Abstract
Background
Digoxin was found to inhibit prostate cancer (PCa) growth via the inhibition of HIF-1α synthesis in a mouse model. We hypothesized that a therapeutic dose of digoxin could inhibit human PCa growth and disease progression.
Methods
An open label, single arm pilot study was performed. Patients (pts) with non-metastatic, biochemically relapsed PCa with prostate specific antigen doubling time (PSADT) of 3–24 months and no hormonal therapy within the past 6 months were enrolled. All pts had testosterone > 50 ng/dL at baseline. Digoxin was taken daily with dose titration to achieve a target therapeutic level (0.8 – 2 ng/ml); patients had routine follow-up including cardiac monitoring with 12-lead electrocardiograms (ECGs) and digoxin levels. The primary endpoint was the proportion of pts at 6 months post-treatment with a PSADT ≥ 200% from the baseline. HIF-1α downstream molecule vascular endothelial growth factor (VEGF) was measured in plasma.
Results
Sixteen pts were enrolled and 14 pts finished the planned 6 months of treatment. Twenty percent (3/15) of the pts had PSA decrease >25% from baseline with a medium duration of 14 months. At 6 months, 5 of 13 (38%) pts had PSADT ≥ 200% of the baseline PSADT and were continued on study for an additional 24 weeks of treatment. Two patients had durable PSA response for more than 1 year. Digoxin was well tolerated with possible relation of one grade 3 back pain. No patients had evidence of digoxin toxicity. The digoxin dose was lowered in 2 patients for significant ECGs changes (sinus bradycardia and QT prolongation), and there were probable digoxin-related ECG changes in 3 patients. Plasma VEGF was detected in 4 (25%) patients.
Conclusions
Digoxin was well tolerated and showed a prolongation of PSDAT in 38% of the patients. However, there was no significant difference comparing that of similar patients on placebo from historical data. Digoxin at the dose used in this study may have limited benefit for patients with biochemically relapsed prostate cancer.
Keywords: digoxin, prostate cancer, prostate specific antigen, PSA doubling time, HIF-1, VEGF
BACKGROUND
Biochemically-relapsed, non-metastatic, hormone-naïve prostate cancer represents a unique disease state 1, 2. As many as 70,000 men per year in the United States fall into this category where a rising prostate specific antigen (PSA) is the only manifestation of illness 2, 3. In men with PSA progression after local therapy, management options include salvage radiation, surveillance, dietary intervention, clinical trial participation or initiation of androgen deprivation therapy (ADT) 4, 5. However, no definite survival benefit has been demonstrated with the early institution of ADT in this population. Given the adverse effects of ADT, many men choose to avoid or delay medical castration for a rising PSA alone. Developing alternative options for patients with “PSA-only” prostate cancer (PCa) is therefore highly desirable.
Clinical trials have been conducted using a variety of non-hormonal therapies to treat PSA recurrent PCa. These reagents include granulocyte-macrophage colony-stimulating factor6, High dose vitamin D using weekly oral calcitriol 7, Cox-2 inhibitor Celecoxib.8, peroxisome proliferator-activated receptor gamma agonist Rosiglitazone9, tyrosine kinase inhibitor imatinib10, copper/zinc-superoxide dismutase inhibitor ATN-22411, matrix metalloproteinase inhibitor marimastat12, antiangiogenic/immunomodulatory drug lenalidomide13, pomegranate extract 14, and DNA methylation inhibitor disulfiram15 etc. With different mechanisms, these agents showed some biological activities but still no drugs are available to treat PCa in this state.
Recently, two independent drug library screenings for inhibitors of PCa revealed digoxin as a potent inhibitor of PCa cell growth 16. Screening for agents inhibiting hypoxia induced factor -1α (HIF-1α) transcriptional activity identified twenty drugs as potential HIF-1α inhibitors 16. Eleven of these drugs were cardiac glycosides, including digoxin, ouabain, and proscillaridin A, which inhibited HIF-1α protein synthesis and expression of HIF-1α target genes in cancer cells. In vitro 50 nM ouabain or proscillaridin A inhibited HIF-1α expression at both 20% and 1% O2 conditions in the human PCa cell line. This study also demonstrated that inhibition of HIF-1α expression was the critical mechanism by which digoxin and other cardiac glycosides inhibited the PCa growth in tumor xenografts model.
