Abstract
Approximately 50 million people in the world suffer from epileptic seizures. Reliable early seizure detection could bring significantly beneficial therapeutic alternatives. In recent decades, most approaches have relied on scalp EEG and intracranial EEG signals, but practical early detection for closed-loop seizure control remains challenging. In this study, we present preliminary analyses of an early detection approach based on intracortical neuronal multiunit activity (MUA) recorded from a 96-microelectrode array (MEA). The approach consists of (1) MUA detection from broadband field potentials recorded at 30 kHz by the MEA; (2) MUA feature extraction; (3) cost-sensitive support vector machine classification of ictal and interictal samples; and (4) Kalman-filtering postprocessing. MUA was here defined as the number of threshold crossing (spike counts) applied to the 300 Hz – 6 kHz bandpass filtered local field potentials in 0.1 sec time windows. MUA features explored in this study included the mean, variance, and Fano-factor, computed across the MEA channels. In addition, we used the leading eigenvalues of MUA spatial and temporal correlation matrices computed in 1-sec moving time windows. We assessed the seizure detection approach on out-of-sample data from one-participant recordings with six seizure events and 4.73-hour interictal data. The proposed MUA-based detection approach yielded a 100% sensitivity (6/6) and no false positives, and a latency of 4.17 ± 2.27 sec (mean ± SD) with respect to ECoG-identified seizure onsets. These preliminary results indicate intracortical MUA may be a useful signal for early detection of human epileptic seizures.
I. INTRODUCTION
Epilepsy is one of the most common neurological disorders, affecting approximately 1% of the entire population [1, 2]. It is characterized by seemingly unpredictable recurrent seizures that have a significant negative impact on autonomy and quality of life. Reliable early seizure detection, as well as seizure prediction, could bring tremendous therapeutic benefits to people with epilepsy. Many attempts have been made to achieve reliable early detection, most of them using scalp electroencephalogram (EEG) and intracranial EEG (iEEG) [3], but their practical efficacy remains variable at best.
In this study, we explore the feasibility of an early seizure detection approach based on intracortical MUA, recorded from a 4×4-mm2 96-microelectrode array on a platform (96-MEA; the NeuroPort System, Blackrock Microsystems, Salt Lake City, UT USA) [4, 5].
II. METHODS
A. Data Description and Approach Outline
Approval of this study was granted by Institutional Review Boards at Partners Healthcare (Massachusetts General and Brigham and Women’s Hospitals) and Brown University, and a participant was enrolled after informed consent. The participant was a 52-year-old female and had a history of epilepsy with complex partial seizures and occasional secondary generalized seizures. Along with standard electrocorticography (ECoG) electrodes to monitor her brain state for clinical purposes, a 96-MEA was implanted in her left middle temporal gyrus. The distance from the MEA to the nearest ECoG electrode that captured seizure onsets was approximately 2 cm. Refer to [6] for more details.
The framework used in this study, outlined in Figure 1, was composed of (1) MUA extraction from broadband intracortical neural signals, (2) MUA feature extraction, (3) classification using cost-sensitive support vector machines (SVMs), and (4) postprocessing with Kalman-filtering. The approach was tested on out-of-sample data, with double cross-validation [7, 8]. Data from one participant, including six epileptic seizure events and 4.73-hour of interictal (between seizures, presumably normal) time, were used. Preictal (5-min period preceding a seizure event) and postictal (2-hour period after a seizure) time segments were not included in the interictal data.
Figure 1.

Outline of the framework for early detection of human epileptic seizures using intracortical multiunit activity.
B. MUA Extraction
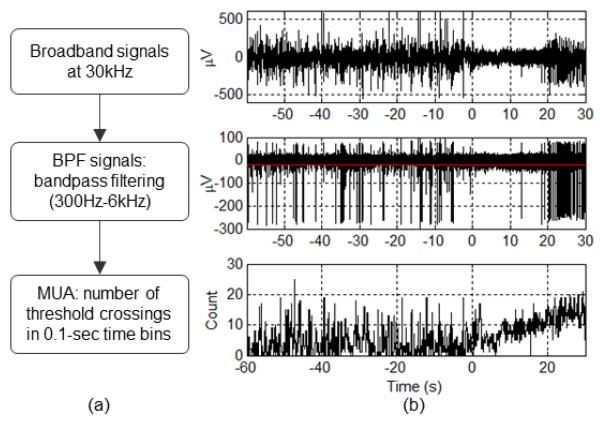
Intracortical neural signals were recorded broadband (0.3 Hz – 7.5 kHz) from a 96-MEA and sampled at 30 kHz. To extract MUA from broadband signals, we used bandpass filtering (300 Hz – 6 kHz) [9], followed by counting threshold crossings in 0.1-sec time bins (see Figure 2). The threshold was defined as −3 SD (standard deviations) of clipped BPF signals (BPF signals outside ±10 SD were identified as artifacts and clipped). Time-varying threshold values were computed in 10-sec moving time windows. Threshold values in 10-sec windows were replaced with ones in the preceding window when the corresponding BPF magnitude square (power) was above 3 SD. We further smoothed the threshold values in a time-causal manner by applying a 5-min moving average.
