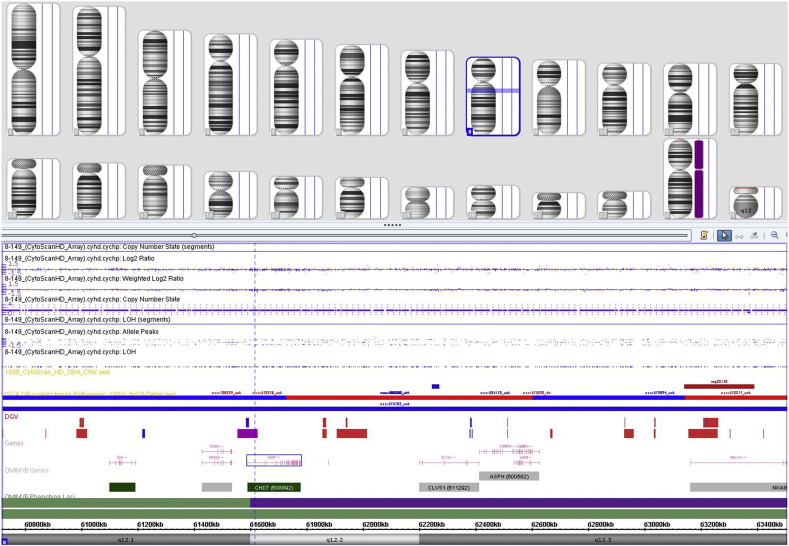
Results of the genomic imbalance analysis were negative in the case (Fig. 1), with no evidence of copy number variation (including deletions and duplications).
Fig. 1.
Results of the microarray analysis.
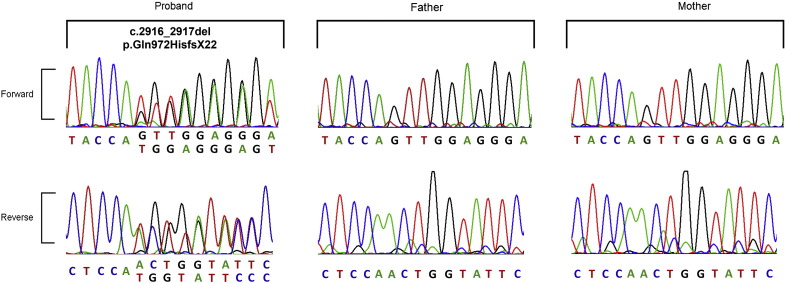
A new heterozygous, two-base deletion of CHD7 located in exon11 was identified in this case (Fig. 2). The c.2916_2917del mutation causes a reading frame shift starting from residue Q972, while the new reading frame ends with a premature stop codon 21 positions downstream (p.Q972HfsX22). Sequence alignment of CHD7 in 8 species shows that the region containing the mutation is extremely conserved, suggesting the indispensability of these residues and importance of CHD7 in vertebrates (Fig. 3).
Fig. 2.
Sequencing result of the patient's CHD7 gene. The chromatogram showed a two-base (GT) deletion in exon 11 of the patient's CHD7 gene. This mutation introduced a frame-shift resulting in a truncated protein with a premature stop codon 21 positions downstream (p.Q972HfsX22).
Fig. 3.
Sequence alignment of CHD7 protein in 8 species. CHD7 sequences used for phylogenetic analysis were obtained from the NCBI conserved domain database.