In the longitudinal Physicians' Health Study (PHS) of 47,759 health professionals, a significantly decreased relative risk for PCa was observed among individuals taking digoxin for cardiac indications after adjustment for age (P<0.001), multiple variables (diabetes mellitus, physical activity, body mass index, height, family history, race, pack-years smoked, and dietary factors; P<0.004), or multiple variables plus other medications (P<0.008) 17. A prospective clinical study using digoxin for the treatment of PCa is thus performed.
Designing a clinical trial for PSA-only disease is a challenge. Though change in PSA kinetics is commonly used to determine the treatment response, there is no uniform consensus for a study endpoint. Fifty percent of PSA reduction 6, 7 or PSA doubling time (PSADT) prolongation over baseline 6–9, 14, 18 is often used in clinical trials. In placebo controlled studies, 20 – 31% of placebo-treated participants had favorable PSA outcomes, defined as post-treatment PSADT of more than 200% of baseline PSADT over 6 months8. To compare the historical data, we defined the prolongation of PSADT of equal to or more than 200% of baseline as positive PSA outcome. In this pilot study, we hypothesized that a six month trial of a therapeutic dose of digoxin could inhibit human PCa growth and disease progression and achieve a positive PSA outcome.
PATIENTS AND METHODS
Eligibility
Patients with histologically confirmed adenocarcinoma of prostate with evidence of biochemical relapse after local therapy were eligible. Patients had no detectable disease as assessed by physical examination and radiographic measures (bone scan and CT of the abdomen/pelvis) within 8 weeks of study entry. Confirmed biochemical progression was defined as three rises in PSA levels, with each PSA determined at least 4 weeks apart, and each PSA value increase ≥0.2 ng/ml with PSA doubling time (PSADT) of 3 – 24 months. PSADT was calculated using the natural log of 2 (0.693) divided by the slope of the relationship of log PSA against time (in months). The last PSA level prior to enrollment had to be ≥2 ng/mL for patients with primary radiation therapy or PSA ≥1 ng/ml if radical prostatectomy was performed.
Patients had to be ≥18 years old, with life expectancy >6 months and Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and with adequate hepatic, renal (creatinine < 1.5 mg/dL) and marrow function, serum testosterone > 50 ng/dL, no uncontrolled inter-current illness, no cardiac conduction disorders such as history of sinus node disease and AV Block, accessory AV Pathway (Wolff-Parkinson-White Syndrome), no recent history of myocardial infarction, no active other malignancy, no history of malabsorption syndromes.
Prior chemotherapy was allowed as long as the requirements for adequate organ and marrow function were met. Patients who had radiotherapy within 3 months or other investigational agents within 28 days prior to the first dose of digoxin were excluded. Patients, who had hormone therapy within 6 months, were receiving steroids for active concurrent illness, any estrogen-like agents, or any hormonally active over-the-counter compounds or 5-α reductase inhibitors, were excluded.
The institutional review board approved the study. All participants gave written informed consent.
Study drug and design
The study drug Digoxin is commercially available and dispensed to study participants by the Jefferson Investigational Drug Service (IDS) pharmacy. All patients were started at 250 mcg daily first week and digoxin level was checked. While waiting for the result, digoxin was given 125 mcg daily the second week and serum level was obtained again. The doses of week 3 and after were titrated to the serum digoxin level of 0.8 – 2 ng/ml for total of 6 cycles (4 weeks/cycle). The maximal dose of digoxin was 500 mcg orally daily. ECG and digoxin were performed every 2 weeks for first cycle and then every 2 cycles and whenever the digoxin dose was adjusted. Digoxin dose adjustments downward were made if thought to be necessary by cardiology. PSA was measured every 2 cycles during the study. Patients were allowed to continue another 6 cycles if the primary endpoint of PSADT >200% baseline was met. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAEv3).
Baseline and follow-up evaluation
Baseline evaluation included history and physical examination, ECG, complete blood count, comprehensive metabolic profile, serum testosterone, and serum PSA. Bone scan, abdominal and pelvic CT scans were obtained within 8 weeks prior to study entry and then every 6 cycles. At least 3 PSA levels, drawn ≥28 days apart, were required in order to calculate the pre-treatment PSADT. PSA levels were obtained at the beginning of every 2 cycles and all values were used for kinetic calculation. PSA slope was calculated as the linear regression line of the natural log of PSA against time. At the end of 6 cycles of treatment, patients were taken off the study if the primary endpoint was not met or evidence of radiographic metastatic disease.
