Abstract
The concept of “probiotic” is generally attributed to Dr. Ilya Mechnikov, who hypothesized that longevity could be enhanced by manipulating gastrointestinal microbes using naturally fermented foods. In 2001, a report of the FAO and WHO (2001 Oct, http://www.who.int/foodsafety/publications/fs_-management/en/probiotics.pdf) proposed a more restrictive definition of probiotic, as follows: “a live micro-organism which, when administered in adequate amounts, confers a health benefit on the host.” As such, answering the fundamental question posed here—“Is human milk a probiotic?”—requires first grappling with the concept and meaning of the term probiotic. Nonetheless, one must also be convinced that human milk contains bacteria. Indeed, there are scores of publications providing evidence of a paradigm shift in this regard. Variation in the human-milk microbiome may be associated with maternal weight, mode of delivery, lactation state, gestation age, antibiotic use, and maternal health. Milk constituents (e.g., fatty acids and complex carbohydrates) might also be related to the abundance of specific bacterial taxa in milk. Whether these bacteria affect infant health is likely, but more studies are needed to test this hypothesis. In summary, a growing literature suggests that human milk, like all other fluids produced by the body, indeed contains viable bacteria. As such, and recognizing the extensive literature relating breastfeeding to optimal infant health, we propose that human milk should be considered a probiotic food. Determining factors that influence which bacteria are present in milk and if and how they influence the mother’s and/or the recipient infant’s health remain basic science and public health realms in which almost nothing is known.
Keywords: human milk, bacteria, microbiota, microbiome, probiotics
Introduction
In 1908, the Nobel Prize in Physiology or Medicine was awarded to Drs. Ilya Mechnikov (1845–1916) and Paul Ehrlich7 (1854–1915) “in recognition of their work on immunity” (1). Although generally known for his research related to phagocytosis and often referred to as the “father of natural immunity,” Mechnikov’s later years were largely devoted to studying the relations between lactobacilli-rich yogurts, gastrointestinal microbiota, and health (mainly longevity). Indeed, Mechnikov fervently advocated the consumption of fermented dairy products, specifically the health benefits of replacing indigenous gastrointestinal microbiota with those in these foods (2, 3). Because of this, Mechnikov is often considered to be the originator of the current concept that intentionally fermented (naturally microbe-containing; now considered “probiotic”) foods can positively affect health. However, the word probiotic [a composite of the Latin preposition pro (for) and the Greek adjective βιωτικóϕ (biotic), the latter deriving from the noun βιοϕ (bios, “life”)] has undergone a substantial evolution of meaning over the years (3). Nonetheless, probiotic foods are generally thought to be those that contain live bacteria that positively influence health.
In this article, we examine the overarching question “Is human milk a probiotic food?” In other words, can the ingestion of bacteria found in human milk positively affect the health of the recipient—in this case, a breastfed infant? Although sufficient data do not currently exist to completely answer this question—indeed, the study of the human milk microbiome is in its fledgling stage—the goal of this article is to convince the perhaps skeptical reader of 3 somewhat paradigm-shifting concepts: 1) milk produced by healthy women contains live bacteria, 2) variability in these organisms might be responsive to environmental (host-related) factors, and 3) milk (mammary) microbiota might potentially offer health benefits to both the producer (the mother) and the consumer (the infant). Once these concepts are accepted, deciding whether the term probiotic should be applied to human and/or other species’ milk will be left to the judgment of each reader, because this conclusion must be at least partially relegated to both forthcoming research and scientific semantics.
Current Status of Knowledge
Defining “probiotic”—a historical perspective.
Unless foods have been sterilized via some sort of treatment (e.g., heat, irradiation), it is safe to say that they contain bacteria. Indeed, the public all-too-well knows that food-borne bacteria can cause disease. However, the practice of purposefully treating foods with live bacteria has long been used as a form of food preservation; as such, these types of food-borne bacteria and other types of microorganisms (e.g., yeasts) used in food preservation are generally health promoting, allowing the consumption of nutrient-rich foods (e.g., yogurts, pickles, wine) long after their shelf lives if they were unpreserved. However, the added health benefits of consuming fermented, microbe-containing foods were not scientifically recognized until the late 19th and early 20th centuries when Ilya Mechnikov, a Russian biologist, made the observation that Bulgarian peasants who ingested large amounts of “soured milks” lived longer than many other populations in northern Europe. From this observation, Mechnikov hypothesized that the consumption of naturally fermented dairy products could result in a health-promoting shift in the bacteria present in the large intestine (1–5).
In the English translation of his treatise entitled Essais Optimistes (6) concerning aging, Mechnikov provided the following, somewhat guarded, conclusion concerning naturally fermented foods and health.
