Abstract
Background/Aims
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenolic compound (stilbene) and a phytoalexin. The purpose of this study was to determine the mechanism which mediated the resveratrol-induced relaxation of cholecystokinin octapeptide- or KCl-induced tension in male guinea pig gallbladder strips.
Methods
Gallbladder strips were prepared and suspended in in vitro chambers filled with Krebs-Henseleit solution. The strips were attached to force displacement transducers, and the changes in tension were recorded on a polygraph. All reagents were added directly into the chambers.
Results
To determine if intracellular Ca2+ release mediated the resveratrol-induced relaxation of cholecystokinin octapeptide-induced tension, 2-aminoethoxydiphenylborane (2-APB) was used. 2-APB significantly (P < 0.01) decreased the amount of RSVL-induced relaxation. To determine if protein kinase A (PKA) mediated the resveratrol-induced relaxation, PKA inhibitor 14-22 amide myristolated (PKA-IM) was used. PKA-IM had no effect on resveratrol-induced relaxation. Neither KT5823, NG-methyl-L-arginine acetate salt, a nitric oxide synthase inhibitor, nor fulvestrant had a significant effect on the amount of resveratrol-induced relaxation. Genistein, a protein tyrosine kinase inhibitor, significantly (P < 0.01) increased the RSVL-induced relaxation. To determine if protein kinase C mediated the RSVL-induced relaxation, the protein kinase C inhibitors bisindolymaleimide IV and chelerythrine Cl- were used together, and a significant (P < 0.05) increase in resveratrol-induced relaxation was observed. The pretreatment of the strips with resveratrol significantly (P < 0.001) decreased the amount of KCl- and cholecystokinin octapep-tide-induced tension.
Conclusions
Resveratrol-induced relaxation is mediated by its effects on L-type Ca2+ channels and intracellular Ca2+ release.
Keywords: Calcium channels; Gallbladder; Muscle, smooth; Phytoalexins; Resveratrol
Introduction
Resveratrol (3,5,4′-trihydroxystilbene, RSVL) is a phytoalexin produced by plants in response to damage, particularly in grapevines and peanuts.1,2 RSVL has been shown to be a non-flavonoid phytoestrogen, and to act as an estrogen receptor agonist in MCF-7 cells transiently transfected with estrogen-responsive reporter constructs.3 RSVL has similar effects in breast cancer cells expressing mutant and wild-type estrogen receptors.4 Levenson et al5 found that RSVL acted as an estrogen receptor agonist in breast cancer cells stably transfected with estrogen receptor α.
RSVL can modulate nitric oxide (NO) levels by its action on both endothelial nitric oxide synthase (eNOS) and cytokine-inducible nitric oxide synthase (iNOS). RSVL can cause NO-mediated relaxation of precontracted endothelium-intact rat aorta through an increase of NO via eNOS.6–9 RSVL decreased Ca2+ sensitivity but did not affect KCl-stimulated intracellular Ca2+ increases in vascular smooth muscle of rat aorta.10 RSVL also increases the amount of cGMP in sheep coronary arteries. This triggers vasorelaxant responses even in endothelium-disrupted arteries.11 RSVL prevented bradykinin-induced contraction of rat urinary bladder smooth muscle by the inhibition of Ca2+ influx.12 Wang et al13 showed that RSVL had an inhibitory effect on guinea pig gallbladder contractility. The present study determined which mechanisms mediated the relaxation effect of RSVL on cholecystokinin octapeptide (CCK) and KCl-induced tension in male guinea pig gallbladder strips.
