Abstract
Prebiotics are ingredients selectively fermented by the intestinal microbiota that promote changes in the microbial community structure and/or their metabolism, conferring health benefits to the host. Studies show that β (1–4) galacto-oligosaccharides [β (1–4) GOS], lactulose and fructo-oligosaccharides increase intestinal concentration of lactate and short chain fatty acids, and stool frequency and weight, and they decrease fecal concentration of secondary bile acids, fecal pH, and nitroreductase and β-glucuronidase activities suggesting a clear role in colorectal cancer (CRC) prevention. This review summarizes research on prebiotics bioassimilation, specifically β (1–4) GOS, and their potential role in CRC. We also evaluate research that show that the impact of prebiotics on host physiology can be direct or through modulation of the gut intestinal microbiome, specifically the probiome (autochtonous beneficial bacteria), we present studies on a potential role in CRC progression to finally describe the current state of β (1–4) GOS generation for industrial production.
1. Introduction
1.1. Prebiotics: Definition and scope of review
Prebiotics are “nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health”[41]. The gut bacteria play an active role in the digestion of carbohydrates, a fermentative process that yields short chain fatty acids (SFCA) like acetic, propionic and butyric acids, and various gases like hydrogen, methane and CO2. The concept of prebiotics is relatively recent; however, the differences between non-digestible and digestible carbohydrates, and the methods to quantify them were established early in the last century. Digestible carbohydrates were considered those available for digestion and absorption in the small intestine that rapidly increased blood glucose levels[83] (had a high glycemic index) whereas non-digestible carbohydrates were not digested and hence had a low glycemic index. The methods for determination of digestible carbohydrates target the reducing sugars, including sucrose and starch as a measure of the available carbohydrates in food products. The non-digestible carbohydrates are reported as the amount of insoluble residue, corrected for protein and ash (reviewed by McCleary[84]).
Prebiotics are short chain oligosaccharides resistant to digestion in the upper gastrointestinal tract; thus, they can reach the colon undigested, to selectively stimulate the growth of the beneficial members of the intestinal the microbiota or probiome[6] that carry functional β-galactosidases and/or β-glucosidases. The most recent definition of prebiotics states that “a dietary prebiotic is an ingredient selectively fermented that results in specific changes in the composition and/or activity of the gastrointestinal (GI) microbiota thus conferring benefit(s) upon host health”[42]. Three commercially available dietary ingredients: galacto-oligosaccharides (GOS), lactulose, and fructo-oligosaccharides (FOS) have been used as food additives in Japan and Europe. In the United States, the Food and Drug Administration (FDA) has not stated health claims for probiotics or prebiotics but requires a notification of safety when applying for commercialization of a new dietary ingredient. Additionally, the FDA Centers for Biologic Evaluation and Research and Drug Evaluation and Research (CBER and CDER, respectively) published a draft document regarding the need to file an Investigational New Drug application when doing human research[1] in which is mentioned that, according to the National Center for Complementary and Alternative Medicine (NCCAM), prebiotics are included in the domain called “biologically based practices”, which “includes, but is not limited to, probiotics, botanicals, animal-derived extracts, vitamins, minerals, fatty acids, amino acids, proteins, whole diets, and functional foods”. Prebiotics commercialized in the US that have submitted notification to be considered as new dietary ingredients (NDI), and have GRAS (generally regarded as safe status) status for use in foods and term infant formulas, include Vivinal GOS® (Friesland Foods Domo®) and Oligomate 55N/55NP (Yakult Pharm. Ind. Co.). Vivinal GOS® is generated using the β-galactosidase from Bacillus circulans while Oligomate, which has been used as a food ingredient in Japan for several years, is generated using whole cells of Sporobolomyces singularis overexpressing its own β-hexosyl transferase. Figure 1 shows the proportion of components in commercial and a non-commercial, enriched GOS formulation recently developed[28]. The commercial β (1–4) galacto-oligosaccharides [β (1–4) GOS] formulations contain approximately 50% β-(1–4) GOS, and residual glucose, lactose and galactose, carbohydrates that may enhance growth of non-probiomic bacteria. The host and the microbial physiological responses to prebiotics, which are poorly digested by endogenous enzymes and fermented by the intestinal microbiota containing functional β-galactosidases and/or β-glucosidases are referred as “prebiotic effects” and have been extensively documented in humans and animals[55, 75, 94].
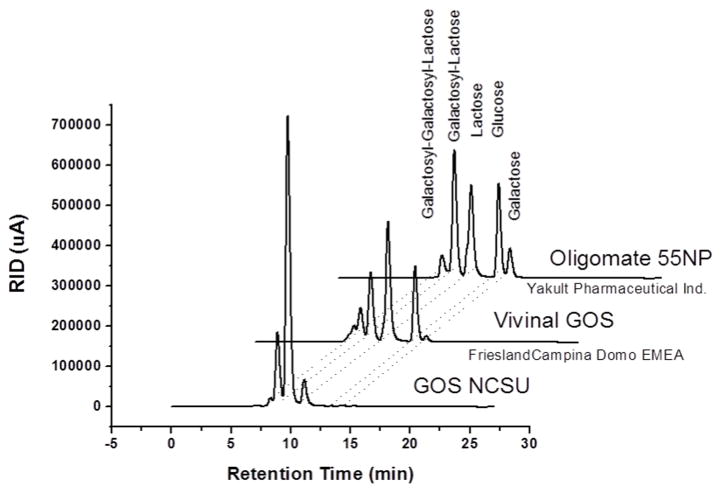
Figure 1.
Purity comparison of residual sugars (lactose, galactose and glucose) and β (1–4) GOS (Gos-3, Gos-4, Gos-5) present in GOS NCSU, Oligomate 55NP (Yakult), Vivinal GOS (Friesland Campina Dome). The enzymes used to generate these products were β-hexosyl-transferase (GOS NCSU and Oligomate 55NP) and β-galactosidase (Vivinal GOS).
1.1.1. Galacto-oligosaccharides (GOS)
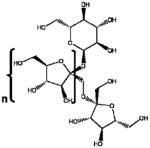
GOS are the most inexpensive alternative often added to infant formulas to mimic the beneficial effects of the oligosaccharides present in human breast milk and is one of the most extensively evaluated prebiotic. They are considered prebiotics as was originally defined by Gibson and Roberfroid[41]: they are not absorbed in the upper part of the gastrointestinal tract, they are specific substrates for one or a group of beneficial bacteria of the probiome (resulting in the modulation of the intestinal microbiota in favor of a healthier composition), and have beneficial systemic effects on the host. Technically, GOS can have α- or β-configurations by the nature of the glycosidic bonds between the sugar molecules (Table 1). The majority of published scientific articles use the term GOS when referring to β (1–4) GOS, while the abbreviation TOS has been alternatively employed[31]. β (1–4) GOS are generally produced by enzymatic transglycosylation using β-galactosidases or β-glucosidases and have a generic formula of β (1–4) [DGalactose]n-D-Glucose where n ranges between 3 and 10 sugar moieties[104]. Due to their glycosidic bond, they are not metabolized in the small intestine reaching the colon intact, where they serve as substrate for specific members of the microbiota capable of hydrolyzing the galactose-glucose bonds[106]. These carbohydrates and the carbohydrate fragments formed from the hydrolysis of the complex polymeric substances are further transformed by the butyrate-producers in the colon (see below a more detailed description of the effect of GOS on the intestinal microbiota). Products of GOS metabolism include SCFAs, lactate, acetate, and gases in proportions depending upon the α- or β-configuration of the sugars[31]. Bifidobacterium species are the most studied members of the probiome able to metabolize GOS, FOS, and human milk oligosaccharides (HMOs). Consequently, an increased abundance of bifidobacteria is the most reported effect of GOS and this is termed “bifidogenic effect” [29, 130].
Table 1.
Chemical structure, synthesis methods, and enzymes required for biotransformation of different oligosaccharides.
| Oligosaccharide | Chemical Structure | Glicosidic Linkages | Synthesis | Enzymes required for biotransformation | |
|---|---|---|---|---|---|
| β (1–4) GOS | GalactosylLactose |
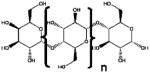
|
β (1,4) glicosidic linkages | Biological | β-Galactosidases and/or β-Glucosidases |
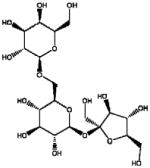
| Lactulose | Lactulose |
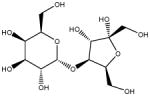
|
β (1,4) glicosidic linkages | Chemical | β-Galactosidases |
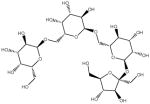
| FOS | Fructo-oligosaccharides |
|
α (1,2) glicosidic linkages | Biological | Fructosidase |
| α (1–6) GOS | Galactosyl-sucrose Raffinose |
|
α (1,2) and α (1,6) glicosidic linkages | Biological | Fructosidase and α –Galactosidases |
| Galactosyl-sucrose Stachyose |
|
α (1,2) and α (1,6) glicosidic linkages | Biological | Fructosidase and α –Galactosidases |
1.1.1.1. GOS and CRC prevention
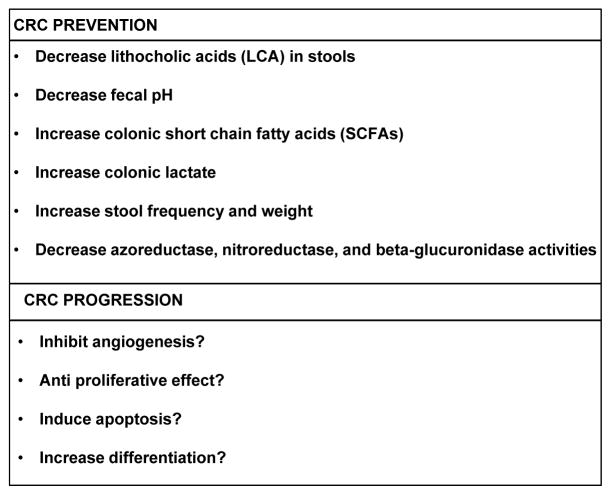
There is a strong genetic component in the development of colorectal adenomas or cancers, which in conjunction with environmental factors including diet and lifestyle have a major impact on risk (reviewed in [7]). Lifestyle aspects related to increased risk of CRC include elevated body mass index (BMI), obesity, and low physical activity[61, 80]. With regards to diet, elevated risk of CRC has been associated with high consumption of red and processed meat, refined grains, sweets, and alcohol, and a low consumption of fruits and vegetables[5, 115]. In this review, we examined the scientific literature and identified a number of factors, consisting of genetic and environmental parameters conducive to CRC, which can potentially be modulated by prebiotics to prevent gathering of the perfect storm. We have not included all chemopreventive agents of CRC like aspirin and other NSAIDs, multivitamins, hormones, and others[24], instead we assessed the literature specifically to identify research studies that suggest a role of GOS in CRC risk and prevention (Figure 2). The following sections list specific parameters with a role in CRC that could be modulated by prebiotics.
