Abstract

Residential exposure can dominate total exposure for commercial chemicals of health concern; however, despite the importance of consumer exposures, methods for estimating household exposures remain limited. We collected house dust and indoor air samples in 49 California homes and analyzed for 76 semivolatile organic compounds (SVOCs)—phthalates, polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and pesticides. Sixty chemicals were detected in either dust or air and here we report 58 SVOCs detected in dust for the first time. In dust, phthalates (bis(2-ethylhexyl) phthalate, benzyl butyl phthalate, di-n-butyl phthalate) and flame retardants (PBDE 99, PBDE 47) were detected at the highest concentrations relative to other chemicals at the 95th percentile, while phthalates were highest at the median. Because SVOCs are found in both gas and condensed phases and redistribute from their original source over time, partitioning models can clarify their fate indoors. We use empirical data to validate air-dust partitioning models and use these results, combined with experience in SVOC exposure assessment, to recommend residential exposure measurement strategies. We can predict dust concentrations reasonably well from measured air concentrations (R2 = 0.80). Partitioning models and knowledge of chemical Koa elucidate exposure pathways and suggest priorities for chemical regulation. These findings also inform study design by allowing researchers to select sampling approaches optimized for their chemicals of interest and study goals. While surface wipes are commonly used in epidemiology studies because of ease of implementation, passive air sampling may be more standardized between homes and also relatively simple to deploy. Validation of passive air sampling methods for SVOCs is a priority.
Introduction
Exposures at home can dominate total exposure for a variety of consumer product chemicals, including some flame retardants, phthalates, and pesticides.1−4 These observations are consistent with Wambaugh et al.’s study that found consumer products to be a strong predictor of biological levels, based on a high throughput exposure model of over 1900 commercial chemicals.5 These studies show the importance of being able to predict exposures to consumer product chemicals but also highlight the limited measurement data and gaps in our understanding of these exposure pathways.6 Given the vast number of chemicals in commercial use, improving models to predict exposure levels and pathways is a priority in order to evaluate and manage health risks.
Fate and transport models have been widely developed and validated to estimate exposure from ambient emissions, but models relevant to indoor exposures are less developed. In a recent comparison of available exposure models, researchers identified the lack of data concerning near-field exposures to consumer product chemicals as a major gap in knowledge needed for better exposure-based chemical prioritization.7 Specifically, exposure measurements from indoor environments are needed to improve and validate models for near-field exposure.
Many consumer product chemicals of current and emerging health concern are classified as semivolatile organic compounds (SVOCs), including flame retardants, phthalates, pesticides, and perfluorinated compounds. SVOCs are found in both the gas and condensed phases and redistribute from their original source over time to indoor air, house dust, and other indoor surfaces.8 Their distribution in the indoor environment determines how people are exposed, so characterizing this distribution informs model development, sampling approaches, and strategies for intervention to reduce exposure.
A few exposure studies have measured a broad range of SVOCs indoors. We previously analyzed house dust and indoor air samples from 120 homes on Cape Cod for 89 semivolatile endocrine disrupting compounds (EDCs), including phthalates, alkylphenols, parabens, flame retardants, polychlorinated biphenyls (PCBs), and pesticides.9 We extended this research to 50 homes in northern California10 and found that indoor air concentrations were substantially higher than outdoor concentrations for most of the 104 SVOCs measured, indicating that indoor sources dominate total exposure.11 Building on this work, Blanchard et al. recently reported concentrations of 57 SVOCs in air and dust in 30 French homes.12 Comprehensive residential exposure measurement studies such as these are resource intensive, so environmental health researchers and chemical regulators have called for validated methods for estimating exposures.4,6,13,14 In response, Weschler and Nazaroff developed a series of equilibrium partitioning models based on physical-chemical properties to describe the fate of SVOCs in indoor environments.8,15−18
In this paper, we analyze relationships between simultaneously measured air and dust levels in the 49 northern California (CA) homes. The dust measures are reported here for the first time. We use indoor air and dust measures to evaluate the equilibrium partitioning models developed by Weschler and Nazaroff. This validation supports U.S. Environmental Protection Agency (EPA) efforts to develop high throughput exposure models.5−7,19 In addition, we use these partitioning models and our measurement data to demonstrate how chemical behavior can be anticipated based on chemical properties, and to provide other researchers with guidelines for efficient sampling design. For example, we demonstrate how these partitioning models can be used to select sampling methods based on chemical properties and to predict concentrations in different media.
Methods
Sampling and Analytical Methods
The California Household Exposure Study (CAHES) collected indoor and outdoor air and house dust in 2006 from 50 nonsmoking homes in Richmond and Bolinas, California. Richmond is a predominately low income urban community on the northeast coast of San Francisco Bay, whereas Bolinas is more rural and located on the Pacific coast north–northwest of San Francisco. Additional information about the study communities and participant selection can be found elsewhere.10 We were able to collect 49 dust samples from the 50 study homes. We previously reported concentrations of polybrominated diphenyl ethers (PBDEs) and 36 other flame retardants in dust samples collected in a subset of homes from this study (n = 16) in 2006 and then again in 2011.20 Indoor and outdoor air samples were also collected and have previously been reported.11 Briefly, air samples comprising gas and particle-bound phases were collected using parallel 160 mm URG personal pesticide samplers (Universal Research Glassware (URG); Chapel Hill, NC) at a target flow rate of 8–9 L/min over 24 h. Each sampler contained a 10 μm at 4 L/min impactor-equipped inlet followed by a 25 mm quartz fiber filter and 3 g XAD-2 sandwiched between two 1 13/16 in. polyurethane foam plugs. We collected respirable particulate (PM2.5, particulate matter less than 2.5 μm in diameter) on Teflon filters at a flow rate of approximately 5 L/min in 42 homes.
Dust samples were collected using a 9 A Eureka Mighty-Mite vacuum cleaner modified to collect dust into a 19 × 90 mm2 cellulose extraction thimble (Whatman, Inc.; Clifton, NJ) within a polytetrafluoroethylene Teflon crevice tool. Dust sampling began immediately following termination of the air sampling. Sample collection was accomplished by slowly dragging the crevice tool just above the surface of rugs, upholstery, wood floors, windowsills, ceiling fans, and furniture in the primary living areas of the home for ∼30 min. At the completion of sampling, thimbles containing the collected dust were removed and placed in precleaned, certified glass jars with Teflon-lined lids and stored at −4 °C prior to shipping to the laboratory.
Chemical analysis of the dust and air samples was conducted at the Southwest Research Institute in San Antonio, TX. Samples were stored for less than 6 months at < −4 °C prior to extraction and analysis. A total of 79 compounds were targeted in the dust samples, including pesticides, phthalates, polycyclic aromatic hydrocarbons (PAHs), PBDEs, and polychlorinated biphenyls (PCBs). Dust samples passed through a <150 μm sieve prior to analysis. Approximately 0.5 g of dust (median) was analyzed by GC/MS in selected ion monitoring mode after Soxhlet extraction using 6% diethyl ether in hexane, concentration to 2.5 mL, florisil column cleaning, and final concentration to 2 mL in 1% ether. Air samples were extracted across the entire URG setup and represent both gas and particle-bound phases. Additional details on extraction and analytical techniques are provided elsewhere.9,21
Quality assurance/quality control (QA/QC) measures were conducted to ensure accuracy and reliability of measurements. To estimate precision, we split three dust samples. To evaluate contamination from the laboratory, we analyzed solvent blanks (n = 3). Matrix spikes (n = 3) and surrogate recoveries were also used to characterize accuracy, compound recovery from the matrix, and extraction efficiency. Additional QA/QC information and results are presented in Supporting Information (SI).