Materials and Methods
The Animal Care Committee-Health Sciences of the University of Alberta approved the experiments performed by issuing protocol number (#275) on February 6, 2014. The experiments used male Hartley guinea pigs (200–375 g body weight) which were killed by decapitation. The gallbladder was removed, cleansed, and placed in Krebs-Henseleit solution that was gassed with 95% O2 and 5% CO2. The composition of the Krebs-Henseleit solution was NaCl, 115 mM; KCl, 5 mM CaCl2, 2.1 mM; MgSO4, 1.2 mM; NaH2PO4, 1.2 mM; NaHCO3, 25 mM; and glucose, 11 mM. Strips (1.5 × 0.5 cm) were made from each gallbladder which were maintained in Sawyer-Bartlestone chambers filled with Krebs-Henseleit solution, maintained at 37°C, and gassed with 95% O2 and 5% CO2. An optimum resting tension of 0.7 g was determined previously and used in the study.14,15
A Grass 7D polygraph (Grass Instruments Co., Quincy MA, USA) and Grass FT03 force displacement transducers were used to measure the force developed by the gallbladder strips. Isolated strips were equilibrated in the chambers for 45 minutes prior to determining their suitability for use. Each chamber had 2 μM (final concentration) of atropine added in every experiment, 3 minutes prior to 1 nM CCK. The tension measurement was followed by 3 changes of Krebs-Henseleit solution. The test was repeated twice with 25 minutes between tests. A repeatable minimum active tension of 0.5 g had to be generated by the strips before use. All agents used were added directly to the chambers. All concentrations are reported as the final concentration in the chambers.
Several series of experiments were performed to examine the effects of RSVL on the tension generated by the gallbladder strips. CCK (1 nM) produced a stable long lasting tension after 3 minutes. This steady tension lasted at least 10 mintes.14,15 In order to determine if RSVL could relax CCK- or KCl-induced tension, concentration response curves were generated. The strips were exposed to 1 concentration of RSVL (10, 50, 100, or 250 μM), the response was observed until the relaxation reached a steady level (approximately 5 minutes), the Krebs-Henseleit solution was changed 3 times, and the strips were allowed to recover for 30 minutes before testing different concentrations of RSVL. The concentration of RSVL (75 μM) was selected for use in subsequent experiments as it produced a reproducible relaxation. The same procedure was followed to generate a concentration response curve using 40 mM KCl instead of 1 nM CCK. The use of KCl to directly depolarize smooth muscle is a standard procedure.
The concentration of each blocker used in the experiments was 5 times the half maximal inhibitory concentration (IC50). In order to determine if the Ca2+ released from the endoplasmic reticulum mediated the RSVL-induced relaxation 2-aminoethoxydiphenylborane (2-APB,125 μM), a cell permeable inhibitor of inositol 1,4,5-triphosphate (IP3)-induced Ca2+ release, was added to the chambers 10 minutes prior to the CCK. The CCK was then added to the chambers. When the tension reached a steady level, 75 μM RSVL was added to the chambers. The amount of relaxation was observed. The amount of relaxation observed when RSVL only was used, was then compared to the amount of relaxation observed when the strips were treated with 2-APB and RSVL. This procedure was followed with each agent used.
Protein kinase A inhibitor 14–22 amide myristolated (a protein kinase inhibitor, PKA-IM; 180 nM), KT5823 (a protein kinase G [PKG] inhibitor, 585 nM), and genistein (a tyrosine kinase inhibitor, 10 μM) were added to the chambers 5 minutes prior to the addition of CCK.
The PKC inhibitors, chelerythrine Cl− (5 μM) and bisindolymaleimide IV (BisIV, 0.5 μM), were used together to determine the effects of PKC on RSVL-induced relaxation of CCK- or KCl-induced tension. They were added to the chambers 5 minutes prior to either CCK or KCl. NG-methyl-L-arginine acetate salt (20 μM) was used to determine if NO mediated the RSVL -induced relaxation. Fulvestrant (10 μM), an estrogen receptor blocker, was used to determine if RSVL might act via estrogen receptor activation.
In order to determine if RSVL inhibited extracellular Ca2+ entry, 40 mM KCl was used to induce tension in the strips. After the amount of tension generated by the 40 mM KCl was recorded, the Krebs-Henseleit solution was changed 3 times and the strips allowed to equilibrate for 25 minutes. The 75 μM RSVL was then added to the chambers 3 minutes prior to the addition of 40 mM KCl. The amount of tension generated was recorded and compared to that observed when the KCl was added to the chambers with no RSVL. The same experiments were performed using CCK, i.e., adding RSVL 3 minutes prior to the addition of CCK.
