Abstract
Background:
Post-operative cerebrospinal fluid (CSF) leak in posterior fossa surgery remains a significant source of morbidity. TissuePatchDural (TPD), a novel impermeable adhesive membrane, was used to reinforce dural closure. A comparison with one of the most commonly used dural sealing devices, DuraSeal, has been made.
Methods:
A retrospective, single-center study was conducted on 161 patients who underwent elective posterior fossa surgery. On surgeon's opinion, when a primary watertight closure was not possible, they received TPD or DuraSeal to reinforce dural closure.
Results:
Out of 161 patients analyzed, 115 were treated with TPD and 46 with DuraSeal. The post-operative leaks related purely to TPD or DuraSeal failure were recognized in 3 (2.6%) and 5 (10.86%) cases, respectively (P = 0.015). The presence of pre- and post-operative risk factors was associated with an increased incidence of CSF leak in both groups. TPD showed a better control in patients without these risk factors (P = 0.08). The incidence of CSF leak in patients who underwent posterior fossa surgery by craniectomy was statistically lower in TPD group compared to DuraSeal group (3.22% vs 17.8%, respectively; P = 0.008)
Conclusions:
TPD seems to be a safe tool for use as an adjunct to standard dural closure in posterior fossa surgery, particularly in patients without pre- or post-oper ative risk factors, in those who did not develop hydrocephalus, and who underwent craniectomy. The CSF leak rate in TPD group was found to be lower or within the range of the more advanced alternative dural closure strategies, including polyethylene glycol (PEG)-based sealant.
Keywords: Cerebrospinal fluid leak, dural sealant, DuraSeal, posterior fossa surgery, TissuePatchDural
INTRODUCTION
Cerebrospinal fluid (CSF) leak still remains a significant source of morbidity in neurosurgery, particularly after posterior fossa surgery. Current treatments aim to promote wound healing by reducing CSF pressure (CSF lumbar drainage or repeated spinal taps) and to prevent infections by administering intravenous antibiotics to the patient. Failure of these treatments potentially requires further surgical intervention. In spite of these treatments, infections, meningitis, encephalitis, and pseudomeningocele formation may complicate the post-operative course and result in permanent damage or delay the beginning of adjuvant therapy in oncologic cases.[13]
Although technological advances in neurosurgical techniques have reduced the occurrence of post-operative CSF leak, the incidence in posterior fossa surgery can be as high as 17%.[8,14,19,20,22,24,33,40,44,48,51,60] Furthermore, the costs related to treating patients affected by this complication have been estimated to be 141% greater than that of patients without a CSF leak.[23]
Various approaches have been taken to overcome this issue by the use of a variety of techniques or products to achieve a watertight dural closure. These have included meticulous primary dural closure, lumbar drainage, the use of autologous grafts such as fat or pericranium, dural replacements, Gelfoam (Pfizer, New York, NY, USA), DuraGen (Integra LifeSciences Corporation, Plainsboro, NJ, USA), and fibrin glue, among others. In recent years, DuraSeal (Covidien, Mansfield, MA, USA), a synthetic absorbable hydrogel containing polyethylene glycol (PEG)-based polymers, has gained in popularity as a dural sealant.
A number of studies have reported a reduction in the incidence of CSF leakage, but only one study considered the incidence of CSF leaks following posterior fossa surgery.[3,10] In this study, Than et al. demonstrated the efficacy of DuraSeal compared to fibrin glue.[59]
TissuePatchDural (TPD) (Tissuemed Ltd, Leeds, UK) is a self-adhesive, absorbable surgical membrane indicated for adjunctive prevention of CSF leak in neurosurgery.
In this retrospective, non-randomized, single-center study, the authors compared the safety and effectiveness of this novel sealant film (TPD) with DuraSeal, a synthetic liquid formulation, in reducing the incidence of both incisional CSF leak and pseudomeningocele following posterior fossa surgery.
MATERIALS AND METHODS
Patient population
This study is a retrospective, single-center clinical investigation conducted on 545 patients who underwent elective posterior fossa surgery in a 30 month period (January 2009-June 2011), with a 6-month follow-up period for each patient. Informed consent was obtained from all patients. Pre-operative admission criteria included adult patients undergoing clean elective surgical procedures. Previous radiotherapy (RT), previous surgery, and chronic corticosteroid therapy were not considered the exclusion criteria. Intra-operative inclusion criteria were a wide cisternal and/or ventricular opening and failure to obtain a watertight primary closure with a standard dural microsuture (leakage evidenced by subdural irrigation of the surgical cavity prior to completion of suturing, followed by Valsalva maneuver and/or positive pressure ventilation to test the completed primary suture closure). On surgeon's opinion, patients received either TPD or DuraSeal to reinforce dural closure. In the period between January 2009 and June 2011, 161 consecutive patients treated at the authors’ institution and who met the inclusion criteria described above were selected. Of these 161 patients (108 females and 53 males; mean age 45 years, range 8-78 years), 115 were treated with TPD and 46 with DuraSeal.
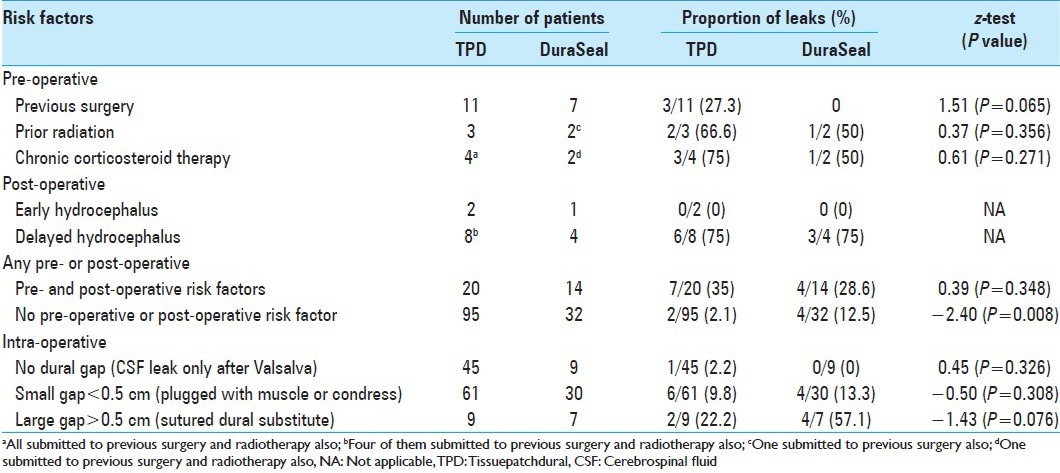
For analysis, patients were stratified according to the presence of: (1) pre-operative risk factors, including (a) chronic corticosteroid therapy, b) previous surgery, and (c) radiation therapy; (2) intra-operative risk factors, including (a) no dural gap (CSF leakage on Valsalva maneuver and/or positive pressure ventilation), (b) small gap (<5 mm) plugged with muscle or collagen, (c) need for duroplasty); and (3) post-operative risks factors, including a) early and (b) late post-operative hydrocephalus [Table 1].