Figure 2.
Summary of the potential impact of β (1–4) GOS on CRC prevention and progression according to published scientific literature.
1.1.1.1.1. Bile acids: decreased concentration of secondary bile acids and increased concentration of primary bile acids?
In the intact intestinal tract, bacterial biotransformations of conjugated bile acids include deconjugation of bile acids (CBAs) to liberate free primary bile acids (cholic acid [CA] and chenodeoxycholic acid [CDCA]), oxidation of hydroxy groups at C-3, C-7 and C-12 with formation of oxo bile acids, and reduction of these oxo groups to either alpha- or beta-configuration[57]. Bacterial bile salt hydrolases (BSH, EC 3.5.1.24) are responsible for deconjugation of CBAs. The enzyme hydrolyzes the amide bond and liberate the glycine/taurine moiety from the steroid[11]. Quantitatively, the most important bacterial biotransformation is the 7 α-dehydroxylation of CA and CDCA, to yield the secondary bile acids deoxycholic (DCA) and lithocholic (LCA) acids. A pioneer study by Reddy and collaborators[118] showed that the secondary bile acids sodium cholate or sodium chenodeoxycholate increased N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced adenocarcinomas and adenomas in germ-free rats, and mainly adenomas in conventional female F344 rats. A study by the same group reported that patients with colon cancer excreted high levels of fecal secondary bile acids and cholesterol metabolites compared to healthy individuals[117]. Likewise, studies have reported that patients with colorectal adenomas had also increased fecal DCA and LCA[119] and serum DCA[10]. A more recent meta-analysis of 20 studies including a total of 1,226 individuals, aimed to review observational studies that examined the relationship between fecal bile acids and CRC or adenoma, reported that patients with adenomas and CRC had a higher concentration of total bile acids in stools. According to this analysis, patients with CRC (but not adenoma patients) had significantly increased concentrations of CDCA. One study reported markedly higher concentrations of fecal DCA in CRC patients[119], while most of the other studies included in the meta-analysis reported either no differences between CRC and control patients or higher concentrations of DCA in controls[151]. DCA excretion in patients with adenoma was significantly higher than controls. Conversely, LCA excretion was significantly higher in CRC patients, but not in adenoma patients[151]. An early study on high-risk CRC patients (individuals that had had adenomas removed) showed no significant differences in concentration of total bile acids in stools compared to controls[96]. No human studies have investigated the concentration or excretion of bile acids in patients with CRC or adenomas fed GOS. However, healthy men that consumed 15 g/day of either GOS, FOS or inulin excreted lower concentrations of fecal LCA and DCA, although values only reached statistical significance in the FOS and inulin groups for DCA[158].
1.1.1.1.2. Decreased fecal pH
Short chain fatty acids (SCFAs) are responsible for the neutralization of bases and the acidification of colon contents (reviewed by Newmark and Lupton[100]). Most SCFAs are generated by colon microbial fermentation of undigested dietary carbohydrates. A study investigating the effects of dietary cellulose and GOS on the development of dimethylhydrazine-induced CRC in rats fed low, medium, or high-fat diets showed that the pH in the cecum of animals fed high-GOS diets was 5.8, significantly lower than animals fed cellulose-diets, which had a pH ranging from 6.4 to 6.6[163]. According to data reviewed by Newmark and Lupton[100], the pH within the colon luminal environment can affect the composition and metabolic activity of the microbiota, the absorption of luminal components like SCFAs and minerals, the metabolism of drugs and carcinogens, enzymatic reactions such as those catalyzing secondary bile acid formation, and mucosal cell proliferation.
1.1.1.1.3. Increased SCFAs
Perhaps the most studied effect of prebiotics consumption is the increase of the major end-products of carbohydrate fermentation, SCFAs in the colon, of which butyrate has been extensively studied for its role in CRC prevention and progression[51, 71, 134], and that have been reported in studies in vitro[140], in animal models[62, 73, 109], and in human infants[35]. A fairly constant ratio of acetate > propionate > butyrate (in a molar ratio of approximately (60:20:20) has been reported in population surveys and measured in the intestinal contents of victims of sudden death, which however can be altered by dietary changes[26, 165]. The role of SCFAs in the intestine include nutrition of the host colonic epithelium, modulation of the colonic pH, intracellular pH and cell volume, and regulation of proliferation and gene expression[8, 36, 90, 100]. Consequences of increased concentration of colonic SCFAs include decreased pH, which influences composition of the microbiota, decreased solubility of bile acids, increased absorption of minerals, and reduced absorption of ammonia (reviewed in [165]), all of which have a general correlation with SCFAs’ anticarcinogenic properties, particularly attributed to butyrate. A study conducted in 1,2-dimethylhydrazine (DMH)-treated rats fed either wheat bran, guar gum, or oat bran showed significantly fewer tumors in the rats fed wheat bran compared with those fed guar or oat bran, with the lowest tumor mass observed in rats fed wheat bran. These results suggested that fiber associated with high butyrate concentrations in the distal large bowel might be protective against CRC, while soluble fibers that do not raise distal butyrate concentrations may not be protective. It also suggested a relationship between butyrate production in vivo and suppression of tumor formation[86].
1.1.1.1.4. Increased lactate
Prebiotic consumption leads to a transient increase in lactate and butyrate in the colon and has been deemed of importance for CRC prevention[40]. However, utilization of GOS by butyrate generating (butyrogenic) bacteria has not been reported. The proposed mechanism by which prebiotics increase butyrate concentration is by cross-feeding (or syntropy) between different members of the colon microbiota[31]. In fact, GOS has been shown to increase microbial abundance of bifidobacteria and lactobacilli in the colon[29, 77], which can produce acetate, lactate, formate, ethanol, and succinate[39, 157], but have not been stated to produce butyrate. Therefore, the butyrogenic effect of prebiotics requires colonic accumulation of butyrate, which in turn needs a syntrophy (cross-feeding) mutualistic relationship between microbial production of lactate and its biotransformation to butyrate[12, 33, 132]. In fact, GOS consumption increased lactic acid concentration in fecal samples of healthy young men from an average of 266 mg/100 g to 379 mg/100 g of dry feces, although differences did not reach statistical significance[158].
1.1.1.1.5. Increased stool frequency and weight
Large bowel transit time or frequency of bowel movements is one of the most important factors affecting the structure and function of the colonic microbiota[78]. Moreover, low frequency of bowel movements (i.e. increased stool retention times) and reduced fecal weight have been associated with higher CRC risk. The best-documented influence of slow colonic transit is on bile acid metabolism by increasing DCA and raising cholesterol saturation of bile[72]. Bulking of the stool accelerates its transit, reducing the exposure time to irritants and carcinogens[27]. In an animal study, effects of dietary cellulose and GOS on the development of DMH-induced CRC were assessed in rats fed low-, medium- or high-fat diets[163]. In animals fed the high-GOS diet, the cecal content was significantly increased in weight and significantly decreased in pH.
1.1.1.1.6. Decreased azoreductase, nitroreductase, and β-glucuronidase activities
Nitroreductases and azoreductases reduce nitro- and azo- components to aromatic amines through highly reactive reactions that generate mutagens and carcinogens while β-glucuronidases hydrolyze glucuronic acid conjugates of heterocyclic amines also yielding reactive metabolites that can cause damage to colonic mucosal cells[44]. In 1976, Goldin and Gorbach reported that the effect of diet on nitroreductase, azoreductase, and β-glucuronidase activities in male rats shifted from a diet with high vegetable and grain content to a diet consisting predominantly of beef[44]. The animals fed predominantly beef had significantly higher levels of these activities, which have been implicated in the conversion of procarcinogens into carcinogens[43, 124]. Moreover, over expression of nitroreductases has been reported in a Clostridium paraputrificum strain isolated from patients with colon cancer compared to clostridia from healthy individuals[99]. A research study showed that β-glucuronidase and nitroreductase activities were reduced in rats fed GOS, while β-glucosidase activity was increased[126], and the same trend was observed in humans[158]. More research on an actual correlation between these activities and CRC is clearly needed.
1.1.1.2. GOS and CRC progression
Although two animal studies have suggested that GOS was protective against the development of colorectal tumors, as demonstrated by an inhibitory effect on tumor incidence, multiplicity and size, regardless of the fat content of the diet [163, 164], studies on GOS and a potential role in CRC progression are lacking. One study reported that angiogenesis, the formation of new blood vessels from the preexisting vasculature and an obligatory event for the growth and progression of solid tumors beyond the size limit imposed by simple diffusion for nutrients, was inhibited by sulfated GOS. The study revealed that sulfated GOS with a low degree of polymerization (even pentasaccharides) were potent angiogenesis inhibitors with an influence on fibroblast growth factor FGF-2[65]. Of potential interest are the studies aimed to determine if human milk oligo-saccharides (HMOs), which are structurally similar to GOS, had a role in modulation of the neonatal intestinal development and enterocyte function. One study reported that exposure of the intestinal cell lines HT-29, HIEC, and Caco-2 to HMOs significantly inhibited cell proliferation[67]. Specifically, neutral oligosaccharides had an anti-proliferative effect, a significant induction of apoptosis, and a minor increase in differentiation, and acidic oligosaccharides showed inhibition of the intestinal cell proliferation via induction of alkaline phosphatase activity. Moreover, HMOs induced growth arrest of intestinal cells by modulation of epidermal growth factor receptor (EGFR) signal pathways and cell cycle-associated gene expression[66].
1.1.2. Other prebiotics
1.1.2.1. Lactulose
Lactulose (4-0-β-d-galactopyranosyl-d-fructofuranose) is an artificial disaccharide composed of fructose and galactose, bonded together by a β (1–4)-glycosidic bond. The production and physiological effects of this disaccharide have been reviewed by Schuster-Wolff-Buhring et al.[135] and Panesar et al.[110]. Originally generated by intensive heating of lactose, several studies have demonstrated that lactulose induces growth of Bifidobacterium both in vitro[30, 108] and in vivo[14, 98, 159] but reports on the same effect on strains of Lactobacillus are mixed, with studies reporting either stimulation of Lactobacillus[127] or no significant differences in their growth rates[14, 50]. Besides the prebiotic, or more specifically, bifidogenic effect, lactulose has been shown to enhance colonic motility which correlates with its traditionally use as laxative in the treatment of constipation[139, 147, 150]. Additionally, a lower pH in the colon due to selective fermentation of this compound has been linked to an enhanced intestinal solubility of minerals which resulted in its improved absorption[138, 156]. Similarly ascribed to the lowering of the intestinal pH[129] and generation of butyrate, one early study showed that lactulose decreased the pH of rat stools. Thus, rats with low pH stool had significantly fewer colon tumors after injections of 1,2-dimethylhydrazine (DMH) than rats treated with DMH alone[129]. Other studies have shown that lactulose decreased the incidence of aberrant crypt foci (ACF) and aberrant crypts generated in azoxymethane (AOM)-treated rats[23] and that lactulose at a dietary level of 0.3% significantly decreased the degree of DNA damage induced in cells of the colonic mucosa by DMH in rats harboring human gut microbiota[125].
