Programmed death ligand-1 (PD-L1) expression in nonclear-cell RCC (non-ccRCC) and its association with clinical outcomes are unknown. In this study, we report that PD-L1 expression occurs in patients with non-ccRCC depending on histology subtype and tumour cell membrane versus immune cell scoring. In addition, we showed that patients with PD-L1-positive tumours appear to have worse clinical outcomes in non-ccRCC .
Keywords: renal cell carcinoma, nonclear-cell renal cell carcinoma, benign kidney tumors, PD-L1, PD-1 inhibitors, immunotherapy
Abstract
Background
Programmed death ligand-1 (PD-L1) expression in nonclear-cell RCC (non-ccRCC) and its association with clinical outcomes are unknown.
Methods
Formalin-fixed paraffin-embedded (FFPE) specimens were obtained from 101 patients with non-ccRCC. PD-L1 expression was evaluated by immunohistochemistry in both tumor cell membrane and tumor-infiltrating mononuclear cells (TIMC). PD-L1 tumor positivity was defined as ≥5% tumor cell membrane staining. For PD-L1 expression in TIMC, a combined score based on the extent of infiltrate and percentage of positive cells was used. Baseline clinico-pathological characteristics and outcome data [time to recurrence (TTR) and overall survival (OS)] were correlated with PD-L1 staining.
Results
Among 101 patients, 11 (10.9%) were considered PD-L1+ in tumor cells: 2/36 (5.6%) of chromophobe RCC, 5/50 (10%) of papillary RCC, 3/10 (30%) of Xp11.2 translocation RCC and 1/5 (20%) of collecting duct carcinoma. PD-L1 positivity (PD-L1+) in tumor cells was significantly associated with higher stage (P = 0.01) and grade (P = 0.03), as well as shorter OS (P < 0.001). On the other hand, PD-L1 positivity by TIMC was observed in 57 (56.4%) patients: 13/36 (36.1%) of chromophobe RCC, 30/50 (60%) of papillary RCC, 9/10 (90%) of Xp11.2 translocation RCC and 5/5 (100%) of collecting duct carcinoma. A trend toward shorter OS was observed in patients with PD-L1+ in TIMC (P = 0.08). PD-L1+ in both tumor cell membrane and TIMC cells were associated with shorter TTR (P = 0.02 and P = 0.03, respectively).
Conclusion
In non-ccRCC, patients with PD-L1+ tumors appear to have worse clinical outcomes, although only PD-L1 positivity in tumor cells is associated with higher tumor stage and grade.
introduction
Renal cell carcinoma (RCC) has been widely recognized as a heterogeneous disease encompassing different histological subtypes [1]. Clear-cell RCC (ccRCC) is the most common subtype and accounts for more than 80% of the tumors that arise from the renal epithelium [2]. The remaining renal epithelial malignancies, collectively named as nonclear-cell RCC (non-ccRCC), include several subtypes such as papillary RCC (10%–15%), chromophobe RCC (5%), and the more rare forms, which include as Xp11.2 translocation RCC, unclassified RCC, and collecting duct carcinoma, among others [3].
In RCC, surgery can be curative for localized disease [4]. However, about 30% of patients treated with nephrectomy will still develop systemic metastases. The risk of recurrence has been associated with clinical and pathological factors such as tumor–node–metastasis (TNM) staging and Fuhrman nuclear grading [5]. Several reports suggested that localized non-ccRCC is more likely to have a favorable prognosis than ccRCC [6]. Paradoxically, some series showed that when metastatic, some types of non-ccRCC such as papillary and Xp11.2 translocation RCC [7, 8], may have an aggressive clinical course and a shorter overall survival (OS).
Immunotherapy strategies have been used for decades in patients with advanced RCC, with prolonged survival being seen in a very small proportion of patients treated with interferon-α or high-dose interleukin (IL)-2 therapy [9]. Based on the important role of angiogenesis in ccRCC, single-agent therapies blocking the vascular endothelial growth factor (VEGF) or its receptors, as well as the mammalian target of rapamycin (mTOR) produced significant clinical benefit in the majority of metastatic ccRCC, resulting in a median OS of 20–30 months, compared with ∼13 months reported with traditional immunotherapy [10, 11]. Because of their relatively low prevalence and their distinct biology, patients with non-ccRCC have typically been excluded from the pivotal clinical trials of antiangiogenic and tumor targeted agents [12]. Although some series have suggested that these drugs may also have activity in patients with non-ccRCC, more effective therapies for this patient population are needed [6, 13–15].