Table 1.
Pre-, intra-, and post-operative risk factors in the 161 patients submitted to surgical procedures for infratentorial pathologies
All patients underwent daily post-operative wound examination in order to assess the occurrence of subgaleal CSF collection, incisional CSF leak, any inflammatory reaction, or wound infection. To monitor post-operative complications, a brain CT or MR scan was performed within 48 h in all patients who underwent lesion resection and occipito-axial decompression for Chiari malformation. Patients with neurovascular conflicts who underwent microvascular decompression were only scanned if they displayed clinical symptoms. In case of leak or subcutaneous liquid collections, a biochemical testing for β-2 transferrin was performed to confirm CSF presence. The wound was then examined at 2 weeks and 6 months after surgery. In addition, in the period from 2 weeks to 2 months, patients were instructed to contact the hospital in case of wound leak and/or subgaleal collection. Demographic data and operative and outcome data were collected and analyzed.
In this study, the term “CSF leak” was used for both pseudomeningocele and incisional CSF leaks. The etiology of these complications is leakage of CSF from the subarachnoid space into the extradural compartment. When the skin incision has adequately healed, a pseudomeningocele may develop; otherwise, an incisional CSF leak may occur. Both situations should be considered as a failure of the dural sealant to prevent CSF from leaking to the extradural compartment.[59]
TissuePatchDural
TPD [Figure 1] is a sterile, synthetic, self-adhesive, absorbable surgical sealant and barrier. It is a thin transparent film available in four different sizes ranging from 25 × 50 mm to 100 × 100 mm. It is a multilayered device comprising alternate layers of poly (lactide-co-glycolide) and a proprietary adhesive TissueBond™. Poly (lactide-co-glycolide) is a resorbable membrane that provides reliable strength for temporary wound support. TPD is not a dural replacement/substitute. According to data provided by the manufacturer (Tissuemed Ltd, Leeds, UK) see the product instructions for use), the material achieves its adhesive properties by virtue of the initial tack provided by poly (acrylic acid) and poly (vinyl pyrrolidone) functional groups and longer term adhesion via nucleophilic substitution reaction between N-hydroxysuccinimide (NHS) and amines. TPD remains in position while it slowly degrades until substantially reabsorbed facilitating tissue in-growth and wound healing.
Figure 1.
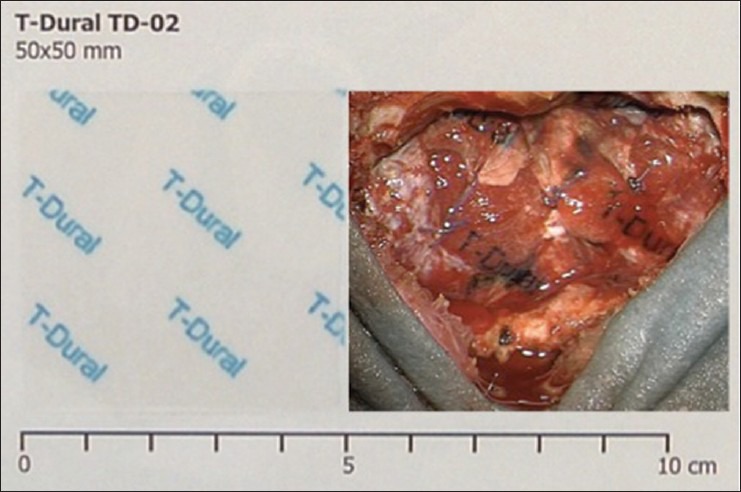
TissuePatchDural film (50 × 50 × 0.04 mm) before (left side) and after activation and adhesion to the sutured dural surface in infratentorial craniotomy (right side)
DuraSeal
DuraSeal is a synthetic absorbable dural sealant, which can be used to support primary dural closure. As described in the manufacturer's insert, DuraSeal is an US Food and Drug Administration (FDA) approved product that is indicated as an adjunct to sutured dural repair during cranial surgery to provide a watertight closure. More recently, it has received FDA approval for use in spinal surgery. It contains PEG, a non-toxic and biocompatible polymer. When water-soluble functionalized PEG is mixed with trilysine (a small molecule amine with reactive linkages), the solutions combine to form the sealant gel that can be sprayed or layered onto the site of dural repair. The cross-linking of PEG and trilysine molecules creates a 3-dimensional hydrogel structure that gradually hydrolyzes (water gradually degrades the cross-linked bonds in a uniform fashion just like absorbable sutures). According to data provided by the manufacturer, it is cleared from the site in 4-8 weeks, which is enough time to allow healing.
Directions and precautions include its avoidance with other hemostatic agents or sealants and the requirement to achieve adequate hemostasis before its application. The DuraSeal manufacturer's insert also includes “contraindications, warnings, and exclusion criteria” concerning its use. One major warning states, “Do not apply DuraSeal hydrogel to confined bony structures where nerves are present since neural compression may result due to hydrogel swelling. The hydrogel may swell up to 50% of its size in any dimension.” Contraindications to its use include a history of allergy, penetration of an air sinus, renal/hepatic/immune dysfunction, head trauma, and infection; it is also to be avoided with hydrocephalus, a ventricular drain, or lumbar drain.
Technique
TPD or DuraSeal was used after standard sutured closure of the dura mater, when subdural irrigation and Valsalva maneuver confirmed the presence of a visible CSF leak. When air-filled cavities within cranial bones were opened, e.g. mastoid air cells, muscle or wax was used as a sealant. Small dural gaps (<5 mm in diameter), when present, were plugged with muscle (when available) or collagen sheet (Condress®; Abiogen Pharma S.p.A., Ospedaletto (Pi), Italy) before the application of the TPD or DuraSeal, in order to create a barrier to CSF leak and to avoid the adhesion of the sealant to the cerebral tissue. In 16 cases, a large defect in the dura mater was present and required reconstruction with various dural substitutes (Duraform™, Codman and Shurtleff, Inc., Berkshire UK; DuraGen®, Integra LifeSciences Corporation; Tutopatch®, Tutogen Medical GmbH, Neunkirchen, Germany). Care was taken to ensure the dural surface was as dry as possible, with excess blood or fluid removed to avoid premature activation and ensure optimal attachment of both products to the surface of the tissue. When the TPD was applied, wet cottonoids/swabs were used to soften the film and increase conformability. Gentle digital pressure for at least 60 s assisted the TPD to adhere to the tissue surface by virtue of the bioadhesive properties of TissueBond™. Following preparation of the DuraSeal kit (involving hydration of component powder by mixing vials), the reconstituted DuraSeal sealant was applied to the sutured dura via a bespoke syringe provided by the manufacturer.