Statistical Methods
For each analyte, the method reporting limit (MRL) was defined as either the analytical detection limit or the 90th percentile of the solvent blank concentrations, whichever was larger. Values reported by the laboratory but below the MRL were not included in the detection frequencies but were treated as estimated values to visualize distributions. Summary statistics use blank-corrected values, whereas modeling uses uncorrected values.
Data tended to be left censored due to detection limits from the laboratory analysis, which means that Pearson or Spearman correlation estimates using arbitrary substitutions for nondetects (e.g., detection limit/2) will result in poor correlation estimates. Therefore, we calculated Kendall’s τ rank correlation coefficients, adjusted for censored data,22 to explore linear relationships between dust and air concentrations, with p-values obtained from 10 000 bootstrap replications. Kendall τ correlation estimates were calculated for air and dust analytes with at least three detected paired concentration values.
Partitioning Theory
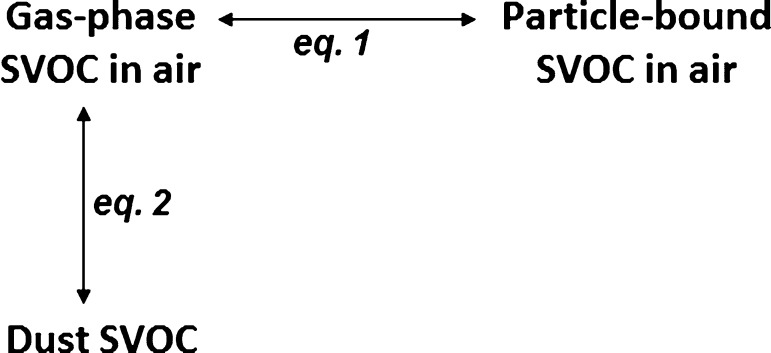
We used equilibrium partitioning concepts to explore relationships between concentrations in air and mass fractions in household dust (see Figure 1). Weschler and Nazaroff tested this model using aggregate data (e.g., published means and medians) available in the literature.15 Partitioning theory tells us that gas-phase chemicals will readily and predictably partition between airborne particles, surfaces, and house dust in the home. Air measurements in this study combined gaseous and particle phases; however, for equilibrium conditions, partitioning applies to gas-phase air concentrations and mass fractions in dust. We estimate gas-phase concentrations (Cg, μg/m3) from measured total air concentrations in a manner similar to Weschler and Nazaroff15 using the following equation:
| 1 |
where Ct is the measured total air concentration (μg/m3), TSP is the total suspended particles or average indoor concentration of particles (μg/m3), ρpart is the density of airborne particles, assumed to be 1 × 106 g/m3, fom_part is the fraction of organic matter associated with particles, assumed to be 0.4 (unitless), and Koa is the octanol-air equilibrium partitioning coefficient. We assumed the same values for particle density and fraction organic matter as Weschler and Nazaroff.15 Values for log Koa, the ratio of the concentration of a chemical in octanol (a surrogate for organic matter) to the concentration in air at equilibrium were obtained from the KOAWIN program in Estimation Programs Interface (EPI) Suite Version 4 developed by U.S. EPA and Syracuse Research Corporation (see SI Table S2).23 Estimated rather than experimental Koa values were used because authoritative experimental values were not available for all SVOCs we measured.
Figure 1.
Schematic illustration of SVOCs in air and dust indoors. Equations 1 (partitioning between gas-phase and total SVOCs in air) and 2 (partitioning between gas-phase air and dust) are described in the text.
We estimated home-specific TSP—concentration of particles with the same cut-point as particles represented in total SVOC air concentrations—from PM2.5concentrations measured for each home. In this study, the approximate particle size cut-point, based on URG sampler design and average flow rate, was 6.9 μm. Since we measured indoor PM2.5 for the duration of SVOC air sample collection, we used this measurement to estimate average particle concentration for <6.9 μm particles using published data. Specifically, we estimated concentrations of particulate matter across multiple size fractions from a study conducted in the greater Boston area.24 Using these data, we developed a linear regression model between measured PM2.5 and PM6, the closest size fraction to 6.9 available. We applied measured PM2.5 data from this study (median = 5.6 μg/m3) to the linear model [log(PM6 concentration, μg/m3) = 0.43 + 0.89log(PM2.5 concentration, μg/m3)] derived from the Long et al. data to estimate average indoor mass of PM6 in the CA residences (SI Table S3). When residence-specific PM2.5 concentrations were not available (n = 8), we used the estimated average PM6 concentration for the study (12 μg/m3).
In a manner similar to work by Weschler and Nazaroff, we used SVOC air concentrations to predict dust concentrations (Xdust; μg/g) assuming equilibrium partitioning between gas-phase air concentrations and settled dust:
| 2 |
where Cg is mass concentration of gas-phase SVOC (ng/m3), fom_dust is the fraction of dust that is organic matter (unitless), and ρdust is the density of dust (typically in the range of 1–2.5 × 106 g/m3). As Weschler and Nazaroff assert, this theoretical relationship assumes equilibrium partitioning that is dominated by the physical-chemical process of absorption in the organic fraction of dust and that octanol is an appropriate chemical model for the organic matter in dust, as it relates to sorption.15 Like Weschler and Nazaroff, we assumed house dust to have an organic matter fraction of 0.2 and a density of 2 × 106 g/m3.15 We compared predicted and measured dust concentrations using regression models with median concentrations for each chemical.
Results and Discussion
Measured Dust Concentrations
Overall, we detected 58 target analytes in house dust, which represents a long-term reservoir for chemicals in the residential environment. Table 1 presents CAHES house dust concentrations and includes all chemicals analyzed, even if they were not observed above the MRL. Phthalates and flame retardants are particularly abundant; DEHP, BBP, PBDE 99, PBDE 47, and DBP were the five chemicals with the highest concentrations at the 95th percentile. We found flame retardants at some of the highest concentrations in the world; whereas, several phthalates—DEHP and DBP—and PAHs were generally found at lower levels than reported elsewhere (see Discussion below). PCB and pesticide levels were not substantially different compared to other available studies. Chemicals that have been banned for years (e.g., DDT) persist in homes where exposure continues because of limited degradation. SI Figure S2 shows measured air and dust concentrations for chemicals concurrently detected in both media. Below, we summarize the dust concentrations by chemical group, and compare concentrations measured in this study to the Cape Cod Household Exposure Study (1999–2001), which used the same sampling and analytical methods. We also compare concentrations to levels reported in other peer-reviewed studies, but note that differences may be due to different sampling methods as well as geographic and temporal variation in chemical use.