The levels and clinical significance of PD-L1 expression in non-ccRCC subtypes is still unknown. In this study, we sought to examine PD-L1 expression and its association with clinical outcome in a large series of patients with non-ccRCC.
methods
patients and samples
One hundred one patients with non-ccRCC (chromophobe RCC, papillary RCC, collecting duct carcinoma and Xp.11.2 translocation RCC) treated surgically at two institutions: Brigham and Women's Hospital (BWH) and Mayo Clinic were indentified. For comparative purposes, 20 patients with oncocytoma or angiomyolipoma treated in the same institutions were also evaluated. Formalin-fixed paraffin-embedded (FFPE) blocks were retrieved and corresponding slides from all cases were re-reviewed by an expert genitourinary pathologist (SS) at BWH. Baseline clinico-pathological characteristics such as age, gender, tumor size, Fuhrman grade, pathological TNM stage at time of surgery and follow-up data were retrospectively collected for patients with non-ccRCC. Uniform data collection templates were used to ensure consistent data. Institutional Review Board approval was obtained before data acquisition and tumor staining.
immunohistochemistry
PD-L1 expression was evaluated by immunohistochemistry using a mouse monoclonal anti-PD-L1 antibody (405.9A11) developed in Dr Gordon Freeman's laboratory (Dana-Farber Cancer Institute, Boston, MA) (Figure 1) . The immunohistochemical assay was extensively validated using FFPE cell line controls known to be positive or negative for PD-L1 expression by flow cytometry [16]. Four-micron-thick tumor sections were stained with an anti-PD-L1 antibody concentration of 3.25 µg/ml, on a Benchmark XT autostainer (Ventana Medical System, Tucson, AZ) with standard antigen retrieval (CC1 buffer, pH8.0, #950-124, Ventana). UltraView Universal DAB Detection kit (#760-500, Ventana) was used according to the manufacturer's instruction. Counterstaining was carried out as part of the automated staining protocol using hematoxylin (#760-2021, Ventana). After staining, slides were then washed in soap water and distilled water, dehydrated in graded alcohol and xylene, mounted and cover slipped.
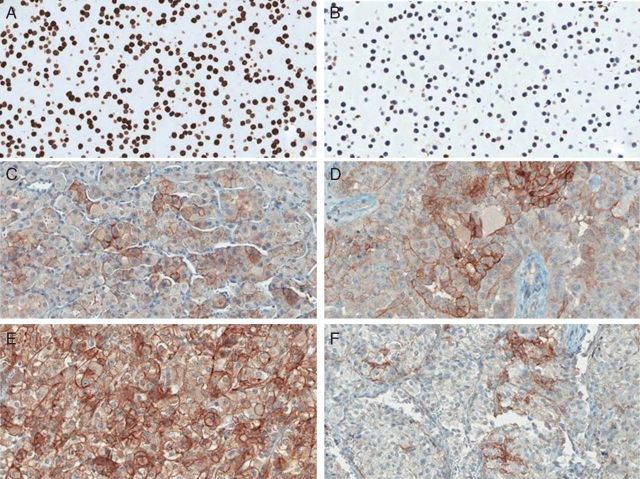
Figure 1.
PD-L1 expression in FFPE samples stained with anti-PD-L1 antibody (clone 405.9A11). (A) Positive cell line control; (B) Negative cell line control; (C) Chromophobe RCC; (D) Papillary RCC. (E, F) Xp11.2 translocation RCC. Positive staining is present in tumor cells membrane in panels (C), (D) and (E). In panel (F), tumor cells are negative and tumor-infiltrating immune cells are positive for PD-L1.
quantification of PD-L1 expression on tumor cell membrane
Membranous expression in tumor cells was quantified semiquantitatively by two independent pathologists (SS and MC) blinded to clinical outcome. PD-L1 tumor positivity was defined as ≥5% tumor cell membrane staining.