The following agents were purchased from Sigma (St. Louis, MO, USA): CCK, atropine, NG-methyl-L-arginine acetate salt, fulvestrant, and bisindolymaleimide IV. The following agents were purchased from Calbiochem (LaJolla, CA, USA): PKA-IM, KT5823, chelerythrine Cl−, genistein, and 2-APB. Trans-resveratrol was purchased from Cayman Chemical (Ann Arbor, MI, USA). All agents were dissolved in either distilled water or dimethyl sulfoxide. The amount of dimethyl sulfoxide (10 μL) added to the chambers was determined to have no effect on the strips.
Statistical Methods
Statistical comparisons were done using the paired t test. Results are expressed as mean ± SE Differences among mean values with P < 0.05 were considered significant. The number of gallbladders (animals) used in each experiment are indicated by “n”. Each gallbladder was used to prepare 4 strips; hence, an n of 4 means up to 16 strips were used.
Results
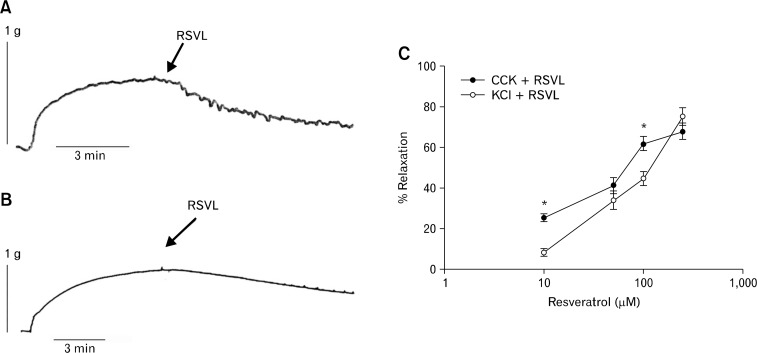
RSVL induced a concentration dependent relaxation in both CCK- and KCl-induced tension (Fig. 1). RSVL at 10 μM and 100 μM significantly (P < 0.01, n = 20) relaxed the CCK-induced tension more than KCl-induced tension. When 75 μM or 250 μM RSVL was used, there was no significant difference in the amount of RSVL-induced relaxation when CCK and KCl were compared. There was no significant difference between the amount of CCK-induced tension and the KCl-induced tension (1 nM CCK, 0.86 ± 0.05 g vs 40 mM KCl, 0.88 ± 0.06 g; n = 8).
Figure 1.
Effect of resveratrol (3,5,4′-trihydroxystilbebe, RSVL) on cholecystokinin octapeptide (CCK)- or KCl-induced tension. (A) A data trace showing the relaxation caused by RSVL on CCK-induced tension in a male guinea pig gallbladder strip. (B) A data trace showing the relaxation caused by RSVL on KCl-induced tension in a male guinea pig gallbladder strip. (C) RSVL (10 μM and 100 μM) produced significantly (*P < 0.01, n = 20) more relaxation in the CCK-induced tension (filled circles) than KCl-induced tension (open circles). When 75 μM or 250 μM RSVL was used, there was no significant difference in the amount of RSVL-induced relaxation when CCK and KCl were compared. The significance was determined by paired t tests.
