Abstract
Professor Shu Chien is a world-renowned leader and founder of Bioengineering. In particular, he has made seminal contributions to advancing our systematic and insightful understanding of how cells perceive their physical/mechanical environment and coordinate cellular functions. In this review, as part of a tribute to Prof. Shu Chien's scientific achievement, we summarize the research progress in understanding the physiology of bone cells interacting with different mechanical/physical environments during bone tissue regeneration/repair. We first introduce the cellular composition of the bone tissue and the mechanism of the physiological bone regeneration/repair process. We then describe the properties and development of biomaterials for bone tissue engineering, followed by the highlighting of research progresses on the cellular response to mechanical environmental cues. Finally, several latest advancements in bone tissue regeneration and remaining challenges in the field are discussed for future research directions.
Keywords: Mechanical Environment, Bone repair, Tissue engineering, scaffolds, Bone cells
1. Introduction
Bones are important structural components of vertebrates that are composed of 60% hydroxyapatite, 10% water and 30% collagen proteins. Bones play important roles in providing mechanical support for locomotion, protecting vital organs, and regulating the metabolism of calcium and phosphorus. The lifelong execution of these functions requires a healthy bone system [1]. However, millions of people worldwide suffer from bone defects due to various reasons, including trauma, tumor, bone diseases, congenital defects and aging. These defects are increasingly becoming the majority of the clinical cases in orthopedics. Accordingly, bone repair has been the focus of many research activities related to clinical therapies.
Recent advances in bone repair research have highlighted hybrid cell-material structures for tissue engineering, which provide a unique self-repair capacity with minimally invasive surgery. These structures are seeded with stem cells from the patients themselves, usually derived from bone marrow, to allow the process of proliferation and differentiation into different bone cells under in vitro conditions [2]. This process of new bone tissue regeneration is managed by the bone cells under a tightly controlled microenvironment including both chemical and mechanical stimuli, e.g. the chemical and physical characteristics of the scaffold [3, 4].
Accordingly, we provide an overview of the bone cell physiology and repair process in this review, with specific attentions focused on the effect of the scaffold’s chemical and physical properties on bone cell functions.
2 Bone Cells and Repair Mechanisms
2.1 Bone Tissue and Cellular Composition
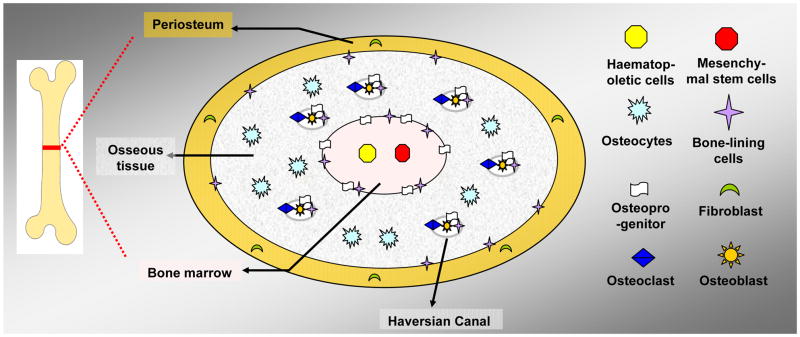
Bone structures contain cells derived from different sources [3]. The multicellular structures of bones consist of four parts, extending from inside to outside: bone marrow, endosteum, porous mineralized osseous tissue, and periosteum (a membrane that lines along the outer surface of bones) (Figure 1).
Figure 1.
The basic multi-cellular structure of the bone depicted in a simple diagram. The bone is remodeled by cells derived from different sources, which are collectively called the basic multi-cellular units (not including the blood vessels and nervous tissue).
The bone marrow contains haematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). The endosteum, located at the walls of the bone marrow cavity and the mesh of cancellous bones, contains the progenitor of osteoblasts (specialized cells capable of matrix secretion and mineralization) and osteoclasts (specialized in bone resorption). In the osseous tissue, there is abundant mineralized bone matrix and five kinds of cells: (1) osteoprogenitors, including the progenitors of osteoblasts and osteoclasts, (2) osteoblasts, (3) osteocytes (a derivative of osteoblasts), (4) osteoclasts, and (5) bone-lining cells (inactive osteoblasts which can be re-activated by mechanical stimulus) [3, 5, 6]. Among these bone cells, osteocytes and bone-lining cells are the most abundant. The osteocytes are located in the spaces within the bone matrix, while bone lining cells cover all bone surfaces. The other three kinds of cells reside at the trabecular surface in cancellous bone or on the surface of the Haversian canals in the osseous tissues of compact bone [3, 6, 7]. Periosteum forms a layer outside of the osseous tissue. Both periosteum and endosteum play important roles in bone formation and growth and the bone remodeling process [8].
2.2 Physiological Processes of Bone Resorption, Formation, and Repair
According to Albright’s metabolic balance theory [9], the structure of human bone tissue is constantly maintained at a steady state by the dynamic balance between the bone resorption and bone remodeling processes. These two processes occur coordinately at different locations, governed by the bone dynamic balance, with the resorption of old bones followed by replacement with new bones [9, 10]. An imbalance between bone resorption and formation is known to be closely associated with serious bone diseases, such as osteoporosis (OP) and osteoarthritis (OA) [3, 11, 12].
The resorption of pre-existing bone by mature osteoclasts is followed by the formation of new bone by osteoblasts. This coupling of resorption and regeneration processes, under a dynamic balance, occurs throughout a bone’s lifespan. For example, a mature bone continuously remodels with an estimated rate of 10% turnover every year [8, 10]. Therefore, in bone tissue engineering, a designed scaffold material should allow a similar resorption/regeneration process to occur.
During the bone repair process, HSCs differentiate into committed osteoclast progenitor cells, or pre-osteoclasts, which enter the healing site through circulation and fuse with each other to become mature osteoclasts [3]. Meanwhile, MSCs can migrate to the healing site and differentiate into chondrocytes (producing the cartilage) and osteoblasts. The defective portions of bones are removed by osteoclast-mediated resorption and replaced by new bone matrix secreted and mineralized by osteoblasts. These new bone tissues are maintained by the mineralized osteocytes and bone-lining cells [3, 8, 10]. Osteoclasts can also regulate the differentiation of osteoblast precursors and the migration of hematopoietic stem cells and secrete cytokines in inflammatory and neoplastic processes [13].