Following sealant application, patients were again assessed for intra-operative CSF leakage through the sealed durotomy with a second Valsalva maneuver for 10 s. An intra-operative watertight closure was obtained in all patients who received either sealant. The wounds were closed in layers. A subgaleal or subfascial drain was inserted as necessary and prophylactic antibiotic therapy was administered during and after surgery in accordance with standard of care.
Statistical analysis
Statistical analysis was undertaken using Prism 4 version 4.0a (GraphPad Software, Inc., San Diego, CA, USA). Durotomy size, the requirement for duroplasty or plugging of dural gaps with muscle or collagen, chronic corticosteroid therapy, previous surgery, radiation therapy, and post-operative hydrocephalus were all evaluated as variables that had the potential to influence the rate of CSF leak.
The two-proportion z-test was used to assess the difference in proportion of patients developing CSF leak between those in whom TPD or DuraSeal was used. Statistical significance was set at one-tailed P < 0.05 level as our hypothesis was that the proportion of patients developing CSF leak should be lower in those patients in whom TPD was used. The following groups were tested: Pre- and post-operative risk factors, intra-operative risk factors, presence of hydrocephalus, and absence of hydrocephalus. Separate analyses were also carried out in patients who underwent different surgical approaches: Proportion of patients with CSF leak was tested in the subgroup of patients that underwent craniotomy or craniectomy. A further separate analysis was also carried out in patients with or without a dural gap after primary closure: Proportion of patients with CSF leak was tested in the subgroup of patients in which TPD or DuraSeal was applied. Finally, we compared the total CSF leak proportion between patients undergoing craniotomy or craniectomy, irrespective of the use of TPD or DuraSeal; in this case, significance was set at two-tailed P < 0.05 level as we had no specific hypothesis on the direction of proportions to test.
RESULTS
TissuePatchDural
Overall, TPD was used in 115 posterior fossa surgeries. The mean follow-up was 8.4 months (range 4-34 months). CSF leak was detected in 9 (0 incisional leaks and 9 pseudomeningoceles) out of 115 cases (7.8%). CSF leak presented as rhinorrhea in one patient and as subgaleal CSF collection in eight. Table 1 provides baseline patient data including pre-operative risk factors (chronic corticosteroid therapy, previous surgery, radiation therapy), intra-operative data (durotomy size, requirement for duroplasty or for plugging dural gaps with muscle or collagen), and post-operative risk factors (early or delayed hydrocephalus). A significant difference in the proportions was found only for patients who had no pre- or post-operative risk factors: Those in the TPD group had lower incidence (2.1%) of CSF leak than those in the DuraSeal group (12.5%). In general [Table 2], lower incidence of CSF leak was observed in patients treated with TPD (7.8%) than in those treated with DuraSeal (17.4%).
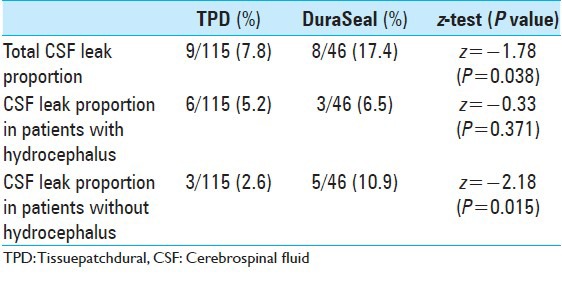
Table 2.
Two-proportion z-test to assess differences in CSF leak incidence in TPD and DuraSeal total groups, and in patients with or without hydrocephalus
Of the nine patients with a CSF leak, six (5.21%) presented with associated post-operative hydrocephalus that was treated using a ventriculo-peritoneal shunt (n = 4) or with endoscopic third ventriculostomy (n = 2). We, therefore, concluded that post-operative leaks related purely to TPD failure were recognized in 3 out of 115 cases (2.6%). Of these three patients, one patient with a rhinoliquorrhea after a retrosigmoid approach for microvascular decompression required surgical revision, while the other two patients healed with conservative treatment (CSF lumbar drain). There was no significant (P < 0.05) statistical difference in the incidence of CSF leaks between the three risk factor groups (pre-, intra-, and post-operative).
Furthermore, in one patient treated with TPD (0.87%), where a dural gap was not visible at the end of dura suturing, a CSF leak was evident in the post-operative period. Also, when a definite/visible dural gap was present, despite the use of muscle, condress, or a dural substitute, the incidence of post-operative CSF leakage was higher (gap dimension < 0.5 cm = 9.8%; gap > 0.5 cm = 22.2%) [Table 1]. In one patient with no leak, a wound infection was observed and required revision surgery. No correlation with the use of TPD was found. No device-related adverse effects were observed.
DuraSeal
DuraSeal was used in 46 posterior fossa surgeries. The mean follow-up was 8.1 months (range 4-34 months). CSF leak was detected in 8 out of 46 cases (17.4%) (2 incisional leaks and 6 pseudomeningoceles) [Table 2]. CSF leak presented consistently as a subgaleal CSF collection. Table 1 provides baseline patient data including pre-operative, intra-operative, and post-operative risk factors.
Out of eight patients with a CSF leak, three (6.52%) presented with associated post-operative hydrocephalus treated with a ventriculo-peritoneal shunt. Therefore, we concluded the post-operative leaks related purely to DuraSeal failure were recognized in 5 out of 46 cases (10.86%). Of these five patients, three were treated conservatively (lumbar drain); the other two patients required further surgery due to incisional CSF leaks after microvascular decompression and atlo-axial decompression for Arnold–Chiari type II disease. No significant difference in CSF leak rate could be found in the three risk factor groups.
Of these eight patients, one patient received RT and chronic corticosteroid therapy. No patients with a CSF leak had undergone prior surgery [Table 1]. Furthermore, no patient without a gap at the dural closure presented a CSF leak. As observed in the TPD group, when a dural gap was present, the incidence of CSF leak was higher (gap dimension < 0.5 cm = 13.3%; gap > 0.5 cm = 57.1%) despite the use of muscle, condress, or a dural substitute [Table 1].
No further DuraSeal-related adverse effects were observed.
Feasibility of TPD application
The fragility of TPD means that careful handling of the film is required in certain applications. TPD can be cut to the required shape and size. It can also be applied to confined bone structures without compressive effects. The dural surface requires some preparation before TPD application, to ensure the site is free from excess blood and fluid. Delicate digital pressure for 60-120 s allows TPD to adhere properly. During the Valsalva maneuver, the integrity of the seal is confirmed under the microscope by checking that the dura is pulsating without fluid leakage. Furthermore, hemostasis is rechecked as there is a risk of dural bone detachment if excessive pressure is applied.