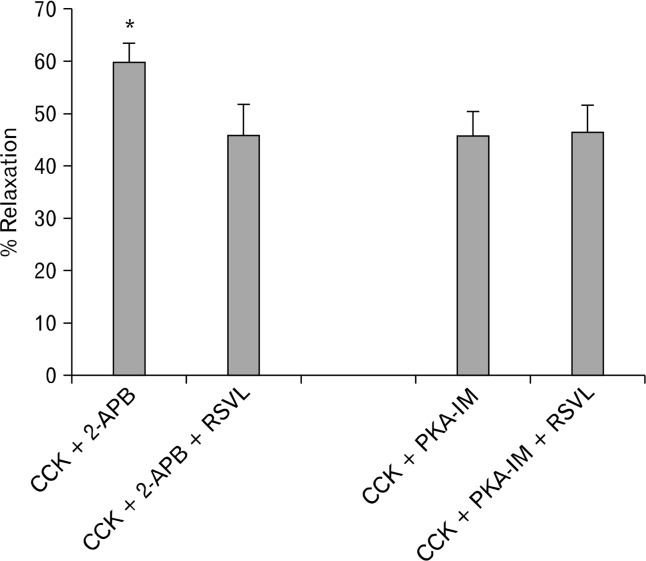
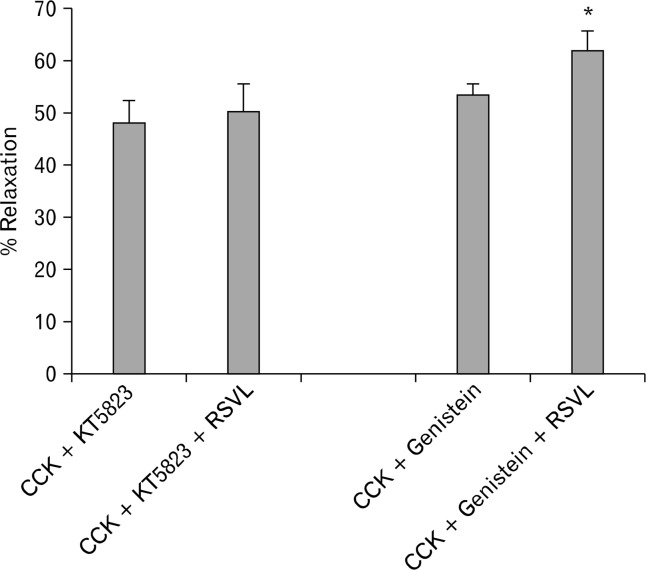
The use of 2-APB, an inhibitor of IP3-induced Ca2+ release, caused a significant (P < 0.001) decrease in the amount of CCK-induced tension (0.79 ± 0.05 g vs 0.31 ± 0.03 g, n = 6). When the strips were treated with 2-APB, there was a significant (P < 0.05) decrease in the amount of relaxation observed between the strips not treated and treated with 2-APB (59.7 ± 3.6% vs 45.9 ± 5.6%, n = 6; Fig. 2). When PKA-IM was used, no significant effect was observed on the amount of CCK-induced tension (0.75 ± 0.05 g vs 0.8 ± 0.05 g, n = 5); nor was there a significant effect on the amount of RSVL-induced relaxation (45.7 ± 4.5% vs 46.5 ± 5.0%, n = 5). Treatment of the strips with KT5823, a PKG blocker, significantly (P < 0.01) increased the amount of CCK-induced tension (0.77 ± 0.06 g vs 0.86 ± 0.06 g, n = 4). KT5823 did not have a significant effect on the amount of RSVL-induced relaxation of CCK-induced tension (48.1 ± 4.0% vs 50.3 ± 5.2%, n = 4; Fig. 3).
Figure 2.
Effect of intracellular Ca2+ release or protein kinase A (PKA) inhibition on resveratrol (3,5,4′-trihydroxystilbene, RSVL)-induced relaxation. When the strips were treated with 2-aminoethoxydiphenylborane (2-APB), there was a significant (*P < 0.05) decrease in the amount of relaxation observed between the strips not treated and treated with 2-APB (n = 6). When the PKA inhibitor 14–22 amide myristolated (PKA-IM), was used, no significant effect was observed on the amount of cholecystokinin octapeptide (CCK)-induced tension; nor was there a significant effect on the amount of RSVL-induced relaxation (n = 6).
Figure 3.
The effect of protein kinase G (PKG) blocking or protein tyrosine kinase blocking of resveratrol (3,5,4′-trihydroxystilbene, RSVL)-induced relaxation. KT5823 (585 nM), a PKG inhibitor, did not have a significant effect on the amount of RSVL-induced relaxation of cholecystokinin octapeptide (CCK)-induced tension (n = 4). Genistein (10 μM) significantly (*P < 0.01) increased effect on the amount of RSVL-induced relaxation of CCK-induced tension (n = 4).