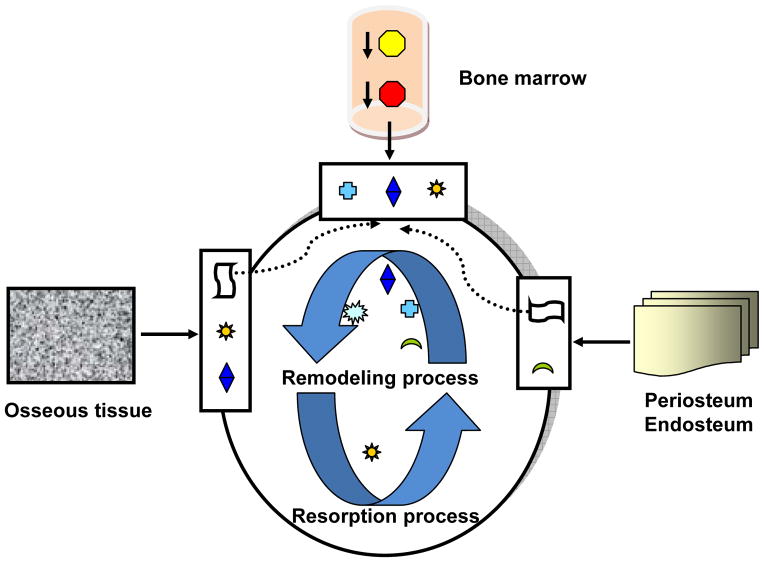
The regeneration process of damaged bone tissue can hence be summarized in Figure 2 [8, 14–16], with multiple well-coordinated steps: (1) The regeneration of periosteum: the fibroblasts within the periosteum start to divide in order to generate enough cells to form new periosteum, closing the defective gap at the surface; (2) The resorption process: The HSCs from bone marrow differentiate into pre-osteoclasts, which enter the circulation, migrate to the defect location and fuse to form mature osteoclasts, under the influence of the osteoprogenitor cells from osseous tissue, periosteum and endosteum [8]. The mature osteoclasts are multinuclear, macrophage-like cells, which can start the resorption process of the calcified matrix fragments at the edge of defective bone under the influence of osteoblasts; (3) The remodeling process: After resorption, MSCs and osteoprogenitor cells are stimulated to migrate toward the healing site and differentiate into chondrocytes and osteoblasts to form new bone tissue. The new bone tissues are composed of cartilage osteoid matrices, which are later replaced by mineralized mature osteocytes. This newly formed bone tissue is usually called ‘woven bone’, which still needs to be remodeled by the repeating resorbing and regeneration processes [8].
Figure 2.
The bone resorption and remodeling process, depicted in a simplified diagram (not depicted is the role of vascularization). There are a variety of cells involved in the regeneration process, including HSCs, MSCs, osteoprogenitor cells, osteoblasts, osteocytes, osteoclasts and fibroblasts, which are originated from bone marrow, osseous tissues, periosteum, and endosteum (based on Simmons DJ 1990; Rydziel S 1994; Silva GA. 2007).
2.3 Mesenchymal Stem Cells
Mesenchymal stem cells are precursor cells derived from any tissue of mesenchyme origin which are uniquely capable of both differentiation (to produce mature daughter cells that carry out particular functions) and self-renewal (to sustain and replenish the stem cell pool). They play critical roles in the establishment of tissues during development and sometimes are retained into adulthood, where they support ongoing replacement of short-lived cells as well as injury-induced regeneration of daughter cells [17–20]. In particular, MSCs are at the center stage of bone tissue engineering because they are multipotent cells capable of self-renewal and differentiation into multiple tissues (including bone, cartilage, muscle, fat, skin, and connective tissue). These capabilities can be applied clinically as a new therapy for treating a variety of bone diseases.
Recent research advancements about MSCs at the cell biology level have delineated the mechanisms that regulate the differentiation of the MSCs [3, 8, 10, 21–23]. These studies have identified that extracellular biochemical stimuli, including hormones, cytokines (e.g. BMPs, the TGF-β family, VEGF, IGF-I, FGFs, IRAP, IL-4, PDGF), and mechanical stimuli, can regulate the differentiation and activity of MSCs [15]. Intracellular signaling molecules (e.g. LMP-1, Smad1, 5 and 8, FAK, Ca2+) can regulate gene expression through activating transcription factors, such as Runx2, Brachyury, or Sox-9. Therefore, these regulation signaling molecules can be targeted to facilitate the differentiation of MSCs for bone therapy [24–26]. For example, it has been shown that electrical stimulation can regulate calcium oscillation and hence promote osteogenesis involving HMSCs. Physical properties such as cell shape can also determine the fate of HMSCs. Flat and spread cells underwent osteogenesis via RhoA activation and up-regulation of actomyosin tension while round cells became adipocytes, via RhoA inhibition [27]. At the transcription level, the transcription factor Sox9 is indispensable for chondrogenesis, while Runx2 is essential for osteoblast differentiation and Wnt signaling appears to switch the chondrocyte fate into osteoblasts [10]. Therefore, the understanding of these cell signaling pathways can provide a foundation for the design of bone repair therapies that mimic natural tissue formation [21, 24].
3 Biomaterials for Bone Tissue Engineering
Although small-scale bone fractures can be easily self-repaired, large-scale bone defects (i.e., comminuted fracture, osteoporotic and cartilaginous tissues) are difficult to be self-healed via regeneration and remodeling [21]. In this case, surgery is often required to implant a bone graft or artificial materials at the site of defect [28]. According to statistics, there are more than 2 million operations requiring bone substitutes each year in the United States alone and the demand continues to rise drastically. For example, there will be a 174% increase for first-time total hip replacements, and a 673% increase for first-time total knee replacements by the year 2030 (AAOS 2006c). Such statistics does not include the steadily growing number of revision surgeries resulting from the average lifespan (only 10 to 15 years) of an orthopedic implant [21]. Clearly, with increasing clinical needs, bone implants are in high demand, with a significant socioeconomic impact worldwide.