High concentrations of secondary bile acids in feces, blood, and bile have been linked to cholesterol gallstone disease and colon cancer[85]. Consumption of lactulose (12 weeks, 60 g/day) in patients with adenomas led to decreased colonic absorption of secondary bile acids, especially DCA. Other outcomes included decreased intestinal transit and fecal pH and increased stool frequency and weight[155]. A study by Roncucci et al.[121] reported that lactulose (20 g/day) taken daily was effective in reducing the recurrence rate of colon adenomas. Conversely, lactulose consumption did not affect the rectal mucosal proliferation in individuals with a family history of CRC[122].
1.1.2.2. Fructo-oligosaccharides (FOS)
FOS are short to medium length chains of β-D fructans in which fructosyl units are bound by a β (2-1) osidic linkage[120]. β (2-1) fructans include inulin and FOS, and occur naturally in onion, chicory, garlic, asparagus, banana, artichoke, and other vegetables[75]. A study in 6 healthy volunteers given FOS for 11 days demonstrated that 89% of the ingested FOS was not metabolized in the small intestine, and none was excreted in stools, indicating that the portion reaching the colon was completely fermented by the colonic microbiota[92]. Unlike lactulose, FOS can be fermented by both Bifidobacterium and Lactobacillus strains[64, 87, 123] and studies have shown that FOS consumption results in increased numbers of Bifidobacterium[17] and Lactobacillus[131, 148] species. Interestingly, a relatively long term study in rats fed a basal low-fiber diet or the same diet containing 9 g/100 g of FOS for 2, 8 or 27 weeks showed that the increased concentrations of Lactobacillus and lactic acid in the cecum were abolished at 8 weeks; however, cecal SCFA concentration and molar butyrate ratio were higher in rats fed FOS at all-time points[69]. The prebiotic effect of FOS is dose dependent[17], also associated with a decrease of fecal pH in some animal models[13, 22] (results are inconclusive in humans[15, 16]), and increased production of lactic acid and SCFAs[34, 123].
Two animal models: the male Sprague-Dawley rat and ApcMin/+ mouse, which are heterozygous for a non-sense mutation of the Apc gene, the murine homologue of APC[95], are most commonly used to study the impact of dietary interventions on CRC. Studies in rats showed that FOS reduced the number of aberrant crypt foci in the colon of DMH-treated male Sprague-Dawley rats fed a 60 g FOS/kg diet for 35 days[56], and inhibited ACF formation and crypt multiplicity in AOM-treated male F344 rats (although at a lesser level than inulin)[116]. This end result was also demonstrated in rats but only when FOS was fed in combination with celecoxib (a non-steroidal anti-inflammatory drug)[20] or soybeans[46]. The alternative animal model currently used for preclinical testing of chemopreventive agents is the ApcMin/+ mouse model, although one major drawback of this mouse model is the occurrence of tumors predominantly in the small intestine and not the colon. Corpet and Pierre[25] pointed out that most of the studied chemopreventive agents have a similar efficacy on large and small intestinal tumors, with specific exceptions that include NSAIDs (more protective in small intestine). Conversely, resveratrol, folic acid, uroguanlylin, selenium in broccoli, and FOS have a more prominent protective effect on the colon (reviewed in [25]). The FOS results are explained by the fact that FOS is not metabolized in the small intestine but fermented by the microbiota in the colon. Pierre et al.[112] reported that short-chain (sc) FOS decreased the number of colon tumors in ApcMin/+ mouse. Moreover, the histopathological examination of large tumors in the colon suggested a delay in the transition from adenomas to carcinomas in animals fed scFOS compared to controls, with adenomas being as numerous as adenocarcinomas in this group, whereas adenocarcinomas predominated in the other diets. The same group reported that scFOS also stimulates the local immune system with up-regulation of IL-15 by scFOS in the colon of Apc +/Min mice[9].
FOS impact on CRC studies in humans are scarce and contradictory at best. A study with healthy human volunteers revealed that FOS ingestion (12.5 g/day) led to an increase in fecal bifidobacterial counts and β-fructosidase activity but had no significant effect on fecal total anaerobes, pH, activities of nitroreductase, azoreductase, and β-glucuronidase, and concentrations of bile acids and neutral sterols (metabolic indexes potentially involved in colonic carcinogenesis)[15]. In a different study, 94 subjects with small adenomas (<10 mm in diameter), larger adenomas, or no adenomas were fed 5 g of scFOS twice daily for 3 months. The study showed that long-term consumption of the prebiotic significantly increased fecal butyrate levels in patients with colon adenomas to the baseline level of adenoma-free patients. Additionally, scFOS consumption reduced fecal concentration of LCA, a secondary bile acid, in subjects without adenomas[18].
The above discussed research indicates that impact of prebiotics on the gut microbiome and parameters of CRC prevention is not exclusive of GOS. However, research shows that differences exist between the different prebiotics, lactulose with a more pronounced laxative effect and a lesser impact on the gut Lactobacillus population.
1.2. Modulation of the intestinal microbiota by GOS: Who can metabolize GOS in the intestinal tract? How is GOS metabolized by bacteria?
The vast majority of the intestinal bacteria have predominantly saccharolytic metabolisms; as a consequence, the availability of carbohydrates is almost certainly the most important factor controlling the composition and metabolic activities of the gut microbiota, despite the presence of large numbers of amino acid fermenting bacteria and syntrophic species in the colon. The bifidogenic effect of GOS has been documented in infants consuming formula containing polydextrose and β (1–4) GOS, which showed increased abundance of total Bifidobacterium species as well as specifically B. longum and B. infantis[133], and in infants consuming formula supplemented with β (1–4) GOS and FOS (in a 9:1 ratio)[52, 128], which showed not only increased abundance of bifidobacteria in stools of infants that consumed the prebiotics, but also increased abundance of lactobacilli and decreased numbers of Clostridium difficile.
It cannot be assured that GOS will be fermented only by probiomic bacteria in the intestinal tract or that the products of fermentation will not benefit growth or activity of potential pathogens. However, the study by Davis et al.[29] done in 18 healthy adult human volunteers, whom were administered β (1–4) GOS during 16 weeks showed that only a few taxa other than bifidobacteria, were impacted by GOS. Statistically significant decreases were observed for the family Bacteroidaceae and the genus Bacteroides while abundance of Coprococcus comes and Faecalibacterium prausnitzii was significantly increased at doses of 5 and 10 g/day. Conversely, a recent study using an in vitro model of the colon, the TIM-2 system[91], and 13C-labeled GOS showed an increased abundance of several Bifidobacterium species being the most affected B. bifidum and B. catenulatum. β (1–4) GOS increased also abundance of Lactobacillus species (specifically L. gasseri and L. salivarius), and commensal, potentially pathogenic bacteria, including members of the family Enterobacteriaceae and Klebsiella species. In contrast, species of Bacteroides and Prevotella decreased in numbers, as well as Faecalibacterium prausnitzii (indicated with its former name, Fusobacterium prausnitzii in the publication), and species of Eubacterium, Ruminococcus and Lactococcus[77]. The study, which used the pooled fresh stools of 8 healthy Dutch adult volunteers to create a standardized microbiota to use as inoculum for the study, also showed that production of SCFA was higher in the GOS pool, with higher acetate and lower propionate production, and slightly lower levels of butyrate. Additionally, levels of i-butyrate, i-valerate and ammonia (originated from protein fermentation) were lower in the GOS pool[77].
Metabolism of prebiotics in the colon is influenced by the respective sugar monomers, the degree of polymerization, and the type of glycosidic bonds[146]. It is accepted that GOS can resist hydrolysis by salivary and intestinal digestive enzymes but they are sensitive to hydrolysis by bacterial enzymes in the colon[113]. Although technically GOS could be hydrolyzed by the human intestinal β-galactosidase, hydrolysis is normally limited due to very weak enzymatic activities[76, 97]. Different enzymatic systems are involved in the hydrolysis of β-GOS, generally metabolized by β-galactosidases (β-Gal, EC 3.2.1.23), commonly known as lactases[21], since β-Gal enzymes are also responsible for the hydrolysis of terminal non-reducing β-D-galactose residue of the disaccharide lactose (4-O-β-galactopyranosyl-D-glucopyranose). Bacterial β-Gal enzymes are quite ubiquitous. They are present in Proteobacteria, Firmicutes, Actinobacteria, the CFB (Bacteroidetes-Chlorobi-Fibrobacteres) group, Verrucomicrobia, Spirochaetes, Victivallaceae, Thermotogales, Chloroflexi, Acidobacteriales, and over 350 species more. β-Gal enzymes are of biotechnological relevance due to their uses to synthetize GOS. In this section of the review, we will discuss how bacteria use β-Gal enzymes for GOS hydrolysis. In E. coli, β-Gal enzymes have two catalytic activities. They and hydrolyze lactose to galactose plus glucose, or convert lactose to another disaccharide, allolactose, which in turn induces the lac operon[82]. Due to the fact that the same structural β (1–4) bonds exist in lactose and GOS, the same enzyme is responsible for their hydrolysis. Lactose and GOS are evolutively related and represent the highest proportion of carbohydrates in the breast milk of mammals being the first and most natural source of nutrients for the newborn. Consequently, the most extensively characterized β-Gal enzymes are those from Bifidobacterium species. B. bifidum and some strains of B. longum subsp. longum exhibit a dedicated pathway for degrading type I HMOs, that involves liberation of lacto-N-biose type I (LNB) and galacto-N-biose type I (GNB) from their natural substrates by extracellular enzymes (endo-α-N-acetylogalactosaminidase[38] and/or lacto-N-biosidase[162]), transport and subsequent cleavage by the lacto-N-biose phosphorylase LnpA. The products of this process are α-galactosylphosphate, which enters glycolysis, and N-acetylhexosamines, which enter the aminosugar metabolic cycle[105]. Strains of B. longum subsp. infantis characterized so far have shown no presence of lacto-N-biosidase homologs[74]. A recent study by Yoshida et al.[166] showed that B. longum subsp. infantis can directly incorporate lacto-N-tetraose (LNT) and hydrolyze it via a specific β-Gal enzyme. The authors identified two different β-Gal enzymes (Bga42A and Bga2A) responsible for the degradation of type-1 and type-2 HMOs respectively.