Table 1. Summary Statistics for Semivolatile Organic Compounds in Household Dust in California Homes (μg/g) (n = 49).
| compound | abbrev. | %>MRL (MRL)b | min. | median | 95th percentile | max.e |
|---|---|---|---|---|---|---|
| phthalates | ||||||
| benzyl butyl phthalatec | BBP | 98 (0.2) | -- | 19 | 220 | 330 |
| bis(2-ethylhexyl) adipate | DEHA | 100 (0.04) | 1.1 | 5.1 | 14 | 24 |
| bis(2-ethylhexyl) phthalatec | DEHP | 100 (0.4) | 50 | 140 | 460 | 800 |
| di-n-butyl phthalatec | DBP | 98 (0.9) | -- | 11 | 35 | 56 |
| di-n-hexyl phthalate | DHP | 96 (0.04) | -- | 0.66 | 5.7 | 110 |
| di-n-octyl phthalate | DOP | 100 (0.02) | 0.42 | 1.6 | 3.9 | 9.4 |
| di-n-pentyl phthalated | DPeP | 12 (0.04) | -- | -- | 0.59 | 2.2 |
| di-n-propyl phthalate | DPP | 2 (0.06) | -- | -- | -- | 0.095 |
| dicyclohexyl phthalated | DCP | 16 (0.04) | -- | -- | 7.4 | 13 |
| diethyl phthalatec | DEP | 96 (0.1) | -- | 2.1 | 6.3 | 85 |
| diisobutyl phthalatec | DIBP | 100 (0.2) | 1.1 | 4.4 | 12 | 320 |
| flame retardants | ||||||
| polybrominated diphenyl ether 47 | PBDE47 | 100 (0.03) | 0.11 | 2.7 | 39 | 110 |
| polybrominated diphenyl ether 99 | PBDE99 | 100 (0.03) | 0.098 | 3.8 | 47 | 170 |
| polybrominated diphenyl ether 100 | PBDE100 | 94 (0.04) | -- | 0.68 | 9.1 | 31 |
| tris(2,3-dibromopropyl) phosphated | TrisBP | 8 (0.01) | -- | -- | 0.032 | 0.072 |
| polychlorinated biphenyls | ||||||
| polychlorinated biphenyl 52d | PCB52 | 35 (0.02) | -- | -- | 0.13 | 0.32 |
| polychlorinated biphenyl 105d | PCB105 | 33 (0.02) | -- | -- | 0.18 | 0.27 |
| polychlorinated biphenyl 153d | PCB153 | 55 (0.02) | -- | 0.022 | 0.32 | 0.54 |
| polycyclic aromatic hydrocarbons | ||||||
| acenaphthened | AcNThe | 24 (0.02) | -- | -- | 0.033 | 0.034 |
| acenaphthylene | AcNThy | 0 (0.02) | -- | -- | -- | -- |
| anthracened | Anth | 29 (0.02) | -- | -- | 0.043 | 0.064 |
| benzo(a)anthracened | BaA | 86 (0.02) | -- | 0.047 | 0.13 | 0.2 |
| benzo(a)pyrened | BaP | 90 (0.007) | -- | 0.085 | 0.19 | 0.26 |
| benzo(b&j)fluoranthened | BbjFluAn | 98 (0.01) | -- | 0.14 | 0.33 | 0.35 |
| benzo(k)fluoranthened | BkFluAn | 92 (0.007) | -- | 0.06 | 0.17 | 0.37 |
| benzothiophene | BThPhe | 0 (0.03) | -- | -- | -- | -- |
| chrysene/iso-chrysened | Chrys | 96 (0.1) | -- | 0.15 | 0.34 | 0.45 |
| dibenz(a,e)pyrene | DBaePyr | 2 (0.03) | -- | -- | -- | 0.057 |
| dibenz(a,h)anthracened | DBahA | 39 (0.02) | -- | -- | 0.081 | 0.079 |
| 3,6-dimethyl phenanthrene | DMPhenan | 2 (0.02) | -- | -- | -- | 0.023 |
| fluoranthene | FluAn | 100 (0.007) | 0.078 | 0.18 | 0.39 | 0.62 |
| fluorened | Flu | 76 (0.007) | -- | 0.023 | 0.057 | 0.086 |
| indeno(1,2,3-cd)pyrened | IcdPyr | 57 (0.02) | -- | 0.072 | 0.18 | 0.18 |
| 1-nitropyrene | 1NPyr | 0 (0.04) | -- | -- | -- | |
| phenanthrenec | Phenan | 98 (0.009) | -- | 0.19 | 0.4 | 0.62 |
| pyrene | Pyr | 100 (0.007) | 0.064 | 0.18 | 0.42 | 0.48 |
| dibenzothiophene | DBTPhe | 2 (0.03) | -- | -- | -- | 0.85 |
| 4,6-dimethyl dibenzothiophened | DMDBTPhe | 20 (0.03) | -- | -- | 0.26 | 1 |
| 2-methyl dibenzothiophened | 2MDBTPhe | 22 (0.03) | -- | -- | 0.27 | 2.1 |
| 1-methyl phenanthrened | 1MPhenan | 96 (0.01) | -- | 0.05 | 0.2 | 0.38 |
| 2-methyl phenanthrened | 2MPhenan | 98 (0.007) | -- | 0.083 | 0.27 | 0.38 |
| 3-methyl phenanthrened | 3MPhenan | 96 (0.01) | -- | 0.081 | 0.3 | 0.58 |
| 9-methyl phenanthrened | 9MPhenan | 96 (0.01) | -- | 0.058 | 0.3 | 0.69 |
| pesticides | ||||||
| alachlor | Alach | 0 (0.04) | -- | -- | -- | -- |
| aldrin | Aldr | 0 (0.04) | -- | -- | -- | -- |
| atrazine | Atraz | 0 (0.02) | -- | -- | -- | -- |
| bendiocarbd | Bendio | 4 (0.1) | -- | -- | -- | 0.35 |
| carbaryl | Carb | 14 (0.06) | -- | -- | 0.85 | 1.8 |
| carbofuran | Crbfur | 0 (0.04) | -- | -- | -- | -- |
| α-chlordaned | aChlor | 61 (0.02) | -- | 0.02 | 0.16 | 0.2 |
| γ-chlordaned | gchlor | 61 (0.02) | -- | 0.021 | 0.15 | 0.2 |
| chlorothalonil | Chorth | 43 (0.02) | -- | -- | 0.53 | 1.2 |
| chlorpyrifosd | ChlPy | 51 (0.02) | -- | 0.022 | 0.24 | 0.61 |
| cyanazine | Cyan | 0 (0.06) | -- | -- | -- | -- |
| cypermethrin | Cyper | 16 (0.1) | -- | -- | 7.5 | 140 |
| 4,4′-dichlorodiphenyldichloroethaned | DDD | 59 (0.02) | -- | 0.027 | 0.21 | 0.32 |
| 4,4′-dichlorodiphenyldichloroethylened | DDE | 76 (0.01) | -- | 0.046 | 0.17 | 0.29 |
| 4,4′-dichlorodiphenyltrichloroethane | DDT | 86 (0.02) | -- | 0.33 | 1.9 | 2.4 |
| diazinond | Diaz | 16 (0.02) | -- | -- | 0.26 | 5.3 |
| dicofol | Dico | 0 (0.04) | -- | -- | -- | -- |
| dieldrin | Dield | 0 (0.04) | -- | -- | -- | -- |
| endrin | Endr | 0 (0.04) | -- | -- | -- | -- |
| ethyl parathion | Parath | 0 (0.1) | -- | -- | -- | -- |
| heptachlor | Hept | 0 (0.02) | -- | -- | -- | -- |
| lindane | Lind | 2 (0.04) | -- | -- | -- | 0.41 |
| malathion | Malth | 0 (0.02) | -- | -- | -- | -- |
| methoxychlord | MX | 57 (0.04) | -- | 0.073 | 0.92 | 1.9 |
| methyl parathion | MePthion | 0 (0.04) | -- | -- | -- | -- |
| metolachlor | Metol | 0 (0.02) | -- | -- | -- | -- |
| nitrofen | Nitrof | 0 (0.04) | -- | -- | -- | -- |
| cis-permethrin | cPerm | 98 (0.02) | -- | 0.87 | 17 | 160 |
| trans-permethrin | tPerm | 98 (0.03) | -- | 1 | 28 | 280 |
| piperonyl butoxide | PipBO | 88 (0.02) | -- | 0.14 | 8.3 | 110 |
| o-phenyl phenold | oPPh | 96 (0.01) | -- | 0.082 | 0.52 | 0.65 |
| prometon | Prom | 0 (0.04) | -- | -- | -- | -- |
| propoxurd | PrPx | 57 (0.08) | -- | 0.12 | 1.5 | 2 |
| simazine | Simz | 0 (0.04) | -- | -- | -- | -- |
| trifluralin | Trifl | 0 (0.02) | -- | -- | -- | -- |
| 4-nitrotoluene | 4NT | 0 (0.04) | -- | -- | -- | -- |
-- indicates insufficient number of detects to calculate summary statistic.
