Abstract
Background:
Central neurocytoma is an uncommon benign tumor of the central nervous system. A section of these tumors have unusual aggressiveness and are termed as “atypical central neurocytomas,” the definition of which is debated. Many studies in the available literature define them as tumors with elevated MIB-1 labeling index (MIB-1 LI) >2%, while some associate them with higher values of MIB-1 LI or those with histological atypical features. Newer parameters also have been identified and correlated with MIB-1 LI to differentiate atypical from benign neurocytoma cases. A recent analysis of the atypical neurocytoma cases with malignant behavior revealed their increased tendency of spread through the cerebrospinal fluid causing craniospinal axis dissemination. However, limited studies document the appropriate indications and usefulness of additional therapeutic modalities, such as upfront craniospinal irradiation (CSI) or adjuvant chemotherapy, in countering the aggressive behavior of such tumors.
Case Description:
We present two such rare cases of atypical neurocytoma with elevated MIB-1 LI, of 3% and 4%, respectively, without histological atypia. Since there is insufficient evidence documenting advantages of any additional measures in the adjuvant management of atypical cases, both patients were treated with localized cranial radiotherapy alone, as per the evidence available in the literature currently.
Conclusion:
We propose that future studies must aptly redefine these atypical neurocytomas with malignant potential and provide guidance to identify aggressiveness of these tumors early in the course of management. Lastly, strong evidence to provide specific adjuvant therapy is also warranted.
Keywords: Atypical neurocytoma, cerebrospinal fluid dissemination, chemotherapy, craniospinal irradiation, malignant, MIB labeling index, radiotherapy
INTRODUCTION
Central neurocytoma (CN) is a rare benign tumor of the central nervous system, which is composed of small round cells with neuronal differentiation. Hassoun et al. described this tumor for the first time in the year 1982.[5] Previously these tumors were described as intraventricular ependymomas or sometimes oligodendrogliomas. CNs constitute nearly 50% of the supratentorial intraventricular tumors in adults but account for only 0.1–0.5% of all brain neoplasms.[11] CNs frequently arise from bipotential precursor cells in the fornix or walls of the lateral ventricles or septum pellucidum and are mainly located in the midline supratentorially, more commonly on the right side.[7] Few cases of extraventricular neurocytomas have also been reported in the literature.[4] CNs predominantly occur in young adults around third decade (ranging from 2 to 70 years), and have higher incidence in Asian population when compared with the Caucasians.
Histology of the benign CN shows monotonous round tumor cells with minimal cytoplasm (empty “halo” appearance), oval nuclei with fine granular chromatin (“salt and pepper” appearance) and micronuclei in the background of fibrillary matrix and reactive astrocytes.[5,7,11] Typically there are no mitoses, nucleoli, or infiltrating margins. Immunohistochemically (IHC) these tumors are strongly positive for markers of neuronal differentiation – synaptophysin, neuron specific enolase, focal glial fibrillary acidic protein (GFAP) and negative for oligodendrocyte transcription factor, p53 immunoexpression. The latest World Health Organization (WHO) classification categorizes CN as a grade II tumor.[4,7,13,14]
Majority of CNs typically have a benign course, with a 10-year survival of 90% and local control rate of 74%.[9] Approximately one-fourth of these benign tumors that have more aggressive behavior are termed as “Atypical central neurocytomas.”[3,9,13,17] This rare subgroup of CNs constitutes tumors with elevated MIB labeling index (MIB-1 LI) greater than 2% or 3% and/or associated histological atypia. Histological picture may show adverse changes such as tumor with infiltrative margins, increased mitotic activity, cellular pleomorphism, endothelial proliferation, presence of necrosis, or vascular proliferation.[4,10,17] Such tumors have a strong tendency of local recurrence or craniospinal dissemination.[3,9,10,13,17] These tumors with malignant behavior have a 10-year survival of 63% and local control rate of 46%.[9] Critical analysis of 19 published cases of CNs with malignant behavior revealed an estimated mean survival of 27.9 months (range 5–46), and a mean progression-free survival (PFS) of 15.3 months (range 2–36) in this group of patients.[11]