Correlative studies
Plasma was collected before and after 2, 4, and 6 cycles of treatment and stored at −80° for evaluation at study completion Plasma VEGF was measured at Yale School of Medicine by suspension array system using multiplexing technology.
Statistical analysis
The primary efficacy endpoint of this pilot phase II study is the proportion of patients with post treatment PSADT ≥ 200% of baseline without evidence of metastatic disease at 6 months after treatment with digoxin. The study was designed to show that treatment with digoxin could result in significantly higher than historical rate up to 30% of positive PSADT outcome. Exact binomial 95% confidence interval was computed for actually observed post-treatment rate of positive PSADT outcome.
All patients who received one dose of digoxin were included for safety analysis and considered evaluable for toxicity and safety.
RESULTS
Patient characteristics
Total 16 patients were enrolled in the study. Two patients did not finish 6 cycles treatment. One patient concerned the rising PSA and withdrew from the study. Another patient developed severe back pain after 3 cycles’ treatment. It was not cancer related and the etiology was not clear. We thought this was possibly related to study drug and took him off the study. On retrospective review, one patient did not meet eligibility criteria (baseline PSADT <3 months). Although he is included in the data analysis for toxicity, he is not included in the statistical analysis of PSA kinetics (efficacy evaluation). Table 1 lists the patient characteristics upon study entry.
Table 1.
Patient characteristics at study entry (n = 16)
| Age (yr) | Average | 66.5 |
| Range | 48–83 | |
| Race (%) | White (14/16) | 87.5% |
| Non-white (2/16) | 12.5% | |
| Gleason score sum (%) | ≥ 7(13/16)[GS 8 4 pts] | 81.3% |
| < 7 (3/16) | 18.7% | |
| Baseline PSA (ng/mL) | Mean+/−SD | 3.9 +/− 2.1 |
| Median | 3.5 | |
| Range | 1.5–8.2 | |
| PSA doubling time Prior to treatment | Average | 8.3 Months |
| Range | 2.7 – 20.4 Months | |
| Prior treatment | Prostatectomy only (1/16) | 6.25% |
| Radiotherapy only (3/16) | 18.75% | |
| Prostatectomy and salvage radiotherapy (9/16) | 56.25% | |
| Radiotherapy and hormonal therapy (1/16) | 6.25% | |
| Prostatectomy and salvage radiotherapy and hormonal therapy (2/16) | 12.5% | |
| Intermittent hormonal therapy (4/16) | 25% |
PSA kinetics
PSA Doubling Time
The PSA kinetics was calculated within the treatment time point of 6 cycles. We compared the changes of PSADT in our patients with those from the historical data in the similar patient population. In the published study, 30% of placebo treated patients had positive PSA outcome 8 . In our study, there were 5 out of 13 patients (38%) (95% CI (0.166 – 1.000) who met the primary endpoint and the PSADT increased to > 200% of baseline PSADT. Using one-sided Binomial exact test for proportions, assuming that up to 30% of patients may have positive PSADT outcome because of placebo effects, there is no significant difference between our treatment group and historical data (p = 0.35). (See table 2)
Table 2.
PSA kinetics
| Percentage of patients with post 6 month treatment PSADT ≥ 200% of baseline PSADT | 5/13 (38%) (CI 16.6 – 100%) | P=0.35 | |
| 30%* | |||
| PSA velocity (ng/ml/year) | 1.91 (CI 1.17 – 2.65) (Before treatment) | 6.07 (CI 2.4 – 12.6) (After treatment) | P=0.078 |
Historical placebo controlled study data
PSA velocity
The statistical analysis is accomplished using mixed effect model, with fixed effects of time and treatment and patient-specific random effects of time and treatment. We assumed diagonal covariance structure for patient-specific random effect, as well as unstructured covariance for random error. The time unit in this analysis is every 365 days. Before treatment, mean PSA velocity is 1.91 ng/ml/year (95% CI (1.165 – 2.648). After treatment mean PSA velocity increases to 6.07 ng/ml/year (95% CI (2.402 – 12.581), with border line significance (p = 0.078) (see Table 2). Six patients showed slowed down PSA increment and 3 patients even decreased PSA after treatment. Figure 1 shows the maximum PSA percent changes after 6 or more cycles of treatment using Waterfall plot.