Genistein significantly (P < 0.01) increased effect on the amount of RSVL-induced relaxation of CCK-induced tension (53.4 ± 2.2% vs 61.8 ± 3.8%, n = 4; Fig. 3). There was no significant difference in the amount of CCK-induced tension (0.79 ± 0.06 g vs 0.74 ± 0.06 g).
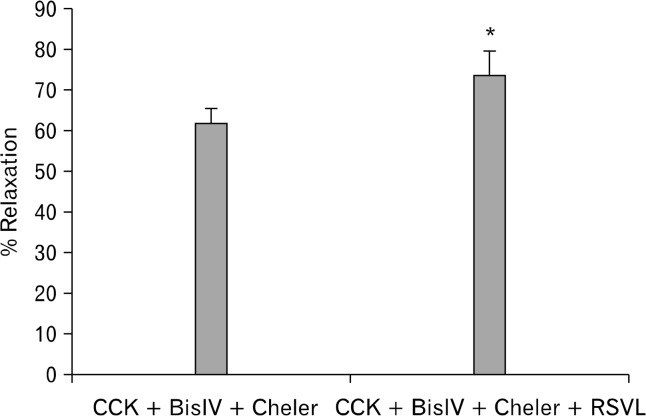
When the PKC blockers, BisIV and chelerythrine Cl− were used in combination, a significant increase (P < 0.05) in the amount of RSVL-induced relaxation (61.8 ± 3.5% vs 73.7 ± 5.6%, n = 4; Fig. 4) was observed; however, there was no significant effect on the amount of CCK-induced tension (0.64 ± 0.04 g vs 0.68 ± 0.04 g).
Figure 4.
The effect of protein kinase C (PKC) blocking on resveratrol (3,5,4′-trihydroxystilbene, RSVL)-induced relaxation. The use of the PKC inhibitors bisindolymaleimide IV (BisIV, 0.5 μM) and chelerythrine Cl− (Cheler, 5 μM) significantly (P < 0.05; n = 4) increased the amount of RSVL-induced relaxation.
The use of NG-methyl-L-arginine acetate salt, a NO synthase blocker, did not have a significant effect on either the CCK-induced tension or the RSVL-induced relaxation (48.1 ± 3.4% vs 44.6 ± 3.2%, n = 4). The use of the estrogen receptor blocker fulvestrant did not have a significant effect on the amount of CCK-induced tension (CCK + RSVL, 0.84 ± 0.09 g vs fulvestrant + CCK + RSVL, 0.81 ± 0.08 g; n = 5). Fulvestrant also had no significant effect in the amount of RSVL-induced relaxation (CCK + RSVL, 50.9 ± 3.8% vs fulvestrant + CCK + RSVL, 50.2 ± 5.2%; n = 5).
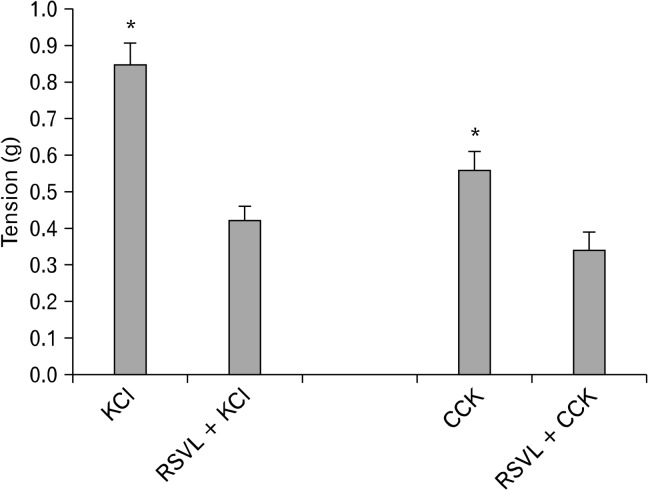
When RSVL was added to the chambers 3 minutes prior to the addition of KCl, there was a significant decrease (P < 0.001) in the amount of tension generated (0.85 ± 0.06 g vs 0.42 ± 0.04 g, n = 5. Fig. 5). When 75 μM RSVL was added to the chambers 3 minutes prior to the addition of CCK (1 nM), there was a significant (P < 0.001) decrease in the amount of tension generated (0.56 ± 0.05 g vs 0.34 ± 0.05 g, n = 3; Fig. 5).