MRL = method reporting limit (defined as either the analytical detection limit or the 90th percentile of the solvent method blanks, whichever is larger).
Values subject to blank correction by subtracting the median blank concentration.
Indicates that elevated nondetect values (due to analytical interferences) are included in the summary statistics presented.
Nondetect with elevated detection limit (due to analytical interferences) excluded.
Phthalates
All 11 target phthalates were detected in house dust samples. Most phthalates were detected in >90% of the dust samples, with the exception of DPeP, DPP, and DCP. The median concentrations of phthalates were lower than those measured in Cape Cod in 1999–2001 for all but one phthalate, DIBP (range 1.1 to 320, median 4.4 μg/g).9 Concentrations of DBP, BBP, DEHP, and DEP were substantially lower at the median than those observed in 30 Berlin apartments tested in 2000 and 200125 and Canadian homes collected between 2007 and 2010,26 but similar to U.S. concentrations reported in 2013 by Shin et al.27 Kolarik et al. and Blanchard et al. recently summarized house dust concentrations of DEHP, BBP, and DBP across different countries.12,28 DEHP and DBP dust concentrations in our study were generally lower than those reported in other countries; whereas, median BBP concentrations in this study fall within the wide range of concentrations reported in the literature.12,28,29
Flame Retardants
PBDEs (PentaBDE congeners) were detected in nearly 100% of dust samples at levels higher than levels reported outside of California, likely the result of California’s unique furniture flammability standard, and at levels that may pose a health risk, especially for children.20,30,31 The concentrations we detected were similar—within a factor of 2—to other California studies,29,32 and we have previously published detailed analysis and discussion of these findings, including an analysis of time trends in relation to phase out of PentaBDE and its substitution with other flame retardant chemicals.20
Polychlorinated Biphenyls
Three PCBs were targeted in this analysis: PCB 52, PCB 105, and PCB 153. PCBs were detected at higher concentrations in the Cape Cod study versus CAHES, but were more frequently detected in CAHES because the MRL was approximately 10 times lower (MRL = 0.02 μg/g) than in the Cape Cod study.33 Median levels of PCB 153 in this study were higher than the geometric mean (GM) and arithmetic mean (AM) from dust in 212 California residences collected in 2001–2006.34 Ninety-fifth percentile concentrations of PCB 105 and 153 in the CAHES were also higher than concentrations reported in the review by Roberts et al.35
Polycyclic Aromatic Hydrocarbons
PAHs were detected frequently in dust samples, with 13 PAHs having detection frequencies >75%. Dust concentrations of benz(a)anthracene, benzo(a)pyrene, and pyrene in this study were approximately 10 times lower at the median than the reported concentrations in the Cape Cod study;9 however, benz(a)anthracene and benzo(a)pyrene concentrations are comparable to samples collected in Los Angeles using similar methods.36 While the concentrations of PAHs in settled house dust in a review of 18 published studies revealed substantial variation, summarized GMs were generally 2–3 times higher than those observed in this study.37
Pesticides
Eighteen of the 36 pesticides analyzed were detected. Several historic use pesticides, including DDT, DDE, DDD, chlordane, and methoxychlor, were detected in the majority of homes. Common pyrethroids in household pesticides, cis- and trans-permethrin, were detected in almost all dust samples (98%). The maximum dust concentrations for historic use pesticides were generally substantially lower than those in the Cape Cod study. Methoxychlor, an organochlorine insecticide banned between the sampling periods for the Cape Cod study and CAHES in 2003,38 was detected at concentrations approximately 3 times lower at the median (0.24 versus 0.07 μg/g) in this study and approximately 7 times lower at the maximum (12.9 versus 1.9 μg/g). Bendiocarb, voluntarily canceled in 1999,39 had a substantially lower maximum concentration compared to the Cape Cod study (40.7 versus 2.3 μg/g). DDE concentrations measured in the CAHES study were higher than those measured in vacuum bags collected from Davis, CA apartments; however, the chlordane concentrations were comparable.29
Relationships between Indoor Air and Household Dust
We used measured dust and air data to describe the relationship between air and dust concentrations in the home and to determine if one measure can be used to predict the other, which would simplify and reduce costs related to residential exposure assessments. Seventy-six compounds were analyzed in both dust and air: 11 phthalates, 3 PBDEs, 3 PCBs, 24 PAHs, and 36 pesticides. Of these, 60 were detected in either dust or air; 40 were simultaneously detected in dust and air in at least one home; and 17 were detected in greater than 50% of air and 50% of dust samples (SI Figure S2).