Figure 2.
MUA extraction. (a) MUA extraction steps. (b) Resulting signals from each step in (a). The red line in the middle plot indicates the threshold. We set the threshold to −3 SD of the BPF signals. In this example, although the firing rate of the unit with large-amplitude action potentials decreases during the initial stages of the seizure, MUA increases throughout. Seizure onset at time zero.
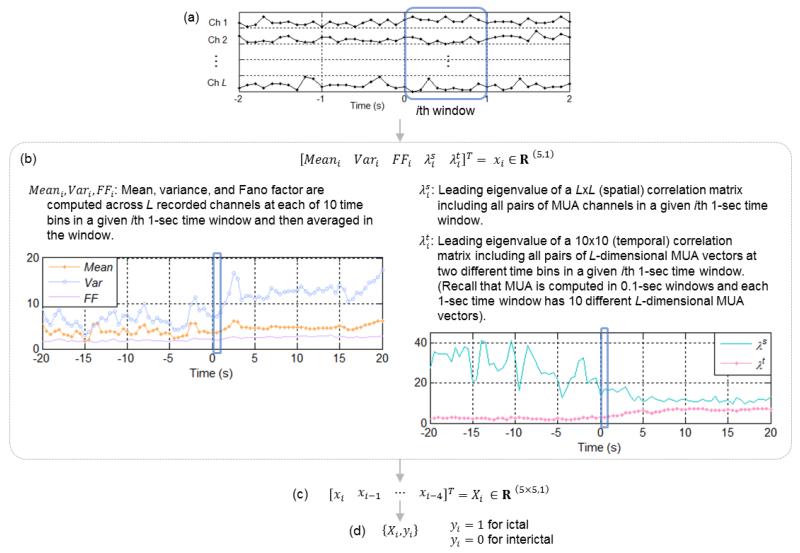
C. MUA Feature Extraction: Across-Channel Mean, Variance, Fano Factor, and Leading Eigenvalues of Spatial and Temporal Correlation Matrices
Visual examinations on MUA time traces over ictal (with a seizure) periods, as shown in Figure 3 (a), pointed out that certain MUA measures computed across channels may capture sudden and inhomogeneous changes at or right after seizure onsets. We computed the mean, variance, and Fano factor of MUA across all recorded channels, and averaged them in 1-sec time windows with 0.5-sec overlap. In addition, we explored spatial and temporal correlation matrices of MUA in 1-sec windows, using Spearman’s rank correlation coefficients. Leading eigenvalues of spatial and temporal correlation matrices may capture early ictal changes in spatial and temporal statistical dependence of MUA in 1-sec windows (see Figure 3 (b)). As a final step, the mean, variance, Fano factor and the leading eigenvalues, computed on causal 5-consecutive 1-sec moving time windows, were concatenated to form a single MUA-feature vector, as comparable to [10, 11]. MUA features were normalized into z-scores, and binary-labeled into either the interictal or ictal group.
Figure 3.
MUA feature extraction. (a) MUA time trace in L recorded channels. Seizure onset at time zero. (b) 5 measures are computed in a 1-sec moving time window with 0.5-sec overlap between consecutive windows. (c) Final MUA features obtained by concatenating the 5 measures in time-causal 5-consecutive windows. (d) Binary labeling for classification.
D. Cost-Sensitive SVM Classification
Because ictal samples were much fewer than interictal ones, as is typical in seizure data, we used cost-sensitive support vector machines (SVMs, software package LIBSVM [12]) to handle this data imbalance [7, 8]. The radial basis function (RBF) kernel was used. The cost-sensitive parameter, determined as the ratio between ictal and interictal samples [13], corresponded to 96.4 in our dataset.