Figure 5.
The effect of resveratrol (3,5,4′-trihydroxystilbene, RSVL) on cholecystokinin octapeptide (CCK)- or KCl-induced tension. When RSVL (75 μM) was added to the chambers 3 minutes prior to the addition of KCl there was a significant decrease (*P < 0.001) in the amount of tension generated (n = 5). When 75 μM RSVL was added to the chambers 3 minutes prior to the addition of CCK (1 nM), there was a significant (*P < 0.001) decrease in the amount of tension generated (n = 3).
Discussion
RSVL is a non-flavonoid phytoestrogen that can act as an estrogen receptor agonist in MCF-7 cells and breast cancer cells.3,4 Strips of either male or female guinea pig gallbladder strips are sensitive to both 17 β-estradiol and progesterone. Both 17 β-estradiol and progesterone relaxed both CCK- and KCl-induced tensions using different signaling pathways.16–18 The current experiments demonstrated that RSVL relaxed both CCK- and KCl-induced tensions in a concentration-dependent manner. The use of fulvestrant demonstrated that this effect of RSVL was not mediated through estrogen receptors which had been suggested by other researchers.3–5
El Mowafy11 showed that RSVL increased cGMP in sheep coronary arteries which led to an endothelium-independent vaso-dilation. In the guinea pig gallbladder strips the use of KT5823, a PKG antagonist, had no significant effect on the amount of RSVL-induced relaxation.
RSVL acted through NO release from endothelium-intact blood vessels.6–9 The use of NG-methyl-L-arginine acetate salt reported here showed that inhibiting NO synthase had no significant effect on the RSVL-induced relaxation.
BisIV and chelerythrine Cl− are PKC inhibitors. Using BisIV and chelerythrine Cl− in together produced consistent results.14,15 The PKC inhibitors significantly increased the amount of RSVL-induced relaxation. Wu et al,19 using the patch-clamp technique, demonstrated that gallbladder contraction required increasing Ca2+ influx through L-type Ca2+ calcium channels via a PKC pathway. Zhu et al20 showed that sulfated CCK prompted contraction of guinea pig proximal colon smooth muscle strips via activation of the IP3-PKC signal transduction pathway. Activation of PKC leads to an increase in L-channel opening which would lead to a decrease in RSVL-induced relaxation. Blocking PKC with BisIV and chelerythrine Cl− would lead to a decrease in L-channel opening which would lead to an increase in RSVL-induced relaxation. This is what was observed in the guinea pig gallbladder strips. In addition, using PKA-IM did not have a significant effect on the amount of RSVL-induced relaxation. Thus, the PKA did not mediate the RSVL-induced relaxation.
Protein tyrosine kinase may have a role in gallbladder smooth muscle contractility. In guinea pig gallbladder strips, genistein, a protein tyrosine kinase inhibitor, significantly decreased the progesterone-induced relaxation, with no effect on the CCK-induced tension.16 Alcón et al21 demonstrated that vanadate activated the tyrosine kinase pathway in guinea pig gallbladder smooth muscle. When genistein was used in the current study, genistein significantly increased the amount of RSVL-induced relaxation. Genistein has been shown to inhibit peristalsis in guinea pig small intestine by inhibiting intracellular signaling cascades involving protein kinases that act on the contractile apparatus in smooth muscle.22 The genistein-induced inhibition of protein tyrosine kinase in addition to the actions of RSVL may have led to the increased relaxation.