Correlation between Air and Dust
We first estimated correlations between measured air and dust concentrations for each compound with sufficient pairs of detected concentrations. We show these correlations on scatterplots sorted by log Koa, which is a predictor of air-dust partitioning (SI Figure S3). Of the 34 analytes with sufficient data for comparison, 25 had significant positive Kendall’s τ correlation estimates (p < 0.05). Sorting correlation estimates by log Koa did not reveal any clear patterns across chemical classes. Phthalates with significant correlation estimates were DEP, DIBP, DBP, and BBP. Three phthalates with the highest log Koa values (DHP, DEHP, and DEHA) were not correlated. The only flame retardant with sufficient data for comparison (PBDE 47) was significantly positively correlated. Correlation estimates for PCB52 and PCB105 were similar; however, the estimate for PCB52, which has a lower log Koa, was significant (p = 0.03), while the estimate for PCB105 was only marginally significant (p = 0.08). PAHs with significant correlations were as follows: acenaphthene; fluoranthene; phenanthrene; dibenzothiophene; 3-, 9-, and 1-methylphenanthrene; 2-methyl dibenzothiophene; and fluoranthene. PAHs without significant correlations were as follows: anthracene; 2-methylphenanthrene; pyrene; and 4,6-dimethyl dibenzothiophene. Pesticides with significant positive correlation estimates were as follows: chlorothalonil, o-phenylphenol, chlorpyrifos, α-chlordane, diazinon, DDE, γ-chlordane, DDT, cis-permethrin, and piperonyl butoxide. Propoxur is the only pesticide without a significant correlation.
Dust/Air Ratios
To see the relative concentrations in house dust and air across chemicals, we calculated ratios of measured dust and total (measured) air concentrations for 40 compounds with simultaneous detects (SI Figure S4). Ratios span 6 orders of magnitude, with compounds with lower log Koa values having smaller ratios and compounds with higher log Koa values having higher ratios. There was a moderately strong correlation (ρ ≈ 0.8) between log Koa and ratio of dust to air concentrations. Also, compounds with higher log Koa values (>10) had lower detection frequencies in air, except for the two phthalates DEHP and DEHA, which are so abundant that they were detected in most air and dust samples.
Exposure Rate by Pathway
We also estimated the relative importance of dust exposure versus other household exposure pathways by calculating exposure rates (ng/day) for dust ingestion, dermal exposure through dust adherence, and inhalation of indoor air (equations in SI). We assumed an inhalation rate of 14.9 m3/day, an absorption fraction for air of 0.5, a dust ingestion rate of 0.064 g/day, a gastrointestinal absorption fraction of 0.9, a dermal loading of 3.55 g/m2, a dermal transfer coefficient of 0.06 m2/h, and a dermal absorption fraction of 0.05 averaged over a 70 year lifespan.40,41 We calculated the fraction exposure to dust relative to total indoor exposure for 40 compounds with simultaneous detects (SI Figure S5). Dermal exposure via gas-phase absorption16,42 is not accounted for in this model, potentially underestimating the air exposure contribution, especially for compounds with lower Koa values. For lower molecular weight compounds, such as fluorene and DEP, inhalation is the main route of exposure; whereas, for compounds with larger log Koa values such as DEHP and DEHA, exposure to dust (dermal and ingestion) dominates. However, for the majority of the SVOCs with a wide range of log Koa values, exposure to dust and air are both important.
Partitioning between Gas and Particle Phase in Air
Our analysis of dust:air concentrations in relation to log Koa (SI Figure S4) shows that chemicals with high log Koa tend to be more abundant in dust, while those with low log Koa are more abundant in air. We next used the Weschler and Nazaroff partitioning model to see whether indoor air and dust relationships are predictable so that one can be estimated from the other. To do this, we used the partitioning model to separate the measured air concentrations into gas-phase and particle-phase concentrations. SI Figure S6 shows the relationship of estimated gas to measured total air concentration by log Koa. Using our data, we see that measured total air concentrations comprise mostly gas-phase concentrations (gas-phase to total air concentration ratios near 1) for log Koa values up to approximately 10, consistent with Weschler and Nazaroff and Schossler et al.15,18 At higher log Koa values, there is increased variability in the contribution of gas-phase to total air concentrations, and, at the highest log Koa values, total air concentrations comprise mostly particulate phase concentrations. This is expected since higher log Koa values indicate greater partitioning to the octanol phase, a proxy for organic matter in particulates.
Relationship of Gas Phase Air to Dust Concentrations
Looking at the relationship between estimated gas-phase air concentrations and dust concentrations, we found a significant positive correlation across all compounds (τ =0.37, p < 0.05). A mixed-effects model of log dust and log gas-phase air concentrations with chemical designated as a random effect also revealed a significant relationship between air and dust (β = 0.34, p < 0.001). The estimated R2 value for the mixed-effects model (R2 = 0.05) indicates that a substantial portion of variation in the gas-phase air concentrations cannot be explained by measured dust concentrations alone. We expanded the mixed-effects model to include log Koa. As expected based on the apparent relationship between dust/air concentrations and log Koa (SI Figure S4), there is a significant negative association between log Koa estimates and log gas-phase air concentrations. Approximately 48% of the variation in air concentrations is explained by dust and log Koa estimates. SI Figure S7 shows dust and gas-phase air concentrations categorized by rounded log Koa values. Compounds with lower relative log Koa values (5–7) tend to lie toward the upper left portion of the graph, with higher relative air concentrations and lower relative dust concentrations; whereas, compounds with higher relative log Koa values (10–12) tend to lie toward the bottom right portion of the graph, with lower relative air concentrations and higher relative dust concentrations.
Partitioning Model Validation
This is the first use of a SVOC partitioning model applied to a range of SVOCs measured simultaneously in indoor air and house dust in the same study. We used our air and dust measurements for 17 chemicals to evaluate Weschler and Nazaroff’s air-dust partitioning model (eq 2). We used our estimated gas-phase concentrations to predict dust concentrations and then compared predictions to measured dust concentrations. We predicted dust concentrations for 17 compounds, mostly PAHs and phthalates, detected in at least 50% of air and 50% of dust samples (Figure 2). The predicted and measured concentrations are well correlated (R2 = 0.8), with a slope slightly greater than one (β = 1.4). On the basis of the intercept (−0.4), the predicted dust concentrations are, on average, 2.5 times lower than measured concentrations. In fact, almost all of the compounds are under-predicted at the median with the exception of DEHA, DEHP, and chlorpyrifos. When DEHP and DEHA are excluded from the model, the model fit improves (R2 = 0.85), but the predicted concentrations are, on average, now 6 times lower than the measured concentrations (results not shown). These data were also visualized by plotting the ratios of predicted to measured dust concentrations sorted by log Koa (SI Figure S8). There is variability in the ratios of predicted and measured for each compound, with over a 10-fold range for some compounds. Also apparent is the under-prediction for PAHs and variability for phthalates.
Figure 2.

Predicted dust concentrations (micrograms/gram) versus measured dust concentrations (micrograms/gram) for 17 SVOCs with at least 50% detection frequency in both indoor air and dust. Predictions made using measured air concentrations in the same homes. Dashed line represents 1:1 line or perfect prediction. Individual points shown (unshaded symbols); however, regression model fit to median concentrations (large shaded circles).
The model performed reasonably well by predicting 80% of the variability in dust concentrations. On average, dust levels were under-predicted for most chemicals and this is particularly true for PAHs. There are several possible explanations for this: (1) sampling artifacts that result in underestimate of air concentrations; (2) oxidation of PAHs on the air samples over the 24 h sampling period; (3) the origin of the measured dust particles since particles of ambient origin may have higher levels of PAHs relative to other chemicals with primarily indoor sources; and (4) sorption behaviors of PAHs to dust particles, which is dependent upon the organic carbon vs black carbon nature of the particle. Phthalates were both over- and under-predicted in dust depending on the compound. DEHP and DEHA, which have log Koa values >12, were overpredicted, whereas the other phthalates—DEP, DIBP, DBP, and BBP—which have log Koa values <10, were under-predicted.
