Abstract
After hematopoietic stem cell transplantation (HSCT), successful engraftment and immune recovery is necessary to protect the patient from relapse and infection. Many studies highlight the importance of conventional αβ T cell recovery after HSCT but the impact of γδ T cell recovery has not been well described. Here, we investigate the recovery of γδ T cells in 102 pediatric patients with acute leukemia in first clinical remission that underwent an allogeneic HSCT at St. Jude Children’s Research Hospital from 1996-2011. The mean age of the patients was 10.5 ± 5.9 years (range, 0.6-25.2) and the mean follow up of the survivors was 2.7±1.8 years (range 0.12-6.0). Diagnoses included 59% patients with ALL and 41% patients with AML. Multivariate analysis demonstrated significant impact of the maximum number of CD3+, CD4+ and CD8+ T cells and donor source on the γδ T cell recovery (P<0.0001, P<0.0001, P<0.0001 and P <0.004; respectively). Univariate and multivariate model found the number of γδ T cells after HSCT to be associated with infections (P = 0.026 and P = 0.02, respectively). We found the probability of infections for patients with an elevated number of γδ T cells was significantly lower compared to patients with low or normal γδ T cells after HSCT (18% vs. 54%; P=0.025). Bacterial infections were not observed in patients with elevated γδ T cells. Lastly, event free survival was significantly higher in patients with enhanced γδ T cell reconstitution compared to patients with low/normal γδ T cell reconstitution after HSCT (91% vs. 55%; P=0.04). Thus, γδ T cell may play an important role in immune reconstitution after HSCT.
INTRODUCTION
Delayed immune reconstitution after hematopoietic stem cell transplantation (HSCT) increases transplant related-mortality (TRM) due to relapse and infection. Prompt recovery of T cells, specifically CD4+ T cells, has been shown to significantly decrease the risk of infections (1). Most studies focus on αβ T cells and the impact of γδ T cell reconstitution in infections after HSCT has not been well investigated (2, 3). Unlike conventional αβ T cells, γδ T cells are described as “bridging” adaptive and innate immunity. The specific receptors on γδ T cells detect unconventional antigens such as phosphorylated microbial metabolites and lipids, non-classical MHC-I molecules and unprocessed proteins (4-6) γδ T cells are concentrated within epithelial and mucosal surfaces to maintain epidermal integrity of the skin and intestinal epithelium (7-10). Studies suggest that tissue-specific antigens are recognized by γδ T cells resulting in immune responses protecting these potential sites of pathogen entry into the body (11, 12). Furthermore, increasing evidence indicates γδ T cells also possess potent innate antitumor activity (13-15). A report by Gertner-Dardenne et al. found that γδ T cells were effective in targeting AML by the perforin and granzyme pathway in vitro and in the mouse model (14). Lamb et al reported the increased frequency of γδ T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor HSCT for leukemia (16). Godder et al. showed that adults with acute leukemia with higher numbers of γδ T cells after HSCT had a significant increase in leukemia-free survival compared to patients with low or normal γδ T cells (17). Thus, in the partially mismatched, related donor HSCT, the beneficial associations between γδ T cells and outcome have been reported following HSCT.(2) (16) (17).
Reconstitution of γδ T cell repertoire diversity after allogeneic HSCT suggest that peripheral expansion of mature T cells in the graft is one of the main pathway of γδ T cell recovery in adults.(18) The recognition of γδ T cells being a non-alloreactive lymphocyte with potential anti-infectious and antitumor properties has lead to the use of γδ T cells in immunotherapy (19-21) Currently, αβ T cell depletion method to engineer a HSC graft that retains monocytes, dendritic cells, NK cells and γδ+ T lymphocytes are used in hope that it might improve the outcome of HSCT (22, 23).
Here we report the first detailed study of γδ T cell reconstitution after HSCT in pediatric patients. Since γδ T cells are known to have protective roles during various types of infections (9), we evaluated infections as well as outcome. We found that γδ T cell recovery during the first year following HSCT correlated with a reduced incidence of infection. Furthermore, an increased number of γδ T cells correlated with a greater event free survival in the first year following HSCT. Further prospective studies evaluating larger number of patients will be needed to determine a stronger correlation between γδ T cell reconstitution and overall survival.
METHODS
Patient
Data were collected retrospectively on 102 consecutive patients with acute leukemia in first clinical remission (CR) that underwent a HSCT from 2006-2011 at St. Jude Children’s Research Hospital. All patients and/or their parents or guardians provided written informed consent for their participation and all research was conducted under institutional review board approved protocols. Patients were excluded if they had secondary leukemia or they had undergone previous HSCT. The preparative regimen, graft source/manipulation and GVHD prophylaxis is detailed in Table S1. Patients undergoing MURD or MRD HSCT received a preparative regimen with cyclophosphamide with mesna (120mg/kg), total body irradiation (TBI) (12 Gy) and anti-thymoglobulin (ATG). Patients undergoing MURD or MRD HSCT with a non-TBI regimen received a preparative regimen with targeted busulfan, cyclophosphamide (200mg/kg) and ATG. Patients undergoing a UCB HSCT received a preparative regimen with fludarabine (75mg/m2), cyclophosphamide (120 mg/kg) and TBI (1320 cGy). Patients undergoing a HAPLO HSCT received a preparative regimen with thioptepa (10mg/kg), melphalan (120mg/m2) and fludarabine (200mg/m2) or clofarabine (200-250 mg/m2). HAPLO patients received an ex vivo T cell depleted graft using the Miltenyi CliniMACS system with a T cell dose ≤ 1.0 × 105 CD3+ cells/kg.