The film also adhered to bone surfaces and often oversized TPD was used in order to bridge over the bone edges.
Feasibility of DuraSeal application
The application of Duraseal was found to be easier than that of TPD. It was delivered as a liquid via a syringe system and onto the sutured dura. The swelling of this hydrogel was taken into account during application, as it is reported to cause compression of neural structures under the dura mater. Typically this mass effect could be observed on post-operative MRI scans, although in this series, its presence did not result in any clinical symptoms.
Craniectomy vs Craniotomy
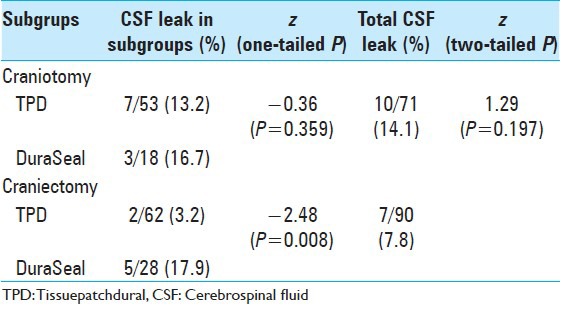
Table 3 reports the z-test results for patients who underwent craniectomy versus craniotomy. There was no statistically significant difference (P = 0.197) in the incidence of CSF leaks in patients who underwent craniectomy compared to craniotomy (7.8% and 14.1%, respectively). Considering the incidence of CSF leak in the craniectomy group, a lower leak rate was observed when TPD was used (3.22% vs 17.8%, respectively; P = 0.008). Parallel to this, no differences were observed in the subgroup of patients that underwent craniotomy (P = 0.359).
Table 3.
Two-proportion z-test to assess differences in CSF leak incidence in patients submitted to craniotomy or craniectomy
Comparison between TPD and DuraSeal groups and analysis of specific risk factors
CSF leak rate was significantly lower (P = 0.038) in patients treated with TPD (7.8%) than in those treated with DuraSeal (17.4%), specifically in those without hydrocephalus, where the z-test was strong (z = −2.18; P = 0.015)
The presence of pre-operative (chronic corticosteroid therapy, previous surgery, and radiation therapy) or post-operative (early and late hydrocephalus) risk factors was associated with a higher incidence of CSF leakage. The difference in proportion was significant only in the TDP group: 35% of patients with pre- or post-operative risk factors developed CSF leak versus 2.1% of those without risk factors (z = 4.98; P < 0.001). In the DuraSeal group, the proportions were 28.6% and 12.5%, respectively (z = 1.32; P = 0.187).
In the TPD group, 11 patients had previous surgery, 3 had prior RT, and 4 received chronic corticosteroid therapy. In addition, two patients presented with early hydrocephalus and eight with late hydrocephalus (four of this cohort had previous surgery and RT). In summary, a total of 20 patients presented with pre- and/or post-operative risk factors. Seven of these developed a CSF leak (35%). Of the remaining 95 cases without pre- and/or post-operative risk factors (95), only 2 patients developed a CSF leak (2.1%) [Table 1].
In the DuraSeal group, seven patients had previous surgery, two had received RT (one of them had been also submitted to previous surgery), and two were under chronic corticosteroid therapy (one of these patients had been also submitted to previous surgery and RT). In addition, one patient presented with early hydrocephalus and four with late hydrocephalus. Therefore, a total of 14 patients presented with pre- and/or post-operative risk factors. Four of these developed a CSF leak (28.6%). Of the remaining 32 cases without pre- and/or post-operative risk factors, 4 patients developed a CSF leak (12.5%) [Table 1].
On evaluating the influence of pre and post-operative risk factors between patients treated with TPD and DuraSeal, we noticed a similar incidence of leakage (35% vs 28.5%, respectively; P = 0.348). In the absence of pre- and post-operative risk factors, the incidence of CSF leaks in the TPD group was statistically significantly lower (P = 0.008) compared to that in DuraSeal group (2.1% and 12.5%, respectively). Excluding patients with hydrocephalus, the incidence of CSF leak was significantly lower in the TPD group than in the DuraSeal group (2.6% and 10.86%, respectively; P = 0.015) [Table 2].
No statistical differences were found between the two groups with regard to pseudomeningocele, presence of hydrocephalus, or presence of rhinoliquorrea [Table 2].
Considering the size of dural gap (CSF leakage only on Valsalva maneuver, small gap <5 mm plugged with muscle or collagen, large gap >5 mm requiring duroplasty), the absence of a clear dural gap following standard microsuture was associated with prevention of CSF leakage after TPD (z = −1.79; P = 0.036), but not in the subgroup of patients in which DuraSeal was applied (z = −1.53; P = 0.063) [Table 1].
DISCUSSION
CSF leak after posterior fossa surgery can occur in 2-17% of cases and remains an unsolved problem since the time of Cushing.[8,12,13,14,19,20,22,24,33,40,44,48,51,60] This high variability depends on numerous factors such as surgical approach and location, general and local conditions (size and location of dural resection, previous radiation therapy, immunodepression, corticosteroid therapy, uncontrolled diabetes, renal or hepatic dysfunction, etc.), surgical technique, and CSF leak diagnostic methods (radiologic or clinical diagnosis).[3,4,6,9,11,14,16,18,23,30,31,35,39,41,43,50,54,57,63] CSF leak remains a potentially life-threatening complication due to the risk of meningoencephalitis. Interventions to treat CSF leaks include repeated surgery, insertion of lumbar drain, and administration of antibiotics to address non-healing wounds. All these factors can result in prolonged hospitalization and the cost of CSF leakage has been estimated to be 141% greater than that for uncomplicated cases.[23] Our study showed, consistent with our hypothesis, that the use of TPD was associated with a lower incidence of CSF leak compared to that of DuraSeal in this cohort of patients. Moreover, the use of TPD resulted in a lower probability of developing CSF leak in patients without pre- or post-operative risk factors, those who did not develop hydrocephalus, and those who underwent craniectomy.
Since the early days of neurosurgery, watertight dural closure has been advocated in order to avoid CSF leakage.[21] Although recently it has been claimed that watertight dural closure is not mandatory in supratentorial procedures, direct watertight suturing of the dural defect has been and is generally attempted in every neurosurgical procedure.[2,15]
In the elderly, the dura can be particularly fragile and adherent to the bone. Furthermore, when opened, it tends to shrink due to dehydration and as a consequence of using bipolar coagulation. The biochemical features of dura mater make it an elastic membrane where needle piercing creates pinholes. To aid dural sealing under problematic conditions where watertight closure is desirable (but difficult to achieve), different materials have been used alone or in combination [Table 4].[5,7,10,25,28,46,55,59,61,66,67,68]
Table 4.