Figure 1.
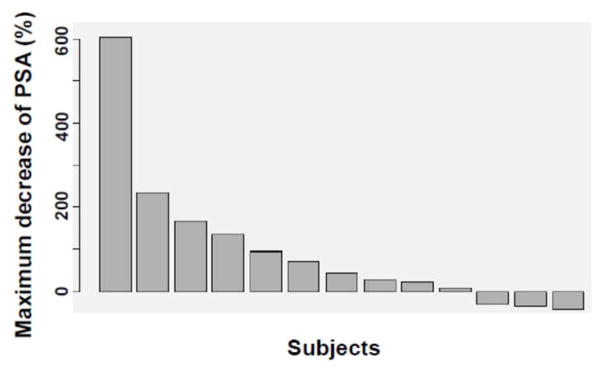
Waterfall plot of PSA declines per subject in this study, with each bar representing one patient’s lowest percent decline after 6 – 12 months’ treatment. (n = 13).
Table 3 summarizes the general outcomes of 16 study patients with attempted treatment. As demonstrated, three patients (20%) had PSA decrease >25% from baseline with a medium duration of 14 months (6, 14, and 18 + months). One of them left the town after 6 months treatment and 2 patients were on study for 1 year and continued digoxin after the study (duration of 14 months and 18+ months). One African American patient had history of Gleason Score 7 (4+3) PCa. He had prostatectomy and salvage radiotherapy. His baseline PSADT 8.19 months and day 1 PSA was 2.4 ng/mL. Currently he is still on digoxin with most recent PSAs between 1.1 – 1.2ng/ml (50% of baseline value). He is now on treatment for 18 months.
Table 3.
Cardiac and other ≥ Grade 2 Adverse Events
| n (%) | |
|---|---|
| Bradycardia, asymptomatic | 2 (12.5%) |
| EKG changes* | 3 (18.75%) |
| Hyperglycemia | 1 (6.25%) |
| Lower back pain¥ | 1 (6.25%) |
8/16 showed dig effects, 3/16 ST stagging but no concerning arrhythmias or other ECG abnormalities to suggest digoxin toxicity.
possible AE
Toxicity
Similar to its tolerability in cardiac patients at levels in the therapeutic range, digoxin was well tolerated in patients with prostate cancer. The most frequent non-hematological adverse events were gastrointestinal discomfort such as possible reduced appetite. One patient developed acute onset lower back pain with unclear etiology during cycle 3 treatment (Table 4). Digoxin was held for possible association but his back pain re-occurred when digoxin was restarted. He was then taken off the study for possible toxicity.
Table 4.
Tabular Summary of the Outcome of Patients With treatment by digoxin for 6 months (n=16)
| % change in PSA from baseline | |
|---|---|
| Greater than 50% increase | 6 |
| Increase of 0 –50% | 5 |
| Decrease of 0 –50% | 3 |
| Greater than 50% decrease | 0 |
| Removed from study | 2* |
| Radiographic disease progression | 1 |
1 withdrew because of rising PSA, 1 taken off study for questionable treatment related grade 3 back pain
The target therapeutic range goal (0.8 – 2 ng/ml) digoxin level used in this study is higher than that used for cardiology indications such as rate control in atrial fibrillation or systolic heart failure. Patients were seen by cardiologist at the oncologist’s discretion either before or during the study if there was an abnormal baseline ECGs or prior cardiac history. The median dose of digoxin in this study was 375 mcg daily. Digoxin dose was reduced in 2 patients per cardiology recommendation for possible digoxin side effects. One patient has baseline right bundle-branch block and sinus bradycardia. At the dose of 500 mcg daily with digoxin level of 0.6 ng/ml, he developed left upper arm pain which could be an angina equivalent. His dose of digoxin was thus reduced to 375 mcg daily. There were no obvious ECG changes such as ST abnormalities. One other patient had dose adjustment from 500 mcg to 375 mcg daily due to sinus bradycardia and increased QT interval (QTc still within normal limit). Of the 16 patients, 8 had ECG changes that could be consistent with digoxin effect: 5 patients had minor or borderline changes (U waves, minor PR prolongation, borderline ST segment abnormalities, and borderline T wave flattening) and 3 patients had more notable ST segment abnormalities (ST sagging) that were likely digoxin-related. There were no concerns for digoxin toxicity throughout the study.