quantification of PD-L1 expression in tumor-infiltrating mononuclear cells
The extent of tumor-infiltrating mononuclear cells (TIMC) (i.e. lymphocytes and macrophages) was assessed in hematoxylin and eosin-stained slides and recorded as absent (0), focal (1), mild (2), moderate (3) and marked (4). The percentage of PD-L1-positive TIMC was evaluated independently by two pathologists (SS and MC), according to three categories (0% = 0, <5% = 1, ≥5% = 2). An adjusted score representing PD-L1 expression was calculated multiplying the percentage of TIMC that stained positive for PD-L1 and the extent of mononuclear cell infiltration.
statistical analysis
The primary objective of this study was to characterize the PD-L1 expression in patients with non-ccRCC and to correlate the levels of expression with clinico-pathological features as well as disease outcomes. Two end points were analyzed: (i) TTR, defined as time from diagnosis to the date of development of metastatic disease; (ii) OS, defined as time from diagnosis to death. In the absence of an event, the end points were censored at last follow-up time. Patient and tumor characteristics were summarized descriptively. PD-L1 tumor positivity was defined as ≥5% tumor cell membrane staining. For PD-L1 expression in TIMCs, any score greater than zero was considered positive. Comparisons between PD-L1 expression and clinico-pathological features were evaluated using χ2 or Fisher's exact test (when sample size was small) for categorical variables and Wilcoxon rank-sum test for continuous variables. Kaplan–Meier method estimated the distribution of TTR and OS by the PD-L1 positivity. Cox proportional regression assessed the associations with hazard ratio (HR) and 95% conference interval (CI). PD-L1 expression in patients with benign tumors was reported descriptively and correlations with clinico-pathological features as well as outcome variables were not carried out.
All statistical computations were carried out using SAS v.9.2 (SAS Institute, Inc., Cary, NC) and a P value (two-sided) <0.05 was considered statistically significant.
results
patients and tumor characteristics
Characteristics of patients with non-ccRCC are outlined in Table 1. The study cohort included a total of 101 patients with non-ccRCC. The histological subtypes included chromophobe RCC (n = 36), papillary RCC (n = 50) and Xp11.2 translocation RCC (n = 10) and collecting duct carcinoma (n = 5). The median follow-up time was 5 years [interquartile range (IQR): 3.5–6.2], and the median age was 59 years (range 24–81 years). For non-ccRCC, TNM clinical stages I, II, III and IV at diagnosis were identified in 54, 19, 18 and 9 patients, respectively. Additionally, 47 patients had high Fuhrman grade (III or IV) and 53 had low Fuhrman grade (I or II). In one tumor sample, the definition of tumor grade was not precisely possible and it was not reported. The median tumors' size was 4.7 cm (range 2.8–7.7 cm).
Table 1.
Non-ccRCC patient characteristics
| Characteristic | Total (N = 101) |
|
|---|---|---|
| No. of patients | % | |
| Gender | ||
| Male | 55 | 54 |
| Female | 46 | 46 |
| Stage | ||
| 1 | 54 | 53 |
| 2 | 19 | 19 |
| 3 | 18 | 18 |
| 4 | 9 | 9 |
| Unknown | 1 | 1 |
| Fuhrman grade | ||
| I/II | 53 | 52.4 |
| III | 38 | 37.6 |
| IV | 9 | 9 |
| Unknown | 1 | 1 |
| Histology | ||
| Chromophobe | 36 | 36 |
| Papillary | 50 | 49 |
| Translocation | 10 | 10 |
| Collecting duct carcinomas | 5 | 5 |
| Metastatic disease | ||
| No | 78 | 77.2 |
| Yes | 23 | 22.8 |
| PD-L1 expression in tumor cells membrane | ||
| <5% (negative) | 90 | 89.1 |
| ≥5% (positive) | 11 | 10.9 |
| PD-L1 expression in tumor-infiltrating mononuclear cells (TIMC) | ||
| Score = 0 (negative) | 44 | 43.6 |
| Score > 0 (positive) | 57 | 56.4 |
| Median | Min, max | |
| Age at Dx (years) | 59 | 24–81 |
| Tumor size (cm) | 4.7 | 0.6–30 |
For comparative purposes, 20 patients with benign kidney tumors were also evaluated for PD-L1 expression. The histological subtypes included oncocytoma (n = 13) and angiomyolipoma (n = 7). The median tumor's size was 3.2 cm (range 1.9–5.6 cm).