Comparison of studies with different types of dural sealants
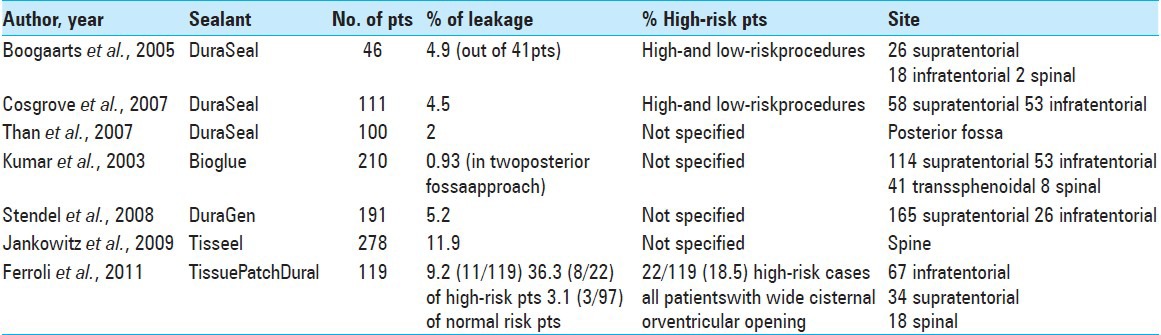
The first duraplasty was performed in 1895 by Abbe, who used rubber sheeting for defect closure.[1] Since then, the search for the optimal closure technique has continued and a variety of materials have been proposed, including autografts (fascia, pericranium, fat, and muscle), allografts (such as cadaveric dura), xenografts [processed whole tissues or highly engineered collagen matrices such as pericardium or DuraGen (Integra)], and synthetic grafts [such as polytetrafluoroethylene Gore and Associates Inc., Newark, DE, USA) and polyester urethane (Neuropatch; B. Braun Melsungen AG, Germany)].[6,14,26,27,29,34,39,42,45,49,56,57,58,61,64,66] Clinical use has identified various negative effects; for some, the preparation and watertight suturing is time consuming, for others inflammatory and foreign body reaction are common; allografts and autografts can be compromised by disease and are therefore not always available; synthetic dural substitutes are associated with a high percentage of infections and a still high percentage of failure.[32,36,47,58,62,65,66]
In addition to grafts, other materials may be used to augment dural closure [Table 4]. Fibrin sealants including Tisseel (Baxter, Deerfield, IL, USA) have been commercially available since the 1990s. They consist of human-derived fibrinogen and thrombin and are classified as thromobogenic hemostats designed to treat low level (not severe) of brisk arterial or venous bleeding.[52,53,62] The disadvantages of fibrin sealant include time-consuming preparation. Furthermore, there have been reports that fibrin sealants can precipitate acute immune responses; chronically, they are associated with adhesion formation and infection.[23]
In recent years, DuraSeal (Covidien), a synthetic absorbable hydrogel containing PEG, has gained popularity. The studies performed have reported a decrease in the incidence of CSF leak compared with that of traditional dural closure techniques [Table 4].[5,10,59,67] In a prospective study, Cosgrove evaluated the safety and efficacy of DuraSeal in patients undergoing elective cranial surgery with documented CSF leakage after sutured dural repair.[10] The authors reported a safe and effective watertight closure when the DuraSeal was used as an adjunct to sutured dural repair. The post-operative incisional CSF leak rate was 1.8%, but in excess of 50% of procedures were supratentorial.
The report by Than et al. specifically considered the efficacy of DuraSeal in a series of patients undergoing posterior fossa surgery; in this study, they prospectively collected the data of 100 patients undergoing posterior fossa surgery with this PEG hydrogel to augment the dural closure.[59] These results were compared with a retrospective cohort of 100 patients treated with fibrin glue. The incidence of incisional CSF leak was 2% in the DuraSeal group and 10% in the fibrin glue group (P = 0.03). If one combines the number of patients who had a pseudomeningocele (8 in the DuraSeal group and 5 in the fibrin sealant group; P = 0.4) with those who had an incisional CSF leak (2 and 10 for DuraSeal and fibrin sealant groups, respectively; P = 0.03), there is no difference between the two groups (10 and 15%, respectively; P > 0.5). As highlighted by Steinbok in his reported response to Than's publication, the higher incidence of incisional CSF leaks in the fibrin glue group may be related to the closure type of the skin or of the subcutaneous tissues, and not to the ability of DuraSeal to provide a leak-proof seal of the dura.[59]
In our study, we were not be able to find any statistical difference between TPD and DuraSeal, including analysis of both incisional CSF leaks and pseudomeningocele together or alone.
Boogaarts et al. prospectively treated 46 patients with DuraSeal in combination with autologous materials.[5] Over a 3-month follow-up period, they reported one CSF leak following a supratentorial craniotomy and one pseudomeningocele after posterior fossa surgery. There were no infections and no other adverse events related to the hydrogel. In this series, the authors excluded patients affected by hydrocephalus and patients who had a neurosurgical procedure within the previous 12 months, as well as RT or chemotherapy. Furthermore, out of 46 patients, only 18 (39%) patients underwent posterior fossa surgery, with an incidence of CSF leak of 5.5%.
In our previous report (Ferroli et al.), we analyzed the influence of pre-operative risk factors (chronic corticosteroid therapy, previous surgery, and radiation therapy), intra-operative risk factors (size of dural gap), and post-operative risks factors (early post-operative hydrocephalus) on the appearance of CSF leak.[17] We concluded that the presence of pre-operative and post-operative risk factors was one of the factors negatively influencing the ability to achieve watertight closure. In fact, of the 22 patients in whom pre-and/or post-operative risk factors were present, 8 developed a CSF leak (36.3%). Conversely, in the remaining cases without pre- and/or intra-operative risk factors, only three patients (3.1%) developed a CSF leak. In the present series, we observed that in the absence of pre- and post-operative risk factors, TPD had a better protective effect on the CSF leak development. The small size of the two samples and the difference in the sample sizes might explain the lack of statistically significant difference in CSF development in the presence of pre- and post-operative risk factors.
Considering the impact of the risk factors in our previous report, we have excluded patients with post-operative hydrocephalus from the analysis. With this exclusion, the incidence of CSF leak (incisional and pseudomeningocele together) was significantly lower in the patients treated with TPD compared to those treated with DuraSeal (2.6% vs 10.9%, respectively; P = 0.015) [Table 1].