Structure modeling
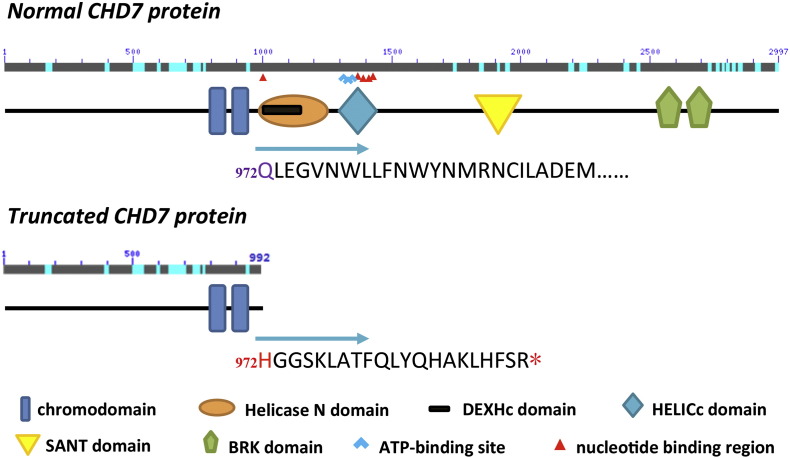
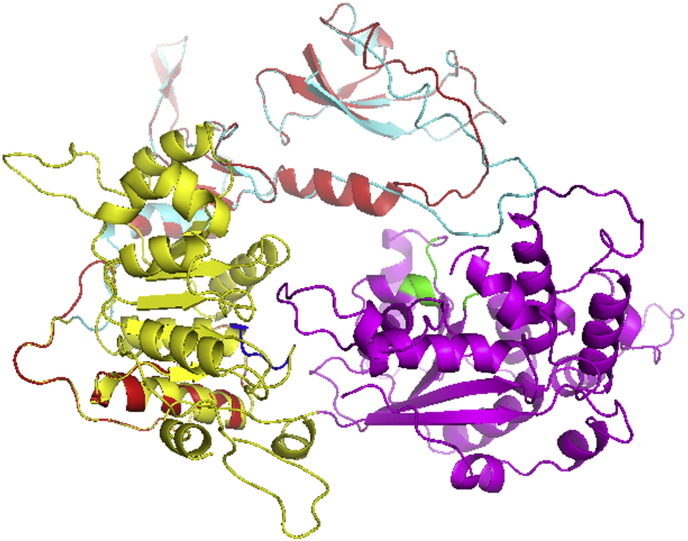
According to the analysis on the primary sequence of CHD7, wild type CHD7 protein is comprised of a pair of chromo (chromatin organization) domains, a helicase domain [ATPase domain, containing DEXDc and Helicase C (HELICc) domains], a SANT (SWI3, ADA2, N-CoR and TFIIIB) domain and a pair of BRK (BRN and KIS) domains (Janssen et al., 2012) (Fig. 4). The c.2916_2917del mutation identified in the patient causes an amino acid change at residue 972 (Q → H), which is located in the helicase domain (containing the ATP-binding region). Furthermore, the consequent frame shift due to the small deletion led to a premature stop codon 21 position downstream Q972 (at residue 993), resulting in a truncated protein with nearly two thirds of the critical functional domains being lost, including the ATP-binding region, the nucleotide binding region, the putative ion binding site, the phosphorylation site, and the BRK domain. The predicted three-dimensional structure of wild type and truncated CHD7 protein (including the chromo- and helicase domains, amino acids from 800aa–1600aa) were depicted in Fig. 5, indicating that the mutant CHD7 induced by c.2916_2917del in the patient only maintains the chromodomains but lacks all the other functional domains.
Fig. 4.
A schematic representation of the wild type and mutant CHD7 protein in the present case. Domains are depicted approximately to scale. Adapted from human reference sequence (NP_060250.2) and (Janssen et al., 2012).
Fig. 5.
The 3D structure model of human CHD7 protein. Wild type CHD7: amino acids 800aa-1600aa; truncated CHD7: amino acids 800aa–992aa. The chromodomains, DEXDc and HELICc are colored cyan, yellow and magenta, respectively. The ATP-binding site and nucleotide binding region are colored blue and green, respectively. The truncated protein is colored red.
Discussion
The reported incidence of CHARGE syndrome is 0.1–1.2/10,000 depending on professional recognition, and most of the cases are sporadic (Blake and Prasad, 2006). Clinical phenotype of CHARGE syndrome is highly variable. Standard diagnostic criteria contain 4 major criteria and 7 minor criteria. Herein, we reported a patient representing both typical and atypical symptoms of CHARGE (Table 1) and clinically diagnosed as CHARGE syndrome. Typical symptoms in this case included choanal atresia, coloboma, characteristic ear anomalies, cardiovascular malformations, genital hypoplasia, growth deficiency and developmental delay, while atypical symptoms included exophthalmos, tracheal stenosis and laryngeal cartilage dysplasia. Targeted treatments were introduced to the patient according to the findings above. Remarkable achievements were observed after accurately treatments: hearing loss was significantly improved after a cochlear implant surgery, and recurrent respiratory tract infection was controlled after an ASD occlusion surgery. The mother was advised to do gene diagnosis during her 2nd pregnancy, which was proved to be useful. No mutation has been identified in the CHD7 gene of the fetus, and after an uncomplicated full term pregnancy, a healthy baby was delivered safely.
The difficulty for clinical diagnosis of CHARGE syndrome is how to differentiate from the 22q deletion syndrome, Kabuki syndrome, Velo-cardio-facial syndrome (VCFS), Cat Eye syndrome, retinoic acid embryopathy, VACTERL association and PAX2 abnormalities, which have many similar behavioral and clinical features with CHARGE. Therefore, gene analysis is the most accurate and definitive method for differential diagnosis (Blake and Prasad, 2006).