The role of extracellular Ca2+ entry to initiate CCK-induced tension was reported by Clas et al23 and Parkman et al24 Nifedi-pine almost completely abolished spontaneous interdigestive gall-bladder contractile activity and decreased resting gallbladder tone. This suggested that extracellular Ca2+ entry was important in gallbladder motiltity. Quinn et al25 reported that extracellular Ca2+ entry was required for intracellular Ca2+ release which was required for the sustained contraction of the gallbladder. Balemba et al26 demonstrated that spontaneous electrical rhythmicity in guinea pig gallbladder smooth muscle cells depended upon Ca2+ entry via voltage-dependent Ca2+channels and then on Ca2+mobilization from the sarcoplasmic reticulum via IP3 channels. KCl was used to directly depolarize the gallbladder strips. RSVL, when added to the chambers prior to KCl, significantly decreased the amount of KCl-induced tension. Therefore, blocking of L-type Ca2+ channels mediated part of the RSVL-induced relaxation. RSVL had the same effect on CCK-induced tension. Since RSVL significantly decreased the amount of both KCl- and CCK-induced tension when added to the chambers prior to either agonist, it suggested that RSVL exerts its effect in part by blocking L-type Ca2+ channels. It may also block intracellular Ca2+ release. Using 2-APB, an inhibitor of IP3-induced Ca2+ release, caused a significant decrease in the amount of RSVL-induced relaxation. This suggested that part of the RSVL effect was mediated through intracellular Ca2+ release.
The sustained phase of CCK-induced tension requires extracellular Ca2+ entry.16 The current study demonstrated that RSVL relaxed both CCK- and KCl-induced tension in male guinea pig gallbladder strips. No second messenger systems studied produced a significant effect on the RSVL-induced relaxation. The adding of RSVL to the in vitro chambers before either CCK or KCl produced a decrease in the amount of tension generated which was significant. If the amount of tension generated by either agonist is significantly decreased when RSVL is added before either CCK or KCl and no second messenger system had an effect on the RSVL-induced relaxation, which was significant, then it can be concluded that RSVL exerts its effects by blocking L-type Ca2+channels. The results of these experiments are similar to those reported for vascular smooth muscle cells and strips.17,27 In conclusion, the relaxation effect of RSVL was mediated primarily by its effects on both L-type Ca2+channels and intracellular Ca2+ release.
Footnotes
Financial support: None.
Conflicts of interest: None.
ORCID: Loren W Kline, http://orcid.org/0000-0002-4735-6296.
References
- 1.Langcake P, Pryce RJ. The production of resveratrol by vitus vinifera and other members of the vitaceae as a response to infection or injury. Physiol Plant Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- 2.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 3.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehm BD, Levenson AS, Liu H, et al. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: role of AF-1 and AF-2. J Steroid Biochem Mol Biol. 2004;88:223–234. doi: 10.1016/j.jsbmb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Levenson AS, Gehm BD, Pearce ST, et al. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER. Int J Cancer. 2003;104:587–596. doi: 10.1002/ijc.10992. [DOI] [PubMed] [Google Scholar]
- 6.Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265(2 Pt 2):H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 8.Andriambeloson E, Kleschyov AL, Muller B, Beretz A, Stoclet JC, Andriantsitohaina R. Nitric oxide production and endothelium-dependent vasorelaxation by wine polyphenols in rat aorta. Br J Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flesch M, Schwarz A, Bohm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275(4 Pt 2):H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- 10.Buluc M, Demirel-Yilmaz E. Resveratrol decreases calcium sensitivity of vascular smooth muscle and enhances cytosolic calcium increase in endothelium. Vascul Pharmacol. 