A potential explanation for imprecise prediction is inaccurate Koa values. We used estimated log Koa values obtained from the KOAWIN program in EPISuite. These estimated values likely have the largest potential uncertainty in the prediction models.15 While the overall accuracy of Koa estimation is good (R2 = 0.957),43 estimates may be quite discrepant for specific chemicals. For example, the estimated log Koa value for BBP from KOAWIN is 9.018; however, Weschler and Nazaroff, using the SPARC online calculator report an estimated log Koa value for BBP of 11.6.15 These different Koa values lead to substantially different dust concentration predictions (over 300-fold). Similarly, perhaps the under-predictions for PAHs result from systematic under-predictions of log Koa values for PAHs due to algorithm limitations for these types of chemicals.
Partitioning Model As a Tool for Study Design
We then used the partitioning model to predict total air concentrations for a range of plausible dust concentrations and log Koa values (SI Figure S9). This prediction model can support study design by indicating detection limits that are needed to generate detectable values. For example, lower molecular weight phthalates (e.g., DEP) are typically detected in dust at central tendency concentrations ranging from 10 to 100 μg/g and have log Koa values of 7–9. Predicted air concentrations would range from 100 to >10 000 ng/m3.
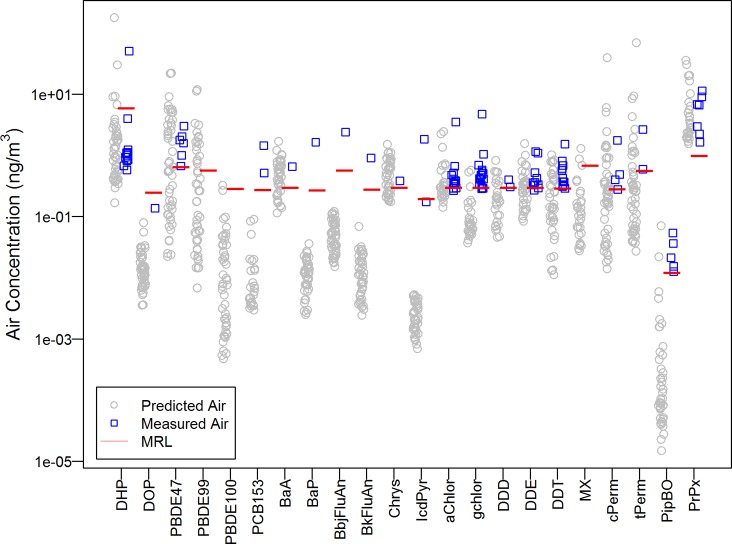
Finally, we used partitioning theory to predict air concentrations for 22 compounds detected in the majority of dust samples but detected less frequently in air (<50%) (Figure 3). We can use the predictions to evaluate reporting limit requirements for future studies. For all but one compound (propoxur), the minimum predicted air concentration was lower by 2- to 100-fold than the MRL in the CAHES. In general, we captured the upper end of the predicted air concentration distributions. Instances when we did not detect chemicals in air despite predictions greater than the MRL may result from model failure or analytical issues. Model failure includes violations of the equilibrium assumption or sorption assumptions, whereas analytical issues may arise from poor absorption onto sampling matrix or desorption during sampling or storage.
Figure 3.
Predicted air concentration (nanograms/cubic meter) from measured dust and measured air concentration for chemicals with at least 50% detection frequency in dust. Predictions are presented next to measured concentrations to compare range of concentrations expected and observed. Predictions are only made using detected dust concentrations. Red line represents study-specific maximum method reporting limit (MRL) for each chemical (MRL calculated on mass basis; variations in MRL attributed to volume differences). Note log-scale.
Limitations
While a partitioning model provides some important insights into the dynamics of SVOCs indoors and provides opportunities to make more accurate exposure estimates using fewer measurements, there are some limitations to this work. First, in this analysis, we modeled only compounds, mostly phthalates and PAHs, detected in ≥50% of air and ≥50% of dust samples. This is because we wanted to use central tendency estimates based on observed rather than estimated data and because if we include all individual points in the model, we bias toward chemicals with the highest detection frequencies. While we were able to use house-specific estimates for total suspended particles (TSP) in estimating the gas-phase concentrations, we had to assume values for dust and particle density and fraction organic matter.
There are also some limitations with the partitioning model concept. Since the partitioning model assumes equilibrium between air and dust, short-term variation in air concentrations, usually measured over hours or days, may not be reflected in dust measurements, which are thought to be stable over months to years.44 Similarly, chemicals with high Koa may not partition readily enough to achieve equilibrium. Also, by using the octanol-air partition coefficient, we assume that octanol is representative of organic matter in dust and particles, and that chemicals will readily partition to the organic matter. This assumption may not hold for chemicals found in nonorganic material in the dust, such as observed by Webster et al. for BDE 209.45
Implications and Recommendations
Our analysis has implications for modeling exposure pathways as well as for sampling study design. For example, chemicals with large Koa values (>10), especially if they are not found at relatively high concentrations like phthalates, may be best measured using dust sampling or air sampling that captures both the gas and particulate phases. In contrast, chemicals with lower Koa values (<10) would be readily measured in air samples. For many SVOCs with Koa values between 5 and 10, partitioning theory suggests, and our analysis confirms, that measurements in one media (e.g., air) can be used to predict concentrations in other media (e.g., dust) reasonably well. Our estimate of the relative importance of dust versus air exposure pathways also revealed that one particular medium does not dominate total exposures. If a researcher is interested in a large number of chemicals with a wide range of physical-chemical properties, then either air or dust sampling may be able to provide information on exposure source concentrations and route-specific exposures.
Researchers need reliable measurement methods that represent household exposures to SVOCs; however, a standard approach has not been established. Many comprehensive exposure studies have used active air sampling, which collects gas and particle-phase air, and field-technician vacuum dust collection; however, both methods rely on trained personnel and require electricity, making them infeasible in some situations and more costly, particularly in large cohort studies. Many large health studies that have a residential exposure measure for SVOCs are using dust wipes in conjunction with biological samples, despite relatively little validation.4,13,46 Dust wipe samples involve wiping a specified area to collect dust and surface SVOCs and analytical results are presented as chemical mass per area. There are several disadvantages to dust wipes. First, dust wipes cannot be collected as duplicates because once a particular surface has been wiped another sample cannot be retrieved, requiring that a second surface must be wiped. Second, discretion in selecting wipe locations leads to a lack of standardization, since dust loading as well as chemical concentrations may vary across the surface. Studies of the variations in dust wipe samples have mostly focused on lead, with one studying finding coefficients of variation for dust wipes in different areas of the home ranging from 0.55 to 1.53.47 Cost, standardization, precision, and relevance to exposure and/or health effect all need to be considered when selecting a residential exposure method.