The traditional bone repair procedure involves the use of autografts (from the patient iliac crest) and allografts (from the cadaver bone) [29]. The autograft has been considered the gold standard in the reconstruction of bone defects until now, because it has structural stability and natural osteogenic ability [30, 31]. Although the autograft and allograft procedures have been fairly successful, there are serious limitations such as limited supply of donor bone tissue, unpredictable rejection characteristics, and infection [2, 29]. Particularly, large bone defects are a major clinical problem since autologous bone grafts are not available in up to 40% of these patients [32]. Therefore, there is a pressing need for more reliable and abundant bone substitutes to replace or repair bone defects in clinics.
3.1 Three Generations of Bone Materials
In past decades, with the development in the fields of materials and biomaterials, a series of bone graft substitutes have been produced. There are currently over 100 approved bone replacement materials in Germany alone [31], through which three different generations have been evolved [33, 34]: the first-generation “bioinert” materials; the second-generation “bioactive and biodegradable” materials; and the third-generation “cell- and gene-activating” materials (designed to stimulate specific cellular responses at the molecular level with the aim of developing materials that, once implanted, will stimulate the body to heal by itself). These three generations of materials are shown in Table 1 as follows [21, 27, 33–36].
Table 1.
Summary of three generations of biomaterials for bone repair
| Different Generations of biomaterials | Time | Characteristics | Composition |
|---|---|---|---|
| First generation | 1960s–1970s | Bioinert | Metal: stainless steel, Ti and Ti alloys Ceramic: alumina, zirconia Polymer: silicone rubber, polyethylene( PE), acrylic resins,polyurethanes, polypropylene (PP) and polymethylmethacrylate(PMMA). |
| Second generation | 1980s–2000s | Bioactive, Biodegradable | Metal: surface coated with bioactive components: Hydroxylapatite (HA) and Bioglasses (BGs) Ceramic: BGs, Calcium phosphate(CaPs), HA Polymer: polylactide( PLA), polyglycolide(PGA), polydioxanone (PDS),chitosan(CS) |
| Third generation | From 2000s | Cell-, gene-activating | A hybrid consist of scaffolds, cells, and active factors |
During the 1960s and 1970s, the first-generation materials were developed with the simple goal of achieving “a suitable combination of physical properties to match those of the replaced tissue with a minimal toxic response in the host” [34]. In 1980, around 2 to 3 million prosthetic parts were implanted in patients in the United States, which enhanced these patients’ life quality for 5 to 25 years with such “inert” biomaterials [34, 37]. In the mid-1980s, the second generation “bioactive” materials were developed and applied in clinics for orthopedic and dental applications. By the 1990s, bioactive composites, including hydroxyapatite particles, have become important in the repair and replacement of bones [34, 35, 37]. Although some of the above first and second generation substitutes have been successfully applied to replace or repair bone defects in clinics, they are limited in part due to the fact that they are man-made, and thus do not respond to physiological loads or biochemical stimuli. For most first and second generation biomaterials, stress shielding effects can result from the mismatch of the mechanical properties between the host bone and the implant. In addition, the implant surface properties can inhibit the regeneration of new tissue, which can lead to loosening of the implant from host bones. This became the main reason for orthopedic implant failure, with one third to one half of prostheses failing within 10 to 25 years [34]. New strategies are hence needed to further improve the repair and regeneration of bone tissues.
During the first decade of the 21st century, the new third generation, “cell- and gene-activating” biomaterials (also called bone tissue engineering materials), have been tailored into extracellular matrix (ECM) scaffolds. This allows the bone progenitor cells to seed on scaffolds for proliferation and differentiation in vitro, thus better mimicking naturally surrounding tissue before being implanted into the patients [35]. Bone tissue engineering has gained increasing recognition to treat bone defects, since it can stimulate new bone tissue regeneration in the host by inducing bone cell adhesion and proliferation. This provides a more effective approach than the traditional methods [29, 35]. To date, one of the most advanced bone tissue engineering methods is to transplant hybrid cell-material constructs into patients, which incorporate cells, a 3D porous scaffold, and bioactive factors as an integrated bone graft substitute. This hybrid construct is a typical third-generation “cell- and gene-activating” material (Figure 3) [2, 29, 34, 38] . After a minimally invasive surgery to insert the hybrid construct to the diseased or injured site, the self-healing process occurs by stimulating the specific response of cells at a molecular level, activating specific gene expression to regulate regeneration, and gradually replacing the missing bone with newly formed tissue [34, 39]. Thus, the new bone repair therapy can be simply defined as the ‘science of persuading the body to heal by its intrinsic repair mechanisms’ [8, 40].
Figure 3.
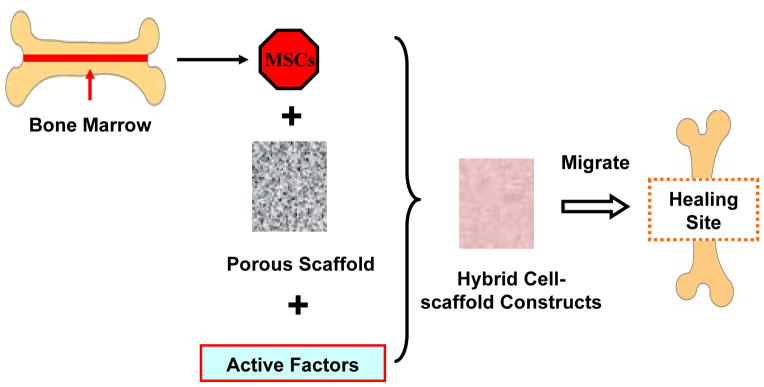
A simplified diagram of bone tissue engineering. The MSCs derived from the bone marrow are seeded into 3D porous scaffold materials along with some transcription factors and growth factors. The cells then continue to differentiate and grow in the hybrid cell-scaffold constructs. Upon maturity, the cells may migrate from the engineered scaffold to the healing site of patients.