“If it be true that our precocious and unhappy old age is due to poisoning of the tissues (the greater part of the poisoning coming from the large intestine inhabited by numberless microbes), it is clear that agents which arrest intestinal putrefaction must at the same time postpone and ameliorate old age. This theoretical view is confirmed by the collection of facts regarding races which live chiefly on soured milk, and among which great ages are common. However, in a question so important, the theory must be tested by direct observations. For this purpose the numerous infirmaries for old people should be taken advantage of, and systematic investigations should be made on the relation of intestinal microbes to precocious old age, and on the influence of diets which prevent intestinal putrefaction in prolonging life and maintaining the forces of the body. It can only be in the future, near or remote, that we shall obtain exact information upon what is one of the chief problems of humanity.” (pp. 182–3)
With this clear plea for further, well-designed scientific study (especially related to the relation between fermented foods and aging), Mechnikov secured his place as the rightful “father of probiotic theory,” launching the field of research related to potential health-promoting effects of fermented foods and microbe-containing supplements. It is important to recognize that Mechnikov’s belief was that “typical” gastrointestinal microbiota are generally harmful, but their replacement with those found in fermented foods (e.g., lactic acid bacteria) is beneficial to health. This and other specific beliefs related to if and how fermented foods affect health have undergone substantial shifts over the past century. A brief timeline describing the evolution of the concept and semantics related to probiotics and health is provided in Figure 1 and described briefly here.
FIGURE 1.
A century of concepts and definitions. Selected timeline and evolution of concepts and definitions related to the relation between fermented foods and the term probiotic.
After Mechnikov’s death in 1916 and the proposition that his “Bulgarian bacillus” (most likely what is now called Lactobacillus delbrueckii subsp. bulgaricus) found in soured dairy products may not survive gastric transit (and therefore could not possibly affect health), researchers began instead studying isolates of Lactobacillus acidophilus prepared as dietary supplements rather than delivered in naturally fermented foods (7). In addition, scientists began to investigate therapeutic outcomes of single-strain, bacteria-containing supplements that were related to quality of life (e.g., constipation) instead of prolongation of life (8). Bacterial strains used in these studies were often isolated from feces, not food. As such, the concept of “probiotic” was broadened to include acute health effects of consuming a single bacterial organism that may or may not have been isolated from foods and that was delivered via supplement.
Research related to the role of diet in modulating the gastrointestinal microbiota was fueled again in the 1950s with the development of germ-free animals, a research technique used first in 1895 by Nuttal and Thierfelder (9). But again, the initial focus of many of these studies was based on the belief that, in general, gastrointestinal microbiota were harmful and that replacing them with selected strains would promote health. However, during this period of scientific endeavor, work (mostly in the agriculture sector) began to show that oral administration of antibiotics to laboratory production animals could result in increased morbidity and mortality, helping shape the belief that some endogenous taxa confer health benefits to the host (e.g., 10, 11). Of course, evidence supporting the overarching hypothesis that variation in gastrointestinal microbiota community structure can predispose the host to either health or disease has burgeoned in the late 20th and early 21st century, driving exponentially increasing numbers of studies including the federally funded Human Microbiome Project (reviewed in 12).
As with basic assumptions underlying the relations among food, gastrointestinal microbiota, and health, the use of the term probiotic has also evolved. Lilley and Stillwell (13) actually coined the term probiotic in 1965 to describe factors produced by microorganisms that influence growth of other microorganisms. In 1971, Sperti (14) used the term to describe tissue extracts that could promote microbial growth. In 1974, Parker (15) repainted the term probiotic (noun) with a broad brush as “organisms and substances which contribute to intestinal microbial balance.” Clearly, this usage of the term was expansive—arguably including everything from fermented foods and microbe-containing supplements to medications and other substances (e.g., dietary fiber) that might directly or indirectly influence gastrointestinal microbial community structure and diversity. In 1989, Fuller (16) proposed the following, quite different, definition of probiotic: “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance.” This proposed definition returned the focus back to consumption of live bacteria, but in this case in the form of a food supplement rather than that found in naturally fermented foods. This shift was likely because his focus was on agricultural animals rather than free-living humans. Recognizing the need for a less restrictive meaning, Fuller (17) in 1995 proposed a new definition: “a probiotic is a preparation consisting of live microorganisms or microbial stimulants which affects the indigenous microflora of the recipient animal, plant or food in a beneficial way.” Note, however, that he specifically included the “traditional fermented milks such as yogurt” in this definition for historical reasons.
Most recently, an even more restrictive definition of probiotic was proposed in a report of a joint FAO and WHO Consultation convened in 2001 to evaluate the health and nutrition properties of probiotics in food. The publication (18) resulting from the first meeting of this group defined a probiotic (adjective) organism as a “live micro-organism which, when administered in adequate amounts, confers a health benefit on the host.” Although this report clearly states that “the opinions expressed in this report are those of the participants at the Consultation and do not imply any opinion on the part of FAO and WHO,” this definition is generally said to be supported by these international agencies and used globally to define the term and its use in labeling and marketing. Because this definition refers to both a dosage and a health benefit, both currently need to be defined and scientifically supported for a food or supplement to be deemed a proper “probiotic” product. As such, the term is now considered more of a health claim than a nutrient content claim.
In summary, and critical to being able to reconcile whether human milk should be considered a probiotic food, one must first consider which perturbation of the concept/term probiotic to use. Should Mechnikov’s concept of a microbe-containing food that helps “outcompete” disease-promoting gastrointestinal microbiota be used? Should one use Parker’s somewhat inclusive concept of organisms and substances that contribute to intestinal microbial balance? Or should today’s widely held view requiring specific strains, dosages, and demonstrable health benefits prevail? This scientifically relevant, semantic dilemma will be revisited in the concluding section of this article.