Evaluation
Successful engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count ≥ 500 cells/µl. Graft failure was defined as the absence of engraftment after 42 days following HSCT. Relapse was defined as the presence of the patient’s initial diagnosis of leukemia in the peripheral blood or bone marrow after having reached clinical remission following HSCT. Event free survival (EFS) was defined as any death, graft failure, or disease relapse following HSCT. Graft versus host disease (GVHD) was defined, diagnosed and staged based on National Institutes of Health (NIH) consensus criteria. Patients were surveyed weekly with galactomannan assay and viral polymerase chain reaction (pcr) as previously described (24). Additionally, cultures for suspected pathogens were obtained when clinically indicated. Diagnosis of infection was defined when a positive culture or pcr for a pathogen was found in association with a clinical illness.
Statistical analysis
A Cox-proportional hazard model was performed stepwise using backward selection to determine variables with significant relationship to overall survival. Univariate and multivariate logistic regression analyses were performed to identify variables associated with infections. A generalized linear model (GLM) was performed to determine variables with a significant relationship to the number of γδ T cell as a continuous or grouped variable. For the continuous variable analysis, covariates were evaluated with time being fixed. For the grouped variables, patients with γδ T cell number greater than one standard deviation above the mean value of γδ T cell in the cohort on two consecutive lab draws within the first year after HSCT were grouped as “elevated”. The remainder of the patients were grouped as “low or normal “ in γδ T cell number. The mean value was determined from the distribution of γδ T cell number in the entire patient cohort before day 365 ± 20. Using this discrimination, we applied Fisher’s exact test to evaluate differences between groups in gender, disease diagnosis, disease state, survival, relapse, GVHD, types of donors, infection and type of infections seen in patients. The Wilcoxon rank sum test was used to evaluate for differences between the two groups in patient age, and days to engraftment within the first 100 days and to determine the relationship of chronic GVHD to their γδ T cell count at the time of diagnosis. The Kruskal-Wallis rank sum test was used to evaluate the relationship of clinical stage of acute GVHD to γδ T cell numbers. The cumulative incidences of acute and chronic GVHD following HSCT, relapse, and infection for the two groups were estimated and compared as described by Kalbfleisch and Prentice (25) and Gray (26). Overall survival (OS) and EFS for the two patient groups were estimated according to the Kaplan-Meier method (27) and were compared using the log-rank test. OS end point was death and EFS end points were death, relapse, or graft failure following HSCT. All statistical analyses were performed with SAS software version 9.2 and Prism v5.0b.
RESULTS
Patients
From 2006 to 2011, γδ T cell data on 102 patients with acute leukemia in first CR who underwent an allogeneic HSCT at St. Jude Children’s Research Hospital were evaluated retrospectively. The mean age of the patients was 10.5 ± 5.9 years (range, 0.6-25.2) and the mean follow up of the survivors was 2.7±1.8 years (range 0.12-6.0). There were 57% males and 43% females. Diagnoses included 59% patients with ALL and 41% patients with AML. Patients received a matched unrelated donor (MURD) (41%), matched related donor (MRD) (23%), haploidentical (HAPLO) (31%) or an umbilical cord blood (UCB) (5%) (Table S1).
Variables associated with γδ T cells reconstitution after HSCT
First, we evaluated the effect of various patient characteristics and the association with γδ T cell reconstitution after HSCT. Specifically, donor, age, gender, diagnosis, and GVHD prophylaxis, GVHD, immune reconstitution, infection, relapse and survival were evaluated. Using univariate and multivariate analysis, we found a significant impact of donor source on the γδ T cell recovery (P=0.005 and P=0.006, respectively) (Table 1). Similarly, we found the number of CD3+, CD4+ and CD8+ T cells to be significantly associated with γδ T cell recovery using both univariate (P<0.001, P<0.001 and P=0.01; respectively) and multivariate analyses (P<0.001, P<0.001 and P<0.001; respectively). Since γδ T cell are a subset of CD3+ T cells, we anticipated the positive correlation of γδ T cell and total CD3+ T cells after HSCT (Estimate =0.4). However, the maximum number of CD4+ and CD8+ T cell were inversely associated with γδ T cell recovery in the multivariate model (Estimate −0.3 and −0.4).
Table 1.