In summary, it is clear that the new generation of bone repair strategy utilizes biomaterials to support cells. These new materials must be biodegradable, nontoxic, resorbable, and have proper physical properties.
3.2 Mesenchymal Stem Cells in Bone Tissue Engineering
Bone tissue engineering and regeneration approaches for therapies often involve isolating and culturing MSCs in vitro, followed by implantation into the defect site. These strategies typically harvest MSCs from the patient’s body (including bone marrow stroma, muscle, skin, fat, and gingiva) to avoid immunological complications. MSCs have been isolated from bone marrow and applied in bone, cartilage, and ligament repair [21, 24, 41–43]. In 1991, Caplan first proposed that the isolation, mitotic expansion, and site-directed delivery of autologous stem cells can govern the rapid and specific repair of skeletal tissues [44]. Later, Quarto et al. published the first clinical paper to report the successful repair of large bone defects by using autologous bone marrow stromal cells in 2001 [41]. Schimming first demonstrated in 2004 that periosteum derived osteoblasts can form lamellar bone within three months after transplantation [45].
The ultimate goal of bone tissue regeneration is the effective and efficient formation of new bones. The inducible expression systems are therefore important because they can directly regulate the proliferation and differentiation of osteoblasts and osteoclasts [46–50]. As early as 1965, Urist first proposed that bone tissue contains specific growth factors capable of inducing bone formation in ectopic sites [51]. Later in 1974, he discovered bone morphogenetic protein (BMP). BMP can bind to the extracellular receptor and ultimately promote gene expression and the development of bone cells from MSCs [52]. Later, specialized cocktail additives have been developed to induce the differential specific bone cells. For example, a chondrogenic cocktail containing TGF-beta, ITS+ Premix, and dexamethasone has been used to induce the MSC chondrogenesis [53, 54]. In a recent study, MSCs were mixed with matrigel, seeded on 3D-woven poly(ε-caprolactone) (PLC) scaffolds and cultured in a bioreactor with bi-directional medium perfusion. The MSCs and a tightly woven PLC scaffold formed functional tissue cartilage with mechanical properties similar to those of normal articular cartilage after 21 days of culture with or without perfusion [53].
To further enhance the long-term growth and differentiation of MSCs, genetic engineering approaches have been developed to introduce target genes encoding functional proteins into MSCs by viral or non-viral carriers[21, 55]. In addition to their osteogenic roles in tissue engineering, MSCs have also been used as delivery machinery of bioactive and immunosuppressive molecules in regenerative medicine with some success [24, 56, 57]. It remains important to further investigate the molecular regulation of the MSCs and to provide more guidance toward their clinical applications. Specifically, the biochemical and physical environment has profound effects on the differentiation and functional regulation of MSCs which remain to be further studied.
4. Physical/Mechanical Environmental Effects on Bone Cell Functions
In the process of regenerative bone tissue engineering, MSCs initially exit from their niche and migrate toward their target location where they engraft and differentiate according to their microenvironment [58]. This bone formation process is largely affected by the physical/mechanical properties of the environment. Therefore, analysis of the differentiation process must take into account the physical/mechanical environment. Currently, the underlying principles involved in the mechanical-regulation of MSC differentiation remain unclear [59].
4.1 The Effect of Porosity and Pore Size of Scaffold
Scaffolds are designed to support the cells, guide their differentiation by controlling their shapes and acting as a temporary synthetic extracellular matrix (Figure 3). As such, they are crucial in tissue engineering for bone regeneration.
Three-dimensional (3D) bone tissue engineering scaffolds have been designed to mimic the features of the in vivo environment [38]. Porous scaffolds can be beneficial for bone tissue formation since they allow for migration, metabolism and vascularization of bone cells [60]. In addition, scaffolds with porous surfaces allow for better interfacing of the implant biomaterial to the host microenvironment [61]. While scaffolds with higher porosity and pore size result in better interaction with the surrounding tissue (and thus in better in vivo in growth of the bone cells), they will also result in diminished mechanical properties. Therefore, scaffolds must be carefully designed to match their mechanical properties with those of the host tissue [61–63]. Bone has heterogeneity at different body parts and among different individuals, therefore it will also be important to optimize the bone material for individual repair. The optimization of porosities and pore sizes in vitro has been done with a range of porosities from 25% to 94% and pores sizes from 6 μm to 900 μm. The results show that higher porosities, >80%, and a pore diameter distribution in the range of 200 μm-400 μm are optimal for new bone tissue regeneration [64–67]. In an earlier work, the minimum pore size for a scaffold was considered to be about 100 μm due to cell size for the migration and transport requirements [68]. However, subsequent studies found that pore sizes above 200 μm are recommended, due to enhanced formation of new bone and vascularization [69]. Karageorgiou et al. further confirmed that small pores are better for inducing osteochondral formation before osteogenesis, while large pores facilitate well-vascularized osteogenesis without preceding cartilage formation [61].
Further studies are, however, still needed for the systematic understanding of porosity and pore sizes effects on the osteogenic outcome of different scaffolds [61].
4.2 The Effect of Physical Properties of Scaffold Surface
Recently, the roles of implant surface have been actively investigated, since the surface is in direct contact with host living tissues. Current orthopedic implant surfaces still need improvement, possibly due to the lack of appropriate cell adhesion (the physiochemical linkage and protein interactions between cells and the implant surface) and implant-tissue integration (the bonding between the implant and surrounding living tissue). These are the main reasons leading to clinical complications that shorten the lifespan of current orthopedic implants [70].
Although the chemical properties of the extracellular matrix are well-known regulators of cellular responses, physical properties of the matrix in a 3D scaffold can also play important roles in regulating the initial response of bone cells around the implant [59, 71]. In fact, MSCs are able to sense the roughness, stiffness and texture of their surroundings and then use this information to decide their fate during differentiation [59]. Other studies have also shown that the cell-matrix interactions are largely influenced by the surface roughness and topography [70, 72–74]. According to these results (Table 2), some inherent properties of the surface, particularly surface energy, physico-chemical properties, and roughness, strongly affect the protein adsorption process, and therefore, the interactions between the cells and their environment. The surface properties also have an effect on cell adhesion, protein arrangement and proteoglycans of the cell's extracellular matrix [75].