Correlative studies
Plasma VEGF was measured at baseline and every 2 cycles. Only 4 patients (25%) had detectable VEGF at baseline. The change of VEGF during the digoxin treatment is shown in Figure 2. Both patients who were on digoxin for more than 1 year had undetectable VEGF at baseline.
Figure 2.
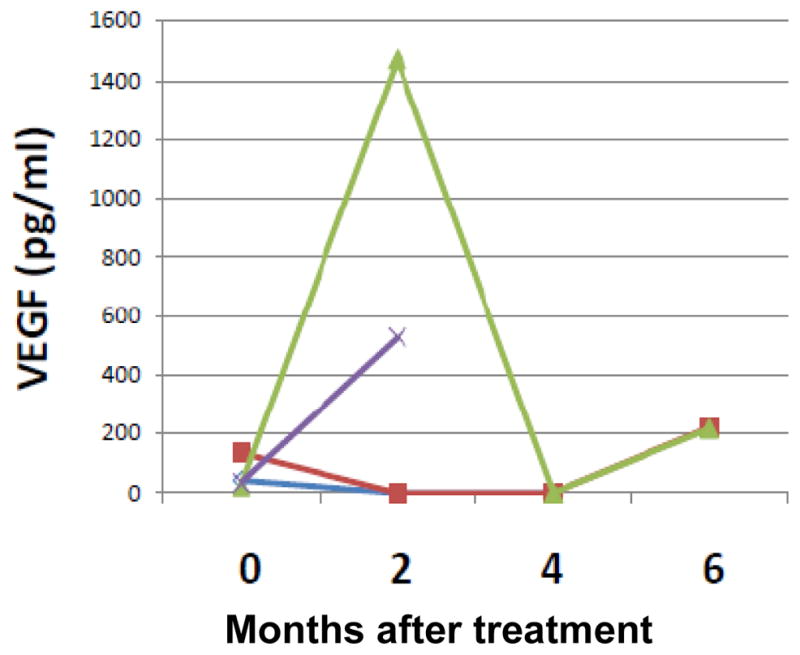
Plasma VEGF changes during the treatment course. Only 4 patients had detectable VEGF. One colored line represents the changes of VEGF over time in one patient.
DISCUSSION
Based on preclinical and epidemiological data, we designed this pilot phase II study in order to examine clinical activity of digoxin for the treatment of recurrent PCa at an early stage. Men with an isolated PSA recurrence after initial local treatment represent an ideal population for the evaluation of novel therapies based on minimal disease state, indolent natural history, and preference to avoid the adverse effects of hormone therapy. The evaluation of novel agents in this setting, however, is hampered by the lack of convenient validated end points. Overall or progression-free survival end points are impractical because of the long interval between an initial PSA increase and development of metastases or death 19. Although PSADT has not been adequately evaluated as a clinical trial end point, change in PSADT may be more sensitive to detect biologic activity than traditional PSA response criteria. It is frequently used in the recent clinical trial setting to suggest clinical efficacy 8, 9. In this study, we measured benefit from digoxin by the proportion of patients with positive PSA outcome, defined as PSADT > 200% of baseline. Statistical analysis was made to compare our data with historical data to determine the possible efficacy of digoxin for the treatment of PCa with rising PSA.
We found no significant difference of positive PSA outcome between digoxin treated patients and placebo treated patients reported in the literature 8. PSA velocity was not decreased after the treatment of digoxin. Although we cannot exactly compare our data with historical data, our results suggests that the activity from digoxin treatment cannot be established yet. In a placebo-controlled trial evaluating the effect of celecoxib on PSADT in a similar patient population, 20% of 40 men in the placebo group had post-treatment PSADT ≥ 200% of baseline PSADT8. A separate study of rosiglitazone versus placebo in a similar population showed that 31% of men in the placebo group had a post-treatment PSADT >200% of baseline 9. In our study, 5/13 patients (38%) had PSADT >200% of baseline after 6 months of treatment. We need to point out that there are differences in baseline PSADT characteristics in our study compared to these prior trials (e.g. PSADTs were between 6 and 24 months8 or <24 months9 in the other studies, but PSADTs between 3 – 24 months in the current study), and therefore direct comparisons of our study with the prior trials is not possible. Therefore, in the absence of a placebo-control, there is limitation to interpret the significance of the PSA changes reported here.