In 2002, Gnanalingham et al. analyzed the difference between the complication rates in children who underwent posterior fossa surgery by craniotomy or craniectomy.[22] They reported that craniectomy was associated with an increased incidence of post-operative CSF leak (odds ratio 10.8, 95% CI 1.3–90.6; P = 0.03). We were not be able to find the same result (P = 0.2) [Table 3]. Interestingly, in the group of patients who underwent craniectomy, the incidence of CSF leak was significantly lower in patients treated with TPD compared to that in whom DuraSeal was used (3.22% vs 17.85%, respectively; P = 0.03) [Table 3], showing a protective effect of TPD.
There are clearly some limitations in this study. The sample sizes are small and not matched, and this has an impact on the outcome of the statistical analysis. Furthermore, it is a retrospective evaluation of patients submitted to different procedures by different surgeons in a limited period of time. Despite these limitations, the safety profile of the product is good and no adverse reactions, directly related to TPD, were evident throughout the follow-up period. The ability of TPD to achieve watertight closure following use in a population presenting a high-risk of CSF leakage is promising. Its non-inferiority to DuraSeal, a widely used and FDA-approved product, merits further prospective, randomized, multicenter investigations.
CONCLUSIONS
TPD seems to be safe when used as an adjunct to standard dural closure in posterior fossa surgery, in particular in patients without pre- or post-operative risk factors, those who did not develop hydrocephalus, and in those who underwent craniectomy. With the intrinsic limitation of this retrospective study, the rate of CSF leak in high-risk posterior fossa surgery was found to be within the range of the more advanced alternative dural closure strategies and lower, albeit not statistically significant, than in patients treated with a commonly used PEG-based liquid sealant.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/171/146154
Contributor Information
Marco Schiariti, Email: schiariti.m@istituto-besta.it.
Francesco Acerbi, Email: acerbi.f@istituto-besta.it.
Morgan Broggi, Email: morgan.broggi@istituto-besta.it.
Giovanni Tringali, Email: giovanni.tringali@istituto-besta.it.
Alberto Raggi, Email: gbroggi@gmail.com.
Giovanni Broggi, Email: paolo.ferroli@istituto-besta.it.
Paolo Ferroli, Email: alberto.raggi@istituto-besta.it.
REFERENCES
- 1.Abbe R. Rubber tissue for meningeal adhesions. Trans Am Surg Assoc. 1895;13:490–1. [Google Scholar]
- 2.Barth M, Tuettenberg J, Thomé C, Weiss C, Vajkoczy P, Schmiedek P, et al. Is it necessary? A prospective randomized trial in patients with supratentorial craniotomies. Neurosurgery. 2008;63(4 Suppl 2):S352–8. doi: 10.1227/01.NEU.0000310696.52302.99. [DOI] [PubMed] [Google Scholar]
- 3.Berjano R, Vinas FC, Dujovny M. A review of dural substitutes used in neurosurgery. Crit Rev Neurosurg. 1999;9:217–22. doi: 10.1007/s003290050136. [DOI] [PubMed] [Google Scholar]
- 4.Biroli F, Fusco M, Bani GG, Signorelli A, Esposito F, de Divitiis O, et al. Novel equine collagen-only dural substitute. Neurosurgery. 2008;62(3 Suppl 1):S273–4. doi: 10.1227/01.neu.0000317404.31336.69. [DOI] [PubMed] [Google Scholar]
- 5.Boogaarts JD, Grotenhuis JA, Bartels RH, Beems T. Use of a novel absorbable hydrogel for augmentation of dural repair: Results of a preliminary clinical study. Neurosurgery. 2005;57(1 Suppl):S146–51. doi: 10.1227/01.neu.0000164384.05351.59. [DOI] [PubMed] [Google Scholar]
- 6.Boop FA, Chadduck WM. Silastic duraplasty in pediatric patients. Neurosurgery. 1991;29:785–8. doi: 10.1097/00006123-199111000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Boudreaux B, Zins JE. Treatment of cerebrospinal fluid leaks in high-risk patients. J Craniofac Surg. 2009;20:743–7. doi: 10.1097/scs.0b013e3181a2efea. [DOI] [PubMed] [Google Scholar]
- 8.Bryce GE, Nedzelski JM, Rowed DW, Rappaport JM. Cerebrospinal fluid leaks and meningitis in acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1991;104:81–7. doi: 10.1177/019459989110400115. [DOI] [PubMed] [Google Scholar]
- 9.Caroli E, Rocchi G, Salvati M, Delfini R. Duraplasty: Our current experience. Surg Neurol. 2004;61:55–9. doi: 10.1016/s0090-3019(03)00524-x. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove GR, Delashaw JB, Grotenhuis JA, Tew JM, van Loveren H, Spetzler RF, et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg. 2007;106:52–8. doi: 10.3171/jns.2007.106.1.52. [DOI] [PubMed] [Google Scholar]
- 11.Costantino PD, Wolpoe ME, Govindaraj S, Chaplin JM, Sen C, Cohen M, et al. Human dural replacement with acellular dermis: Clinical results and a review of the literature. Head Neck. 2000;22:765–71. doi: 10.1002/1097-0347(200012)22:8<765::aid-hed4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Cushing H. Experiences with the cerebellar astrocytomas: A critical review of seventy six cases. Surg Gynecol Obstet. 1931;52:129–204. [Google Scholar]
- 13.Cushing H. Surgery of the head. In: Keen WW, editor. Surgery, Its Principles and Practice. Vol. 3. Philadelphia: WB Saunders; 1908. pp. 17–276. [Google Scholar]
- 14.Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 2006;104(1 Suppl):S16–20. doi: 10.3171/ped.2006.104.1.16. [DOI] [PubMed] [Google Scholar]
- 15.Della Puppa A, Rossetto M, Scienza R. Use of a new absorbable sealing film for preventing postoperative cerebrospinal fluid leaks: Remarks on a new approach. Br J Neurosurg. 2010;24:609–11. doi: 10.3109/02688697.2010.500413. [DOI] [PubMed] [Google Scholar]
- 16.Djordjevic Z, Milosavlijevic B. Postoperative cerebrospinal fluid fistulas and their treatment. J Neurosurg Sci. 1974;18:109–11. [PubMed] [Google Scholar]