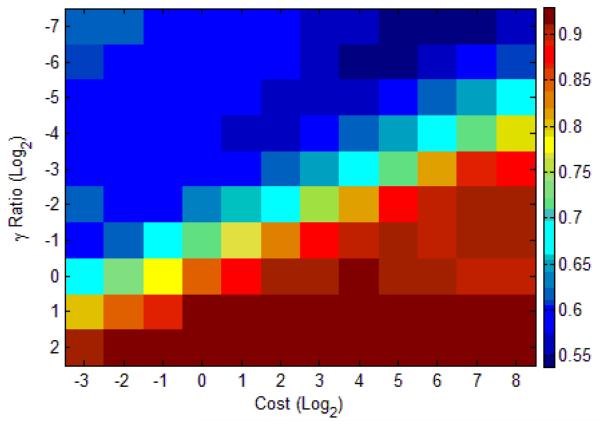
SVMs with the RBF kernel are vulnerable to the over-fitting problem [8]. To address this issue, we used a double cross-validation scheme [7, 8]. Our double cross-validation scheme included 5-fold cross-validation for in-sample optimization with a training dataset and leave-one-seizure-out cross-validation [10] for out-of-sample evaluation with a test dataset. We used the F-score as a single measure to evaluate optimality of classification mapping in in-sample optimization as well as to take the imbalanced condition into account [14] (see Figure 4).
Figure 4.
In-sample optimization with F-score via 5-fold cross-validation with a training dataset. A range of costs (C’s, penalties) and γ’s in the RBF kernel (proportional to a default value in LIBSVM) defines a grid where the optimal parameter pair, i.e. the pair that maximizes the F-score, is chosen. In this example, the pair of C = 21 and γ = 22 was selected (maximum F-score = 0.93). The SVM is trained using the optimal pair of parameters with the training dataset, and then evaluated on an untouched test dataset.
E. Postprocessing with Kalman Filtering
Cost-sensitive SVM classification, by penalizing more false negatives more than false positives (FPs), may sporadically produce undesired FPs. To address this issue, we applied a postprocessing step consisting of Kalman filtering of SVM decision values (real number outputs). The state-space model used in the Kalman filter was comparable to one in [7]. We set the ratio between the variances of the process and observation noise to 2−10.
III. RESULTS
We evaluated the proposed MUA-based approach for early seizure detection in one-participant dataset that included 6 seizures and 4.73-hour interictal data. We assessed our framework in two ways: event-wise, i.e. a seizure occurrence was considered one event; and sample-wise, i.e. a single feature was classified into an ictal or interictal class. In event-wise detection rate, the framework produced 100% sensitivity (6/6), no FP, and a 4.17 ± 2.27 sec (mean ± SD) detection latency with respect to ECoG-identified seizure onsets. TABLE I presented the sample-wise detection rate before and after postprocessing, including confusion matrices, sensitivity, specificity, and positive and negative predictive numbers.
TABLE I.
SAMPLE-WISE DETECTION RATE BEFORE AND AFTER POSTPROCESSING
| Actual ictal | Actual interictal | ||
|---|---|---|---|
| Classified as ictal |
291 | 21 | Pos. pred. value = 0.933 |
| Classified as interictal |
69 | 34023 | Neg. pred. value = 0.998 |
| Sens. = 0.808 | Spec. = 0.999 | ||
|
| |||
| (a) Before postprocessing | |||
|
| |||
| Actual ictal | Actual interictal | ||
|
| |||
| Classified as ictal |
279 | 0 | Pos. pred. value = 1.000 |
| Classified as interictal |
81 | 34044 | Neg. pred. value = 0.998 |
| Sens. = 0.775 | Spec. = 1.000 | ||
|
| |||
| (b) After postprocessing | |||
IV. DISCUSSION
MUA recorded via microelectrode arrays results from a mixture of multiple neurons spiking near each electrode [5]. There are various different ways to extract MUA from MEA recordings [9]. We defined MUA as the number of threshold crossings in 0.1-sec time bins applied to the bandpass filtered broadband recorded field potentials. Different MUA definitions may result in different seizure detection rates.
Our preliminary analyses indicate that multi-channel intracortical MUA may be useful for early seizure detection. We plan to extend our analyses by including several participants and various types of epileptic seizures. In addition, we plan to explore early seizure detection approaches that combine MUA with various other types of neural signals, including single-unit activity and local field potentials.