Paradigm shift: human milk is not sterile.
Regardless of the definition used, for milk to be considered a probiotic food it must contain live bacteria or other microorganisms. Historically, milk of all mammals was thought to be a sterile fluid unless contaminated during collection or storage or produced by a diseased gland, such as during mastitis. This belief was largely due to both perspective and methodologic limitations related to the selective use of culture-dependent techniques to detect only bacteria known to be pathogenic. These studies (e.g., 19–21) suggested that most milk samples were sterile but that milk collected from mastitic women contained primarily Staphylococcus aureus and to a lesser extent streptococci. Nonetheless, culturable bacteria were often not detected in milk samples collected from mastitic women. Consequently, experts have long questioned the prevailing dogma related to “infectious” vs. “noninfectious” mastitis (i.e., bacterial vs. nonbacterial), the precise role of bacteria in the etiology of breast inflammation, and the implications this might have for or against antibiotic use in affected women (e.g., 22–24).
More recently, the use of culture-independent techniques that do not rely on taxa-specific growth media but instead identify bacterial groups on the basis of variation in specific DNA segments has led to a paradigm shift in this regard. In other words, these studies using molecular techniques overwhelmingly support the existence of a rich and diverse community of bacteria in milk, regardless of whether the milk was produced by healthy women or those with mastitis. A list of studies that used these molecular techniques (often in combination with carefully conducted, culture-dependent techniques) to investigate the microbiota in human milk are provided in Table 1 and described briefly here. Although these studies have provided unprecedented insights into the full range of bacteria that are present in milk (in particular, anaerobic taxa that are exceptionally difficult to culture but may play important roles in the colonization and health of an infant’s gastrointestinal tract), it should be noted that there are several limitations of using only culture-independent approaches. For instance, the viability of bacteria cannot be assessed, in most cases bacterial load is not determined and/or its accuracy may be compromised by gene copy number, and there is the possibility of DNA contamination from laboratory personnel and molecular biology reagents. For these reasons and others, marrying both culture-dependent and culture-independent analyses will be important as the field moves forward.
TABLE 1.
Chronologic overview of selected studies using culture-independent methods (often combined with culture-dependent methods) to identify bacteria in human milk1
| Study (reference) | Population | Identification methods | Major taxa (or type) identified | Comments |
| Martín et al., 2003 (25) | • Spain | Cultured for lactic acid bacteria, RAPD analysis | Lactic acid bacteria found in all milk samples, specifically Lactobacillus gasseri and Enterococcus faecium | Methods chosen to specifically identify lactic acid–producing bacteria; bacterial types found in milk similar to those found in infant feces but not breast skin |
| • n = 8 | ||||
| • 4 d postpartum | ||||
| Grönlund et al., 2007 (50) | • Finland | Real-time PCR | Bifidobacteria detected in all milk samples (median count of 1.4 × 103 bacterial cells/mL) with the Bifidobacterium longum group being most frequently detected | Primers specifically designed to detect/identify bifidobacteria; allergic mothers had lower amounts of bifidobacteria in their milk compared with nonallergic mothers; infants of allergic mothers also had lower amounts in their feces |
| • n = 61 mothers and infants | ||||
| • At least 1 person in family at high risk of allergy | ||||
| • Maternal feces, infant feces, and milk collected | ||||
| Collado et al., 2009 (28) | • Spain | First use of qPCR to assess presence of different bacteria genera or clusters | All samples contained Staphylococcus, Bifidobacterium, Lactobacillus, and Streptococcus; most contained Enterococcus and Clostridium cluster XIVa-XIVb; some contained Bacteroides and Clostridium cluster IV. | Most abundant was Streptococcus |
| • n = 50 | ||||
| Solís et al., 2010 (58) | • Spain | Cultured for lactic acid bacteria and bifidobacteria coupled with 16S rRNA sequencing and RAPD | Streptococcus (dominated by Streptococcus salivarius) most predominant followed by Lactobacillus and Bifidobacterium | Whereas bacterial counts increased over time in infant feces, they decreased in milk; bacterial counts lower in milk produced by women given antibiotics; bifidobacterial strains similar between milk and infant feces |
| • n = 20 mothers and infants | ||||
| • day 1, 10 d, 1 mo, and 3 mo postpartum | ||||
| Albesharat et al., 2011 (42) | • Syria | Cultured for lactic acid bacteria coupled with RAPD, 16S rRNA sequencing, and matrix-assisted laser desorption ionization-time of flight-mass analysis | Lactic acid bacteria isolated from all samples; Lactobacillus, Enterococcus, Pediococcus, Streptococcus, Staphylococcus, and Weisella present in milk. | Lactobacillus plantarum, Lactobacillus fermentum, Pediococcus pentosaceus, and Lactobacillus brevis found in milk, fermented foods, infant feces, milk, and maternal feces |