Univariate and Multivariate Analysis of Patient Characteristics and Association with yδ T cells after HSCT
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| Variable | Category | Estimate | P-value | Lower 95% CI | Upper 95% CI |
| Donor | 0.005* | ||||
| MURD | 0.000 | ||||
| MRD | 0.071 | 0.003 | 0.025 | 0.118 | |
| CORD | −0.051 | 0.194 | −0.128 | 0.026 | |
| HAPLO | 0.031 | 0.138 | −0.010 | 0.073 | |
| Age | −0.002 | 0.148 | −0.005 | 0.001 | |
| Gender | Female vs. Male | 0.024 | 0.199 | −0.013 | 0.061 |
| Diagnosis | ALL vs. AML | 0.003 | 0.862 | −0.034 | 0.041 |
| GVHD Prophylaxis | CSA & MMF | −0.010 | 0.758 | −0.075 | 0.055 |
| MTX & FK | 0.004 | 0.845 | −0.035 | 0.043 | |
| Acute GVHD | Yes vs. No | 0.020 | 0.293 | −0.018 | 0.058 |
| Chronic GVHD | Yes vs. No | 0.043 | 0.122 | −0.012 | 0.098 |
| Immune Recovery | CD3 | 0.035 | <0.001* | 0.020 | 0.050 |
| CD4 | 0.096 | <0.001* | 0.057 | 0.136 | |
| CD 8 | 0.026 | 0.011* | 0.006 | 0.046 | |
| NK | 0.050 | 0.048* | 0.001 | 0.100 | |
| CD19 | 0.006 | 0.679 | −0.022 | 0.033 | |
| Infection | Yes vs. No | 0.044 | 0.018* | 0.008 | 0.080 |
| Infection Groups | 0.097 | ||||
| None | 0.034 | 0.128 | −0.010 | 0.078 | |
| Bacterial | −0.018 | 0.493 | −0.070 | 0.034 | |
| Fungal Viral |
−0.059 0.000 |
0.530 | −0.246 | 0.127 | |
| Relapse | Yes vs. No | 0.027 | 0.245 | −0.019 | 0.074 |
| Survival | Yes vs. No | 0.054 | 0.005* | 0.017 | 0.091 |
|
| |||||
|
Multivariate Analysis
| |||||
| Effect | Donor | Estimate | Pr > |t| | Lower | Upper |
|
| |||||
| Donor | 0.006* | ||||
| CORD | −0.03 | 0.19 | −0.09 | 0.02 | |
| HAPLO | 0.025 | 0.13 | −0.008 | 0.05 | |
| MRD | 0.053 | 0.004 | 0.01 | 0.08 | |
| MURD | 0 | ||||
| Infection | Yes | −0.001 | 0.406 | −0.04 | 0.01 |
| Immune Recovery | CD3 | 0.415 | <0.0001* | 0.282 | 0.548 |
| CD4 | −0.343 | <0.0001* | −0.482 | −0.205 | |
| CD 8 | −0.395 | <0.0001* | −0.526 | −0.264 | |
| NK | 0.024 | 0.238 | −0.016 | 0.065 | |
P
values indicate statistical significance observed
γδ T cells are inversely correlated with the incidence of infection after HSCT
Many studies have documented that poor T cell recovery after HSCT is linked to infectious complications (4). However, the impact of γδ T cell recovery on the incidence of infection after HSCT in pediatric patients has not been well described. Research has shown that γδ T cells play a role in pathogen clearance (28), thus we used a logistic regression model to estimate the probability of developing an infection based on other measured variables, including the number of T (CD3, CD4, CD8, γδ), B and NK cells as continuous variables. Univariate and multivariate analysis found the number of γδ T cells after HSCT to be associated with infections (P = 0.026 and P=0.02, respectively). However, infection became not statistically significant when expanding the analysis to evaluate the effect of various donor sources and immune component. Sub-analysis of patients undergoing MRD/MURD and HAPLO/CORD HSCT are provided in Table S2-5. Although similar variables impacted γδ T cells and the risks for infections, the number of patients in each sub-groups were too small for analysis to reach statistical significance. However, in the combined cohort analysis, the data demonstrate that there is a significant association between the incidence of infections and the number of γδ T cells after HSCT.
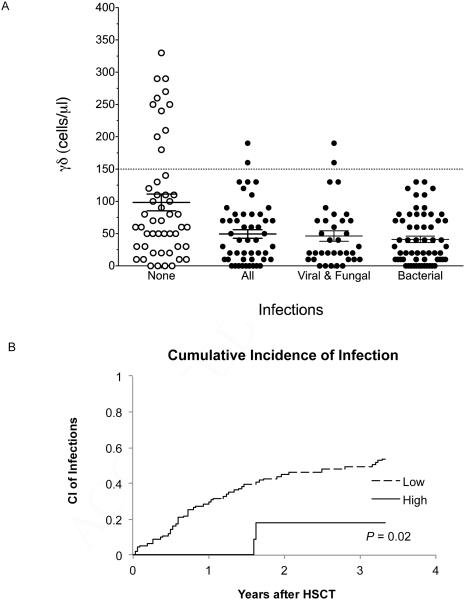
Next, we evaluated the risk of infections to the number of γδ T cells as a grouped variable, as previously described by Godder et al. (17). During the first year after HSCT, 11% of patients had at least 2 consecutive measurements greater than one standard deviation above the mean γδ T cell numbers (≥ 150 cells/µl). The remaining patients (89%) had low/normal γδ T cell numbers (<150 cells/µl). The groups were similar with respect to age, sex, disease and donor source (P = 0.34, P = 1, P = 1 and P=0.07; respectively) (Table 2). The patients with elevated γδ T cells had only viral infections (2/2 events) while the low/normal γδ group had viral, bacterial and/or fungal infections (Figure 1A). Using a logistic regression model, we found the cumulative incidence of infection was significantly lower for patients with an elevated number of γδ T cells compared to patients with low or normal γδ T cells after HSCT (0.53 vs. 0.18%; P=0.02) (figure 1B). In summary, the incidence of infection after HSCT was significantly higher in the low/normal γδ T cell group compared to the elevated group.