β-Galactosidases have been isolated and characterized from a number of Lactobacillus species, including L. pentosus[79], L. sakei[59], L. delbrueckii subsp. bulgaricus[103], L. plantarum[60], L. reuteri[102] and L. acidophilus[101]. However, the dedicated pathways and enzymes involved in GOS degradation have not been extensively characterized. A transcriptional analysis of genes induced in GOS-supplemented medium showed that in L. acidophilus, GOS specifically induced a cluster of genes encoding intracellular proteins involved with galactose and lactose metabolism, notably the LacS permease implicated in GOS transport[3]. β (1–4) GOS are structurally similar to oligosaccharides present in mammals breast milk and can be synthetically produced from lactose by the transglycosylating activity of β-galactosidases (discussed above) while α-GOS can be found in natural reservoirs such as soybeans[89]. α-GOS (galactosyl-sucrose oligosaccharides) include raffinose, stachyose and verbascose and consist of galactose residues linked α (1–6) to the glucose moiety of sucrose (Table 1). Given that most mammals do not express pancreatic α –Galactosidases (α (1–6) Gal), its digestion is mediated by colonic bacterial enzymes. The fermentation of α (1–6) GOS results in fermentative gases, which can induce abdominal pain and flatulence[114, 144]. Bacterial α (1–6) Galactosidases (EC 3.2.1.22) are clustered into families 4, 27 and 36 of the sequence-based classification of glycoside hydrolases (GH)[48, 49]. A search for EC 3.2.1.22 in the Kyoto Encyclopedia of Genes and Genomes (KEGG)[63] retrieved 1,114 hits, including genes from Bifidobacterium and Lactobacillus, indicating that α–Gal hydrolases are widely distributed in microbial communities. α–Gal enzymes have been identified and characterized in several microorganisms including L. plantarum[143], L. reuteri[153], Thermotoga neapolitana[32], Bacillus stearothermophilus[149], Enterococcus faecium[168], Enterobacter cloacae[107], Citrobacter freundii[141], species of Bifidobacterium[53, 169] and recently, Ruminococcus gnavus, a human intestinal isolate[2]. The R. gnavus α–Gal is encoded by the aga1 gene, which is 2.2 kb in size, monocistronic, and predicted to encode a 743-amino acid (aa) protein, with a 41% similarity to the α–Gal encoded by Thermotoga[2]. In L. plantarum ATCC 8014, α–Gal is encoded by the melA gene. The enzyme has a predicted 738 aa-size and a molecular mass of 84 kDa. Although melA is flanked by two terminators, it is immersed in a gene cluster involved in utilization of α- and β-galactosidases, including rafP, a putative raffinose transporter. No sorting signals were identified in the protein encoded by melA suggesting its presence as a soluble enzyme in the cytoplasm of L. plantarum[143]. According to the KEGG database, α–Gal hydrolyzes terminal, non-reducing α-D-galactose residues in α-D-galactosides, including GOS, galactomannans, galactolipids, and also hydrolyzes α-D-fucosides[145]. A protein search at NCBI (http://www.ncbi.nlm.nih.gov/guide/proteins/) performed in June of 2014, showed 22,588 bacterial entries for α–Gal enzymes, with 6,632 representatives in the Firmicutes phylum (almost 50% of those corresponding to the order Lactobacillales), 7,430 entries in the Proteobacteria (mostly gamma-Proteobacteria), and 4,797 entries in the phylum Actinobacteria (mostly Actinomycetales).
1.3. GOS effects on host physiology and immune system
Numerous research studies have shown that prebiotics have an effect on the intestinal microbiota (discussed above). The anaerobic breakdown of GOS and other prebiotics increase the concentration of fermentation products like lactate and SCFAs, which per se affect the intestinal physiology. The purpose of this section is to summarize available information concerning a direct effect of GOS on the intestinal cells and physiology.
A potential effect of GOS on the intestinal mucus layer has been described in a number studies; however, the molecular mechanisms involved have not been fully elucidated. In the colon, the mucus layer is a bilayered system. The outer layer, which can be removed by suction, appears to be vital in reducing shear stress to the mucosa[4], and can also trap bacterial pathogens. The underlying adherent layer, which cannot be removed by suction, is normally free of bacteria and may act as a size exclusion barrier to prevent translocation of damaging luminal agents [19, 47]. Brownlee et al.[19] showed that rats consuming a diet containing cellulose or a fiber-deficient diet had significantly lower colonic resting mucus thickness. Conversely, rats fed ispaghula (a plant of the genus Plantago commonly used as used as a bulk-forming laxative) had a significantly thicker adherent layer and higher total mucus secretion. The authors hypothesized that there could be a direct molecular interaction between specific dietary fibers and the colonic mucin. Moreover, complex carbohydrates from fiber could be absorbed through colonic antigen-presenting cells resulting in stimulation of mucus production. A study by Zhong et al. (2009) showed that GOS feeding significantly improved intestinal barrier function in rats with severe acute pancreatitis[170]. An earlier study by Meslin et al.[88] showed that consumption of β (1–4) GOS [(referred as trans galacto-oligosaccharides (TOS) in the study] modified the mucin cell distribution in the colon of germ-free rats. The authors hypothesized that the lower number of mucus cells observed for the three types of mucins (neutral, acid, and sulphonated) in the proximal colon and the greater number in the distal colon was related to changes in osmolality produced by β (1–4) GOS. Conversely, β (1–4) GOS had no effect in the colon of conventional animals, but reduced the number of acid mucin containing cells in the cecum, maybe due to higher concentrations of SCFA in the cecum[88]. Interestingly, a study on the small intestine of BALB/c mice showed that protein content of the mucosa of β (1–4) GOS-consuming mice was higher than controls, and β (1–4) GOS ingestion also increased significantly the mucin content compared to controls. However, expression of MUC-2 and MUC-4 encoding the major mucins found in the colon, showed no significant differences between β (1–4) GOS -consuming mice and controls[70], contrarily to a reported increased MUC-3 expression induced by inulin feeding in three-week old Sprague–Dawley rats[111]. Taken together, these findings suggest that β (1–4) GOS might estimulate mucus production; however, the mechanisms involved in such estimulation are not well understood.
The gastrointestinal tract hosts a major part of the body’s immune system: the gut-associated lymphoid tissue. Few reports have studied interactions between GOS and the mucosal immune system of the gut, and, given their effect on the intestinal microbiota, it is not easy to elucidate whether prebiotics exert direct or indirect modulatory effects. A recent study investigated the efficacy of β (1–4) GOS on colitis development in Smad3−/− mice after infection with Helicobacter hepaticus[45]. The study found that β (1–4) GOS reduced the severity of colitis, augmented NK cell function and IL-15 production. This was associated with 1.5-fold increase in fecal bifidobacteria. In a different study, Vaisman et al.[154] investigated the effect of a mixture of FOS, β (1–4) GOS, and acidic oligosaccharides on the number and consistency of stools and on immune system biomarkers in children age 9 to 24 months with acute diarrhea. No significant effects were observed on stool characteristics over the first 10 days of treatment, or cytokine profiles during the first 2–3 days of treatment. In fact, a large variability in magnitude and direction of cytokine secretion was detected in patients included in the study. However, when the authors further divided each group dichotomously according to the percentage of subjects who had an increase, no change or a decrease in the levels of different cytokines over the first 2–3 days of treatment, they observed that levels of TNF-α decreased significantly more in the prebiotics supplemented group. Finally, a study investigated the effects of β (1–4) GOS as potential modulators of the elderly intestinal microbiota and their immune system[161]. In the double-blind, placebo-controlled, crossover study, the authors detected significant increases in phagocytosis, NK cell activity, and IL-10 production and a significant reduction of proinflammatory cytokines (IL-6, IL-1β, and tumor necrosis factor-α). It is clear from the limited number of research studies that more studies in animal models as well as in humans are needed to demonstrate an influence of GOS on the immune system. Especially considering that a number of studies suggest a beneficial effect of FOS[9, 37, 54, 167] and HMOs (reviewed by Vos et al.[160]).
In addition to the impact on the gut microbiome and the host immune system, prebiotics may exert beneficial effects by directly inhibiting the adherence of pathogens to the host epithelial cell surface. The proposed mechanism of action is based on the observation that the structure of free oligosaccharides and glycoproteins of human breast milk can resemble pathogen receptors on intestinal epithelial cells and may act as anti-adhesive molecules that competitively inhibit bacterial adherence[68]. In vitro studies showed that GOS significantly inhibited adhesion of microcolony-forming enteropathogenic Escherichia coli E2348/69 on HEp-2 and Caco-2 cells in a dose dependent fashion[142] and reduced the invasion and adherence of Salmonella Thyphimurium in three dimensionally cultured HT-29-16E cells[136]. Moreover, a research study showed that GOS low molecular weight fractions (primarily tri and tetra-saccharides) in BiMuno® significantly stimulated both pro- and anti-inflammatory cytokines in vitro, a mechanism that may contribute to a reduction in Salmonella Typhimurium colonization ability[137]. Additionally, dietary GOS supplementation in neonates in combination with B. breve and B. vulgatus may be an important factor for suppressing growth of C. perfringens[93].
1.4. Enzymatic synthesis of β (1–4) GOS
For a long time, lactose was considered an inconvenient sub product of the dairy industry and a health issue in dairy products due to lactose intolerance in a growing number of individuals. Therefore, lactose hydrolysis to glucose and galactose using β-galactosidases was a common practice until the beneficial impact of GOS on GI health was acknowledged and methods to recycle lactose into a functional food ingredient [β (1–4) GOS] were envisioned. Prior to that GOS generation during this enzymatic process was usually observed and considered detrimental albeit low accumulation of GOS normally occurs during commercial fermented food preparations[81]. Multiple β-galactosidases have been evaluated to improve GOS synthetic activity; however, since they are predominantly hydrolytic enzymes, their efficiency to generate pure GOS is generally limited. Several reactor designs and operational processes have been designed to improve GOS synthesis efficiency[58, 152], but lactose conversion into β (1–4) GOS never exceeds 50%. Such is the case for the β-galactosidase catalyzing the generation of Vivinal GOS® or the process using Sporobolomyces singularis membrane bound β-hexosyl transferase to catalyze the synthesis of Oligomate 55N/55NP (Figure 1). In the final formulation of these products there are significant concentrations of lactose, glucose, and galactose (sugars that can impact the physiological influence of the prebiotic) and the final β (1–4) GOS composition and purity vary due to fundamental process differences. Therefore, the traditional options are β-galactosidases (glycosyl-hydrolases or galactosydases) versus β-hexosyl transferases (glycosyl-transferases or glucosydases). The soluble β-hexosyl transferase from Sporobolomyces singularis permits a more controllable process able to reach values of 95% β (1–4) GOS with a narrower spectrum of oligosaccharides (mostly β (1–4) GOS-3 and β (1–4) GOS-4) and only a residual small proportion of lactose[28].