| • n = 30 mothers and infants (1 mo–2 y postpartum) | ||||
| • milk, maternal/infant feces, and fermented foods collected | ||||
| Hunt et al., 2011 (33) | • United States | Cultured for common “mastitic” pathogens, pyrosequencing | Most abundant genera were Streptococcus, Staphylococcus, Serratia, and Corynebacteria. High diversity (100–600 OTU in each sample); “core” microbiome: Staphylococcus, Streptococcus, Serratia, Pseudomonas, Corynebacterium, Ralstonia, Propionibacterium, Sphingomonas, Bradyrhizobium | Primers targeted V1–V2 region; analyses at the genus level; high intersubject variability, although relatively consistent microbial “fingerprint” within each woman |
| • n = 16 | ||||
| • ∼22–26 wk postpartum | ||||
| • 3 samples collected from each subject | ||||
| Collado et al., 2012 (47) | • Finland | qPCR | Most abundant genus was Lactobacillus, followed by Bifidobacterium and Staphylococcus (predominated by Staphylococcus aureus, which increased over lactation); Staphylococcus group bacteria were higher and Bifidobacterium and Lactobacillus group bacteria were lower in overweight subjects | Overweight subjects had higher total bacterial counts during first 6 mo of lactation; excessive weight gain associated with higher levels of Staphylococcus group in colostrum and lower counts of Bifidobacterium group at 1 mo; complex interactions among maternal adiposity/weight gain, milk cytokines, and microbiota detected |
| • n = 56 mothers (22 overweight and 34 normal weight) and their infants | ||||
| • 1–2 d (colostrum), 1 mo, and 6 mo postpartum | ||||
| Cabrera-Rubio et al., 2012 (35) | • Finland | Pyrosequencing, qPCR | Weisella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus predominant in colostrum; Leuconostoc, Weisella, Lactococcus, and Staphylococcus predominant in mature milk | Primers targeted V1–V3 region; analyses conducted at the family and genus levels; milk from obese (vs. normal-weight) mothers different and less diverse; milk from mothers who had a cesarean delivery (especially elective) was different (e.g., greater evenness, increased Acinetobacter) from mothers having vaginal births |
| • n = 18 | ||||
| • 0–2 d, 1 mo, and 6 mo postpartum | ||||
| González et al., 2013 (41) | • Mozambique | Cultured for nonfastidious bacteria, yeasts, molds, enterobacteria, fastidious microorganisms, and lactic acid bacteria; qPCR | 44 genera and 124 species identified; most common isolated by culture were staphylococci, streptococci, and lactobacilli; most frequently identified by qPCR were Streptococci, Staphylococcus epidermidis, Enterococci, and Bifidobacterium | Exclusive breastfeeding associated with higher counts of Staphylococcus parasanguis; bacterial diversity and frequency of Lactobacillus spp. higher in HIV-positive women; frequency of Staphylococcus hominis and S. aureus lower in HIV-positive women |
| • n = 55 (29 of whom tested positive for HIV) | ||||
| • ≤14 d, 15–90 d, 91–180 d, and 181–360 d postpartum | ||||
| Jost et al., 2013 (36) | • Switzerland | Cultured using 2 nonselective and 7 selective agar media targeting major gastrointestinal-associated bacteria populations; pyrosequencing; RAPD; Sanger sequencing | High diversity (512 ± 237 OTU and 172 genera); Firmicutes and Proteobacteria predominated, although Actinobacteria and Bacteroidetes were also abundant; Staphylococcus, Streptococcus, Pseudomonas, and Ralstonia most abundant | Primers targeted V5–V6 region; analyses conducted at the phylum and genus levels |
| • n = 7 | ||||
| • 3–6 d, 9–14 d, and 25–30 d postpartum | ||||
| Ward et al., 2013 (38) | • Canada | Illumina sequencing | 360 general identified with those in the Proteobacteria (65%) and Firmicutes (34%) phyla dominating; Pseudomonas and Staphylococcus were most abundant genera, followed by 13 less-abundant taxa | Unable to describe variation among women, because pooled milk (n = 1) was used; milk metagenome less diverse than fecal samples and contained more genes associated with nitrogen metabolism, membrane transport, and stress response; milk metagenome rich in immunomodulatory DNA motifs |
| • n = 1 (pooled milk from 10 women) | ||||
| • 9–30 d postpartum | ||||
| Khodayar-Pardo et al., 2014 (39) | • Spain | qPCR | Lactobacillus, Streptococcus, and Enterococcus spp. were predominant bacterial groups; total bacteria, Bifidobacterium, and Enterococcus spp. counts increased over lactation | Bifidobacterium spp. concentration consistently higher in milk from term (vs. preterm) births; higher bacteria concentrations in early milk and cesarean (vs. vaginal) deliveries; Bifidobacterium detected more frequently in vaginal (vs. cesarean) deliveries |
| • n = 32 | ||||
| • 1–5 d, 6–15 d, and 17–18 d postpartum | ||||
| Olivares et al., 2014 (49) | • Spain | qPCR | Bifidobacterium spp. detected in all milk samples; Bifidobacterium bifidum and Bifidobacterium breve most prevalent | Primers specifically targeted Bifidobacterium spp; gene copy numbers/mL of Bifidobacterium spp. and Bifidobacterium fragilis group higher in healthy mothers than those with celiac disease |
| • n = 24 (half with celiac disease) | ||||
| • 1 mo postpartum | ||||