For patients with benign or typical CNs, complete resection (CR) or incomplete resection (IR) followed by postoperative radiotherapy are widely accepted treatment modalities.[9,13,14] However, currently there is no accepted standard treatment regimen for atypical CNs. Since a randomized clinical trial is not possible for such rare cases, the available therapeutic recommendations are from the case reports, small case series, and meta-analysis of reported studies. Gross total excision or CR is the best available therapeutic approach for atypical CNs. Localized cranial radiotherapy has been shown to improve local control (70% vs 7% at 5 years) and survival (78% vs 43% at 5 years) after IR of atypical neurocytomas as against IR alone.[14] The benefit of adjuvant radiotherapy after IR in the improvement of local control and survival is more for patients with atypical neurocytoma when compared with the benign neurocytoma.[9,13,14] Diverse chemotherapeutic agents have been tried in some cases; however, the role of chemotherapy in the adjuvant management of CNs is uncertain at this time.[1,6,8]
Besides local recurrence, atypical CNs are known to disseminate through cerebrospinal fluid (CSF) causing ventricular or spinal metastases.[2,3,11] The role of whole craniospinal axis irradiation (CSI) to treat CSF dissemination has been described in the literature presently, but is not recommended in the initial management of atypical CNs to prevent recurrences.[11]
We describe two cases of CNs with high proliferation index and further discuss the pertinent literature in the management of atypical neurocytomas.
CASE REPORTS
Case 1
A 23-year-old male with no significant previous problems presented with one year history of headaches that worsened over 3 days prior to presentation. History of 5-6 episodes of vomiting and blurring of vision was present. There was no history of seizures or other neurological deficits. On examination, he was conscious, dull but obeying commands, with no focal motor or sensory deficits except lateral rectus muscle paresis bilaterally, and papilledema. Computed tomography (CT) scan showed tumor in the right lateral ventricle, which was isodense on plain CT, nonenhancing with few areas of calcification, with asymmetrical ventriculomegaly. Magnetic resonance imaging (MRI) brain revealed large intraventricular tumor in right lateral ventricle at the foramen Munro level [Figure 1]. The lesion was crossing the midline displacing septum pellucidum and had inferior extension into the 3rd ventricle. Tumor was heterogeneously isointense on T1W, hyperintense on T2W images, with small cystic areas, nonenhancing on contrast. There was asymmetrical ventriculomegaly with right side being larger than the left side. On February 2, 2014 patient underwent right frontal parasagittal craniotomy, and near-total decompression of the tumor and external vascular drain placement by interhemispheric transcollosal approach. Intraoperatively the tumor was grayish, soft, friable, succable. His postoperative recovery was uneventful with no fresh neurological deficits, except for paranoid delusions for which psychiatric consultation was obtained.
Figure 1.
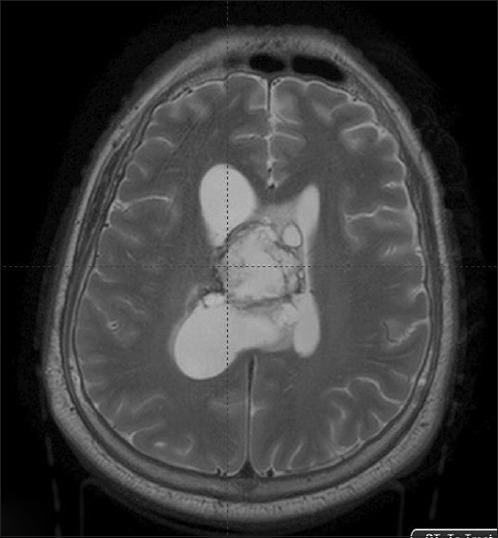
Axial section of MRI images – Case 1
Postoperative histopathological report showed monomorphic round cells arranged in sheets and with honey-comb pattern [Figure 2]. Cells had moderate amount of clear cytoplasm, with centrally placed nuclei. There was no evidence of microvascular proliferation, abnormal mitotic activity, or focal areas of necrosis. Multiple foci of calcification were seen. Immunohistochemistry was strongly positive for synaptophysin [Figure 3]. Features were consistent with CN, WHO grade II. Since the MIB labeling index was 4%, the tumor was referred to as “Atypical Central Neurocytoma” with high recurrence potential [Figure 4].