Passive sampling, or diffusive sampling without the aid of a pump, using semipermeable membrane devices, polyurethane foam (PUF), or other matrices, has been explored by a few researchers, generally demonstrating that they have potential as low-cost versatile measurement tools for SVOCs.48−53 Despite the promise of this technology, there has been limited development or application of passive indoor sampling as an exposure tool for environmental health studies. Our findings that gas-phase air concentrations can be used to predict total air and house dust concentrations support the potential utility of this approach. A next step is to compare measured air concentrations from passive air samples with measured dust concentrations to investigate and validate this recommendation.
SVOCs from consumer products and building materials are common in residential air and house dust. We found 40 simultaneously detected phthalates, flame retardants, PCBs, PAHs, and pesticides in 49 homes in northern CA, and some chemical classes such as phthalates and PAHs were abundant in both air and dust. Because of their physical-chemical properties, SVOCs redistribute throughout the indoor environment. Resource-intense techniques such as active air sampling and field-technician collected vacuum dust have been used successfully in the past; however, these methods may be too costly for large-scale cohort studies. Simpler methods are needed. We applied a theoretical partitioning model to empirical data measured in this study and found that we can predict dust concentrations reasonably well from measured air concentrations. On the basis of these findings and ease of implementation, we conclude that passive air sampling, in particular, may be a more standardized measure compared to surface wipes, and is less resource intensive to collect than vacuum dust and active air samples. Validation of passive air methods for SVOCs is a priority.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (R25ES013258), New York Community Trust, and the Swain Barber Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Communities for a Better Environment for their collaboration as well as the participants. We thank Carla Perez, Jessica Tovar, Wanna Wright, Andrea Samulon, Allan Just, Sarah Dunagan, Laura Perovich, and Alice Yau for their contributions to study planning, community involvement, data collection and management, and chemical analysis.
Supporting Information Available
Specific details for analytical methods; Quality Assurance/Quality Control methods and results; exposure modeling; measured air and dust concentrations; measured total air concentrations versus dust concentrations with correlation estimates; ratio of dust to air concentrations; relative exposures; ratio of gas-phase air to total air concentrations; gas-phase air versus dust concentrations with log Koa; ratios of predicted to measured dust concentrations; and contour plot of predicted air concentrations. This material is available free of charge via the Internet at http://pubs.acs.org/
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008, 1812–19. [DOI] [PubMed] [Google Scholar]
- Rudel R. A.; Perovich L. J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 431170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi J.; Whyatt R. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008, 1164467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A.; Whyatt R. M. Characterizing exposures to nonpersistent pesticides during pregnancy and early childhood in the National Children’s Study: a review of monitoring and measurement methodologies. Environ. Health Perspect 2005, 11381092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh J. F.; Setzer R. W.; Reif D. M.; Gangwal S.; Mitchell-Blackwood J.; Arnot J. A.; Joliet O.; Frame A.; Rabinowitz J.; Knudsen T. B.; Judson R. S.; Egeghy P.; Vallero D.; Cohen Hubal E. A. High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ. Sci. Technol. 2013, 47158479–8488. [DOI] [PubMed] [Google Scholar]
- Egeghy P. P.; Judson R.; Gangwal S.; Mosher S.; Smith D.; Vail J.; Cohen Hubal E. A. The exposure data landscape for manufactured chemicals. Sci. Total Environ. 2012, 414, 159–166. [DOI] [PubMed] [Google Scholar]
- Mitchell J.; Arnot J. A.; Jolliet O.; Georgopoulos P. G.; Isukapalli S.; Dasgupta S.; Pandian M.; Wambaugh J.; Egeghy P.; Cohen Hubal E. A.; Vallero D. A. Comparison of modeling approaches to prioritize chemicals based on estimates of exposure and exposure potential. Sci. Total Environ. 2013, 458–460, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler C. J.; Nazaroff W. W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42409018–9040. [Google Scholar]
- Rudel R. A.; Camann D. E.; Spengler J. D.; Korn L. R.; Brody J. G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37204543–4553. [DOI] [PubMed] [Google Scholar]
- Brody J. G.; Morello-Frosch R. A.; Zota A. R.; Brown P.; Perez C.; Rudel R. Linking exposure assessment science with policy objectives for environmental justice and breast cancer advocacy: The Northern California Household Exposure Study. Am. J. Public Health 2009, 99Suppl 3S600–S609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel R. A.; Dodson R. E.; Perovich L. J.; Morello-Frosch R.; Camann D. E.; Zuniga M. M.; Yau A. Y.; Just A. C.; Brody J. G. Semivolatile endocrine disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ. Sci. Technol. 2010, 44, 6583–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard O.; Glorennec P.; Mercier F.; Bonvallot N.; Chevrier C.; Ramalho O.; Mandin C.; Bot B. L. Semivolatile organic compounds in indoor air and settled dust in 30 French dwellings. Environ. Sci. Technol. 2014, 4873959–3969. [DOI] [PubMed] [Google Scholar]
- Fenske R. A.; Bradman A.; Whyatt R. M.; Wolff M. S.; Barr D. B. Lessons learned for the assessment of children’s pesticide exposure: Critical sampling and analytical issues for future studies. Environ. Health Perspect. 2005, 113101455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy P. J.; Freeman C. G.; Millette J. R. Dust: A metric for use in residential and building exposure assessment and source characterization. Environ. Health Perspect. 2002, 110, 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler C. J.; Nazaroff W. W. SVOC partitioning between the gas phase and settled dust indoors. Atmos. Environ. 2010, 44303609–3620. [Google Scholar]
- Weschler C. J.; Nazaroff W. W. SVOC exposure indoors: Fresh look at dermal pathways. Indoor Air 2012, 225356–377. [DOI] [PubMed] [Google Scholar]
- Weschler C. J.; Salthammer T.; Fromme H. Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments. Atmos. Environ. 2008, 4271449–1460. [Google Scholar]
- Schossler P.; Schripp T.; Salthammer T.; Bahadir M. Beyond phthalates: Gas phase concentrations and modeled gas/particle distribution of modern plasticizers. Sci. Total Environ. 2011, 409194031–4038. [DOI] [PubMed] [Google Scholar]
- Cohen Hubal E. A.; Richard A.; Aylward L.; Edwards S.; Gallagher J.; Goldsmith M. R.; Isukapalli S.; Tornero-Velez R.; Weber E.; Kavlock R. Advancing exposure characterization for chemical evaluation and risk assessment. J. Toxicol. Environ. Health B Crit. Rev. 2010, 132–4299–313. [DOI] [PubMed] [Google Scholar]