| Urbaniak et al., 2014 (51) | • Canada | Ion Torrent sequencing | Chemotherapy related to lower diversity and altered bacterial profiles: decreased percentage abundances of Acinetobacter and Xanthomonadaceae, and decreased Bifidobacterium and Eubacterium genera | Primers targeted V6 region; analysis conducted at varying taxonomic levels depending on bioinformatics criteria |
| • n = 9 (1 undergoing chemotherapy related to Hodgkin lymphoma) | ||||
| • Longitudinal, prospective sampling | ||||
| Tušar et al., 2014 (40) | • Slovenia | Cultured for lactic acid bacteria, aerobic mesophilic bacteria, lactococci, and enterococci, lactobacilli, bifidobacteria, Escherichia coli¸ Enterococcus ssp., Staphylococcus aureus, and Staphylococcus epidermidis; RAPD; sequence analysis of 16S rRNA | Milk contained lactic acid bacteria, bifidobacteria, and mesophilic aerobic bacteria, of which the last were the most abundant; RAPD analysis revealed numerous Lactobacillus species, Enterococcus faecium, Staphylococcus epidermidid, and Bifidobacterium breve. | Left and right breasts often had similar bacterial taxa but often different quantities |
| • n = 47 | ||||
| • day 30 postpartum | ||||
| Soto et al., 2014 (48) | • Germany and Austria | Cultured for lactobacilli and bifidobacteria before PCR analysis of Lactobacillus and Bifidobacterium species | Lactobacilli and bifidobacteria were found in 40.9% and 10.6%, respectively, of samples by culture-dependent means and 67.5% and 25.6%, respectively, by PCR | Birth method and antibiotic use during pregnancy or lactation reduced the percentage of lactobacilli or bifidobacteria positive milk samples |
| • n = 160 | ||||
| • Mainly 1–4 wk postpartum |
OTU, operational taxonomic unit; RAPD, random amplified polymorphic DNA; rRNA, ribosomal RNA.
Characterizing the bacteria in human milk.
Perhaps the first (25) of the studies that used culture-independent methods to study healthy human-milk microbiota was published in 2003 by Dr. Juan Rodríguez and his research group. This study in 8 healthy, breastfeeding Spanish mothers and their infants tested the hypothesis that human milk is a source of lactic acid bacteria8 and combined targeted culture-dependent and culture-independent methods. Lactobacillus gasseri and Enterococcus faecium were isolated from the milk, mammary areola/skin, the infant’s mouth, and the infant’s feces. However, lactic acid bacteria types in the milk differed from those found on the skin of the breast. The researchers concluded that human milk can be an important source of lactic acid bacteria to the infant, and that these bacteria do not simply result from contamination from the surrounding skin but instead likely have an endogenous origin. Although the origin of milk microbiota is beyond the scope of this article, the reader is directed to another article in this issue related to this topic (26).
In a subsequent and systematic series of elegant studies conducted in the decade after their first report (e.g., 28 and as reviewed in 29–31), Rodríguez and his group provided additional evidence that not only does human milk contain viable lactic acid and other types of bacteria but that oral provision of selected milk-derived bacteria to mastitic women results in the appearance of these bacteria in their milk and is substantially more effective than antibiotic therapy in reducing pain and risk of lactation cessation associated with mastitis (32). These studies provide solid evidence that bacteria residing in the mammary gland are not necessarily harmful; instead, it is likely that there is a dysbiosis of bacterial community structure that leads to maternal disease. Moreover, a rebalancing of this community via oral administration of milk-derived bacteria can restore health—a concept perfectly in line with that of Mechnikov a century earlier. Interestingly, however, this application of consumption of “probiotic” bacteria initially found in human milk involves a unique twist; that is, the health benefit is bestowed on lactating women rather than on breastfed infants.
Building on the work of Rodríguez and his team, several other research groups provided additional evidence that milk produced by healthy women contains bacteria. For instance, utilizing 454-pyrosequencing and primers targeting the V1–V2 hypervariable region of the bacterial 16S ribosomal RNA (rRNA) gene, our group characterized the diversity and temporal stability of bacterial communities in 3 milk samples collected from each of 16 US women over a 4-wk period (33). Ecological analyses of the data at the genus level suggest substantial richness and diversity of bacteria, although only 9 genera were found in all samples. This “core” bacterial community accounted for 52% of all operational taxonomic units and included Staphylococcus, Streptococcus, Serratia, Pseudomonas, Corynebacterium, Ralstonia, Propionibacterium, Sphingomonas, and Bradyrhizobium. These milk samples were also subjected to the currently recommended, culture-dependent methods of the National Mastitis Council’s Laboratory and Field Handbook on Bovine Mastitis (34). Although all were found to have complex microbial community structures via pyrosequencing, 20% of the samples were void of bacterial growth. A potential limitation of this work is that viability and quantity of bacteria could not be determined—only relative abundances of bacterial taxa. Nonetheless, these data provided the first obtained by using next-generation sequencing techniques to characterize the global bacterial community in human milk.