PD-L1 expression in tumor cells and clinico-pathological features
Among 101 patients with non-ccRCC, PD-L1 expression in tumor cell membrane was negative in 90 patients (89.1%) and positive in 11 patients (10.9%). Specifically, PD-L1 positivity in tumor cell membrane was detected in 2 of 36 (5%) chromophobe RCC, 5 of 50 (10%) papillary RCC, 3 of 10 (30%) Xp11.2 translocation RCC and 1 of 5 (20%) collecting duct carcinomas.
PD-L1 positivity in tumor cell membrane was significantly associated with higher TNM stage (P = 0.01) and Fuhrman grade III/IV (P = 0.03) (Table 2). On the other hand, PD-L1 positivity was not correlated with gender, age at diagnosis or tumor size (data not shown).
Table 2.
Correlation of PD-L1 expression and clinico-pathological factors in non-ccRCC
| Characteristic | % Positive tumor cell membrane |
P value | TIMC |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| <5% (negative) (N = 90, 89.1%), n (%) | 5% or more (positive) (N = 11, 10.9%), n (%) | Total (N = 101) | Score = 0 (negative) (N = 44, 43.6%), n (%) | Score >0 (positive) (N = 57, 56.4%), n (%) | Total (N = 101) | |||
| Stage | ||||||||
| 1 | 52 (58) | 2 (20) | 54 (53) | 0.01 | 24 (55) | 30 (54) | 54 (53) | 0.35 |
| 2 | 18 (20) | 1 (10) | 19 (19) | 11 (25) | 8 (14) | 19 (19) | ||
| 3 | 14 (16) | 4 (40) | 18 (18) | 7 (16) | 11 (20) | 18 (18) | ||
| 4 | 6 (7) | 3 (30) | 9 (9) | 2 (5) | 7 (12) | 9 (9) | ||
| Unknown | 0 | 1 (1) | 1 (1) | 0 | 1 (1) | 1 (1) | ||
| Fuhrman grade | ||||||||
| I/II | 51 (57) | 2 (18) | 53 (52.4) | 0.03 | 23 (53) | 30 (53) | 53 (52.4) | 0.11 |
| III | 31 (35) | 7 (64) | 38 (37.6) | 19 (44) | 19 (33) | 38 (37.6) | ||
| IV | 7 (8) | 2 (18) | 9 (9) | 1 (1) | 8 (14) | 9 (9) | ||
| Unknown | 1 (1) | 0 | 1 (1) | 1 (1) | 0 | 1 (1) | ||
| Histology | ||||||||
| Chromophobe | 34 (94.4) | 2 (5.6) | 36 (36) | 0.1 | 23 (63.9) | 13 (36.1) | 36 (36) | 0.001 |
| Collecting duct | 4 (80) | 1 (20) | 5 (5) | 0 (0) | 5 (100) | 5 (5) | ||
| Papillary | 45 (90) | 5 (10) | 50 (49) | 20 (40) | 30 (60) | 50 (49) | ||
| Translocation | 7 (70) | 3 (30) | 10 (10) | 1 (10) | 9 (90) | 10 (10) | ||
TIMC, tumor-infiltrating mononuclear cells.
PD-L1 expression in TIMCs and clinico-pathological features
Overall, the extent of TIMC infiltration was: absent in 11 patients, focal in 27 patients, mild in 31 patients, moderate in 20 patients and marked in 12 patients.
PD-L1 expression in TIMC was negative (score 0) in 44 patients (43.6%). Fifty-seven patients (56.4%) were considered PD-L1+ in the TIMC. Among the cases with PD-L1+ TIMC, 37 patients had expression in <5% of immune cells and 20 patients presented expression in more than 5% of immune cells. There was a significant association of histology subtype and PD-L1 expression levels in TIMC (P = 0.001). Specifically, among patients with PD-L1+, 13 of 36 (36%) had chromophobe RCC, 30 of 50 (60%) had papillary RCC, 9 of 10 (90%) Xp11.2 had translocation RCC and 5 of 5 (100%) had collecting duct carcinoma.
PD-L1 positivity in TIMC was not significantly associated with TNM stage (P = 0.35) or tumor grade (P = 0.11) (Table 2). In addition, PD-L1 positivity in TIMC did not correlate with gender, age at diagnosis or tumor size (data not shown).