Table 2A.
Patient Characteristics and Distribution in the Low/Normal vs. High yδ T cell Group
| Variable | Category | Overall N=102 |
Low/Normal yδ N=91 |
High yδ N=11 |
P-value |
|---|---|---|---|---|---|
| Donor | 0.147 | ||||
| MRD | 22 (21.6%) | 17 (18.7%) | 5 (45.5%) | ||
| MURD | 42 (41.2%) | 40 (44.0%) | 2 (18.2%) | ||
| HAPLO | 32 (31.4%) | 28 (30.8%) | 4 (36.4%) | ||
| UCB | 6 (5.9%) | 6 (6.6%) | 0 (0.0%) | ||
| Age | 0.340 | ||||
| Mean ± SD | 10.5 ± 5.91 | 10.7 ± 5.96 | 9.0 ± 5.60 | ||
| Median (Range) | 10.5 (0.6-25.2) | 10.6 (0.6-25.2) | 10.2 (0.6-16.3) | ||
| Gender | 1.000 | ||||
| Female | 44 (43.1%) | 39 (42.9%) | 5 (45.5%) | ||
| Male | 58 (56.9%) | 52 (57.1%) | 6 (54.5%) | ||
| Diagnosis | 1.000 | ||||
| ALL | 60 (58.8%) | 53 (58.2%) | 7 (63.6%) | ||
| AML | 42 (41.2%) | 38 (41.8%) | 4 (36.4%) | ||
| Acute | 0.891 | ||||
| GVHD | 1-2 | 28 (27.5%) | 26 (28.6%) | 2 (18.2%) | |
| 3-4 | 12 (11.8%) | 11 (12.1%) | 1 (9.1%) | ||
| No | 62 (60.8%) | 54 (59.3%) | 8 (72.7%) | ||
| Chronic | 0.351 | ||||
| GVHD | No | 89 (87.3%) | 78 (85.7%) | 11 (100.0%) | |
| Yes | 13 (12.7%) | 13 (14.3%) | 0 (0.0%) | ||
| Chimerism | 0.93 | ||||
| Mean ± SD | 97.6 ± 5.34 | 97.6 ± 5.57 | 98.3 ± 2.99 | ||
| Median (Range) | 99.5 (64.2-100) | 99.5 (64.2-100) | 99.6 (90.6-100) | ||
| Relapse | 0.687 | ||||
| No | 82 (80.4%) | 72 (79.1%) | 10 (90.9%) | ||
| Yes | 20 (19.6%) | 19 (20.9%) | 1 (9.1%) | ||
| Survival | 0.07 | ||||
| Alive | 66 (64.7%) | 56 (61.5%) | 10 (90.9%) | ||
| Expired | 36 (35.3%) | 35 (38.5%) | 1 (9.1%) | ||
| Infection | 0.02 | ||||
| Yes | 51 (50.0%) | 49 (53.8%) | 2 (18.2%) | (0.08) | |
| No | 51 (50.0%) | 42 (46.2%) | 9 (81.8%) | ||
| Bacterial | 0.06 | ||||
| Infection | Yes | 24 (23.5%) | 24 (26.4%) | 0 (0.0%) | (0.24) |
| No | 78 (76.5%) | 67 (73.6%) | 11 (100.0%) | ||
| Viral | 0.7 | ||||
| Infection | Yes | 26 (25.5%) | 24 (26.4%) | 2 (18.2%) | (1.0) |
| No | 76 (74.5%) | 67 (73.6%) | 9 (81.8%) | ||
| Fungal | 1.00 | ||||
| Infection | Yes | 1 (1.0%) | 1 (1.1%) | (1.0) | |
| No | 101 (99.0%) | 90 (98.9%) | 11 (100.0%) | ||
| GVHD Prophylaxis (0-2 agent) | 1.000 | ||||
| No | 9 (8.8%) | 8 (8.8%) | 1 (9.1%) | ||
| Yes | 93 (91.2%) | 83 (91.2%) | 10 (90.9%) | ||
| GVHD Prophylaxis (3 agent) | 1.000 | ||||
| No | 35 (34.3%) | 31 (34.1%) | 4 (36.4%) | ||
| Yes | 67 (65.7%) | 60 (65.9%) | 7 (63.6%) | ||
| GVHD Treatment (Steroids ± 3rd agent) | 1.000 | ||||
| No | 85 (83.3%) | 76 (83.5%) | 9 (81.8%) | ||
| Yes | 17 (16.7%) | 15 (16.5%) | 2 (18.2%) | ||
| CD3 | <0.001* | ||||
| Mean ± SD | 1.3 ± 1.12 | 1.2 ± 1.04 | 2.4 ± 1.17 | ||
| Median (Range) | 1.0 (0.0-5.2) | 0.8 (0.0-5.2) | 2.4 (1.0-4.9) | ||
| yδ | <0.001* | ||||
| M Mean ± SD | 0.1 ± 0.09 | 0.1 ± 0.05 | 0.3 ± 0.09 | ||
| Median (Range) | 0.1 (0.0-0.6) | 0.1 (0.0-0.2) | 0.3 (0.2-0.6) | ||
| CD4 | 0.010* | ||||
| Mean ± SD | 0.5 ± 0.43 | 0.5 ± 0.38 | 0.9 ± 0.61 | ||
| Median (Range) | 0.4 (0.0-2.0) | 0.4 (0.0-1.6) | 0.8 (0.1-2.0) | ||
| CD8 | 0.001* | ||||
| Mean ± SD | 0.8 ± 0.91 | 0.7 ± 0.89 | 1.4 ± 0.89 | ||
| Median (Range) | 0.5 (0.0-4.4) | 0.5 (0.0-4.4) | 0.8 (0.6-3.2) | ||
| NK | 0.639 | ||||
| Mean ± SD | 0.4 ± 0.37 | 0.4 ± 0.28 | 0.6 ± 0.78 | ||
| Median (Range) | 0.4 (0.0-2.9) | 0.3 (0.0-1.5) | 0.4 (0.1-2.9) | ||
| CD19 | 0.078 | ||||
| Mean ± SD | 0.4 ± 0.68 | 0.3 ± 0.69 | 0.5 ± 0.58 | ||
| Median (Range) | 0.2 (0.0~5.5) | 0.1 (0.0-5.5) | 0.3 (0.1-1.9) | ||
P
values indicate statistical significance observed between the low/normal vs. high yδ T cell group
(P) values for infections were adjusted for multiple comparisons by Bonferroni method.