A similar study was also conducted by Cabrera-Rubio et al. (35), who characterized the milk microbial community at 3 different time points (colostrum and at 1 and 6 mo postpartum) in 18 Finnish women. These investigators used both 454-pyrosequencing and qPCR, and primers targeted the V1–V3 region of the 16S rRNA gene for taxonomic identification at the family and genus levels. Results indicated relatively high amounts of lactic acid bacteria (especially in colostrum) but also high amounts of Staphylococcus, Streptococcus, Veillonella, Leptotrichia, and Prevotella in the milk samples. These investigators did not determine, however, whether there existed unique bacterial “fingerprints” within women across time as was described by us (33). However, they compared their data to our data and reported substantial differences in microbial community make-up. Again, why these differences exist are not known but likely are because of both (real) population and (confounding) methodologic differences that will require additional research to understand.
Jost et al. (36, 37) also published relatively extensive work characterizing the human-milk microbiome using a combination of culture-dependent methods and culture-independent, molecular techniques (454-pyrosequencing; primers targeting the V5–V6 region of the bacterial 16S rRNA gene). For instance, they collected milk from 7 healthy, exclusively breastfeeding Swiss women at 3 times during the first month postpartum. Members of the Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria phyla were predominant, with Staphylococcus, Streptococcus, Pseudomonas, Ralstonia, Bifidobacterium, Blautia, and Bacteroides identified as the most abundant genera. Members of the Enterococcus and Lactobacillus genera were present in 9.5% and 15% of the samples, respectively.
There exist several other recently published reports characterizing the microbiome in milk collected from healthy lactating women. These include a study conducted by Ward et al. (38) who used Illumina next-generation sequencing technology to characterize all of the genetic material (metagenome) of a single milk pool constituted from 10 milk samples collected from 10 breastfeeding women between 9 and 30 d postpartum. Bacteria in milk were classified at the phylum and genus levels. Milk community structure was compared with that of maternal and infant fecal samples at the phylum level; and potentially immunologically related bacterial-DNA motifs were searched for within the pooled milk sample. More than 360 prokaryotic genera were detected with the phyla Proteobacteria and Firmicutes dominating. At the genus level, Pseudomonas and Staphylococcus (both identified as “core” bacterial taxa in reference 33) together contributed the majority of the total community membership, followed by 13 additional less-abundant genera. The milk metagenome was found to be less diverse at the phylum level than that of the feces, and milk contained notable amounts of genetic material associated with nitrogen metabolism, membrane transport, stress response, and immunomodulatory functions. Differences between the findings of Hunt and colleagues (33) and those of Ward et al. (38) are likely related to a constellation of variables, including collection methods (e.g., cleaned vs. uncleaned breast), extraction techniques (e.g., use of bead beating to rupture bacterial cell membranes), sequencing platforms (pyrosquencing vs. Illumina), and unexplored yet potentially important host-specific, environmental differences (e.g., diet).
Khodayar-Pardo et al. (39) also characterized the microbial community structure in 32 Spanish women. Using qPCR, they identified Lactobacillus, Streptococcus, and Enterococcus spp. as the predominant bacterial groups. Tušar et al. (40) also reported a host of Lactobacillus, Enterococcus, Staphylococcus, and Bifidobacterium species in milk collected from 47 Slovenian women, and González et al. (41) found that the most frequent bacterial groups in milk collected from Mozambique women were of the Staphylococcus, Streptococcus, and Lactobacillus genera. In addition, Albesharat et al. (42), using an elegant combination of culture-dependent and culture-independent methods, identified a host of lactic acid bacteria in milk produced by Syrian women.
In conclusion, there is now indisputable evidence that human milk contains a diverse and viable bacterial community, even when it is produced by healthy women with no signs or symptoms of mastitis or other mammary disease. Although bacterial community structure differs between these studies, most suggest that milk contains Staphylococcus, Streptococcus, and Lactobacillus genera. However, differences between studies warrant careful scrutiny to determine if they are due to environmental, behavioral, or genetic differences or are a consequence of methodologic variation. Furthermore, although it is beyond the scope of this article to review the related literature, strong evidence for the presence of bacteria in milk has been published for bovine, ovine, and caprine taxa (e.g., 43–46), confirming that this phenomenon is not unique to humans. As such, the time has come to acknowledge a paradigm shift away from the long-held dogma suggesting that milk is sterile toward recognition of a rich bacterial community in milk. This acknowledgment is the first required step in considering whether human milk should be considered a probiotic food.
Factors associated with variation in milk microbiota.
As described above, using both culture-dependent and culture-independent methods, researchers have in recent years consistently identified a variety of bacterial types in human milk. However, great variation exists among published reports. At this time, very little is known about what factors are responsible for these differences, although it can be assumed that some of them are due to confounding methodologic issues that will need to be addressed coordinately by researchers in this field. Nonetheless, there is some evidence that demographic, environmental, and physiologic variables might influence milk microbial community structure. These variables include geographic location (which encompasses myriad variables including genetics and dietary patterns), time postpartum, delivery mode, maternal adiposity, maternal health (e.g., celiac disease, HIV, mastitis), and medical treatments (e.g., chemotherapy). In fact, as described by Tušar et al. (40), microbial ecology may even differ between breasts within the same woman.