Table 2.
The effect of materials surface on bone cells
| Object | Results | References |
|---|---|---|
| Surface topography | Surface parameter influence the bone cellular proliferation process. | (Sá J.C. et al. 2009) |
| Surface free energy | Surface free energy was a more important characteristic than surface roughness for cellular adhesion and proliferation. | (Hallab N. J. et al. 1995) |
| Wettability |
|
(Ruardy T. G. et al. 1997) (Sá J.C. et al. 2009) (Faghihi S. et al. 2006) |
| Grain orientation (crystallographic texture) | The Crystal orientation of titanium sheets substrate was more favorable for the attachment and proliferation of pre-osteocytes than that of the titanium rod, possibly due to the subsequent increase in wettability and protein adsorption affinity. | (Faghihi S. et al. 2006) |
| Surface roughness |
|
(Mustafa K. et al. 2001) (Hatano K. et al. 1999) (Fumio W. et al. 2009) (Hacking A. S. et al. 2008) |
| Patterned surface |
|
(Thomas C. H. et al. 1997) (Fumio W. et al. 2009) (Puckett S. et al. 2008) |
The irregularity of the material surface can be characterized based on the scales: macroroughness (100 μm-milimeters), microroughness (100 nm-100 μm), and nanoroughness (less than 100 nm). Macroroughness does not seem to have a major impact on cells and it does not restrict cell attachment and spreading. The effect of microroughness is more controversial: micro-rough surfaces, in some cases, increase the bone tissue growth around the implant by facilitating the deposition and better distribution of proteins on the implant’s surface, whereas in other cases they impair the surrounding tissue growth [70–74, 76, 77]. In these cases, the irregular surface textures with different shapes, e.g., pyramids, ridges, grooves, round pores, etc, may have contributed to these conflicted observations [70, 71]. Nanoscale roughness has been shown to be beneficial for osteoblast cell response, including initial cell adhesion, proliferation, and expression of differentiation markers [70, 78]. This observation is consistent with the fact that bone itself is a structure with a nano-rough surface, which mainly consists of 90% type I collagen synthesized by osteoblasts with linear fibrils 300 nm in length and 0.5 nm in diameter [79], and of hydroxyapatite crystals matrices that contain hexagonal unit structures 2~5 nm thick and 20~80 nm long [80]. Accordingly, current research on nanotexture surfaces aims to reproduce the natural nanoroughness of bone using nanotechnology techniques [73, 78, 81]. In the future, studies can also focus on cell substrate interactions at the atomic and molecular level to allow the study of the ultrastructure levels [72, 82].
4.3 The Effect of Environmental Mechanical Properties
Studies have shown that cell proliferation, migration, and differentiation are influenced by mechanical properties of the extracellular matrix [24, 59, 83–85]. For example, Engler et al. found that the differentiation of MSCs was controlled by the stiffness of the two-dimensional substrate matrix which cells were cultured on [59]. This commitment could be reprogrammed during the first week in culture by the addition of soluble induction factors, but not after several weeks in culture. This result was recently confirmed by MSCs cultured in three-dimensional gels [83]. Kong et al. studied the effect of the substrate rigidity on gene transfer and expression [86]. This study utilized fluorescent resonance energy transfer (FRET), an imaging technology extensively used to evaluate and visualize specific intermolecular interactions and intracellular activity in and outside of cells [86–88] to monitor the interaction between cells and the substrate. The results indicate that high substrate stiffness enhances cell proliferation by increasing the endocytosis of the pDNA condensates and their transportation into the nucleus. Increasing the substrate rigidity also facilitated the release of pDNA from the condensates and enhanced the ability of cells to generate traction forces and promote the cell cycle [86]. Conversely, softer substrate or gels had difficulty providing stable anchor sites for cell receptors, limiting the ability of the cells to generate traction forces [59, 86] and uptake exogenous signaling molecules [89]. While the underlying mechanism remains unclear, environmental rigidity may be perceived by the cell via the adhesion molecules to regulate the intracellular stress and subsequently the RhoA/ROCK signaling pathway for the ultimate control of cellular functions [59, 89].
4.4 The Effect of Active Mechanical Loading
Bones can adapt to their surrounding environment by controlling their mass and structure [90, 91]. Consistent with the fundamental role of bones in controlling body movement and providing mechanical support, bone cells are continuously exposed to mechanical loading, such as interstitial fluid shear stress and tensile strain. The predicted range of in vivo fluid shear stress ranges from 0.8 to 3 Pa [92, 93]. This mechanical loading can stimulate bone cells and result in the change of bone mass and structure to maintain optimal skeletal architecture [91]. In fact, mechanical loading at the physiologic level has been shown to cause a significant increase in bone mass in vivo [91, 94]. Prolonged absence of loading can lead to a decrease of bone mass and strength, resulting in osteoporosis (OP) and osteoarthritis (OA) as common diseases in the elderly [12, 91].
Mechanical loading also promotes bone cells to adapt to implants during repair processes, causing osseous integration between host and implant, a prerequisite for the stability of implants [95, 96]. Fluctuating fluid motion can occur between bone cells and implants due to mechanical loading during walking and other daily activities. In addition to ensuring sufficient nutrient supply and waste removal, these fluid motions can apply shear stresses on cells seeded in the scaffold which increase the production and release of growth factors. For example, shear stresses have been shown to initiate a cascade of signaling events, including transient release of calcium from intercellular stores, synthesis and release of prostaglandins (PG) [94] and nitric oxide [93], and phosphorylation of the mitogen activated protein (MAP) kinases ERK-1/2 [94]. Among these signaling events, phosphorylated ERK-1/2 is also essential for the recruitment of MSCs and their subsequent differentiation into bone forming cells [96]. Thus, mechanical fluid loading is considered an important aspect in the development of artificial bone scaffold because it facilitates osteo-integration and osteo-induction [91].