The only pathogenic gene involved in CHARGE syndrome reported to date is CHD7. Heterozygous CHD7 mutations existed in more than 90% of the patients with typical CHARGE syndrome based on the clinical diagnostic criteria (Corsten-Janssen et al., 2013). We documented a de novo heterozygous mutation on CHD7 gene (c.2916_2917del) in the present case, which was a novel small deletion causing a frame-shift in CHD7. The novel variant was expected to lead to a truncated protein and considered to be pathogenic because it is very likely to result in haploinsufficiency.
CHD7 belongs to the chromosome helicase DNA binding (CHD) family which evolutionarily conserved in eukaryotes. Studies on the protein structure of CHD7 indicate that it composes of at least four major domains: N-terminal tandem chromodomains, central helicase domain, DNA binding/SANT domain and two C-terminal BRK domains (de la Cruz et al., 2005, Zentner et al., 2010). It was demonstrated that the chromodomain of CHD7 was able to bind methylated H3K4, therefore creating an open chromatin state at promoters of certain genes (Schnetz et al., 2009). The helicase domain is thought to facilitate ATP-dependent translocation of proteins along DNA, and generate force for movement or destabilization of nucleosomes (Cairns, 2007). Daniel et al. showed that DNA-binding domain is required for Saccharomyces cerevisiae Chd1 to bind and remodel nucleosomes (Ryan et al., 2011). The conserved role of DNA-binding domain in CHD proteins suggests the same function of this domain in CHD7. Although the function of BRK domains is currently unknown, it is interesting to note that mutation that introduces a stop codon in the middle of the second BRK domain causes CHARGE syndrome, suggesting the importance of this region (Lalani et al., 2006). Feng et al. reported that CHD7 stimulates the expression of Sox4 and Sox11 genes in neuron stem cells via remodeling their promoters to an open chromatin state, thus regulating stem cell differentiation (Feng et al., 2013). CHD7 loss of function impaired neuronal differentiation and dendritic development of newborn neurons, providing a possible mechanism of the learning and intellectual disability in 75% of the CHARGE patients (Bergman et al., 2011). The truncated protein in this patient only contains chromodomains of CHD7, which is clearly insufficient for protein function. Although chromodomain itself may still recognize methylated H3K4 and preserve the ability to bind modified nucleosome, the activity of chromatin remodeling is abolished (Fig. 5).
To the best of our knowledge, this is the first CHARGE syndrome in Chinese patients diagnosed by gene analysis, in which a de novo truncated CHD7 mutation was identified. Most of the patients with CHARGE syndrome reported to date, which were identified by genetic diagnosis, were from the western countries, and there are also several cases reported in Korea (Cho et al., 2013) and Japan (Aramaki et al., 2006). However, without genetic diagnosis, CHARGE syndrome in China was difficult to diagnose and often considered as an incurable disease. Before our study, only two cases probably suspected as CHARGE syndrome according to the clinical symptoms were reported, but without further verification (Qu et al., 2010). Therefore, the clinical symptoms and pertinent treatment in the present case, combined with genetic analysis and functional prediction of CHD7, fill the gap of genetic diagnosis of CHARGE syndrome in Chinese patients, and suggests that identifying the underlying genetic defects in patients with CHARGE syndrome is helpful for accurate diagnosis, appropriate treatment, and genetic counseling.
Authors' contributions
XM and YGY critically revised the manuscript, contributed to the study design, analysis, and supervised the research. YGY coordinated clinical diagnosis and collected phenotype information. LBL and TTY drafted and critically revised the manuscript, performed the statistical analysis, and contributed to the interpretation of the data. LLW performed the blood collection, DNA extraction, microarray and data analysis. All authors read and approved the final manuscript.
Acknowledgments
We thank the support from the patient and the family. This study is further supported by the “Major Program of Shanghai Committee of Science and Technology” from The Shanghai Municipal Science and Technology Commission (No. 12411950400). And supported by the “Program of Shanghai Committee of Science and Technology” from The Shanghai Municipal Science and Technology Commission (No. 124119a2601); Health Science grant from the social development branch of Pudong New District (PKJ2012-Y47) to YYG.
We thank Dr. Yiping Shen for providing clinical and molecular diagnosis for this patient.
Contributor Information
Xi Mo, Email: xi.mo@shsmu.edu.cn.
Yongguo Yu, Email: yuyongguo@shsmu.edu.cn.