- 17.Ferroli P, Acerbi F, Broggi M, Schiariti M, Albanese E, Tringali G, et al. A novel impermeable adhesive membrane to reinforce dural closure: A preliminary retrospective study on 119 consecutive high-risk patients. World Neurosurg. 2013;79:551–7. doi: 10.1016/j.wneu.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Filippi R, Schwarz M, Voth D, Reisch R, Grunert P, Perneczky A. Bovine pericardium for duraplasty: Clinical results in 32 patients. Neurosurg Rev. 2001;24:103–7. doi: 10.1007/pl00012392. [DOI] [PubMed] [Google Scholar]
- 19.Fishman AJ, Hoffman RA, Roland JT, Jr, Lebowitz RA, Cohen NL. Cerebrospinal fluid drainage in the management of CSF leak following acoustic neuroma surgery. Laryngoscope. 1996;106:1002–4. doi: 10.1097/00005537-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Fishman AJ, Marrinan MS, Golfinos JG, Cohen NL, Roland JT., Jr Prevention and management of cerebrospinal fluid leak following vestibular schwanoma surgery. Laryngoscope. 2004;114:501–5. doi: 10.1097/00005537-200403000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, et al. Outcomes following purely endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas. J Neurosurg. 2008;109:6–16. doi: 10.3171/JNS/2008/109/7/0006. [DOI] [PubMed] [Google Scholar]
- 22.Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. MRI study of the natural history and risk factors for pseudomeningocele formation following postfossa surgery in children. Br J Neurosurg. 2003;17:530–6. doi: 10.1080/02688690310001627777. [DOI] [PubMed] [Google Scholar]
- 23.Grotenhuis JA. Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol. 2005;64:490–4. doi: 10.1016/j.surneu.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman RA. Cerebrospinal fluid leak following acoustic neuroma removal. Laryngoscope. 1994;104:40–58. doi: 10.1288/00005537-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Jankowitz BT, Atteberry DS, Gerszten PC, Karausky P, Cheng BC, Faugh R, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. 2009;18:1169–74. doi: 10.1007/s00586-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline DG. Dural replacement with resorbable collagen. Arch Surg. 1965;91:924–9. doi: 10.1001/archsurg.1965.01320180058014. [DOI] [PubMed] [Google Scholar]
- 27.Knopp U, Christmann F, Reusche E, Sepehrnia A. A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defects-a comparative animal experimental study. Acta Neurochir (Wien) 2005;147:877–87. doi: 10.1007/s00701-005-0552-0. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Maartens NF, Kaye AH. Evaluation of the use of BioGlue in neurosurgical procedures. J Clin Neurosci. 2003;10:661–4. doi: 10.1016/s0967-5868(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 29.Laquerriere A, Yun J, Tiollier J, Hemet J, Tadie M. Experimental evaluation of bilayered human collagen as a dural substitute. J Neurosurg. 1993;78:487–91. doi: 10.3171/jns.1993.78.3.0487. [DOI] [PubMed] [Google Scholar]
- 30.Leonetti JP, Anderson D, Marzo S, Moynihan G. Prevention and management of cerebrospinal fluid fistula after transtemporal skull base surgery. Skull Base. 2001;11:87–92. doi: 10.1055/s-2001-14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maher CO, Anderson RE, McClelland RL, Link MJ. Evaluation of a novel propylene oxide-treated collagen material as a dural substitute. J Neurosurg. 2003;99:1070–6. doi: 10.3171/jns.2003.99.6.1070. [DOI] [PubMed] [Google Scholar]
- 32.Malliti M, Page P, Gury C, Chomette E, Nataf F, Roux F ×. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: A clinical review of 1 year. Neurosurgery. 2004;54:599–603. doi: 10.1227/01.neu.0000108640.45371.1a. [DOI] [PubMed] [Google Scholar]
- 33.Mangham CA. Complications of translabyrinthine vs. suboccipital approach for acoustic tumor surgery. Otolaryngol Head Neck Surg. 1988;99:396–400. doi: 10.1177/019459988809900408. [DOI] [PubMed] [Google Scholar]
- 34.Mayfield FH. Autologous fat transplants for the protection and repair of the spinal dura. Clin Neurosurg. 1980;27:349–61. doi: 10.1093/neurosurgery/27.cn_suppl_1.349. [DOI] [PubMed] [Google Scholar]
- 35.Messing-Jünger AM, Ibáñez J, Calbucci F, Choux M, Lena G, Mohsenipour I, et al. Effectiveness and handling characteristics of a three-layer polymer dura substitute: A prospective multicenter clinical study. J Neurosurg. 2006;105:853–8. doi: 10.3171/jns.2006.105.6.853. [DOI] [PubMed] [Google Scholar]
- 36.Morikawa T. Tissue sealing. Am J Surg. 2001;182(Suppl 2):29–35S. doi: 10.1016/s0002-9610(01)00774-7. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa S, Hayashi T, Anegawa S, Nakashima S, Shimokawa S, Furukawa Y. Postoperative infection after duraplasty with expanded polytetrafluoroethylene sheet. Neurol Med Chir (Tokyo) 2003;43:120–4. doi: 10.2176/nmc.43.120. [DOI] [PubMed] [Google Scholar]
- 38.Narotam PK, Jose S, Nathoo N, Taylon C, Vora Y. Collagen matrix (DuraGen) in dural repair: Analysis of a new modified technique. Spine. 2004;29:2861–7. doi: 10.1097/01.brs.0000148049.69541.ad. [DOI] [PubMed] [Google Scholar]
- 39.Narotam PK, van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg. 1995;82:406–12. doi: 10.3171/jns.1995.82.3.0406. [DOI] [PubMed] [Google Scholar]
- 40.Nutik SL, Korol HW. Cerebrospinal fluid leak after acoustic neuroma surgery. Surg Neurol. 1995;43:553–7. doi: 10.1016/0090-3019(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 41.Parízek J, Mericka P, Husek Z, Suba P, Spacek J, Nemecek S, et al. Detailed evaluation of 2959 allogeneic and xenogeneic dense connective tissue grafts (fascia lata, pericardium, and dura mater) used in the course of 20 years for duraplasty in neurosurgery. Acta Neurochir (Wien) 1997;139:827–38. doi: 10.1007/BF01411400. [DOI] [PubMed] [Google Scholar]
- 42.Parizek J, Mericka P, Spacek J, Nemecek S, Elias P, Sercl M. Xenogeneic pericardium as a dural substitute in reconstruction of suboccipital dura mater in children. J Neurosurg. 1989;70:905–9. doi: 10.3171/jns.1989.70.6.0905. [DOI] [PubMed] [Google Scholar]
- 43.Parízek J, Mericka P. Duraplasty with pretreated freeze-dried sterilized human dura mater. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1990;33:135–43. [PubMed] [Google Scholar]