Shear stresses can clearly regulate the osteogenic differentiation of human mesenchymal stem cells [97]. In this case, mechanical-induced Ca2+ signal and FAK/Src complex in hMSCs can lead to the activation of the ERK1/2 signaling pathway, which is critical for osteoblastic differentiation via the activation of transcription factors, such as FosB and Runx2 [98]. In fact, the expression level of Runx2, which is essential for the osteogenic differentiation of hMSCs, osteoblasts, and chondroblasts [99], can be regulated not only by ECM, growth factors such as BMPs and FGF-2, and hormones, but also by mechanical loading [100].
Mechanical forces can also affect the differentiation of osteoprogenitor cells at different stages of proliferation, extracellular matrix maturation, and matrix mineralization. Haut Donahue investigated the effect of shear stress in regulating the intracellular calcium mobilization and prostaglandin E2 (PGE2) [101]. The results indicate that bone cells respond to oscillating fluid flow with an increase in intracellular calcium concentration ([Ca2+]i) and PGE2 production when the flow rate is increased. Celil Aydemir, et al. demonstrated that fluid shear stress and tensile strain can result in nuclear translocation of nuclear factor of activated T cells (NFAT) in cells of the osteoblastic lineage [91]. Since NFAT is an inflammation transcription factor well-known to act downstream of the Ca2+/calcineurin pathway, a peptide inhibitor of the calcineurin/NFAT axis was found to block the mechanical stimulation mediated Cox2 induction. Chromatin immunoprecipitation assay further demonstrated the direct interaction between NFAT2 and the human Cox2 promoter region [91]. Bacabac, et al. also investigated bone cell responses to vibration stresses at a wide range of frequencies (5~100Hz) by monitoring the release of NO and prostaglandin E2 (PGE2) and the expression of COX-2 mRNA as important regulators of bone adaptation [93]. The results revealed that NO release is positively correlated with the maximum acceleration rate of the vibration, but has a negative correlation with PGE2 release. COX-2 mRNA expression also increased in a frequency dependent manner, mediated by NO release at high frequencies. These results may lead to a better understanding of diseases involving altered mechanotransduction, such as osteoporosis [102].
Both bone diseases of OP and OA are associated with age increase, possibly due to the imperfection in the bone remodeling process controlled by bone cell responses to mechanical stimuli. However, OP and OA rarely coexist within one patient. Bakker, et al. found a difference in shear-stress-induced responses of OP and OA, measured as the production of nitric oxide (NO) and prostaglandin E2 (PGE2) [12]. The NO-response to shear stress was higher in the OP than in the OA cells, while the PGE2-response was higher in the OA cells [12, 103]. These results should shed new light on our understanding of the underlying mechanisms governing OP and OA diseases.
5. Summary and Perspectives
In the last decade, bone repair therapies have undergone several major advancements: from the direct replacement of defective tissues with first and second generation materials to the application of the third-generation bioactive materials for the induction of the bone self-healing. However, challenging questions still remain unaddressed for the further improvement of functionality and effectiveness of materials. For instance, how to control and optimize the rate of new tissue formation and old tissue resorption; how to maintain the strength during the scaffold’s degradation process; how to develop new biomaterials with controlled physical, mechanical and biochemical properties; how to tailor the materials for specific patients, disease states, and different parts of body; and how to design 3D biologically inspired materials that can mimic or even surpass the natural repair and regeneration processes. In addition, the first and second generation materials cannot be immediately abandoned at the current stage because of their excellent mechanical properties, particularly for serious large bone fractures and joint replacement in clinical therapies. However, in order to improve the bonding of scaffolds with the host bone tissue, there is a crucial need for surface modification techniques, including tailoring surface texture, modifying surface-chemistry of the materials, improving the nonspecific protein adsorption in vivo, and immobilizing precise signaling groups on surfaces.
Furthermore, the current evaluation method of biomaterials for tissue engineering is largely based on traditional biochemical assays such as immunoblotting and immunostaining, which kill the cells and may introduce artifacts or result in loss of information [88]. Therefore, it is also important to develop and apply living cell imaging methods for the evaluation of biomaterials. Integrated with optimally transparent materials, three-dimensional microscopy, and advanced image analysis methods, live cell imaging technologies such as fluorescence resonance energy transfer (FRET) are expected to provide novel insights at the molecular levels into the interaction mechanisms between cells and materials, which should provide a fundamental basis for the development of optimized materials for tissue engineering.
Acknowledgments
This work is supported in part by grants from NIH HL098472, NS063405, NSF CBET0846429 (Y.W.), and National Natural Foundation of China No. 81101154 (X.L., W.X. and B.L.).