2006;44:231–237. doi: 10.1016/j.vph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.El-Mowafy AM. Resveratrol activates membrane-found guanylyl cyclise in coronary arterial smooth muscle and a novel signaling mechanism in support of coronary protection. Biochem Biophys Res Commun. 2002;291:1218–1224. doi: 10.1006/bbrc.2002.6598. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda Y, Nakahara T, Mori A, Sakamoto K, Ishii K. Resveratrol prevents bradykinin-induced contraction of rat urinary bladder by decreasing prostaglandin production and calcium influx. Eur J Pharmacol. 2011;666:189–195. doi: 10.1016/j.ejphar.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Wang LD, Qiu XQ, Tian ZF, Zhang YF, Li HF. Inhibitory effects of genistein and resveratrol on guinea pig gallbladder contractility in vitro. World J Gastroenterol. 2008;14:4955–4960. doi: 10.3748/wjg.14.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline LW, Kaneko T, Benishin CG, Pang PK. Calcitonin gene-related peptide: an inhibitor of gallbladder contraction. Can J Physiol Pharmacol. 1991;69:1149–1154. doi: 10.1139/y91-168. [DOI] [PubMed] [Google Scholar]
- 15.Kline LW, Karpinski E. 17β-Estradiol relaxes cholecystokinin- and KCl-induced tension in male guinea pig gallbladder strips. Steroids. 2011;76:553–557. doi: 10.1016/j.steroids.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Kline LW, Karpinski E. Progesterone inhibits gallbladder motility through multiple signalling pathways. Steroids. 2005;70:673–679. doi: 10.1016/j.steroids.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Kline LW, Karpinski E. Testosterone and dihydrotestosterone inhibit gallbladder motility through multiple signaling pathways. Steroids. 2008;73:1174–1180. doi: 10.1016/j.steroids.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Kline LW, Karpinski E. A comparison of the effects of various sex steroids on cholecystokinin- and KCl-induced tension in female guinea pig gallbladder strips. Gen Comp Endocrinol. 2013;185:37–43. doi: 10.1016/j.ygcen.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Wu ZX, Yu BP, Xia H, Xu L. Emodin increase Ca2+ influx through L-type Ca2+ channel in guinea pig gallbladder smooth muscle. Eur J Pharmacol. 2008;595:95–99. doi: 10.1016/j.ejphar.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Chen L, Xia H, Luo HS. Mechanisms mediating CCK-8S-induced contraction of proximal colon in guinea pigs. World J Gastroenterol. 2010;16:1076–1085. doi: 10.3748/wjg.v16.i9.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcón S, Camello PJ, García LJ, Pozo MJ. Activation of tyrosine kinase pathways by vanadate in gallbladder smooth muscle. Biochem Pharmacol. 2000;59:1077–1089. doi: 10.1016/S0006-2952(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 22.Gharzouli K, Holzer P. Inhibition of guinea pig intestinal peristalsis by the flavonoids quercetin, naringenin, apigenin and genistein. Pharmacology. 2004;70:5–14. doi: 10.1159/000074237. [DOI] [PubMed] [Google Scholar]
- 23.Clas D, Hould FS, Rosenthall L, Arzoumanian A, Fried GM. Nifedipine inhibits cholecystokinin-induced gallbladder contraction. J Surg Res. 1989;46:479–483. doi: 10.1016/0022-4804(89)90164-9. [DOI] [PubMed] [Google Scholar]
- 24.Parkman HP, Pagano AP, Ringold MA, Ryan JP. Effect of modulating voltage-dependent calcium channels on cholecystokinin and acetylcholine-induced contractions of the guinea pig gallbladder. Regul Pept. 1996;63:31–37. doi: 10.1016/0167-0115(96)00023-7. [DOI] [PubMed] [Google Scholar]
- 25.Quinn T, Mollay M, Smyth A, Baird AW. Capacitative calcium entry in guinea pig gallbladder smooth muscle in vitro. Life Sci. 2004;74:1659–1669. doi: 10.1016/j.lfs.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Balemba OB, Salter MJ, Heppner TJ, Bonev AD, Nelson MT, Mawe GM. Spontaneous electrical rhythmicity and the role of the sarcroplasmic reticulum in the excitability of guinea gallbladder smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G655–G664. doi: 10.1152/ajpgi.00310.2005. [DOI] [PubMed] [Google Scholar]
- 27.Yu P, Harnett KM, Biancani P, De Petris G, Behar J. Interaction between signal transduction pathways contributing to gallbladder tonic contractions. Am J Physiol. 1993;265(6 Pt 1):G1082–G1089. doi: 10.1152/ajpgi.1993.265.6.G1082. [DOI] [PubMed] [Google Scholar]