1.5. Conclusion
Prebiotics have been consumed by humans for millennia. Moreover, the general health benefits associated with prebiotic consumption have been clearly recognized in the past century. Nonetheless, it is now, with the advent of next generation sequencing technology and bioinformatics tools that researchers have the opportunity to dissect dietary effects and identify key bacterial players, components of the gut microbiota, influencing fermentation and generation of secondary metabolites. This review presented research studies on GOS and CRC that clearly suggest a potential role in prevention. Moreover, this role is supported by a randomized, double-blind, diet-controlled clinical trial that demonstrated that GOS significantly increased fecal acetate and decreased DCA and beta-glucuronidase activity in healthy adult men[158]. A clear association between GOS and CRC progression however remains to be elucidated.
Highlights.
β (1–4) galacto-oligosaccharides [β (1–4) GOS], lactulose and fructo-oligosaccharides are prebiotics that have clear role in colorectal cancer (CRC) prevention.
β (1–4) GOS increase intestinal concentration of lactate and short chain fatty acids as well as stool frequency and weight, and they decrease fecal concentration of the secondary bile acid lithocholic acid, fecal pH, and nitroreductase and β-glucuronidase activities.
This review summarizes the current state of research on the bioassimilation of prebiotics through modulation of the intestinal microbiota, and the impact of prebiotics on host physiology and immune system.
We also present studies on a potential role in CRC progression and briefly describe the current state of β (1–4) GOS enzymatic synthesis for industrial production.
Acknowledgments
The Microbiome Core Facility is supported in part by the NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK34987. We gratefully acknowledge Eric Altermann and Temitope Keku for critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U. S. Food and Drug Administration. Complementary and Alternative Medicine Products and their Regulation by the Food and Drug Administration. Guidance for Industry. 2006 Available from: http://www.fda.gov/RegulatoryInformation/Guidances/ucm144657.htm?utm_source=fdaSearch&utm_medium=website&utm_term=prebiotics&utm_content=10.
- 2.Aguilera M, Rakotoarivonina H, Brutus A, Giardina T, Simon G, Fons M. Aga1, the first alpha-Galactosidase from the human bacteria Ruminococcus gnavus E1, efficiently transcribed in gut conditions. Research in microbiology. 2012;163(1):14–21. doi: 10.1016/j.resmic.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JM, Barrangou R, Abou Hachem M, Lahtinen S, Goh YJ, Svensson B, Klaenhammer TR. Transcriptional and functional analysis of galactooligosaccharide uptake by lacS in Lactobacillus acidophilus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(43):17785–90. doi: 10.1073/pnas.1114152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2001;280(5):G922–9. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 5.Austin GL, Adair LS, Galanko JA, Martin CF, Satia JA, Sandler RS. A diet high in fruits and low in meats reduces the risk of colorectal adenomas. J Nutr. 2007;137(4):999–1004. doi: 10.1093/jn/137.4.999. [DOI] [PubMed] [Google Scholar]
- 6.Azcarate-Peril MA, Altermann E, Goh YJ, Tallon R, Sanozky-Dawes RB, Pfeiler EA, O’Flaherty S, Buck BL, Dobson A, Duong T, Miller MJ, Barrangou R, Klaenhammer TR. Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Applied and Environmental Microbiology. 2008;74(15):4610–25. doi: 10.1128/AEM.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azcarate-Peril MA, Sikes M, Bruno-Barcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? American journal of physiology. Gastrointestinal and liver physiology. 2011;301(3):G401–24. doi: 10.1152/ajpgi.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basson MD, Sgambati SA. Effects of short-chain fatty acids on human rectosigmoid mucosal colonocyte brush-border enzymes. Metabolism. 1998;47(2):133–4. doi: 10.1016/s0026-0495(98)90208-6. [DOI] [PubMed] [Google Scholar]
- 9.Bassonga E, Forest V, Pierre F, Bornet F, Perrin P, Meflah K, Menanteau J. Cytokine mRNA expression in mouse colon: IL-15 mRNA is overexpressed and is highly sensitive to a fibre-like dietary component (short-chain fructo-oligosaccharides) in an Apc gene manner. Cytokine. 2001;14(4):243–6. doi: 10.1006/cyto.2001.0872. [DOI] [PubMed] [Google Scholar]
- 10.Bayerdorffer E, Mannes GA, Ochsenkuhn T, Dirschedl P, Wiebecke B, Paumgartner G. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut. 1995;36(2):268–73. doi: 10.1136/gut.36.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72(3):1729–38. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Applied and Environmental Microbiology. 2006;72(5):3593–9. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg EL, Fu CJ, Porter JH, Kerley MS. Fructooligosaccharide supplementation in the yearling horse: effects on fecal pH, microbial content, and volatile fatty acid concentrations. Journal of Animal Science. 2005;83(7):1549–1553. doi: 10.2527/2005.8371549x. [DOI] [PubMed] [Google Scholar]
- 14.Bouhnik Y, Attar A, Joly FA, Riottot M, Dyard F, Flourie B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. European journal of clinical nutrition. 2004;58(3):462–6. doi: 10.1038/sj.ejcn.1601829. [DOI] [PubMed] [Google Scholar]
- 15.Bouhnik Y, Flourie B, Riottot M, Bisetti N, Gailing MF, Guibert A, Bornet F, Rambaud JC. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutrition and Cancer. 1996;26(1):21–9. doi: 10.1080/01635589609514459. [DOI] [PubMed] [Google Scholar]
- 16.Bouhnik Y, Raskine L, Simoneau G, Paineau D, Bornet F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutrition journal. 2006;5:8–13. doi: 10.1186/1475-2891-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P, Marteau P, Flourie B, Bornet F, Rambaud JC. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. The Journal of nutrition. 1999;129(1):113–6. doi: 10.1093/jn/129.1.113. [DOI] [PubMed] [Google Scholar]
- 18.Boutron-Rualt MC, Marteau P, Lavergne-Slove A, Myara A, Gerhardt MF, Franchisseur C, Bornet F, Group ES. Effects of a 3-mo consumption of short-chain fructo-oligosaccharides on parameters of colorectal carcinogenesis in patients with or without small or large colorectal adenomas. Nutrition and Cancer. 2005;53(2):160–8. doi: 10.1207/s15327914nc5302_5. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee IA, Havler ME, Dettmar PW, Allen A, Pearson JP. Colonic mucus: secretion and turnover in relation to dietary fibre intake. The Proceedings of the Nutrition Society. 2003;62(1):245–9. doi: 10.1079/pns2003206. [DOI] [PubMed] [Google Scholar]
- 20.Buecher B, Thouminot C, Menanteau J, Bonnet C, Jarry A, Heymann MF, Cherbut C, Galmiche JP, Blottiere HM. Fructooligosaccharide associated with celecoxib reduces the number of aberrant crypt foci in the colon of rats. Reproduction, nutrition, development. 2003;43(4):347–56. doi: 10.1051/rnd:2003028. [DOI] [PubMed] [Google Scholar]
- 21.Campbell AK, Waud JP, Matthews SB. The molecular basis of lactose intolerance. Science progress. 2005;88(Pt 3):157–202. doi: 10.3184/003685005783238408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell JM, Fahey GC, Jr, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. The Journal of nutrition. 1997;127(1):130–6. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- 23.Challa A, Rao DR, Chawan CB, Shackelford L. Bifidobacterium longum and lactulose suppress azoxymethane-induced colonic aberrant crypt foci in rats. Carcinogenesis. 1997;18(3):517–21. doi: 10.1093/carcin/18.3.517. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Samplin-Salgado M, Ryan CT, Dart H, Fisher L, Tokuda A, Rockhill B. Harvard report on cancer prevention, volume 5: fulfilling the potential for cancer prevention: policy approaches. Cancer Causes & Control: CCC. 2002;13(3):199–212. doi: 10.1023/a:1015040702565. [DOI] [PubMed] [Google Scholar]
- 25.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive . Oncology. 2003;12(5):391–400. [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22(9):763–79. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings JH, Bingham SA, Heaton KW, Eastwood MA. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber) Gastroenterology. 1992;103(6):1783–9. doi: 10.1016/0016-5085(92)91435-7. [DOI] [PubMed] [Google Scholar]
- 28.Dagher SF, Azcarate-Peril MA, Bruno-Barcena JM. Heterologous expression of a bioactive beta-hexosyltransferase, an enzyme producer of prebiotics, from Sporobolomyces singularis. Applied and Environmental Microbiology. 2013;79(4):1241–9. doi: 10.1128/AEM.03491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis LM, Martinez I, Walter J, Goin C, Hutkins RW. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One. 2011;6(9):e25200. doi: 10.1371/journal.pone.0025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Souza Oliveira RP, Rodrigues Florence AC, Perego P, De Oliveira MN, Converti A. Use of lactulose as prebiotic and its influence on the growth, acidification profile and viable counts of different probiotics in fermented skim milk. International journal of food microbiology. 2011;145(1):22–7. doi: 10.1016/j.ijfoodmicro.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Djouzi Z, Andrieux C. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br J Nutr. 1997;78(2):313–24. doi: 10.1079/bjn19970149. [DOI] [PubMed] [Google Scholar]
- 32.Duffaud GD, McCutchen CM, Leduc P, Parker KN, Kelly RM. Purification and characterization of extremely thermostable beta-mannanase, beta-mannosidase, and alpha-galactosidase from the hyperthermophilic eubacterium Thermotoga neapolitana 5068. Applied and Environmental Microbiology. 1997;63(1):169–77. doi: 10.1128/aem.63.1.169-177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Applied and Environmental Microbiology. 2004;70(10):5810–7. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Applied and Environmental Microbiology. 2006;72(12):7835–41. doi: 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, Stahl B, Vigi V. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: A review. Acta Pediatrica. 2007;94(s449):22–26. doi: 10.1111/j.1651-2227.2005.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 36.Floch MH. Soluble dietary fiber and short-chain fatty acids: an advance in understanding the human bacterial flora. Am J Gastroenterol. 1990;85(10):1313–4. [PubMed] [Google Scholar]