References
- 1.Kuczumow A, et al. Investigation of chemical changes in bone material from South African fossil hominid deposits. Journal of Archaeological Science. 2010:107–115. [Google Scholar]
- 2.Smith IO, LAS, Liu XH, Ma PX. Nano-structured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(2):226–236. doi: 10.1002/wnan.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 4.Rimondini L, Mele S. Stem cell technologies for tissue regeneration in dentistry. Minerva Stomatol. 2009;58(10):483–500. [PubMed] [Google Scholar]
- 5.Martin RB. Toward a unifying theory of bone remodeling. Bone. 2000;26(1):1–6. doi: 10.1016/s8756-3282(99)00241-0. [DOI] [PubMed] [Google Scholar]
- 6.Mullender MG, Huiskes R. Osteocytes and bone lining cells: which are the best candidates for mechano-sensors in cancellous bone? Bone. 1997;20(6):527–32. doi: 10.1016/s8756-3282(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 7.Buenzli PR, Pivonka P, Smith DW. Spatio-temporal structure of cell distribution in cortical bone multicellular units: a mathematical model. Bone. 2011;48(4):918–26. doi: 10.1016/j.bone.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Silva GA, et al. Materials in particulate form for tissue engineering. 2. Applications in bone. J Tissue Eng Regen Med. 2007;1(2):97–109. doi: 10.1002/term.1. [DOI] [PubMed] [Google Scholar]
- 9.Albright F. The effect of hormones on osteogenesis in man. Recent Prog Horm Res. 1947;1:293–353. doi: 10.1016/b978-1-4831-9840-8.50014-4. [DOI] [PubMed] [Google Scholar]
- 10.Long F. Bone Appétit! Cell. 2009;139:1044–1045. [Google Scholar]
- 11.Zhang H, et al. Proteomics in bone research. Expert Rev Proteomics. 2010;7(1):103–11. doi: 10.1586/epr.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker AD, et al. Different responsiveness to mechanical stress of bone cells from osteoporotic versus osteoarthritic donors. Osteoporos Int. 2006;17(6):827–33. doi: 10.1007/s00198-006-0072-7. [DOI] [PubMed] [Google Scholar]
- 13.Boyce BF, Yao Z, Xing L. Osteoclasts have Multiple Roles in Bone in Addition to Bone Resorption. Crit Rev Eukaryot Gene Expr. 2009;19(3):171–180. doi: 10.1615/critreveukargeneexpr.v19.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr. 2009;19(3):171–80. doi: 10.1615/critreveukargeneexpr.v19.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschaseaux F, Sensebe L, Heymann D. Mechanisms of bone repair and regeneration. Trends Mol Med. 2009;15(9):417–29. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Deng ZL, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–21. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Caplan AI. Mesenchymal stem cells and tissue engineering for orthopaedic surgery. Chir Organi Mov. 2003;88(3):305–16. [PubMed] [Google Scholar]
- 18.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132(4):612–30. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 20.Caplan AI. New era of cell-based orthopedic therapies. Tissue Eng Part B Rev. 2009;15(2):195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gersbach CA, Phillips JE, Garcia AJ. Genetic engineering for skeletal regenerative medicine. Annu Rev Biomed Eng. 2007;9:87–119. doi: 10.1146/annurev.bioeng.9.060906.151949. [DOI] [PubMed] [Google Scholar]
- 22.Satija NK, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16(1):7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 23.Undale AH, et al. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc. 2009;84(10):893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20(5–6):441–8. doi: 10.1016/j.cytogfr.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Satija NK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13(11–12):4385–402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Othman SF, Magin RL. Monitoring tissue engineering using magnetic resonance imaging. J Biosci Bioeng. 2008;106(6):515–27. doi: 10.1263/jbb.106.515. [DOI] [PubMed] [Google Scholar]
- 29.Laurencin CT, et al. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Gazdag AR, et al. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J Am Acad Orthop Surg. 1995;3(1):1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Huber F-X, Arthur NM, Heimann L, Elvira Dingeldein, Héloïse Cavey, Xavier Palazzi, Gaëlle Clermont, Jean-Pierre Boutrand. Evaluation of a novel nanocrystalline hydroxyapatite paste Ostim® in comparison to Alpha-BSM® - more bone in growth inside the implanted material with Ostim® compared to Alpha BSM®. BMC Musculoskeletal Disorders. 2009;10(164):1–11. doi: 10.1186/1471-2474-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider RK, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three- dimensional collagen scaffolds. Biomaterials. 2010;31:467–480. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Navarro M, et al. Biomaterials in orthopaedics. J R Soc Interface. 2008;5(27):1137–58. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295(5557):1014–7. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 35.Hench LL, Thompson I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7(Suppl 4):S379–91. doi: 10.1098/rsif.2010.0151.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mourino V, Boccaccini AR. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010;7(43):209–27. doi: 10.1098/rsif.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hench LL. Biomaterials. Science. 1980;208(4446):826–31. doi: 10.1126/science.6246576. [DOI] [PubMed] [Google Scholar]
- 38.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Vagaska B, et al. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol Res. 2010;59(3):309–22. doi: 10.33549/physiolres.931776. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55(2):141–50. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 41.Quarto R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 42.Lazarus HM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–98. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Pietras K, et al. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3(5):439–43. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 44.Caplan AI. Mesenchymal stem cells. J orthop Res. 1991;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 45.Schimming R, Schmelzeisen R. Tissue-engineered bone for maxillary sinus augmentation. J Oral Maxillofac Surg. 2004;62(6):724–9. doi: 10.1016/j.joms.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 47.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2(4):389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 48.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 49.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4(5):341–8. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–23. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 51.Urist MR. Bone: formation by autoinduction. Science. 1965;150(698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 52.Lutwak L, Singer FR, Urist MR. UCLA conference: Current concepts of bone metabolism. Ann Intern Med. 1974;80(5):630–44. doi: 10.7326/0003-4819-80-5-630. [DOI] [PubMed] [Google Scholar]
- 53.Valonen PK, et al. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials. 2010;31(8):2193–200. doi: 10.1016/j.biomaterials.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin I, et al. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res. 2001;55(2):229–35. doi: 10.1002/1097-4636(200105)55:2<229::aid-jbm1009>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 55.Partridge KA, Oreffo RO. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10(1–2):295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 56.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 57.Meyerrose T, et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62(12):1167–74. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huesa C, Helfrich MH, Aspden RM. Parallel-plate fluid flow systems for bone cell stimulation. J Biomech. 2010;43(6):1182–9. doi: 10.1016/j.jbiomech.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 59.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 60.Kuboki Y, et al. BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. J Biomed Mater Res. 1998;39(2):190–9. doi: 10.1002/(sici)1097-4636(199802)39:2<190::aid-jbm4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 61.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Shi X, et al. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28(28):4078–90. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang W, et al. PHBV microspheres--PLGA matrix composite scaffold for bone tissue engineering. Biomaterials. 2010;31(15):4278–85. doi: 10.1016/j.biomaterials.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 64.Kujala S, et al. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials. 2003;24(25):4691–7. doi: 10.1016/s0142-9612(03)00359-4. [DOI] [PubMed] [Google Scholar]