Figure 1. Reconstitution of γδ T cells and Risk for Infection.
Infection was defined as a positive culture or PCR for a pathogen in association with a clinical illness. Patients were surveyed weekly with galactomannan assay and PCR for CMV, EBV and adenovirus as previously described (24). Additional cultures for suspected pathogens were obtained when clinically indicated. (A) Each point represents a patient and the documented maximum γδ T cell value in the first year after HSCT. The mean value of γδ for patients with no documented infections (?) is compared to the mean value of γδ for patients with documented infections (Ο). Infections are further depicted as bacterial, viral or fungal infections. Error bar represent SEM. (B) The cumulative incidence of infection is shown for patients in the low/normal (--) or high (—) γδ T cell group.
In addition, information regarding the percent of lymphocytes and number of CD3, αβ and γδ T cells over time was evaluated in patients with either low/normal or high γδ T cells (Figure S). Although there was a significant difference in the percent of γδ T cells between the low/normal and high γδ group from day 56 (2.3% vs. 3.5%; P=0.02), we found there was no significant difference in the percent of αβ T cells or the CD3 T cells. Similar comparisons were made with the total number of CD3, αβ and γδ T cells between the two groups. The total number of CD3 and αβ T cells were significantly different between the low/normal and elevated γδ T cell group early after HSCT at day 28 (P=0.051 and P=0.01, respectively), but were not significantly different at later time points. Furthermore, the total number of CD3 and αβ T cells were not significantly lower in the high γδ group compared to the low γδ group, suggesting against the hypothesis that lymphopenia was a significant driving force for γδ T cell expansion. Lastly, the potential effect of the lymphopenia due to OKT3 and ATG were evaluated by determining the number of patients in the elevated group who received OKT3 (2 or 18%) or ATG (2 or 18%). Majority of the patients (63%) in the elevated γδ group did not receive ATG or OKT3.
Incidence of Engraftment, Relapse, GVHD and Survival
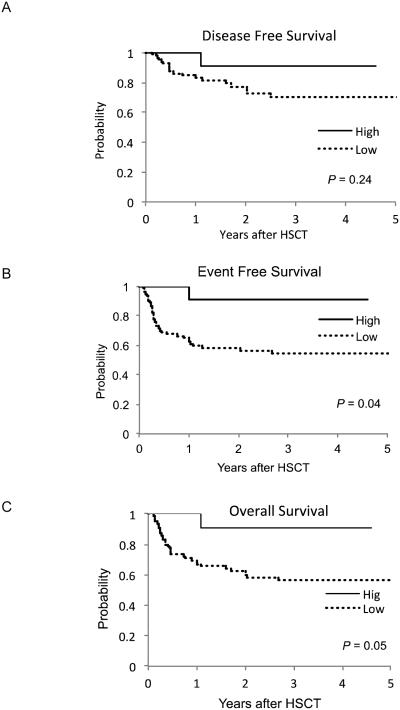
Because infections and survival may be related to engraftment, GVHD or relapse, we determined if there was a significant association between γδ T cell reconstitution and these factors. There were no significant differences seen in the incidence of engraftment or median time to engraftment in the elevated group compared to the low/normal group (19.0±6.1 and 18.2±7.5 days, respectively; P=0.8). The overall median donor chimerism was 99.5%; (range 64.2%-100%) while patients with low/normal γδ T cells was 99.5% (range 62.1%-100%) and patient with elevated γδ T cells was 99.6% (range 90.6% -100%). There was no correlation between chimerism and the low/normal or elevated γδ T cell groups (p = 0.93). The disease free survival between the patients in the elevated γδ T cell group compared to patients in the low/normal γδ T cell group was not significantly different at 1 year (91% vs. 79%; P=0.6) or 5 years after HSCT (91% vs. 74%; P=0.25) (Figure 2A). The median time to relapse for the patients in the low/normal group was 177 ± 185 days (range, 23-746) and the relapse event occurring in the elevated group was on day 365. The event-free survival in patients with an elevated number of γδ T cells compared to those who recovered with low/normal γδ cells was significantly higher (91% vs. 55%; P=0.04) (Figure 2B).