- 37.Forest V, Pierre F, Bassonga E, Meflah K, Menanteau J. Large intestine intraepithelial lymphocytes from Apc+/+ and Apc+/Min mice and their modulation by indigestible carbohydrates: the IL-15/IL-15R alpha complex and CD4+ CD25+ T cells are the main targets. Cancer immunology, immunotherapy: CII. 2005;54(1):78–86. doi: 10.1007/s00262-004-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita K, Oura F, Nagamine N, Katayama T, Hiratake J, Sakata K, Kumagai H, Yamamoto K. Identification and molecular cloning of a novel glycoside hydrolase family of core 1 type O-glycan-specific endo-alpha-N-acetylgalactosaminidase from Bifidobacterium longum. The Journal of biological chemistry. 2005;280(45):37415–22. doi: 10.1074/jbc.M506874200. [DOI] [PubMed] [Google Scholar]
- 39.Garvie EI. Bacterial lactate dehydrogenases. Microbiological reviews. 1980;44(1):106–39. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutrition research reviews. 2004;17(2):259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 41.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. The Journal of nutrition. 1995;125(6):1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 42.Gibson GR, Scott KP, Rastall RA, Tuohy K, Hotchkiss AT. Dietary prebiotics: current status and new definition. IFIS. 2010;7(1):1–19. [Google Scholar]
- 43.Goldin B, Gorbach SL. Alterations in fecal microflora enzymes related to diet, age, lactobacillus supplements, and dimethylhydrazine. Cancer. 1977;40(5 Suppl):2421–6. doi: 10.1002/1097-0142(197711)40:5+<2421::aid-cncr2820400905>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Goldin BR, Gorbach SL. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. Journal of the National Cancer Institute. 1976;57(2):371–5. doi: 10.1093/jnci/57.2.371. [DOI] [PubMed] [Google Scholar]
- 45.Gopalakrishnan A, Clinthorne JF, Rondini EA, McCaskey SJ, Gurzell EA, Langohr IM, Gardner EM, Fenton JI. Supplementation with galacto-oligosaccharides increases the percentage of NK cells and reduces colitis severity in Smad3-deficient mice. The Journal of nutrition. 2012;142(7):1336–42. doi: 10.3945/jn.111.154732. [DOI] [PubMed] [Google Scholar]
- 46.Gourineni VP, Verghese M, Boateng J, Shackelford L, Bhat NK, Walker LT. Combinational Effects of Prebiotics and Soybean against Azoxymethane-Induced Colon Cancer In Vivo. Journal of Nutrition and Metabolism. 2011;2011:868197. doi: 10.1155/2011/868197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson GC. Role of mucus layers in gut infection and inflammation. Current opinion in microbiology. 2012;15(1):57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. The Biochemical journal. 1993;293 (Pt 3):781–8. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henrissat B, Bairoch A. Updating the sequence-based classification of glycosyl hydrolases. The Biochemical journal. 1996;316 (Pt 2):695–6. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez-Hernandez O, Muthaiyan A, Moreno FJ, Montilla A, Sanz ML, Ricke SC. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food microbiology. 2012;30(2):355–61. doi: 10.1016/j.fm.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 51.Hofmanova J, Strakova N, Vaculova AH, Tylichova Z, Safarikova B, Skender B, Kozubik A. Interaction of Dietary Fatty Acids with Tumour Necrosis Factor Family Cytokines during Colon Inflammation and Cancer. Mediators Inflamm. 2014;2014:848632. doi: 10.1155/2014/848632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN Journal of parenteral and enteral nutrition. 2012;36(1 Suppl):95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]
- 53.Holt SM, Teresi JM, Cote GL. Influence of alternansucrase-derived oligosaccharides and other carbohydrates on alpha-galactosidase and alpha-glucosidase activity in Bifidobacterium adolescentis. Letters in applied microbiology. 2008;46(1):73–9. doi: 10.1111/j.1472-765X.2007.02266.x. [DOI] [PubMed] [Google Scholar]
- 54.Hosono A, Ozawa A, Kato R, Ohnishi Y, Nakanishi Y, Kimura T, Nakamura R. Dietary fructooligosaccharides induce immunoregulation of intestinal IgA secretion by murine Peyer’s patch cells. Bioscience, biotechnology, and biochemistry. 2003;67(4):758–64. doi: 10.1271/bbb.67.758. [DOI] [PubMed] [Google Scholar]
- 55.Howard MD, Gordon DT, Pace LW, Garleb KA, Kerley MS. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. Journal of pediatric gastroenterology and nutrition. 1995;21(3):297–303. doi: 10.1097/00005176-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Hsu CK, Liao JW, Chung YC, Hsieh CP, Chan YC. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. The Journal of nutrition. 2004;134(6):1523–8. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- 57.Hylemon PB, Harder J. Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS Microbiol Rev. 1998;22(5):475–88. doi: 10.1111/j.1574-6976.1998.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 58.Illanes A. Whey upgrading by enzyme biocatalysis. Electronic Journal of Biotechnology. 2011;14(6) [Google Scholar]
- 59.Iqbal S, Nguyen TH, Nguyen HA, Maischberger T, Kittl R, Haltrich D. Characterization of a heterodimeric GH2 beta-galactosidase from Lactobacillus sakei Lb790 and formation of prebiotic galacto-oligosaccharides. Journal of Agricultural and Food Chemistry. 2011;59(8):3803–11. doi: 10.1021/jf103832q. [DOI] [PubMed] [Google Scholar]
- 60.Iqbal S, Nguyen TH, Nguyen TT, Maischberger T, Haltrich D. beta-Galactosidase from Lactobacillus plantarum WCFS1: biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydrate research. 2010;345(10):1408–16. doi: 10.1016/j.carres.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 61.Johnson IT, Lund EK. Review article: nutrition, obesity and colorectal cancer. Aliment Pharmacol Ther. 2007;26(2):161–81. doi: 10.1111/j.1365-2036.2007.03371.x. [DOI] [PubMed] [Google Scholar]
- 62.Kanakupt K, Vester Boler BM, Dunsford BR, Fahey GC., Jr Effects of short-chain fructooligosaccharides and galactooligosaccharides, individually and in combination, on nutrient digestibility, fecal fermentative metabolite concentrations, and large bowel microbial ecology of healthy adults cats. J Anim Sci. 2011;89(5):1376–84. doi: 10.2527/jas.2010-3201. [DOI] [PubMed] [Google Scholar]
- 63.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan H, Hutkins RW. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Applied and Environmental Microbiology. 2000;66(6):2682–4. doi: 10.1128/aem.66.6.2682-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasbauer CW, Paper DH, Franz G. Sulfated beta-(1-->4)-galacto-oligosaccharides and their effect on angiogenesis. Carbohydr Res. 2001;330(3):427–30. doi: 10.1016/s0008-6215(00)00305-0. [DOI] [PubMed] [Google Scholar]
- 66.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. British Journal of Nutrition. 2009;101(9):1306–15. doi: 10.1017/S0007114508079622. [DOI] [PubMed] [Google Scholar]
- 67.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. British Journal of Nutrition. 2008;99(3):462–71. doi: 10.1017/S0007114507824068. [DOI] [PubMed] [Google Scholar]
- 68.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annual Review of Nutrition. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 69.Le Blay G, Michel C, Blottiere HM, Cherbut C. Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. The Journal of nutrition. 1999;129(12):2231–5. doi: 10.1093/jn/129.12.2231. [DOI] [PubMed] [Google Scholar]
- 70.Leforestier G, Blais A, Blachier F, Marsset-Baglieri A, Davila-Gay AM, Perrin E, Tome D. Effects of galacto-oligosaccharide ingestion on the mucosa-associated mucins and sucrase activity in the small intestine of mice. European journal of nutrition. 2009;48(8):457–64. doi: 10.1007/s00394-009-0036-8. [DOI] [PubMed] [Google Scholar]
- 71.Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care. 2012;15(5):474–9. doi: 10.1097/MCO.0b013e32835665fa. [DOI] [PubMed] [Google Scholar]
- 72.Lewis SJ, Heaton KW. The metabolic consequences of slow colonic transit. The American journal of gastroenterology. 1999;94(8):2010–6. doi: 10.1111/j.1572-0241.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 73.Li M, Bauer LL, Chen X, Wang M, Kuhlenschmidt TB, Kuhlenschmidt MS, Fahey GC, Jr, Donovan SM. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J Nutr. 2012;142(4):681–9. doi: 10.3945/jn.111.154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Applied and Environmental Microbiology. 2010;76(22):7373–81. doi: 10.1128/AEM.00675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lomax AR, Calder PC. Prebiotics, immune function, infection and inflammation: a review of the evidence. The British journal of nutrition. 2009;101(5):633–58. doi: 10.1017/S0007114508055608. [DOI] [PubMed] [Google Scholar]
- 76.London DR, Cuatrecasas P, Birge SJ, Jr, Segal S. Metabolism of lactose by intestinal mucosa from normal and lactase-deficient subjects. British medical journal. 1967;1(5539):524–6. doi: 10.1136/bmj.1.5539.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maathuis AJ, van den Heuvel EG, Schoterman MH, Venema K. Galacto-Oligosaccharides Have Prebiotic Activity in a Dynamic In Vitro Colon Model Using a C-Labeling Technique. The Journal of Nutrition. 2012;142(7):1205–12. doi: 10.3945/jn.111.157420. [DOI] [PubMed] [Google Scholar]
- 78.Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45(Suppl):S120–7. doi: 10.1097/MCG.0b013e31822fecfe. [DOI] [PubMed] [Google Scholar]
- 79.Maischberger T, Leitner E, Nitisinprasert S, Juajun O, Yamabhai M, Nguyen TH, Haltrich D. Beta-galactosidase from Lactobacillus pentosus: purification, characterization and formation of galacto-oligosaccharides. Biotechnology journal. 2010;5(8):838–47. doi: 10.1002/biot.201000126. [DOI] [PubMed] [Google Scholar]
- 80.Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterol Clin North Am. 2008;37(1):73–82. doi: 10.1016/j.gtc.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez-Villaluenga C, Cardelle-Cobas A, Corzo N, Olano A. Study of galactooligosaccharide composition in commercial fermented milks. Journal of Food Composition and Analysis. 2008;21:540–4. [Google Scholar]
- 82.Matthews BW. The structure of E. coli beta-galactosidase. Comptes rendus biologies. 2005;328(6):549–56. doi: 10.1016/j.crvi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 83.McCance RA, Lawrence RD. Medical Research Council Special Report Series. H. M. Stationary Office; London: 1929. The carbohydrate content of foods. [Google Scholar]
- 84.McCleary BV. Dietary fibre analysis. The Proceedings of the Nutrition Society. 2003;62(1):3–9. doi: 10.1079/PNS2002204. [DOI] [PubMed] [Google Scholar]
- 85.McGarr SE, Ridlon JM, Hylemon PB. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol. 2005;39(2):98–109. [PubMed] [Google Scholar]
- 86.McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34(3):386–91. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mei GY, Carey CM, Tosh S, Kostrzynska M. Utilization of different types of dietary fibres by potential probiotics. Canadian Journal of Microbiology. 2011;57(10):857–65. doi: 10.1139/w11-077. [DOI] [PubMed] [Google Scholar]