References
- Aramaki M., Udaka T., Kosaki R., Makita Y., Okamoto N., Yoshihashi H., Oki H., Nanao K., Moriyama N., Oku S., Hasegawa T., Takahashi T., Fukushima Y., Kawame H., Kosaki K. Phenotypic spectrum of CHARGE syndrome with CHD7 mutations. J. Pediatr. 2006;148:410–414. doi: 10.1016/j.jpeds.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Bajpai R., Chen D.A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C.P., Zhao Y., Swigut T., Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J.E., Janssen N., Hoefsloot L.H., Jongmans M.C., Hofstra R.M., van Ravenswaaij-Arts C.M. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J. Med. Genet. 2011;48:334–342. doi: 10.1136/jmg.2010.087106. [DOI] [PubMed] [Google Scholar]
- Blake K.D., Prasad C. CHARGE syndrome. Orphanet J. Rare Dis. 2006;1:34. doi: 10.1186/1750-1172-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns B.R. Chromatin remodeling: insights and intrigue from single-molecule studies. Nat. Struct. Mol. Biol. 2007;14:989–996. doi: 10.1038/nsmb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.J., Song M.H., Choi S.Y., Kim J., Lee J., Kim U.K., Bok J., Choi J.Y. Genetic analysis of the CHD7 gene in Korean patients with CHARGE syndrome. Gene. 2013;517:164–168. doi: 10.1016/j.gene.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Corsten-Janssen N., Saitta S.C., Hoefsloot L.H., McDonald-McGinn D.M., Driscoll D.A., Derks R., Dickinson K.A., Kerstjens-Frederikse W.S., Emanuel B.S., Zackai E.H., van Ravenswaaij-Arts C.M. More clinical overlap between 22q11.2 deletion syndrome and CHARGE syndrome than often anticipated. Mol. Syndromol. 2013;4:235–245. doi: 10.1159/000351127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz X., Lois S., Sanchez-Molina S., Martinez-Balbas M.A. Do protein motifs read the histone code? Bioessays. 2005;27:164–175. doi: 10.1002/bies.20176. [DOI] [PubMed] [Google Scholar]
- Feng W., Khan M.A., Bellvis P., Zhu Z., Bernhardt O., Herold-Mende C., Liu H.K. The chromatin remodeler CHD7 regulates adult neurogenesis via activation of SoxC transcription factors. Cell Stem Cell. 2013;13:62–72. doi: 10.1016/j.stem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Hargreaves D.C., Crabtree G.R. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N., Bergman J.E., Swertz M.A., Tranebjaerg L., Lodahl M., Schoots J., Hofstra R.M., van Ravenswaaij-Arts C.M., Hoefsloot L.H. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum. Mutat. 2012;33:1149–1160. doi: 10.1002/humu.22086. [DOI] [PubMed] [Google Scholar]
- Jongmans M.C., Admiraal R.J., van der Donk K.P., Vissers L.E., Baas A.F., Kapusta L., van Hagen J.M., Donnai D., de Ravel T.J., Veltman J.A., Geurts van Kessel A., De Vries B.B., Brunner H.G., Hoefsloot L.H., van Ravenswaaij C.M. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J. Med. Genet. 2006;43:306–314. doi: 10.1136/jmg.2005.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani S.R., Safiullah A.M., Fernbach S.D., Harutyunyan K.G., Thaller C., Peterson L.E., McPherson J.D., Gibbs R.A., White L.D., Hefner M., Davenport S.L., Graham J.M., Bacino C.A., Glass N.L., Towbin J.A., Craigen W.J., Neish S.R., Lin A.E., Belmont J.W. Spectrum of CHD7 mutations in 110 individuals with CHARGE syndrome and genotype–phenotype correlation. Am. J. Hum. Genet. 2006;78:303–314. doi: 10.1086/500273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q., Liu J., Ni D., Zhang Q., Yang D., Wang N., Wu X., Han H. Diagnosis and clinical characteristics of congenital anosmia: case series report. J. Otolaryngol. Head Neck Surg. 2010;39:723–731. [PubMed] [Google Scholar]
- Ryan D.P., Sundaramoorthy R., Martin D., Singh V., Owen-Hughes T. The DNA-binding domain of the Chd1 chromatin-remodelling enzyme contains SANT and SLIDE domains. EMBO J. 2011;30:2596–2609. doi: 10.1038/emboj.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz M.P., Bartels C.F., Shastri K., Balasubramanian D., Zentner G.E., Balaji R., Zhang X., Song L., Wang Z., Laframboise T., Crawford G.E., Scacheri P.C. Genomic distribution of CHD7 on chromatin tracks H3K4 methylation patterns. Genome Res. 2009;19:590–601. doi: 10.1101/gr.086983.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner G.E., Layman W.S., Martin D.M., Scacheri P.C. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am. J. Med. Genet. A. 2010;152A:674–686. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]