- 44.Parízek J, Sercl M, Michl A, Mericka P, Nemecek S, Nemecková J, et al. Posterior fossa duraplasty in children: Remarks on surgery and clinical and CT follow-up. Childs Nerv Syst. 1994;10:444–9. doi: 10.1007/BF00303609. [DOI] [PubMed] [Google Scholar]
- 45.Park YK, Tator CH. Prevention of arachnoiditis and postoperative tethering of the spinal cord with Gore-Tex surgical membrane: An experimental study with rats. Neurosurgery. 1998;42:813–23. doi: 10.1097/00006123-199804000-00076. [DOI] [PubMed] [Google Scholar]
- 46.Pomeranz S, Constatini S, Umansky F. The use of fibrin glue in cerebrospinal fluid leakage. Neurochirurgia (Stuttg) 1991;34:166–9. doi: 10.1055/s-2008-1052082. [DOI] [PubMed] [Google Scholar]
- 47.Raul JS, Godard J, Arbez-Gindre F, Czorny A. Use of polyester urethane (Neuro-Patch) as a dural substitute. Prospective study of 70 cases. Neurochirurgie. 2003;49:83–9. [PubMed] [Google Scholar]
- 48.Reyes-Moreno I, Verheggen R. Time-sparing and effective procedure for dural closure in the posterior fossa using a vicryl mesh (Ethisorb) Neurocirugia (Astur) 2006;17:527–31. doi: 10.1016/s1130-1473(06)70316-5. [DOI] [PubMed] [Google Scholar]
- 49.Rosen DS, Wollman R, Frim DM. Recurrence of symptoms after Chiari decompression and duraplasty with nonautologous graft material. Pediatr Neurosurg. 2003;38:186–90. doi: 10.1159/000069097. [DOI] [PubMed] [Google Scholar]
- 50.Sakas DE, Charnvises K, Borges LF, Zervas NT. Biologically inert synthetic dural substitutes. Appraisal of a medical-grade aliphatic polyurethane and a polysiloxane-carbonate block copolymer. J Neurosurg. 1990;73:936–41. doi: 10.3171/jns.1990.73.6.0936. [DOI] [PubMed] [Google Scholar]
- 51.Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): Surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11–23. doi: 10.1097/00006123-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Sawamura Y, Katsuyuki A, Terasaka S. Evaluation of application techniques of fibrin sealant to prevent cerebrospinal fluid leakage: A new device for the application of aerosolized fibrin glue. Neurosurgery. 1999;44:332–7. doi: 10.1097/00006123-199902000-00048. [DOI] [PubMed] [Google Scholar]
- 53.Shaffrey CI, Spotnitz WD, Shaffrey ME, Jane JA. Neurosurgical applications of fibrin glue: Augmentation of dural closure in 134 patients. Neurosurgery. 1990;26:207–10. [PubMed] [Google Scholar]
- 54.Shimizu S, Koizumi H, Kurita M, Utsuki S, Oka H, Fujii K. Duraplasty in the posterior fossa using a boat-shaped sheet of expanded polytetrafluoroethylene. Neurol Med Chir (Tokyo) 2007;47:379–81. doi: 10.2176/nmc.47.379. [DOI] [PubMed] [Google Scholar]
- 55.Sierra DH, Nissen AJ, Welch J. The use of fibrin glue in intracranial procedures: Preliminary results. Laryngoscope. 1990;100:360–3. doi: 10.1288/00005537-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Stendel R, Danne M, Fiss I, Klein I, Schilling A, Hammersen S, et al. Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg. 2008;109:215–21. doi: 10.3171/JNS/2008/109/8/0215. [DOI] [PubMed] [Google Scholar]
- 57.Tachibana E, Saito K, Fukuta K, Yoshida J. Evaluation of the healing process after dural reconstruction achieved using a free fascial graft. J Neurosurg. 2002;96:280–6. doi: 10.3171/jns.2002.96.2.0280. [DOI] [PubMed] [Google Scholar]
- 58.Thammavaram KV, Benzel EC, Kesterson L. Fascia lata graft as a dural substitute in neurosurgery. South Med J. 1990;83:634–6. doi: 10.1097/00007611-199006000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Than KD, Baird CJ, Olivi A. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery. 2008;63(1 Suppl 1):S182–7. doi: 10.1227/01.neu.0000335034.08274.d2. [DOI] [PubMed] [Google Scholar]
- 60.Tubbs RS, Wellons JC, 3rd, Blount JP, Oakes WJ. Posterior atlanto-occipital membrane for duraplasty. Technical note. J Neurosurg. 2002;97(2 Suppl):266–8. doi: 10.3171/spi.2002.97.2.0266. [DOI] [PubMed] [Google Scholar]
- 61.Turgut M, Erkus M, Tavus N. The effect of fibrin adhesive (Tisseel) on interbody allograft fusion: An experimental study with cats. Acta Neurochir (Wien) 1999;141:273–8. doi: 10.1007/s007010050298. [DOI] [PubMed] [Google Scholar]
- 62.van Velthoven V, Clarici G, Auer LM. Fibrin tissue adhesive sealant for the prevention of CSF leakage following transsphenoidal microsurgery. ActaNeurochir (Wien) 1991;109:26–9. doi: 10.1007/BF01405692. [DOI] [PubMed] [Google Scholar]
- 63.Vanaclocha V, Saiz-Sapena N. Duraplasty with freeze-dried cadaveric dura versus occipital pericranium for Chiari type I malformation: Comparative study. Acta Neurochir (Wien) 1997;139:112–9. doi: 10.1007/BF02747190. [DOI] [PubMed] [Google Scholar]
- 64.Vargel I, Tuncbilek G, Mavili E, Cila A, Ruacan S, Benli K, et al. Solvent-dehydrated calvarial allografts in craniofacial surgery. Plast Reconstr Surg. 2004;114:298–306. doi: 10.1097/01.prs.0000131983.48201.e2. [DOI] [PubMed] [Google Scholar]
- 65.Von Wild KR. Examination of the safety and efficacy of an absorbable dura mater substitute (Dura Patch) in normal applications in neurosurgery. Surg Neurol. 1999;52:418–25. doi: 10.1016/s0090-3019(99)00125-1. [DOI] [PubMed] [Google Scholar]
- 66.Warren WL, Medary MB, Dureza CD, Bellotte JB, Flannagan PP, Oh MY, et al. Dural repair using acellular human dermis: Experience with 200 cases: Technique assessment. Neurosurgery. 2000;46:1391–6. doi: 10.1097/00006123-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 67.Weinstein JS, Liu CK, Delashaw JB, Burchiel KJ, van Loveren HR, Vale FL, et al. The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty materials. J Neurosurg. 2010;112:428–33. doi: 10.3171/2009.6.JNS081540. [DOI] [PubMed] [Google Scholar]
- 68.Yoshimoto T, Sawamura Y, Houkin K, Abe H. Effectiveness of fibrin glue for preventing postoperative extradural fluid leakage. Neurol Med Chir (Tokyo) 1997;37:886–90. doi: 10.2176/nmc.37.886. [DOI] [PubMed] [Google Scholar]