ACKNOWLEDGMENT
The authors thank the participant in this study and nurses and physicians at MGH. The contents do not represent the views of the Department of Veterans Affairs or the United States government.
This study was supported by: the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS) under grants R01NS079533 (to WT), K01NS057389 (to WT), and R01NS062092 (to SSC); the Department of Veterans Affairs, Merit Review Award (to WT); the Office of Research and Development, Rehabilitation R&D Service, Department of Veterans Affairs B6453R (to LRH); the Doris Duke Charitable Foundation (to LRH); the Massachusetts General Hospital Deane Institute; and a postdoctoral fellowship from the Epilepsy Foundation (to YSP).
Contributor Information
Yun S. Park, Department of Neuroscience and the Brown Institute for Brain Science, Brown University, Providence, RI 02912 USA (yun_sang_park@brown.edu)
Leigh R. Hochberg, Center for Neurorestoration and Neurotechnology, Rehabilitation R&D Service, Department of Veterans Affairs, Providence, RI 02908 USA; the School of Engineering, Brown University, Providence, RI 02912 USA; and the Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114 USA (leigh_hochberg@brown.edu)
Emad N. Eskandar, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114 USA (eeskandar@partners.org)
Sydney S. Cash, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114 USA (scash@partners.org)
Wilson Truccolo, Department of Neuroscience and the Brown Institute for Brain Science, Brown University, Providence, RI 02912 USA; the Center for Neurorestoration and Neurotechnology, Rehabilitation R&D Service, Department of Veterans Affairs, Providence, RI 02908 USA (phone: 401-863-5282; fax: 401-863-6481; wilson_truccolo@brown.edu).
REFERENCES
- [1].National Institutes of Health Seizures and Epilepsy: Hope Through Research. National Insitute of Neurological Disorders and Stroke. 2004 [Google Scholar]
- [2].England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: Promoting health and understanding.: A summary of the Institute of Medicine report. Epilepsy & Behavior. 2012;25:266–276. doi: 10.1016/j.yebeh.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nasehi S, Pourghassem H. Seizure Detection Algorithms Based on Analysis of EEG and ECG Signals: a Survey. Neurophysiology. 2012 Jun;44:174–186. [Google Scholar]
- [4].Homer ML, Nurmikko AV, Donoghue JP, Hochberg LR. Sensors and Decoding for Intracortical Brain Computer Interfaces. Annual Review of Biomedical Engineering. 2013;15:383–405. doi: 10.1146/annurev-bioeng-071910-124640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wolpaw J, Wolpaw EW. Brain-computer interfaces: principles and practice. Oxford University Press; 2012. [Google Scholar]
- [6].Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, et al. Single-neuron dynamics in human focal epilepsy. Nature Neuroscience. 2011 May;14:635–U130. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Park Y, Luo L, Parhi KK, Netoff T. Seizure prediction with spectral power of EEG using cost-sensitive support vector machines. Epilepsia. 2011 Oct;52:1761–70. doi: 10.1111/j.1528-1167.2011.03138.x. [DOI] [PubMed] [Google Scholar]
- [8].Cherkassky VS, Mulier F. Learning from data: concepts, theory, and methods. Wiley-IEEE Press; 2007. [Google Scholar]
- [9].Stark E, Abeles M. Predicting movement from multiunit activity. Journal of Neuroscience. 2007 Aug 1;27:8387–8394. doi: 10.1523/JNEUROSCI.1321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park YS, Hochberg LR, Eskandar EN, Cash SS, Truccolo W. Int IEEE EMBS Conf Neural Eng. San Diego, CA USA: 2013. Early detection of human epileptic seizures based on intracortical local field potentials; pp. 323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kharbouch A, Shoeb A, Guttag J, Cash SS. An algorithm for seizure onset detection using intracranial EEG. Epilepsy & Behavior. 2012;24:389. doi: 10.1016/j.yebeh.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Transactions on Intelligent Systems and Technology (TIST) 2011;2:27. [Google Scholar]
- [13].Eitrich T, Lang B. Efficient optimization of support vector machine learning parameters for unbalanced datasets. Journal of computational and applied mathematics. 2006;196:425–436. [Google Scholar]
- [14].Sun Y, Kamel MS, Wong AKC, Wang Y. Cost-sensitive boosting for classification of imbalanced data. Pattern Recognition. 2007;40:3358–3378. [Google Scholar]