- 65.Ren J, et al. Repair of mandibular defects using MSCs-seeded biodegradable polyester porous scaffolds. J Biomater Sci Polym Ed. 2007;18(5):505–17. doi: 10.1163/156856207780852578. [DOI] [PubMed] [Google Scholar]
- 66.Christoph R, et al. In vitro proliferation of human osteogenic cells in presence of different commercial bone substitute materials combined with enamel matrix derivatives. Head & Face Medicine. 2009;5(23):1–9. doi: 10.1186/1746-160X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, et al. Effects of structural property and surface modification of Ti6Ta4Sn scaffolds on the response of SaOS2 cells for bone tissue engineering. Journal of Alloys and Compounds. 2010;494(1–2):323–329. [Google Scholar]
- 68.Hulbert SF, et al. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 1970;4(3):433–56. doi: 10.1002/jbm.820040309. [DOI] [PubMed] [Google Scholar]
- 69.Gotz HE, et al. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials. 2004;25(18):4057–64. doi: 10.1016/j.biomaterials.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Puckett S, Pareta R, Webster TJ. Nano rough micron patterned titanium for directing osteoblast morphology and adhesion. Int J Nanomedicine. 2008;3(2):229–41. doi: 10.2147/ijn.s2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sá JC, et al. Influence of argon-ion bombardment of titanium surfaces on the cell behavior. Surface & Coatings Technology. 2009;203:1765–1770. [Google Scholar]
- 72.Faghihi S, et al. The significance of crystallographic texture of titanium alloy substrates on pre-osteoblast responses. Biomaterials. 2006;27(19):3532–9. doi: 10.1016/j.biomaterials.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 73.Tsai SW, Hsu FY, Chen PL. Beads of collagen-nanohydroxyapatite composites prepared by a biomimetic process and the effects of their surface texture on cellular behavior in MG63 osteoblast-like cells. Acta Biomater. 2008;4(5):1332–41. doi: 10.1016/j.actbio.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Chappard D, et al. Sinus lift augmentation and beta-TCP: a microCT and histologic analysis on human bone biopsies. Micron. 2010;41(4):321–6. doi: 10.1016/j.micron.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Schuler M, et al. Biomimetic modification of titanium dental implant model surfaces using the RGDSP-peptide sequence: a cell morphology study. Biomaterials. 2006;27(21):4003–15. doi: 10.1016/j.biomaterials.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hacking SA, et al. The response of mineralizing culture systems to microtextured and polished titanium surfaces. J orthop Res. 2008;26(10):1347–54. doi: 10.1002/jor.20622. [DOI] [PubMed] [Google Scholar]
- 78.Yao C, Storey D, Webster TJ. Nanostructured metal coatings on polymers increase osteoblast attachment. Int J Nanomedicine. 2007;2(3):487–92. [PMC free article] [PubMed] [Google Scholar]
- 79.Webster TJ. Nanophase ceramics: the future orthopdic and dental implant material. Adv Chem Eng. 2001;27:125–166. [Google Scholar]
- 80.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20(2):92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 81.Steinmuller-Nethl D, et al. Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials. 2006;27(26):4547–56. doi: 10.1016/j.biomaterials.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 82.Mustafa K, et al. Determining optimal surface roughness of TiO(2) blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001;12(5):515–25. doi: 10.1034/j.1600-0501.2001.120513.x. [DOI] [PubMed] [Google Scholar]
- 83.Wu C, Zhang Yufeng ZY, Thor Friis, Yin Xiao. Structure-property relationships of silk-modified mesoporous bioglass scaffolds. Biomaterials. 2010;31:3429–3438. doi: 10.1016/j.biomaterials.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 84.Ozcivici E, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6(1):50–9. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pek YS, Wan AC, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31(3):385–91. doi: 10.1016/j.biomaterials.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 86.Kong HJ, et al. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci U S A. 2005;102(12):4300–5. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, et al. Visualizing the mechanical activation of Src. Nature. 2005;434(7036):1040–5. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Shyy JY, Chien S. Fluorescence proteins, live-cell imaging, and mechanobiology: seeing is believing. Annu Rev Biomed Eng. 2008;10:1–38. doi: 10.1146/annurev.bioeng.010308.161731. [DOI] [PubMed] [Google Scholar]
- 89.Kong HJ, et al. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4(6):460–4. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 90.Kwon RY, Jacobs CR. Time-dependent deformations in bone cells exposed to fluid flow in vitro: investigating the role of cellular deformation in fluid flow-induced signaling. J Biomech. 2007;40(14):3162–8. doi: 10.1016/j.jbiomech.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Celil Aydemir AB, et al. Nuclear factor of activated T cells mediates fluid shear stress- and tensile strain-induced Cox2 in human and murine bone cells. Bone. 2010;46(1):167–75. doi: 10.1016/j.bone.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–60. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 93.Bacabac RG, et al. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315(4):823–9. doi: 10.1016/j.bbrc.2004.01.138. [DOI] [PubMed] [Google Scholar]
- 94.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6):1047–55. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Blecha LD, Rakotomanana L, Razafimahery F, Terrier A, Pioletti DP. Targeted mechanical properties for optimal fluid motion inside artificial bone substitutes. JOURNAL OF ORTHOPAEDIC RESEARCH OCTOBER. 2009;27:1082–1087. doi: 10.1002/jor.20836. [DOI] [PubMed] [Google Scholar]
- 96.Blecha LD, et al. Mechanical interaction between cells and fluid for bone tissue engineering scaffold: modulation of the interfacial shear stress. J Biomech. 2010;43(5):933–7. doi: 10.1016/j.jbiomech.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol. 2010 doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- 98.Haasper C, et al. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp Toxicol Pathol. 2008;59(6):355–63. doi: 10.1016/j.etp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Fu H, et al. Osteoblast differentiation in vitro and in vivo promoted by Osterix. J Biomed Mater Res A. 2007;83(3):770–8. doi: 10.1002/jbm.a.31356. [DOI] [PubMed] [Google Scholar]
- 100.Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88(3):446–54. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- 101.Donahue TL, et al. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36(9):1363–71. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 102.McGarry JG, et al. A comparison of strain and fluid shear stress in stimulating bone cell responses--a computational and experimental study. Faseb J. 2005;19(3):482–4. doi: 10.1096/fj.04-2210fje. [DOI] [PubMed] [Google Scholar]
- 103.Blecha LD, et al. Mechanical interaction between cells and fluid for bone tissue engineering scaffold: Modulation of the interfacial shear stress. Journal of Biomechanics. 2010;43:933–937. doi: 10.1016/j.jbiomech.2009.11.004. [DOI] [PubMed] [Google Scholar]