VEGF is expressed by a variety of human solid tumors including PCa 20, 21. It plays a critical role in the pathogenesis and progression of human prostate cancer 22, 23. VEGF is present in both localized and metastatic prostate tumors, and increasing plasma concentration of VEGF correlates with metastatic disease progression 24–26. In patients with metastatic castration-resistant prostate cancer (mCRPC), both plasma and urine VEGF levels are independent predictors of overall survival 27, 28. Since VEGF is one of the downstream molecules of HIF-1a, we measured baseline plasma VEGF levels and their changes after treatment with digoxin. It is possible that tumor derived VEGF level will be decreased when PCa HIF-1a expression is inhibited. From the assay we used, only 4 (25%) patients had detectable VEGF in our patients. Two patients had decreased VEGF after treatment while 2 patients had transient increase of VEGF during the exposure of digoxin. The patient who responded treatment and has been on treatment for more than 18 months did not have detectable VEGF. It is impossible to make any conclusion from this small sample size study about the correlation of VEGF changes with the study drug digoxin and clinical outcomes. We need to mention that there are other diverse mechanisms reported to be involved in cardiac glycoside-mediated regulation of cell proliferation (reviewed in 29, 30). One mechanism of action was thought to be mainly mediated by alterations in intracellular calcium levels 31, 32.
This study has several limitations. First, there was no placebo control in this trial. It has been reported that in similar patient populations, placebo-treated patients can have relative stable disease for many weeks8. In retrospective analyses of the PSA kinetics from patients with biochemically relapsed PCa, the calculated PSADT may naturally increase over time in the absence of therapy and may be influenced by duration of PSA follow-up. Placebo-controlled randomized clinical trials are thus recommended to screen novel agents to mitigate bias because of natural PSADT variability33. Second, the patient population is too broad with the PSADT from 3 to 24 months. These patients could have very different clinical outcomes with this broad range of PSADT 34. A multi-center trial does have the advantage of selecting appropriate patients and finishing study on time.
For study design, surrogate endpoints for future studies evaluating investigational agents in this patient population need to be validated. PSADT and PSA kinetics changes may be acceptable for phase II studies, but would have questionable significance in larger phase III studies in the absence of clinical endpoints (e.g. metastasis-free survival or overall survival). Metastasis-free survival as a surrogate for the survival endpoint is currently being discussed in the clinical research community and may be reasonable for studies requiring prolonged follow-up35. Interestingly, a preliminary evaluation of several PSA measures in the “PSA-only” population seems to suggest that changes in PSA kinetics induced by treatments with a variety of experimental agents correlate significantly with metastatic progression18, although these data require prospective confirmation.
In conclusion, digoxin was well tolerated in PCa patients without obvious cardiac toxicities in this pilot phase II, open labeled single center study. We attempted to assess the efficacy of digoxin on inhibiting PCa progression as measured by PSADT in men with PSA-recurrent non-metastatic PCa. Digoxin showed a prolongation of PSDAT in 38% of the patients, which is not significantly different compared to historical cohorts. However, digoxin may have some activity in a small proportion of patients but it seems there is no association with plasma VEGF level and it is unclear if its activity is related to HIF-1a inhibition. Placebo-controlled, biomarker driven study designs may aid the interpretation of future trials evaluating non-hormonal investigational drugs in this patient population.
Acknowledgments
Funding Support: Thomas Jefferson University Department of Medical Oncology & Grant # IRG - 08-060-04 from the American Cancer Society
We are grateful to patients and their families for participating the trial.