- 88.Meslin JC, Andrieux C, Sakata T, Beaumatin P, Bensaada M, Popot F, Szylit O, Durand M. Effects of galacto-oligosaccharide and bacterial status on mucin distribution in mucosa and on large intestine fermentation in rats. The British journal of nutrition. 1993;69(3):903–12. doi: 10.1079/bjn19930090. [DOI] [PubMed] [Google Scholar]
- 89.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. The American Journal of Clinical Nutrition. 1999;70(3 Suppl):439S–450S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 90.Midtvedt AC, Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr. 1992;15(4):395–403. doi: 10.1097/00005176-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Minekus M, Smeets-Peeters M, Bernalier A, Marol-Bonnin S, Havenaar R, Marteau P, Alric M, Fonty G, Huis in’t Veld JH. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Applied microbiology and biotechnology. 1999;53(1):108–14. doi: 10.1007/s002530051622. [DOI] [PubMed] [Google Scholar]
- 92.Molis C, Flourie B, Ouarne F, Gailing MF, Lartigue S, Guibert A, Bornet F, Galmiche JP. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. The American journal of clinical nutrition. 1996;64(3):324–8. doi: 10.1093/ajcn/64.3.324. [DOI] [PubMed] [Google Scholar]
- 93.Morishita Y, Oowada T, Ozaki A, Mizutani T. Galactooligosaccharide in combination with Bifidobacterium and Bacteroides affects the population of Clostridium perfringens in the intestine of gnotobiotic mice. Nutrition Research. 2002;22:1333–41. [Google Scholar]
- 94.Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, Boehm G. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. Journal of pediatric gastroenterology and nutrition. 2002;34(3):291–5. doi: 10.1097/00005176-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 95.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 96.Mudd DG, McKelvey ST, Norwood W, Elmore DT, Roy AD. Faecal bile acid concentration of patients with carcinoma or increased risk of carcinoma in the large bowel. Gut. 1980;21(7):587–90. doi: 10.1136/gut.21.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mussatto SI, Mancilha IM. Non-digestible oligosaccharides: A review. Carbohydrate Polymers. 2007;68:587–97. [Google Scholar]
- 98.Nagendra R, Vishwanatha S, Rao SV, Ravish SR. Effect of feeding infant formula containing lactulose on intestinal flora in the infant. Indian journal of pediatrics. 1992;59(6):763–6. doi: 10.1007/BF02859419. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura J, Kubota Y, Miyaoka M, Saitoh T, Mizuno F, Benno Y. Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol Immunol. 2002;46(7):487–90. doi: 10.1111/j.1348-0421.2002.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 100.Newmark HL, Lupton JR. Determinants and consequences of colonic luminal pH: implications for colon cancer. Nutrition and Cancer. 1990;14(3–4):161–73. doi: 10.1080/01635589009514091. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen TH, Splechtna B, Krasteva S, Kneifel W, Kulbe KD, Divne C, Haltrich D. Characterization and molecular cloning of a heterodimeric beta-galactosidase from the probiotic strain Lactobacillus acidophilus R22. Fems Microbiology Letters. 2007;269(1):136–44. doi: 10.1111/j.1574-6968.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 102.Nguyen TH, Splechtna B, Steinbock M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D. Purification and characterization of two novel beta-galactosidases from Lactobacillus reuteri. Journal of Agricultural and Food Chemistry. 2006;54(14):4989–98. doi: 10.1021/jf053126u. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen TT, Nguyen HA, Arreola SL, Mlynek G, Djinovic-Carugo K, Mathiesen G, Nguyen TH, Haltrich D. Homodimeric beta-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: expression in Lactobacillus plantarum and biochemical characterization. Journal of Agricultural and Food Chemistry. 2012;60(7):1713–21. doi: 10.1021/jf203909e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nilsson KGI. Enzymatic synthesis of oligosaccharides. Trends in Biotechnology. 1988;6:256–264. [Google Scholar]
- 105.Nishimoto M, Kitaoka M. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Applied and Environmental Microbiology. 2007;73(20):6444–9. doi: 10.1128/AEM.01425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohtsuka K, Tsuji K, Nakagawa Y, Ueda H, Ozawa O, Uchida T, Ichikawa T. Utilization and metabolism of [U-14C]4′ galactosyllactose (O-beta-D-galactopyranosyl-(1----4)-O-beta-D-galactopyranosyl-(1----4)-D-glucopyranose) in rats. J Nutr Sci Vitaminol (Tokyo) 1991;37(2):173–84. doi: 10.3177/jnsv.37.173. [DOI] [PubMed] [Google Scholar]
- 107.Okazaki N, Jue XX, Miyake H, Kuroda M, Shimamoto T, Tsuchiya T. A melibiose transporter and an operon containing its gene in Enterobacter cloacae. Journal of Bacteriology. 1997;179(13):4443–5. doi: 10.1128/jb.179.13.4443-4445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ozer D, Akin S, Ozer B. Effect of inulin and lactulose on survival of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-02 on Acidophilus-Bifidus yoghurt. Food Science and Technology International. 2005;11:19–24. [Google Scholar]
- 109.Pan XD, Chen FQ, Wu TX, Tang HG, Zhao ZY. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B. 2009;10(4):258–63. doi: 10.1631/jzus.B0820261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panesar PS, Kumari S. Lactulose: production, purification and potential applications. Biotechnology advances. 2011;29(6):940–8. doi: 10.1016/j.biotechadv.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Paturi G, Butts CA, Stoklosinski H, Ansell J. Effects of early dietary intervention with a fermentable fibre on colonic microbiota activity and mucin gene expression in newly weaned rats. Journal of Functional Foods. 2012;4(2):520–30. [Google Scholar]
- 112.Pierre F, Perrin P, Champ M, Bornet F, Meflah K, Menanteau J. Short-chain fructo-oligosaccharides reduce the occurrence of colon tumors and develop gut-associated lymphoid tissue in Min mice. Cancer research. 1997;57(2):225–8. [PubMed] [Google Scholar]
- 113.Priebe MG, Vonk RJ, Sun X, He T, Harmsen HJ, Welling GW. The physiology of colonic metabolism. Possibilities for interventions with pre- and probiotics. European journal of nutrition. 2002;41(Suppl 1):I2–10. doi: 10.1007/s00394-002-1101-8. [DOI] [PubMed] [Google Scholar]
- 114.Rackis JJ, Honig DH, Sessa DJ, Steggerda FR. Flavor and flatulence factors in soybean protein products. Journal of agricultural and food chemistry. 1970;18(6):977–82. doi: 10.1021/jf60172a026. [DOI] [PubMed] [Google Scholar]
- 115.Randi G, Edefonti V, Ferraroni M, La Vecchia C, Decarli A. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev. 2010;68(7):389–408. doi: 10.1111/j.1753-4887.2010.00299.x. [DOI] [PubMed] [Google Scholar]
- 116.Reddy BS, Hamid R, Rao CV. Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis. 1997;18(7):1371–4. doi: 10.1093/carcin/18.7.1371. [DOI] [PubMed] [Google Scholar]
- 117.Reddy BS, Sharma C, Simi B, Engle A, Laakso K, Puska P, Korpela R. Metabolic epidemiology of colon cancer: effect of dietary fiber on fecal mutagens and bile acids in healthy subjects. Cancer research. 1987;47(2):644–8. [PubMed] [Google Scholar]
- 118.Reddy BS, Watanabe K, Weisburger JH, Wynder EL. Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer research. 1977;37(9):3238–42. [PubMed] [Google Scholar]
- 119.Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977;39(6):2533–9. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 120.Roberfroid MB, Bornet F, Bouley C, Cummings JH. Colonic microflora: nutrition and health. Summary and conclusions of an International Life Sciences Institute (ILSI) [Europe] workshop held in Barcelona, Spain. Nutr Rev. 1995;53(5):127–30. doi: 10.1111/j.1753-4887.1995.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 121.Roncucci L, Di Donato P, Carati L, Ferrari A, Perini M, Bertoni G, Bedogni G, Paris B, Svanoni F, Girola M, et al. Antioxidant vitamins or lactulose for the prevention of the recurrence of colorectal adenomas. Colorectal Cancer Study Group of the University of Modena and the Health Care District 16. Diseases of the colon and rectum. 1993;36(3):227–34. doi: 10.1007/BF02053502. [DOI] [PubMed] [Google Scholar]
- 122.Rooney PS, Hunt LM, Clarke PA, Gifford KA, Hardcastle JD, Armitage NC. Wheat fibre, lactulose and rectal mucosal proliferation in individuals with a family history of colorectal cancer. The British journal of surgery. 1994;81(12):1792–4. doi: 10.1002/bjs.1800811228. [DOI] [PubMed] [Google Scholar]
- 123.Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Applied and Environmental Microbiology. 2005;71(10):6150–8. doi: 10.1128/AEM.71.10.6150-6158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rowland I. The influence of the gut microflora on food toxicity. The Proceedings of the Nutrition Society. 1981;40(1):67–74. doi: 10.1079/pns19810011. [DOI] [PubMed] [Google Scholar]
- 125.Rowland IR, Bearne CA, Fischer R, Pool-Zobel BL. The effect of lactulose on DNA damage induced by DMH in the colon of human flora-associated rats. Nutrition and Cancer. 1996;26(1):37–47. doi: 10.1080/01635589609514461. [DOI] [PubMed] [Google Scholar]
- 126.Rowland IR, Tanaka R. The effects of transgalactosylated oligosaccharides on gut flora metabolism in rats associated with a human faecal microflora. The Journal of applied bacteriology. 1993;74(6):667–74. doi: 10.1111/j.1365-2672.1993.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 127.Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scandinavian journal of gastroenterology Supplement. 1997;222:45–8. doi: 10.1080/00365521.1997.11720717. [DOI] [PubMed] [Google Scholar]
- 128.Salvini F, Riva E, Salvatici E, Boehm G, Jelinek J, Banderali G, Giovannini M. A specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects. The Journal of nutrition. 2011;141(7):1335–9. doi: 10.3945/jn.110.136747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samelson SL, Nelson RL, Nyhus LM. Protective role of faecal pH in experimental colon carcinogenesis. Journal of the Royal Society of Medicine. 1985;78(3):230–3. doi: 10.1177/014107688507800311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sangwan V, Tomar SK, Singh RR, Singh AK, Ali B. Galactooligosaccharides: novel components of designer foods. Journal of Food Science. 2011;76(4):R103–11. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- 131.Sarmiento-Rubiano LA, Zuniga M, Perez-Martinez G, Yebra MJ. Dietary supplementation with sorbitol results in selective enrichment of lactobacilli in rat intestine. Research in Microbiology. 2007;158(8–9):694–701. doi: 10.1016/j.resmic.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 132.Sato T, Matsumoto K, Okumura T, Yokoi W, Naito E, Yoshida Y, Nomoto K, Ito M, Sawada H. Isolation of lactate-utilizing butyrate-producing bacteria from human feces and in vivo administration of Anaerostipes caccae strain L2 and galacto-oligosaccharides in a rat model. FEMS Microbiology Ecology. 2008;66(3):528–36. doi: 10.1111/j.1574-6941.2008.00528.x. [DOI] [PubMed] [Google Scholar]