Figure 2. Survival Estimates for Patients in Low/Normal and High γδ T cell Group.
The Kaplan-Meier estimates for DFS (A), EFS (B) and OS (C) are compared between the patients with low/normal (--) or high (—) γδ T cell.
There were a total of 36 deaths in the cohort with the cause due to recurrent disease (21), multi-organ failure (5), infection (4), pulmonary hemorrhage (3), hepatic failure (2), or veno-oclusive disease (1). We determined whether γδ T cells reconstitution had a significant effect on OS using a Cox proportional hazards model (Supplemental). In a multivariate analysis using the number of γδ T cells as a continuous variable, we found that the number of γδ T cell along with CD3+ and CD4+ T cell had a significant relationship with OS, (P=0.02, P<0.001 and P=0.02, respectively) (Table 3). Out of the 36 deaths, 35 (97%) occurred in patients with low/normal γδ T cells while 1 (3%) occurred in the group with elevated γδ T cells. The patient in the elevated group died from relapse at 365 days after HSCT. Overall survival using the Kaplan Meier method between the two groups was 91% vs. 61%; P=0.06 (Figure 2C).
DISCUSSION
Early after HSCT, relapse and infections continue to be major complications that significantly impact survival. Studies to improve immune reconstitution have heavily focused on conventional T cells (1) (29, 30) Recent studies have reported the increased frequency of γδ T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor HSCT for leukemia and suggested the beneficial association.(16) Godder et al. showed that adults with acute leukemia with higher numbers of γδ T cells after HSCT had a significant increase in leukemia-free survival compared to patients with low or normal γδ T cells (17). There is growing evidence of the beneficial associations between γδ T cells and outcome of adults who of undergo a partially mismatched, related donor HSCT. (2, 16, 17) This study is the first to suggest that early γδ T cell reconstitution after HSCT decreases the risk of infection and impacts survival in pediatric patients who underwent MRD, MURD and HAPLO HSCT. We initially determined whether patient characteristics of gender, age, disease, preparative regimen, GVHD prophylaxis and donor source were associated with γδ T cell reconstitution. Compared to MURD, patients who had MRD or HAPLO donors had a significant difference in the recovery of γδ T cells after HSCT. The impact of graft source on immune reconstitution after HSCT has been well reported and the number of γδ T cells present in the graft will most likely play a role in immune recovery. (1, 2) One limitation of this study is the γδ T cell content of the graft was not available, restricting the investigation of how graft source impact γδ T cell reconstitution after HSCT. Currents studies are under way to provide detailed information regarding the infused graft product.
Here, we report the effect of γδ T cell recovery after HSCT by evaluating patients undergoing their first HSCT with similar disease (acute leukemia) and disease status (CR1). Furthermore, γδ T cells were evaluated as a continuous variable to correlate the effect on infections and survival. Thereafter, we identified the patients with elevated γδ T cells and confirmed our findings that these patients had decreased infections and increased survival. Since γδ T cells are subset of CD3+ T cells, the direct correlation with total number of CD3+ T cell recovery and γδ T cells was anticipated. It has been presumed that γδ T cell recovery would parallel CD4+ and CD8+ T cell recovery. On the contrary, recovery of γδ T cells was inversely associated with the number of CD4+ and CD8+ T cell after HSCT. This may be due to peripheral proliferation of γδ T-cells during severely lymphopenic periods as previously described in adults patients after HSCT (18, 31). However, analysis of the CD3 and αβ T cells recovery after HSCT did not support the hypothesis that lymphopenia was the driving force for γδ T cell peripheral expansion. Patients with elevated γδ T cells did not have significantly lower CD3 or αβ T cells, although associations with NK and B cells were not thoroughly investigated. Furthermore, some patients in the study received antibodies directed towards lymphocytes such as ATG or Muromonab-CD3 (OKT3), although uni-variate and multivariate analysis did not find significant association with γδ T cell recovery after HSCT. A prospective study that provide information regarding γδ T cell reconstitution along with other immune parameters in a homogeneous population will be provide further insight on the role of γδ T cells after HSCT.
Recently, γδ T cell reactivity towards a microbial metabolite, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) has been demonstrated and indicates a role in activity towards bacterial infections (32). Of note, bacterial infections were not observed in patients with elevated γδ T cells. Increased levels of γδ T cells after HSCT has been attributed to infections (2), but data demonstrating direct increase in antigen specific γδ TCR has not been shown. Studies monitoring γδ T cell receptor (TCR) diversity after HSCT and during active infections are currently underway to better understand the role of γδ T cells. In summary, our results demonstrate that patients who have an enhanced γδ T cell recovery after HSCT are protected with decreased incidence of infections and improved survival.