PD-L1 expression and clinical outcome in non-ccRCC
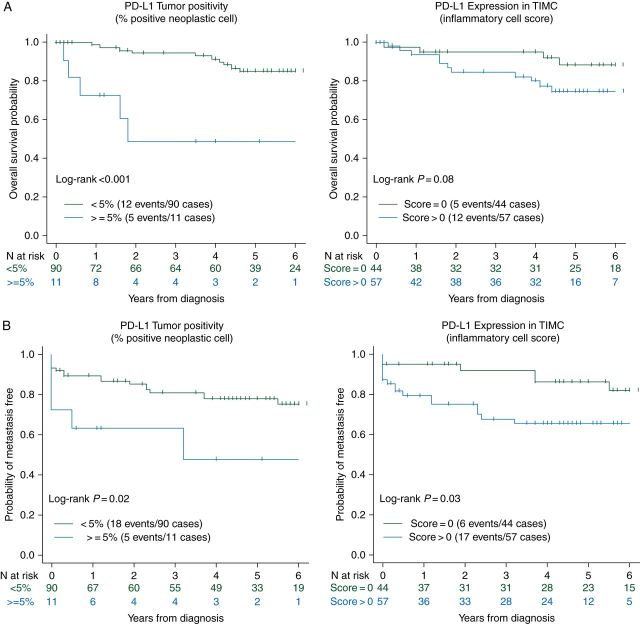
The overall median follow-up of the cohort was 5 years, 17 patients died and 24 patients developed distant metastases. Patients with PD-L1+ in tumor cells were significantly associated with increased risk of death (HR = 6.41, 95% CI 2.17–18.88; P < 0.001) compared with patients with PD-L1 negative in tumor cells. A similar trend was observed when comparing PD-L1 expression in TIMC, but the result was not statistically significant (HR = 2.49, 95% CI 0.86–7.2; P = 0.08) (Figure 2A). In addition, PD-L1+ on tumor cell membrane and TIMC both were associated with lower TTR (P = 0.02 and P = 0.03, respectively) (Figure 2B).
Figure 2.
(A) Correlation of PD-L1 expression and OS (univariate analysis) in non-ccRCC; (B) Correlation of PD-L1 expression and TTR (univariate analysis) in non-ccRCC.
PD-L1 expression in benign kidney tumors
PD-L1 expression in tumor cell membrane was positive in 4 of 13 (30.8%) oncocytomas and 0 of 7 (0%) angiomyolipomas. In addition, 7 of 13 (53.8%) of oncocytoma and 7 of 7 (100%) angiomyolipoma expressed PD-L1 in TIMC (score >0). Correlations with clinico-pathological features as well as outcome variables were not carried out.
discussion
Thompson et al. were among the first to describe the PD-L1 expression in ccRCC. In one study of 196 patients, PD-L1 expression was associated with aggressive features such as higher TNM stage, tumor size or Fuhrman grade and increased risk of cancer-specific mortality [17]. In another study of 306 patients, PD-L1+ was seen in 23% of cases. Similarly, PD-L1+ tumors were more likely to present adverse pathologic features including TNM stage III or IV, higher tumor size and Fuhrman grade III or IV (P < 0.001 for all), and higher risk of cancer-specific mortality (RR = 2.0 95% CI 1.27–3.15, P < 0.003) adjusting for TNM stage and grade [18]. Interestingly, the correlation between PD-L1 expression and adverse prognostic factors as well as OS was identified with PD-L1 expression in both tumor cell membrane and tumor-infiltrating lymphocytes (TILs). Based on these studies, PD-L1 expression may be considered as an independent predictor of poor prognosis in ccRCC [19].
Overcoming this adaptive mechanism of tolerance with therapies blocking the PD-1 or PD-L1 could restore the effectiveness of T-cell responses against tumor cells [20]. A phase I study evaluating the safety and efficacy of the anti-PD-1 monoclonal antibody (nivolumab) in patients with advanced cancer produced encouraging tumor responses in patients with RCC and other malignancies. Moreover, specimens from 42 patients, including 5 patients with RCC were analyzed for PD-L1 expression in tumor cells. Overall, 25 of 42 were considered PD-L1+. Among these 25 patients, 9 (36%) had objective response. On the other hand, none of the patients with PD-L1− expression achieved objective response (P = 0.006). These results supported the hypothesis that PD-L1 may be a promising predictive biomarker of response to agents that target the PD1/PD-L1 axis [21]. Since that landmark study, two other studies in RCC specifically showed that patients with PD-L1+ tumors have numerically higher response to agents that target the PD-L1/PD-1 axis than PD-L1 negative tumors, although it is important to note that responses were seen in PD-L1-negative tumors [22, 23].