- 133.Scalabrin DM, Mitmesser SH, Welling GW, Harris CL, Marunycz JD, Walker DC, Bos NA, Tolkko S, Salminen S, Vanderhoof JA. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. Journal of pediatric gastroenterology and nutrition. 2012;54(3):343–52. doi: 10.1097/MPG.0b013e318237ed95. [DOI] [PubMed] [Google Scholar]
- 134.Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682(1):39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 135.Schuster-Wolff-Buhring R, Fischer L, Hinrichs J. Production and physiological action of the disaccharide lactulose. International Dairy Journal. 2010;20:731–41. [Google Scholar]
- 136.Searle LEJ, Cooley WA, Jones G, Nunez A, Crudgington B, Weyer U, Dugdale AH, Tzortzis G, Collins JW, Woodward MJ, La Ragione RM. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. Journal of Medical Microbiology. 2010;59(12):1428–39. doi: 10.1099/jmm.0.022780-0. [DOI] [PubMed] [Google Scholar]
- 137.Searle LEJ, Jones G, Tzortzis G, Woodward M, Rastall RA, Gibson GR, La Ragione RM. Low molecular weight fractions of BiMuno® exert immunostimulatory properties in murine macrophages. Journal of Functional Foods. 2012;4(4):941–53. [Google Scholar]
- 138.Seki N, Hamano H, Iiyama Y, Asano Y, Kokubo S, Yamauchi K, Tamura Y, Uenishi K, Kudou H. Effect of lactulose on calcium and magnesium absorption: a study using stable isotopes in adult men. Journal of Nutritional Science and Vitaminology. 2007;53(1):5–12. doi: 10.3177/jnsv.53.5. [DOI] [PubMed] [Google Scholar]
- 139.Shafe AC, Lee S, Dalrymple JS, Whorwell PJ. The LUCK study: Laxative Usage in patients with GP-diagnosed Constipation in the UK, within the general population and in pregnancy. An epidemiological study using the General Practice Research Database (GPRD) Therapeutic advances in gastroenterology. 2011;4(6):343–63. doi: 10.1177/1756283X11417483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shen Q, Tuohy KM, Gibson GR, Ward RE. In vitro measurement of the impact of human milk oligosaccharides on the faecal microbiota of weaned formula-fed infants compared to a mixture of prebiotic fructooligosaccharides and galactooligosaccharides. Lett Appl Microbiol. 2011;52(4):337–43. doi: 10.1111/j.1472-765X.2011.03005.x. [DOI] [PubMed] [Google Scholar]
- 141.Shimamoto T, Xu XJ, Okazaki N, Kawakami H, Tsuchiya T. A cryptic melibiose transporter gene possessing a frameshift from Citrobacter freundii. Journal of biochemistry. 2001;129(4):607–13. doi: 10.1093/oxfordjournals.jbchem.a002897. [DOI] [PubMed] [Google Scholar]
- 142.Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infection and Immunity. 2006;74(12):6920–28. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silvestroni A, Connes C, Sesma F, De Giori GS, Piard JC. Characterization of the melA locus for alpha-galactosidase in Lactobacillus plantarum. Applied and Environmental Microbiology. 2002;68(11):5464–71. doi: 10.1128/AEM.68.11.5464-5471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suarez FL, Springfield J, Furne JK, Lohrmann TT, Kerr PS, Levitt MD. Gas production in human ingesting a soybean flour derived from beans naturally low in oligosaccharides. The American journal of clinical nutrition. 1999;69(1):135–9. doi: 10.1093/ajcn/69.1.135. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki H, Li SC, Li YT. Alpha-galactosidase from Mortierella vinacea. Crystallization and properties. The Journal of biological chemistry. 1970;245(4):781–6. [PubMed] [Google Scholar]
- 146.Swennen K, Courtin CM, Delcour JA. Non-digestible oligosaccharides with prebiotic properties. Critical reviews in food science and nutrition. 2006;46(6):459–71. doi: 10.1080/10408390500215746. [DOI] [PubMed] [Google Scholar]
- 147.Tabbers MM, Boluyt N, Berger MY, Benninga MA. Clinical practice: diagnosis and treatment of functional constipation. European journal of pediatrics. 2011;170(8):955–63. doi: 10.1007/s00431-011-1515-5. [DOI] [PubMed] [Google Scholar]
- 148.Takemura N, Ozawa K, Kimura N, Watanabe J, Sonoyama K. Inulin-type fructans stimulated the growth of exogenously administered Lactobacillus plantarum No. 14 in the mouse gastrointestinal tract. Bioscience, biotechnology, and biochemistry. 2010;74(2):375–81. doi: 10.1271/bbb.90794. [DOI] [PubMed] [Google Scholar]
- 149.Talbot G, Sygusch J. Purification and characterization of thermostable beta-mannanase and alpha-galactosidase from Bacillus stearothermophilus. Applied and Environmental Microbiology. 1990;56(11):3505–10. doi: 10.1128/aem.56.11.3505-3510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tamura Y, Mizota T, Shimamura S, Tomita M. Lactulose and Its Application to the Food and Pharmaceutical Industries. Bulletin of the International Dairy Federation No 289. 1993;1993:43–53. [Google Scholar]
- 151.Tong JL, Ran ZH, Shen J, Fan GQ, Xiao SD. Association between fecal bile acids and colorectal cancer: a meta-analysis of observational studies. Yonsei Medical Journal. 2008;49(5):792–803. doi: 10.3349/ymj.2008.49.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Torres DPM, Goncalves MP, Teixeira JA, Rodrigues LR. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Comprehensive Reviews in Food Science and Food Safety. 2010;9(5):438–54. doi: 10.1111/j.1541-4337.2010.00119.x. [DOI] [PubMed] [Google Scholar]
- 153.Tzortzis G, Jay AJ, Baillon ML, Gibson GR, Rastall RA. Synthesis of alpha-galactooligosaccharides with alpha-galactosidase from Lactobacillus reuteri of canine origin. Applied microbiology and biotechnology. 2003;63(3):286–92. doi: 10.1007/s00253-003-1426-0. [DOI] [PubMed] [Google Scholar]
- 154.Vaisman N, Press J, Leibovitz E, Boehm G, Barak V. Short-term effect of prebiotics administration on stool characteristics and serum cytokines dynamics in very young children with acute diarrhea. Nutrients. 2010;2(7):683–92. doi: 10.3390/nu2070683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Berge Henegouwen GP, van der Werf SD, Ruben AT. Effect of long term lactulose ingestion on secondary bile salt metabolism in man: potential protective effect of lactulose in colonic carcinogenesis. Gut. 1987;28(6):675–80. doi: 10.1136/gut.28.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van den Heuvel EG, Mujis T, Van Dokkum W, Schaafsma G. Lactulose stimulates calcium absorption in postmenopausal women. Journal of Bone and Mineral Research. 1999;14(7):1211–16. doi: 10.1359/jbmr.1999.14.7.1211. [DOI] [PubMed] [Google Scholar]
- 157.Van der Meulen R, Adriany T, Verbrugghe K, De Vuyst L. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Applied and Environmental Microbiology. 2006;72(8):5204–10. doi: 10.1128/AEM.00146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Dokkum W, Wezendonk B, Srikumar TS, van den Heuvel EG. Effect of nondigestible oligosaccharides on large-bowel functions, blood lipid concentrations and glucose absorption in young healthy male subjects. European journal of clinical nutrition. 1999;53(1):1–7. doi: 10.1038/sj.ejcn.1600668. [DOI] [PubMed] [Google Scholar]
- 159.Vanhoutte T, De Preter V, De Brandt E, Verbeke K, Swings J, Huys G. Molecular monitoring of the fecal microbiota of healthy human subjects during administration of lactulose and Saccharomyces boulardii. Applied and Environmental Microbiology. 2006;72(9):5990–7. doi: 10.1128/AEM.00233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vos AP, M’Rabet L, Stahl B, Boehm G, Garssen J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Critical Reviews in Immunology. 2007;27(2):97–140. doi: 10.1615/critrevimmunol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 161.Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. The American Journal of Clinical Nutrition. 2008;88(5):1438–46. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 162.Wada J, Ando T, Kiyohara M, Ashida H, Kitaoka M, Yamaguchi M, Kumagai H, Katayama T, Yamamoto K. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Applied and Environmental Microbiology. 2008;74(13):3996–4004. doi: 10.1128/AEM.00149-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wijnands MV, Appel MJ, Hollanders VM, Woutersen RA. A comparison of the effects of dietary cellulose and fermentable galacto-oligosaccharide, in a rat model of colorectal carcinogenesis: fermentable fibre confers greater protection than non-fermentable fibre in both high and low fat backgrounds. Carcinogenesis. 1999;20(4):651–6. doi: 10.1093/carcin/20.4.651. [DOI] [PubMed] [Google Scholar]
- 164.Wijnands MV, Schoterman HC, Bruijntjes JB, Hollanders VM, Woutersen RA. Effect of dietary galacto-oligosaccharides on azoxymethane-induced aberrant crypt foci and colorectal cancer in Fischer 344 rats. Carcinogenesis. 2001;22(1):127–32. doi: 10.1093/carcin/22.1.127. [DOI] [PubMed] [Google Scholar]
- 165.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 166.Yoshida E, Sakurama H, Kiyohara M, Nakajima M, Kitaoka M, Ashida H, Hirose J, Katayama T, Yamamoto K, Kumagai H. Bifidobacterium longum subsp. infantis uses two different beta-galactosidases for selectively degrading type-1 and type-2 human milk oligosaccharides. Glycobiology. 2012;22(3):361–8. doi: 10.1093/glycob/cwr116. [DOI] [PubMed] [Google Scholar]
- 167.Zenhom M, Hyder A, de Vrese M, Heller KJ, Roeder T, Schrezenmeir J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARgamma and peptidoglycan recognition protein 3. The Journal of Nutrition. 2011;141(5):971–7. doi: 10.3945/jn.110.136176. [DOI] [PubMed] [Google Scholar]
- 168.Zhang X, Vrijenhoek JE, Bonten MJ, Willems RJ, van Schaik W. A genetic element present on megaplasmids allows Enterococcus faecium to use raffinose as carbon source. Environmental Microbiology. 2011;13(2):518–28. doi: 10.1111/j.1462-2920.2010.02355.x. [DOI] [PubMed] [Google Scholar]
- 169.Zhao H, Lu L, Xiao M, Wang Q, Lu Y, Liu C, Wang P, Kumagai H, Yamamoto K. Cloning and characterization of a novel alpha-galactosidase from Bifidobacterium breve 203 capable of synthesizing Gal-alpha-1,4 linkage. FEMS Microbiology Letters. 2008;285(2):278–83. doi: 10.1111/j.1574-6968.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 170.Zhong Y, Cai D, Cai W, Geng S, Chen L, Han T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin Nutr. 2009;28(5):575–80. doi: 10.1016/j.clnu.2009.04.026. [DOI] [PubMed] [Google Scholar]