Figure 2.
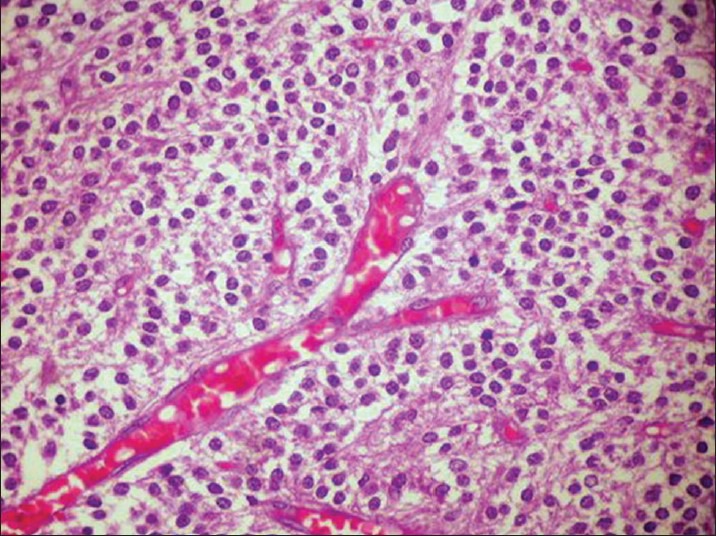
H and E staining showing histological features of neurocytoma – Case 1
Figure 3.
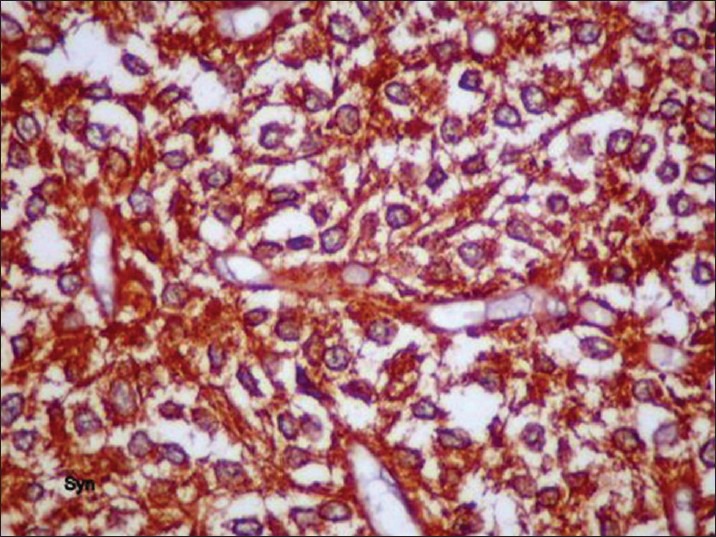
Tumor cells showing strong positivity for synaptophysin
Figure 4.
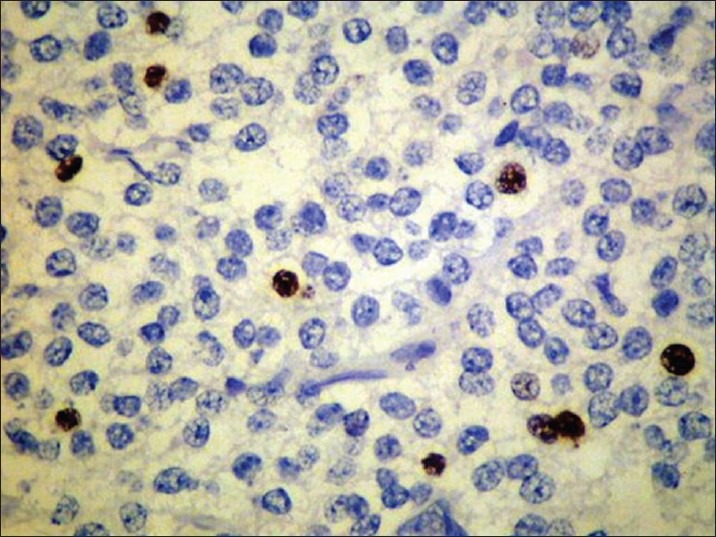
Ki67 immunostaining: proliferative index of 4%
The patient received 54 grays (Gy) of adjuvant local radiotherapy postoperatively, which he tolerated well. Patient was found to be asymptomatic and without any neurological signs at the third month follow-up visit.