Perhaps best documented are the potential influences of time postpartum and delivery mode on microbial community structure. For instance, Cabrera-Rubio et al. (35) reported that, whereas Weisella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus are predominant genera in colostrum of Finnish women, the typical inhabitants of the oral cavity (e.g., Veillonella, Leptotrichia, and Prevotella) become relatively more abundant in mature milk. Milk produced by women who had undergone cesarean delivery (especially elective) was also different (e.g., greater evenness, increased Acinetobacter) from that produced by women who had vaginal delivery. They also found that milk produced by obese mothers [BMI (kg/m2) ≥30] contained a different mix of bacteria and was less diverse than that collected from normal-weight women (BMI ≤25). The lack of dietary records precludes making any conclusions related to the effects of overall nutritional status (e.g., energy balance, micronutrient status, etc.) in this regard. However, because these samples were collected and analyzed in a similar manner in the same laboratory, one can conclude that reported differences related to time postpartum, delivery mode, and BMI are real and not related to collection and laboratory methods. Instead, this variation is likely related to physiologic (e.g., hormonal) changes, use of antibiotics, stress, and other factors yet to be delineated.
In another longitudinal study designed to investigate the potential relations among maternal adiposity, time postpartum, and the milk microbiome, Collado et al. (47) collected milk samples from 56 mothers (22 overweight and 34 normal weight) and their infants at 1–2 d, 1 mo, and 6 mo postpartum. qPCR was used to detect various bacterial genera and groups. They found higher amounts of Staphylococcus group bacteria and lower amounts of Bifidobacterium group bacteria in overweight mothers compared with normal-weight ones. The prevalence of Akkermansia muciniphila–type bacteria was also higher in overweight mothers, and their numbers were related to the IL-6 concentration in colostrum. The authors concluded that a dysbiosis of bacteria in the milk of overweight women (and those who gain excessive weight during pregnancy) may play a role in predisposing their infants to unhealthy weight gain.
Recently, Soto et al. (48) demonstrated a lower occurrence of lactobacilli and bifidobacteria in milk from 160 German or Austrian women who received antibiotics during pregnancy or lactation. Milk from women undergoing cesarean delivery and/or who received anesthesia during delivery tended to be less likely to contain lactobacilli. With the use of PCR, of the most abundant Lactobacillus species, L. salivarius and L. fermentum were detected in >25% of the samples, whereas for Bifidobacteria, B. breve was found in ∼15% of the samples.
Khodayar-Pardo et al. (39) also reported differences in milk microbes over lactation in 32 Spanish women: total bacteria, Bifidobacterium, and Enterococcus spp. counts increased during the first 3 wk postpartum. They also reported that Bifidobacterium spp. concentration was consistently higher in milk produced by women delivering at term (vs. preterm), and higher bacteria concentration was present in early milk in that produced by women who had undergone cesarean (vs. vaginal) deliveries. Bifidobacterium was also detected more frequently in vaginal (vs. cesarean) deliveries. These authors, however, were unable to look at more global microbial changes, because they used targeted PCR analyses rather than next-generation sequencing (e.g., pyrosequencing) as was done by Cabrera-Rubio et al. (35). Conversely, it should be noted that the Finnish study focused on the 10 most-abundant bacterial taxa and therefore did not report potential differences in less abundant groups (e.g., Bifidobacterium). For this reason, it is somewhat difficult to compare results obtained by these high-quality studies.
Albesharat et al. (42) also provided convincing evidence for “sharing” of bacteria species among local, fermented foods, the maternal gastrointestinal tract, milk, and an infant’s gastrointestinal tract. In this study, milk and fecal samples were collected from 30 mother-infant dyads; and traditional plant-, dairy-, and meat-based probiotic foods (e.g., kishk, shanklish, and makdous) were sampled from the local marketplace. A combination of random amplified polymorphic DNA (RAPD), 16S rRNA sequencing, and matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis was used to phenotype and genotype lactic acid bacteria in each sample matrix. Although there were bacterial taxa that were unique to each sample type, Lactobacillus plantarum, Lactobacillus fermentum, Pediococcus pentosaceus, and Lactobacillus brevis were found in all of them, suggesting an intimate microbial interplay among maternal diet, milk microbiota, and the gastrointestinal microbes in breastfed infants and the importance of host factors in colonization and personalization of microbes within a cultural setting.
Several research groups also reported differences in milk microbial communities on the basis of maternal health status. For example, González et al. (41) found higher bacterial diversity and frequency of Lactobacillus spp. in HIV-positive African women and lower frequency of Staphylococcus hominis, and S. aureus in HIV-positive women. These differences are likely not related to sampling or methodologic issues, although one cannot infer causality (or directionality) from these differences. It is possible that factors associated with different microbial ecologies might influence susceptibility to HIV, but it is equally possible that HIV infection can trigger microbial community shifts. Prospective longitudinal human studies coupled with animal models will likely be needed to study this relation in more detail. Similarly, Olivares et al. (49) reported in a cross-sectional study that gene copies (numbers/mL) of Bifidobacterium spp. and B. fragilis group were higher in milk produced by 12 self-reported healthy Spanish mothers than that collected from 12 lactating Spanish women with celiac disease. This shift was accompanied by lower concentrations of immunoprotective compounds (e.g., TGF-β1 and secretory immunoglobulin A) in the milk. Grönlund et al. (50) also found lower counts of Bifidobacterium in milk of allergic women compared with their nonallergic counterparts. Whether these differences are strictly due to the disease or other confounding factors (e.g., gluten in the diet or other “avoidance-type” diets) will require additional research.