The known pleiotropic effects of γδ T cells suggest multiple mechanisms by which γδ T cells may promote survival after HSCT in pediatric populations. Data suggest that γδ T cells have direct anti-tumor function in vitro. Previous reports by Godder et al showed a long term disease free survival in mostly adult patients with elevated γδ T cells and suggested a graft versus leukemia effect as a possible mechanism (17). Though there were fewer relapse in patients with elevated γδ T cells compared to patients with low/normal γδ T cells, especially early after HSCT, the numbers were not significant. In larger studies, the effect of relapse and overall survival may be more significant. In our study, the protective effect against infection during the early post-HSCT period was the strongest correlate observed.
Supplementary Material
Figure S1: Recovery of γδ T cells for different graft source after HSCT The average number of γδ T cells for patients who underwent a HAPLO, MRD OR MURD HSCT is shown for day 28, 56, 84 and 100. The error bar represents the SEM.
Figure S2: T cell Reconstitution for in Low/Normal and High γδ T cell Group The average percent and number of γδ, αβ and CD3 T cells for patients in the low/normal and high γδ group is shown for day 28, 56 and 100. The error bar represents the SEM.
Highlights.
Recovery of γδ T cells is associated with graft source.
Patients with robust γδ T cells have lower risk for infection.
Recovery of enhanced γδ T cells is associated with improved EFS and OS.
Table 2B.
Patient Characteristic and Association with Elevated yδ T cells after HSCT (Logistic Model)
| Variable | Category | Estimate | Pr>Chi-Square | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Donor | 0.256 | . | ||
| CORD | . | . | ||
| HAPLO | 3.169 | 0.981 | 23 E3 (0.00, I) | |
| MRD | 3.891 | 0.976 | 475E3 (0.00, I) | |
| MURD | 2.119 | 0.987 | 80776 (0.00, I) | |
| Age | −0.048 | 0.381 | 0.953 (0.856, 1.061) | |
| Gender | Male vs Female | −0.052 | 0.870 | 0.900 (0.256, 3.164) |
| Diagnosis | AML vs ALL | −0.113 | 0.732 | 0.797 (0.218, 2.916) |
| GVHD Prophylaxis | CSA & MMF | −0.019 | 0.973 | 0.963 (0.109, 8.519) |
| MTX & FK | −0.050 | 0.880 | 0.904 (0.246, 3.327) | |
| ATG ± OKT3 | 0.059 | 0.887 | 1.126 (0.221, 5.742) | |
| Acute GVHD | Yes vs. No | 0.4878 | 0.144 | 1.629 (0.847, 3.131) |
| Chronic GVHD | Yes vs. No | −5.668 | 0.958 | 0.000 (0.00, 1E177) |
| Relapse | Yes vs. No | −0.485 | 0.369 | 0.379 (0.046, 3.147) |
| Survival | Yes vs. No | −0.916 | 0.087 | 0.160 (0.020, 1.305) |
| Infection | Yes vs. No | −0.829 | 0.041* | 0.190 (0.039, 0.931) |
| Infection Groups | 0.718 | . | ||
| Bacterial | . | |||
| Fungal | −4.641 | 0.983 | 1.000 (0.000, 2E246) | |
| None | 5.114 | 0.944 | 17235 (0.000, 33E52) | |
| Viral | 4.169 | 0.954 | 6703 (0.000, 13E52) | |
| Immune | CD3 | 0.729 | 0.003* | 2.073 (1.288, 3.335) |
| Recovery | CD4 | 2.067 | 0.003* | 7.897 (2.007, 31.08) |
| CD 8 | 0.562 | 0.033* | 1.755 (1.048, 2 .939) | |
| CD19 | 0.256 | 0.474 | 1.292 (0.641, 2.604) | |
| NK | 0.988 | 0.134 | 2.684 (0.736, 9.785) |
ACKNOWLEDGEMENTS
This work was supported by the NIH R56 AI091938, American Association for Cancer Research (AACR), Cancer Center Grant P30CA021765, St. Baldrick’s Foundation, Assisi Foundation of Memphis, American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
There are no competing financial interests in relation to the work and authors.