To our knowledge, this is the first study to report PD-L1 expression in non-ccRCC and its correlation with clinical outcome. Consistent with previously published ccRCC studies, PD-L1 expression in tumor cell membrane was correlated with higher Fuhrman grade or TNM stage in patients with non-ccRCC. In addition, on univariate analysis, patients with PD-L1 positivity in tumor cells were significantly more likely to have a shorter OS. Furthermore, a trend for shorter OS was also observed in patients with PD-L1+ TIMC and both PD-L1 positivity on tumor cell membrane and TIMC were associated with lower TTR. Our exploratory multivariate analyses suggest that tumor stage, Fuhrman grade and histology are significant effect modifiers for the association of PD-L1 positivity on clinical outcome (data not shown). Interestingly, we confirm that PD-L1 expression can exist in benign kidney tumors, as previously reported [24]. However, how it could affect the clinical course of this disease remains to be studied and addressed in other studies.
Infiltrating mononuclear cells in RCC release cytokines to either promote tumor growth or impair antitumor immune responses. In addition, high levels of TILs have been associated with an increased risk for cancer progression and death [25]. Similarly, higher expression of PD-L1 in TILs was also associated with aggressive features such as tumor grade and TNM stage in ccRCC [26]. Among non-ccRCC, we did not observe statistically significant association between PD-L1 expression in TIMC and clinico-pathological features or OS. Nonetheless, the percentage of patients who were considered PD-L1+ by this method was overall much higher than with the tumor membrane staining.
In this analysis, we showed that PD-L1 expression in non-ccRCC is heterogeneous and depends on histology. In 2004, the World Health Organization (WHO) classification of renal tumors recognized a new subtype of kidney cancer characterized by translocations involving the transcription factor E3 (TFE3) located on Xp11.2 gene [27]. These tumors share some morphological features with ccRCC and the real incidence of this subtype may be underestimated [8]. Aggressive clinical course in a younger adult population with a female predominance has been reported. Despite anti-VEGF drugs having some activity in these patients, there is no established treatment of patients with metastatic disease [28]. In our study, 3 of 10 patients who had Xp11.2 translocation RCC (30%) exhibited PD-L1+ in tumor cells and 9 of 10 (90%) harbored PD-L1+ TIMC. Collecting duct carcinomas are also a very aggressive disease and up to 40% of patients present with metastatic disease at diagnosis [14]. In our study, 1 of 5 patients expressed PD-L1 on tumor cells and all of them were considered positive in TIMC. Thus, we hypothesize that PD-L1 may play a key role in the biology of Xp11.2 translocation RCC and collecting duct carcinoma and could represent an important therapeutic target for these RCC subtypes for which few therapeutic options are currently available.
Our study has many limitations. First, the non-ccRCC is a very heterogeneous disease. In addition, considering the rarity of Xp11.2 translocation RCC and collecting duct carcinomas, even evaluating a large cohort, a small number of patients with these histologic subtypes have been represented limiting our conclusions. In addition, the majority of tumors did not have metastatic disease, and may underestimate the prevalence of PD-L1 staining in cytoreductive nephrectomies or distant metastases. Given the smaller group size for patients with PD-L1 positive and a small number of events (deaths), a multivariate analysis may not properly adjust the association of PD-L1 expression and clinical outcome for potential confounding factors. Moreover, the relatively short follow-up period may influence the correlation of PD-L1 expression and OS. Intratumor heterogeneity has been described in RCC. Although we have evaluated whole tissue sections, our results may not represent the PD-L1 expression in the entire tumor. Furthermore, the value of Fuhrman nuclear grading has been debated in non-ccRCC. However, it remains widely adopted in the clinical practice [29]. Finally, comparisons with other studies should be done with caution, since many different methodologies and antibodies have been applied to assess PD-L1 expression.
Notably, <10% of patients with nonclear-cell histologies were included on clinical trials of new investigational agents [30]. Our study suggests that patients with non-ccRCC, especially subsets with higher PD-L1 expression by either tumor or immune cells should not be automatically excluded from clinical trials of agents that target the PD-1/PD-L1 pathway.