Case 2
A 28-year-old female, who presented with 10-day history progressive headache and blurry vision, was detected with left ventricular mass lesion in the spiral CT scan. Clinically patient was found to have right sided weakness. MRI revealed a fairly well defined, mixed intense, heterogeneously enhancing lesion with restriction on diffusion weighted imaging (DWI), and with focal areas of cystic degeneration seen in the body and trigone of left lateral ventricle [Figure 5]. Tumor was displacing the interventricular septum and causing compression of right lateral ventricle and moderate dilatation of occipital, temporal horns of left lateral ventricle. Secondary bulging of 3rd ventricle, empty sella, prominence of bilateral perioptic CSF spaces due to increased intracranial pressure were also detected.
Figure 5.
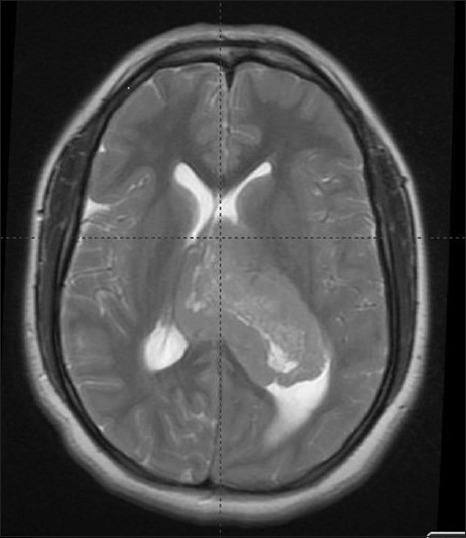
Axial section of MRI images of – Case 2
Patient underwent left precoronal craniotomy and transcortical approach and a near-total excision of lesion with placement of left frontal reservoir. Intraoperatively the tumor was found to be highly vascular, grayish-white, cusa-amenable lesion with infiltration of choroid plexus. Postoperative recovery of the patient was uneventful. Histopathology report showed cellular tumor composed of uniform round cells arranged in sheets and honey comb pattern [Figure 6]. Cells had moderate amount of cytoplasm with centrally placed nuclei. Small round monomorphic nuclei with evenly dispersed stippled chromatin was present. Multiple foci of calcification were noted. There was no evidence of microvascular proliferation, pleomorphism, or mitotic activity. In view of elevated MIB-1 labeling index (MIB-1 LI) of 3%, the tumor was referred to as “Atypical Central Neurocytoma” and to be associated with shorter recurrence-free interval. Postoperatively the patient received adjuvant local radiotherapy to a dose of 60 Gy, which she completed uneventfully. There was significant improvement in her neurological weakness by her first monthly follow-up visit. She remained asymptomatic at 4 months after the completion of the treatment, but with residual right sided motor weakness.
Figure 6.