References
- 1.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moul JW, Banez LL, Freedland SJ. Rising PSA in nonmetastatic prostate cancer. Oncology (Williston Park, NY. 2007;21(12):1436–1445. discussion 1449–1452, 1454. [PubMed] [Google Scholar]
- 3.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163(6):1632–1642. [PubMed] [Google Scholar]
- 4.Paller CJ, Antonarakis ES, Eisenberger MA, et al. Management of patients with biochemical recurrence after local therapy for prostate cancer. Hematology/oncology clinics of North America. 2013;27(6):1205–1219. doi: 10.1016/j.hoc.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaorsky NG, Raj GV, Trabulsi EJ, et al. The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Seminars in oncology. 2013;40(3):322–336. doi: 10.1053/j.seminoncol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Weinberg V, Bok R, et al. Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte-macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol. 2003;21(1):99–105. doi: 10.1200/JCO.2003.04.163. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Lemmon D, Lowe BA, et al. High-dose weekly oral calcitriol in patients with a rising PSA after prostatectomy or radiation for prostate carcinoma. Cancer. 2003;97(5):1217–1224. doi: 10.1002/cncr.11179. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Manola J, Kaufman DS, et al. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24(18):2723–2728. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Manola J, Kaufman DS, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101(7):1569–1574. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 10.Rao K, Goodin S, Levitt MJ, et al. A phase II trial of imatinib mesylate in patients with prostate specific antigen progression after local therapy for prostate cancer. The Prostate. 2005;62(2):115–122. doi: 10.1002/pros.20130. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Zahurak M, Beer TM, et al. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naive prostate cancer. Urologic oncology. 2013;31(5):581–588. doi: 10.1016/j.urolonc.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum E, Zahurak M, Sinibaldi V, et al. Marimastat in the treatment of patients with biochemically relapsed prostate cancer: a prospective randomized, double-blind, phase I/II trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(12):4437–4443. doi: 10.1158/1078-0432.CCR-04-2252. [DOI] [PubMed] [Google Scholar]
- 13.Keizman D, Zahurak M, Sinibaldi V, et al. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(21):5269–5276. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paller CJ, Ye X, Wozniak PJ, et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate cancer and prostatic diseases. 16(1):50–55. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweizer MT, Lin J, Blackford A, et al. Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate cancer and prostatic diseases. 2013 doi: 10.1038/pcan.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platz EA, Yegnasubramanian S, Liu JO, et al. A Novel Two-Stage, Transdisciplinary Study Identifies Digoxin as a Possible Drug for Prostate Cancer Treatment. Cancer discovery. 2011;2011(1):68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonarakis ES, Zahurak ML, Lin J, et al. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. 118(6):1533–1542. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Eisenberger M, D'Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(3):537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 20.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19(4):1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 21.Mao K, Badoual C, Camparo P, et al. The prognostic value of vascular endothelial growth factor (VEGF)-A and its receptor in clinically localized prostate cancer: a prospective evaluation in 100 patients undergoing radical prostatectomy. Can J Urol. 2008;15(5):4257–4262. [PubMed] [Google Scholar]
- 22.Ferrer FA, Miller LJ, Lindquist R, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54(3):567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 23.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143(2):401–409. [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrer FA, Miller LJ, Andrawis RI, et al. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997;157(6):2329–2333. [PubMed] [Google Scholar]
- 25.Duque JL, Loughlin KR, Adam RM, et al. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999;54(3):523–527. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 26.Duque JL, Loughlin KR, Adam RM, et al. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61(5):401–408. doi: 10.1590/s1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 27.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res. 2001;61(6):2533–2536. [PubMed] [Google Scholar]
- 28.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res. 2005;11(5):1815–1820. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 29.Newman RA, Yang P, Pawlus AD, et al. Cardiac glycosides as novel cancer therapeutic agents. Molecular interventions. 2008;8(1):36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Denmeade S, Carducci MA. HIF-1alpha and calcium signaling as targets for treatment of prostate cancer by cardiac glycosides. Current cancer drug targets. 2009;9(7):881–887. doi: 10.2174/156800909789760249. [DOI] [PubMed] [Google Scholar]
- 31.Yeh JY, Huang WJ, Kan SF, et al. Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells. The Journal of urology. 2001;166(5):1937–1942. [PubMed] [Google Scholar]
- 32.McConkey DJ, Lin Y, Nutt LK, et al. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer research. 2000;60(14):3807–3812. [PubMed] [Google Scholar]
- 33.Paller CJ, Olatoye D, Xie S, et al. The effect of the frequency and duration of PSA measurement on PSA doubling time calculations in men with biochemically recurrent prostate cancer. Prostate cancer and prostatic diseases. 2013 doi: 10.1038/pcan.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA : the journal of the American Medical Association. 2005;294(4):433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 35.Schweizer MT, Zhou XC, Wang H, et al. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(11):2881–2886. doi: 10.1093/annonc/mdt335. [DOI] [PMC free article] [PubMed] [Google Scholar]