In summary, PD-L1 expression in tumor and TIMC occurs in patients with non-ccRCC depending on histology subtype and tumor membrane versus immune cell scoring. In addition, PD-L1 positivity on tumors cell membrane was associated with aggressive clinico-pathological features. PD-L1 expression in Xp11.2 translocation RCC and collecting duct carcinomas as well as its correlation with clinical outcomes needs to be prospectively confirmed in larger series. Further evaluation of PD-L1 as a potential predictive biomarker to PD-1/PDL1 inhibitors in non-ccRCC is warranted.
funding
This work was supported in part by DF/HCC Kidney Cancer (SPORE: P50 CA101942-01) to DFM, SS and TKC, and by the Trust Family, the Loker Pinard and the Michael Brigham Funds for Kidney Cancer Research to TKC.
disclosure
TKC: Consultancy: Pfizer, Novartis; Advisory Board: Pfizer, Novartis, Aveo, GlaxoSmithKline, Exelixis; Research: Pfizer; No Speakers bureau. LA: advisory board: Novartis compensated, Pfizer compensated, Amgen compensated, Sanofi compensated; Honoraria: Novartis, Pfizer; Research: Novartis, Pfizer. MBA: Advisory Board: BMS, Merck and Prometheus. GJF: Significant financial interest from DFCI administered patent royalties from BMS, Merck, Roche/Genentech, EMD-Serrono, Amplimmune, Boehringer-Mannheim, CoStim; Scientific founder and scientific board member of CoStim. FSH: Advisory Board Genentech. All remaining authors have declared no conflict of interest.
acknowledgements
APF receives a scholarship from CAPES-CNPq – Brazil.
references
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK. Renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:xiii–xiv. doi: 10.1016/j.hoc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Kidney Cancer–Pathological Classification. Lyon, France: IARC press; 2004. [Google Scholar]
- 4.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 5.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 6.Heng DY, Choueiri TK. Non-clear cell renal cancer: features and medical management. J Natl Compr Canc Netw. 2009;7:659–665. doi: 10.6004/jnccn.2009.0046. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 9.Figlin RA. Renal cell carcinoma: management of advanced disease. J Urol. 1999;161:381–386. doi: 10.1016/s0022-5347(01)61897-4. discussion 386–387. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 11.Sonpavde G, Choueiri TK. Precision medicine for metastatic renal cell carcinoma. Urol Oncol. 2013;32:5–15. doi: 10.1016/j.urolonc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury S, Matrana MR, Tsang C, et al. Systemic therapy for metastatic non-clear-cell renal cell carcinoma: recent progress and future directions. Hematol Oncol Clin North Am. 2011;25:853–869. doi: 10.1016/j.hoc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harshman LC, Choueiri TK. Targeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinoma. Cancer J. 2013;19:316–323. doi: 10.1097/PPO.0b013e31829e3c9a. [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, Dutcher J. Targeted therapies and the treatment of non-clear cell renal cell carcinoma. Ann Oncol. 2013;24:1730–1740. doi: 10.1093/annonc/mdt152. [DOI] [PubMed] [Google Scholar]
- 15.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 16.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 19.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 20.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho DC, Sosman JA, Sznol M, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) 2013. 2013 ASCO Annual Meeting, Chicago, IL.
- 23.Choueiri TK, Fishman MN, Escudier BJ, et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): biomarker-based results from a randomized clinical trial. J Clin Oncol. 2014;32(5s) (suppl; abstr 5012) [Google Scholar]
- 24.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell co-regulatory molecule expression in renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Urology. 2009;74:1359–1364. doi: 10.1016/j.urology.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster WS, Lohse CM, Thompson RH, et al. Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer. 2006;107:46–53. doi: 10.1002/cncr.21951. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 27.Malouf GG, Camparo P, Molinie V, et al. Transcription factor E3 and transcription factor EB renal cell carcinomas: clinical features, biological behavior and prognostic factors. J Urol. 2011;185:24–29. doi: 10.1016/j.juro.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 28.Malouf GG, Camparo P, Oudard S, et al. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): a report from the Juvenile RCC Network. Ann Oncol. 2010;21:1834–1838. doi: 10.1093/annonc/mdq029. [DOI] [PubMed] [Google Scholar]
- 29.Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]