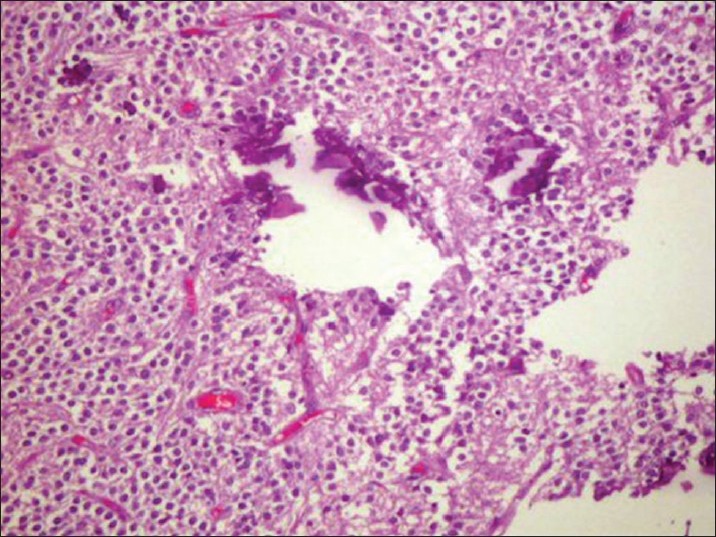
H and E staining showing histological features of neurocytoma – Case 2
DISCUSSION
CNs are rare benign tumors of central nervous system that predominantly occur in young adults and typically arise from lateral ventricles. Typically CNs have a benign course with satisfactory outcome. Although cases with aggressive clinical course and recurrences have been described in the literature, the definition and therapeutic line of management of the atypical CNs is unclear at this time.
Atypical neurocytomas are characterized by an elevated MIB-1 LI with or without atypical histological features. MIB-1 LI is an indicator of monoclonal antibody against the Ki-67 antigen, reflects the proliferating potential of the CN tumors. Soylemezoglu et al. compared clinical outcomes in 36 neurocytoma cases that were observed over a period of 150 months and reported a 63% of tumor recurrence rate among those with MIB-1 LI >2% as against 22% in those with MIB-1 LI <2%.[17] They proposed the term “atypical central neurocytoma” for this subgroup of patients, corresponding to WHO grade II. Rades et al. studied 129 patients in a meta analysis and showed that recurrence rate was 48% for CNs with MIB-1 LI >3% versus 12% for those with LI <3%.[15]
Mozes et al., after a systematic analysis of the literature, reported 19 CN cases that had malignant clinical course and rapid progression.[11] These tumors showed high rates of local recurrence and craniospinal dissemination either prior to the diagnosis or after surgical resection. Among these 19 cases, 12 out of 14 cases with accessible data had MIB-1 LI index >2%. The analysis of these cases showed a higher mean MIB-1 LI of 17.82% (range: 4.4-37.3%) for the patients with first tumor recurrence or dissemination occurring within 12 months. A nonsignificant association was found between initial mean MIB-1 LI of 13.4% and spinal dissemination. Dissemination of these malignantly behaving CNs through the CSF was detected in 17 out of 19 cases (89.4%): Ventricular dissemination in 5 patients, spinal dissemination in 12 patients at an average of 11 and 22 months after initial operation, respectively. One of the two remaining patients had a multifocal disease at presentation and the other had peritoneal dissemination through V-P shunt at 43 months after initial diagnosis.
Evidence of anaplasia in neurocytoma cases has not been consistently established as an indicator of high tumor recurrence. In one study, Mackenzie et al. compared histological atypia, MIB-1 LI, and clinical outcomes in 15 CN patients.[10] They found a significant correlation between the MIB-1 LI (>2%) and the poor outcome of patients, but there was no correlation between histological atypia and clinical outcomes and there was a little correlation between MIB-1 LI and histological atypia. Hence the study proposed the term “proliferating neurocytoma” instead of the terms “atypical” or “anaplastic” to describe these lesions.
On the contrary to the above findings, Qiu-lin et al. suggested a higher cut-off of MIB-1 LI of 10% that correlates with histological atypical features and also with aggressive behavior of CN, and proposed “WHO grade III” to be more appropriate term to characterize these tumors.[12]
A newer study by Sakamoto et al. attempted to estimate the proliferative potentiality of CNs by using preoperative CT scan, DWI, and fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET).[16] The study results did not show significant correlation of morphological appearance of CNs with MIB-1 LI, except strong enhancement of the tumors with high MIB-1 LI. Another important observation was that MIB-1 LI showed linear correlation with both minimum apparent diffusion coefficien (ADCmin) and the maximum standardized uptake value (SUVmax), although only the ADCmin was statistically significant. The study concluded that CNs have a wide variety of morphological appearances radiologically and that ADCmin is a potential proliferative marker to differentiate atypical from benign cases.