REFERENCES
- 1.Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. American journal of hematology. 1997;54(2):131–8. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Cela ME, Holladay MS, Rooney CM, Richardson S, Alexander B, Krance RA, et al. Gamma delta T lymphocyte regeneration after T lymphocyte-depleted bone marrow transplantation from mismatched family members or matched unrelated donors. Bone Marrow Transplant. 1996;17(2):243–7. [PubMed] [Google Scholar]
- 3.Viale M, Ferrini S, Bacigalupo A. TCR gamma/delta positive lymphocytes after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992;10(3):249–53. [PubMed] [Google Scholar]
- 4.Pfeffer K, Schoel B, Gulle H, Kaufmann SH, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. European journal of immunology. 1990;20(5):1175–9. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 5.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunological reviews. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 6.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, et al. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264(5156):267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 7.Burjanadze M, Condomines M, Reme T, Quittet P, Latry P, Lugagne C, et al. In vitro expansion of gamma delta T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma. Br J Haematol. 2007;139(2):206–16. doi: 10.1111/j.1365-2141.2007.06754.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciofani M, Zuniga-Pflucker JC. Determining gammadelta versus alphass T cell development. Nat Rev Immunol. 2010;10(9):657–63. doi: 10.1038/nri2820. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa H, Naito T, Iwanaga T, Takahashi-Iwanaga H, Suematsu M, Hibi T, et al. Curriculum vitae of intestinal intraepithelial T cells: their developmental and behavioral characteristics. Immunological reviews. 2007;215:154–65. doi: 10.1111/j.1600-065X.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 10.Takagaki Y, Decloux A, Bonneville M, Tonegawa S. Diversity of Gamma-Delta T-Cell Receptors on Murine Intestinal Intraepithelial Lymphocytes. Nature. 1989;339(6227):712–4. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- 11.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. 1991;252(5011):1430–2. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 12.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gamma delta T cells. Science. 1998;279(5357):1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh A, Narita M, Watanabe N, Tochiki N, Satoh N, Takizawa J, et al. Anti-tumor cytotoxicity of gamma delta T cells expanded from peripheral blood cells of patients with myeloma and lymphoma. Med Oncol. 2008;25(2):137–47. doi: 10.1007/s12032-007-9004-4. [DOI] [PubMed] [Google Scholar]
- 14.Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, et al. Human V gamma 9V delta 2 T Cells Specifically Recognize and Kill Acute Myeloid Leukemic Blasts. J Immunol. 2012;188(9):4701–8. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 15.Castella B, Vitale C, Coscia M, Massaia M. V gamma 9V delta 2 T cell-based immunotherapy in hematological malignancies: from bench to bedside. Cell Mol Life Sci. 2011;68(14):2419–32. doi: 10.1007/s00018-011-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb LS, Jr., Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C, et al. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother. 1996;5(5):503–9. doi: 10.1089/scd.1.1996.5.503. [DOI] [PubMed] [Google Scholar]
- 17.Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39(12):751–7. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]
- 18.Hirokawa M, Horiuchi T, Kawabata Y, Kitabayashi A, Miura AB. Reconstitution of gammadelta T cell repertoire diversity after human allogeneic hematopoietic cell transplantation and the role of peripheral expansion of mature T cell population in the graft. Bone Marrow Transplant. 2000;26(2):177–85. doi: 10.1038/sj.bmt.1702478. [DOI] [PubMed] [Google Scholar]
- 19.Chatzidimitriou D, Gavriilaki E, Sakellari I, Diza E. Hematopoietic cell transplantation and emerging viral infections. Journal of medical virology. 2010;82(3):528–38. doi: 10.1002/jmv.21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27(6):1328–38. doi: 10.1038/leu.2012.374. [DOI] [PubMed] [Google Scholar]
- 21.Farnault L, Gertner-Dardenne J, Gondois-Rey F, Michel G, Chambost H, Hirsch I, et al. Clinical evidence implicating gamma-delta T cells in EBV control following cord blood transplantation. Bone Marrow Transplant. 2013;48(11):1478–9. doi: 10.1038/bmt.2013.75. [DOI] [PubMed] [Google Scholar]
- 22.Locatelli F, Bauquet A, Palumbo G, Moretta F, Bertaina A. Negative depletion of alpha/beta+ T cells and of CD19+ B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation. Immunology letters. 2013;155(1-2):21–3. doi: 10.1016/j.imlet.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Schumm M, Lang P, Bethge W, Faul C, Feuchtinger T, Pfeiffer M, et al. Depletion of T-cell receptor alpha/beta and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy. 2013;15(10):1253–8. doi: 10.1016/j.jcyt.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Leung W, Campana D, Yang J, Pei DQ, Coustan-Smith E, Gan K, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118(2):223–30. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentice RL, Kalbfleisch JD, Peterson AV, Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–54. [PubMed] [Google Scholar]
- 26.Gray BM, Egan ML, Pritchard DG. Specificity of monoclonal antibodies against group B streptococcus type II and inhibition of their binding by human secretions. Pediatric research. 1988;24(1):68–72. doi: 10.1203/00006450-198807000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53(282):457–81. [Google Scholar]
- 28.Bonneville M, O'Brien RL, Born WK. gamma delta T cell effector functions: a blend of innate programming and acquired plasticity. Nature Reviews Immunology. 2010;10(7):467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 29.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. The New England journal of medicine. 1995;332(3):143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 30.Porter DL. Allogeneic immunotherapy to optimize the graft-versus-tumor effect: concepts and controversies. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2011;2011:292–8. doi: 10.1182/asheducation-2011.1.292. [DOI] [PubMed] [Google Scholar]
- 31.Fukui Y, Oono T, Cabaniols JP, Nakao K, Hirokawa K, Inayoshi A, et al. Diversity of T cell repertoire shaped by a single peptide ligand is critically affected by its amino acid residue at a T cell receptor contact. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13760–5. doi: 10.1073/pnas.250470797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, et al. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2Vdelta 2 TCR. J Immunol. 2008;181(7):4798–806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Recovery of γδ T cells for different graft source after HSCT The average number of γδ T cells for patients who underwent a HAPLO, MRD OR MURD HSCT is shown for day 28, 56, 84 and 100. The error bar represents the SEM.
Figure S2: T cell Reconstitution for in Low/Normal and High γδ T cell Group The average percent and number of γδ, αβ and CD3 T cells for patients in the low/normal and high γδ group is shown for day 28, 56 and 100. The error bar represents the SEM.