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 462413056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel R. A.; Brody J. G.; Spengler J. D.; Vallarino J.; Geno P. W.; Sun G.; Yau A. Identification of selected hormonally active agents and animal mammary carcinogens in commercial and residential air and dust samples. J. Air Waste Manage. Assoc. 2001, 514499–513. [DOI] [PubMed] [Google Scholar]
- Newton E.; Rudel R. Estimating correlation with multiply censored data arising from the adjustment of singly censored data. Environ. Sci. Technol. 2007, 411221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA, Estimation Programs Interface Suite for Microsoft Windows Version 4. In United States Environmental Protection Agency: Washington, DC, 2013. [Google Scholar]
- Long C. M.; Suh H. H.; Koutrakis P. Characterization of indoor particle sources using continuous mass and size monitors. J. Air Waste Manage. Assoc. 2000, 5071236–1250. [DOI] [PubMed] [Google Scholar]
- Fromme H.; Lahrz T.; Piloty M.; Gebhart H.; Oddoy A.; Ruden H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air 2004, 143188–195. [DOI] [PubMed] [Google Scholar]
- Kubwabo C.; Rasmussen P. E.; Fan X.; Kosarac I.; Wu F.; Zidek A.; Kuchta S. L. Analysis of selected phthalates in Canadian indoor dust collected using household vacuum and standardized sampling techniques. Indoor Air 2013, 236506–514. [DOI] [PubMed] [Google Scholar]
- Shin H. M.; McKone T. E.; Nishioka M. G.; Fallin M. D.; Croen L. A.; Hertz-Picciotto I.; Newschaffer C. J.; Bennett D. H. Determining source strength of semivolatile organic compounds using measured concentrations in indoor dust. Indoor Air 2013, 243260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik B.; Naydenov K.; Larsson M.; Bornehag C. G.; Sundell J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ. Health Perspect. 2008, 116198–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H. M.; Park E. K.; Young T. M.; Hammock B. D. Occurance of endocrine-disrupting chemicals in indoor dust. Sci. Total Environ. 2008, 404, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R.; Rudel R. A.; Morello-Frosch R. A.; Brody J. G. Elevated house dust and serum concentrations of PBDEs in California: Unintended consequences of furniture flammability standards?. Environ. Sci. Technol. 2008, 42218158–8164. [DOI] [PubMed] [Google Scholar]
- Zota A. R.; Rudel R. A.; Morello-Frosch R. A.; Brody J. G. Response to comment on “Elevated house dust and serum concentrations of PBDEs in California”: Unintended consequences of furniture flammability standards?. Environ. Sci. Technol. 2009, 43, 2661–2662. [DOI] [PubMed] [Google Scholar]
- Quiros-Alcala L.; Bradman A.; Nishioka M.; Harnly M. E.; Hubbard A.; McKone T. E.; Eskenazi B. Concentrations and loadings of polybrominated diphenyl ethers in dust from low-income households in California. Environ. Int. 2011, 373592–596. [DOI] [PubMed] [Google Scholar]
- Rudel R. A.; Dodson R. E.; Newton E.; Zota A. R.; Brody J. G. Correlations between urinary phthalate metabolites and phthalates, estrogenic compounds 4-butyl phenol and o-phenyl phenol, and some pesticides in home indoor air and house dust. Epidemiology 2008, 196S332–S332. [Google Scholar]
- Ward M. H.; Colt J. S.; Metayer C.; Gunier R. B.; Lubin J.; Crouse V.; Nishioka M. G.; Reynolds P.; Buffler P. A. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ. Health Perspect. 2009, 11761007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W.; Wallace L. A.; Camann D. P.; Dickey P.; Gilbert S. G.; Lewis R. G.; Takaro T. K. Monitoring and Reducing Exposure of Infants to Pollutants in House Dust. Rev. Environ. Contam. Toxicol. 2009, 201, 1–39. [DOI] [PubMed] [Google Scholar]
- Camann D. E.; Colt J. S.; Teitelbaum S. L.; Rudel R. A.; Hart R. M.; Gammon M. D. In Pesticide and PAH Distributions in House Dust from Seven Areas of U.S.A.; Society of Environmental Toxicology and Chemistry (SETAC): Nashville, TN, 2000. [Google Scholar]
- Maertens R. M.; Bailey J.; White P. A. The mutagenic hazards of settled house dust: A review. Mutat. Res.-Rev. Mutat. Res. 2004, 5672–3401–425. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency Methoxychlor Reregistration Eligibility Decision (RED). http://www.epa.gov/oppsrrd1/REDs/methoxychlor_red.htm.
- U.S. Environmental Protection Agency R.E.D. FACTS: Bendiocarb; EPA 738-F-99-010; Office of Prevention, Pesticides and Toxic Substances: 1999.
- Egeghy P. P.; Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J. Expo Sci. Environ. Epidemiol. 2011, 212150–168. [DOI] [PubMed] [Google Scholar]
- U.S. EPA Exposure Factors Handbook; U.S. Environmental Protection Agency: Washington, DC, 2011. [Google Scholar]
- Weschler C. J.; Nazaroff W. W. Dermal uptake of organic vapors commonly found in indoor air. Environ. Sci. Technol. 2014, 4821230–1237. [DOI] [PubMed] [Google Scholar]
- Meylan W. M.; Howard P. H. Estimating octanol-air partition coefficients with octanol-water partition coefficients and Henry’s law constants. Chemosphere 2005, 615640–644. [DOI] [PubMed] [Google Scholar]
- Butte W.; Heinzow B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [PubMed] [Google Scholar]
- Webster T. F.; Harrad S.; Millette J. R.; Holbrook R. D.; Davis J. M.; Stapleton H. M.; Allen J. G.; McClean M. D.; Ibarra C.; Abdallah M. A.; Covaci A. Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ. Sci. Technol. 2009, 4393067–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B.Children’s study faces tough year. Chem. Eng. News September 27, 2010, 2010; pp 43–45. [Google Scholar]
- Sterling D. A.; Roegner K. C.; Lewis R. D.; Luke D. A.; Wilder L. C.; Burchette S. M. Evaluation of four sampling methods for determining exposure of children to lead-contaminated household dust. Environ. Res. 1999, 812130–141. [DOI] [PubMed] [Google Scholar]
- Bartkow M. E.; Booij K.; Kennedy K. E.; Muller J. F.; Hawker D. W. Passive air sampling theory for semivolatile organic compounds. Chemosphere 2005, 602170–176. [DOI] [PubMed] [Google Scholar]
- Gale R. W.; Cranor W. L.; Alvarez D. A.; Huckins J. A.; Petty J. D.; Robertson G. L. Semivolatile organic compounds in residential air along the Arizona–Mexico border. Environ. Sci. Technol. 2009, 43, 3054–3060. [DOI] [PubMed] [Google Scholar]
- Hayward S. J.; Gouin T.; Wania F. Comparison of four active and passive sampling techniques for pesticides in air. Environ. Sci. Technol. 2010, 4493410–3416. [DOI] [PubMed] [Google Scholar]
- Shoeib M.; Harner T. Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 2002, 36194142–4151. [DOI] [PubMed] [Google Scholar]
- Waite D. T.; Droneck N. R.; Tuduri L.; Sproull J. F.; Chau D. F.; Quiring D. V. Comparison of active versus passive atmospheric samplers for some current-use pesticides. Bull. Environ. Contam. Toxicol. 2005, 7461011–1018. [DOI] [PubMed] [Google Scholar]
- Wilford B. H.; Harner T.; Zhu J.; Shoeib M.; Jones K. C. Passive sampling survey of polybrominated diphenyl ether flame retardants in indoor and outdoor air in Ottawa, Canada: Implications for sources and exposure. Environ. Sci. Technol. 2004, 38205312–5318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.