Thus, the definition of the atypical neurocytomas is still evolving and has not been fully established.
A wide variety of treatment approaches were adopted in the 19 atypical CN cases with malignant behavior including surgery, radiotherapy, and chemotherapy.[11] Majority of the patients received subtotal or IR (13 out of 19 cases), whereas gross total or CR was only possible in 4 out of 19 cases due to the diffuse tumor growth toward the adjacent structures. Adjuvant treatment in the form of radiotherapy, chemotherapy, or both was given in 17 out of 19 cases, either initially or at recurrence. The total radiotherapy doses in this scenario were found to be quite variable, ranging from 25 to 66 Gy. Also a wide range of chemotherapeutic agents including Temozolamide were used in 11 out of these 19 cases toward the adjuvant chemotherapy, the value of which is yet to be defined.[1,2,6,8]
The role of whole CSI in the management of atypical CNs is unclear at this time. Although spinal cord dissemination was detected in 12 out of 19 cases with malignant behavior, CSI was performed only in 3 of them, and only after the detection of spinal cord metastases.[11] Even though Mozes et al. considered CSI in their case as a part of initial therapy due to very high MIB-1 LI (25–30%), it was not delivered due to the lack of good literature support. Unfortunately the patient developed spinal dissemination after 36 months of good tumor control, and only then was CSI given. The authors concluded that CN cases with an increased potential for malignant behavior should be identified beforehand and must be strongly considered for maximal tumor resection followed by adjuvant CSI, instead of only adjuvant local radiotherapy. However, the level of MIB-1 LI at which this aggressive treatment approach must be adopted is not specified currently.
Due to the lack of strong evidence for upfront CSI and adjuvant chemotherapy for atypical CNs at this time, both patients were treated only with localized cranial irradiation, to doses of 54 and 60 Gy, respectively, after surgery.
In conclusion, CNs with aggressive or malignant behavior, although rare, have poor clinical outcomes. This category of tumors must be clearly defined and identified early in their course. Studies should be designed to investigate appropriate therapeutic options for this subgroup of patients. The role of chemotherapy, upfront CSI, different dosage regimen of radiotherapy in high risk cases are ought to be examined. Our case report would add to the current literature in this context and aid in future research.
ACKNOWLEDGMENT
Dr. M. Narasimha Rao, Dr.T. Pratap Reddy, Dr. Bhaskar Rao.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/183/147414
Contributor Information
Gangadhar Vajrala, Email: gvajrala@gmail.com.
Piyush K. Jain, Email: drpkjain82@gmail.com.
Shitalkumar Surana, Email: shitalsurana@yahoo.com.
Sailaja Madigubba, Email: drsailaja_mreddy@yahoo.in.
Satish R. Immaneni, Email: isatishrao@gmail.com.
Manas K. Panigrahi, Email: manaspanigrahi@live.com.
REFERENCES
- 1.Brandes AA, Amista P, Gardiman M, Volpin L, Danieli D, Guglielmi B, et al. Chemotherapy in patients with recurrent and progressive central neurocytoma. Cancer. 2000;88:169–74. doi: 10.1002/(sici)1097-0142(20000101)88:1<169::aid-cncr23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Cook DJ, Christie SD, Macaulay RJB, Rheaume DE, Holness RO. Fourth ventricular neurocytoma: Case report and review of the literature. Can J Neurol Sci. 2004;31:558–64. doi: 10.1017/s0317167100003814. [DOI] [PubMed] [Google Scholar]
- 3.Eng DY, DeMonte F, Ginsberg L, Fuller GN, Jaeckle K. Craniospinal dissemination of central neurocytoma. Report of two cases. J Neurosurg. 1997;86:547–52. doi: 10.3171/jns.1997.86.3.0547. [DOI] [PubMed] [Google Scholar]
- 4.Giangaspero F, Cenacchi G, Losi L, Cerasoli S, Bisceglia M, Burger PC. Extraventricular neoplasms with neurocytoma features— A clinicopathological study of 11 cases. Am J Surg Pathol. 1997;21:206–12. doi: 10.1097/00000478-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun J, Gambareli D, Grisoli F, Pellet W, Salamon G, Pellisier JF, et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151–6. doi: 10.1007/BF00690587. [DOI] [PubMed] [Google Scholar]
- 6.Juratli TA, Geiger K, Leimert M, Schackert G, Kirsch M. Atypical central neurocytoma with recurrent spinal dissemination over a period of 20 years: A case report and review of the literature. Case Rep Neurol Med 2013. 2013:925647. doi: 10.1155/2013/925647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane AJ, Sughrue ME, Rutkowski MJ, Tihan T, Parsa AT. The molecular pathology of central neurocytomas. J Clin Neurosci. 2011;18:1–6. doi: 10.1016/j.jocn.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Shravan Kumar C, Sharma DN, Sharma K, Haresh KP, Rath GK. Youngest case of third ventricular anaplastic neurocytoma. Indian J Med Paediatr Oncol. 2009;31:69–71. doi: 10.4103/0971-5851.71660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leenstra JL, Rodriguez FJ, Frechette CM, Giannini C, Stafford SL, Pollock BE, et al. Central neurocytoma: Management recommendations based on a 35-year experience. Int J Radiat Oncol Biol Phys. 2007;67:1145–54. doi: 10.1016/j.ijrobp.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie IR. Central neurocytoma: Histologic atypia, proliferation potential and clinical outcome. Cancer. 1999;85:1606–10. doi: 10.1002/(sici)1097-0142(19990401)85:7<1606::aid-cncr24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Mozes P, Szanto E, Tiszlavicz L, Barzo P, Cserhati A, Fodor E, et al. Clinical course of central neurocytoma with malignant transformation-an indication for craniospinal irradiation. Pathol Oncol Res. 2013;20:319–25. doi: 10.1007/s12253-013-9697-y. [DOI] [PubMed] [Google Scholar]
- 12.Qiu-lin L, Xiao-dong C, Da-yun P. Final diagnosis – Anaplastic central neurocytoma (CN) of both lateral ventricles. 2013. Available from: http://www.path.upmc.edu/cases/case784/dx.html . Last accessed on 2014 Nov 23.
- 13.Rades D, Fehlauer F, Lamszus K, Schild SE, Hagel C, Westphal M, et al. Well-differentiated neurocytoma: What is the best available treatment? Neuro Oncol. 2005;7:77–83. doi: 10.1215/S1152851704000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rades D, Fehlauer F, Schild SE. Treatment of atypical neurocytomas. Cancer. 2004;100:814–7. doi: 10.1002/cncr.20032. [DOI] [PubMed] [Google Scholar]
- 15.Rades D, Schild SE, Fehlauer F. Prognostic value of the MIB-1 labeling index for central neurocytomas. Neurology. 2004;62:987–9. doi: 10.1212/01.wnl.0000115392.21898.e3. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto R, Okada T, Kanagaki M, Yamamoto A, Fushimi Y, Kakigi T, et al. Estimation of proliferative potentiality of central neurocytoma: Correlational analysis of minimum ADC and maximum SUV with MIB-1 labeling index. Acta Radiol. 2014 Jan 29; doi: 10.1177/0284185114521187. doi: 0284185114521187. [DOI] [PubMed] [Google Scholar]
- 17.Söylemezoglu F, Scheithauer BW, Esteve J, Kleihues P. Atypical central neurocytoma. J Neuropathol Exp Neurol. 1997;56:551–6. doi: 10.1097/00005072-199705000-00011. [DOI] [PubMed] [Google Scholar]