Chemotherapy has also been associated with alteration in milk microbiome. Urbaniak et al. (51) used Ion Torrent (Life Technologies) next generation sequencing technology to document prospective, longitudinal global changes in milk microbiota in a single lactating woman undergoing chemotherapy related to Hodgkin lymphoma. Data were compared with those on milk produced by 8 healthy lactating women. Chemotherapy was related to lower diversity and altered bacterial profiles: decreased percentage abundances of Acinetobacter and Xanthomonadaceae and decreased abundances of the members in Bifidobacterium and Eubacterium genera.
In addition to the aforementioned environmental, physiologic, and anthropometric variables that might be related to milk bacterial communities, other milk components might also affect or be influenced by these organisms. Perhaps most obvious in this regard are the oligosaccharides, which have long been touted as being “prebiotic” in terms of encouraging growth of health-promoting bacteria in the recipient infant’s gastrointestinal tract (as reviewed in 52). Interestingly, Bode and his research group also showed that milk oligosaccharide profiles (which are thought to be, at least in part, driven by genetic variation) differ between HIV-infected and HIV-uninfected mothers and are related to the incidence of necrotizing enterocolitis in their preterm infants (53). In addition, in collaboration with the Bode group, we provided in vitro evidence that human milk oligosaccharides can interact with the bacterial communities present in milk—specifically with regard to the growth of Staphylococcus species (54). It is also well established that various FAs common in milk (e.g., conjugated linoleic acid) can modify growth of common milk bacteria (e.g., S. aureus) (55). Furthermore, microbe-microbe interactions are possible in milk, as has been described by Heikkilä and Saris (56). To our knowledge, other relations between milk constituents (including nutrients, hormones/growth factors, immune cells, and cytokines) and milk bacterial communities have yet to be examined.
In conclusion, there is limited evidence that demographic, physiologic, and environmental factors can influence the microbes present in a woman’s milk. As such, it will likely be impossible to describe a single “normal” milk microbiome, because its membership and distribution probably are dependent on other important factors such as environment, dietary habits, delivery mode and other birth attributes, time postpartum, breastfeeding behaviors, and even genetics. It is also conceivable that a microbial community that confers optimal health to infants in one population may differ from that in another population. These differences should be understood and respected before suggesting that there exists a consistent, optimal human-milk microbiome worldwide. Clearly, what is a “normal” milk microbiome is unlikely a one-size-fits-all proposition, and manufacturers of microbe-containing (probiotic?) infant foods should be aware that adding one bacterial strain or a select cocktail of bacterial taxa to their products is likely premature until we understand this concept more thoroughly.
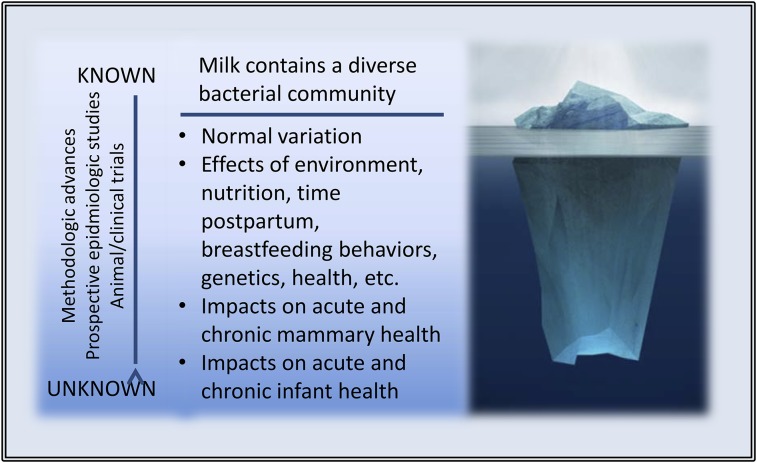
Next Steps—Exploring the Depths beneath the Iceberg
There now exists little, if any, doubt that human milk contains a complex bacterial community. However, what is known about the microbial ecology of human milk and how it affects maternal and infant health is in its preliminary stages at best. As illustrated in Figure 2, there remain myriad important discoveries to be made in this regard. These include the following:
FIGURE 2.
The tip of the human-milk microbiome iceberg, illustrating what is known and unknown concerning the human-milk microbiome. Image credit: ©Fotosearch.com.
Understanding normal variation within a woman and between women, communities, and populations
Appreciating potential effects of environment, nutrition, time postpartum, breastfeeding behaviors, genetics, health, etc., on milk microbial ecology
Exploring effects of variation in microbial community structure on acute and chronic mammary health (and vice versa)
Identifying effects of variation in microbial community structure on acute and chronic infant health (and vice versa)
These discoveries will require significant advances in and standardization of methodologic approaches across research groups, including coordinate culture-dependent and culture-independent approaches; careful, prospective epidemiologic studies in a variety of populations; and well-controlled animal and human intervention trials. In terms of methods standardization, it will be important to determine the best collection techniques (e.g., how/if to clean the breast, manual vs. pump collection, and whether a complete expression is needed), from which milk fraction DNA should be extracted (e.g., whole vs. skim vs. cell pellet, and which extraction method to use), which primer or primers work best for 16S rRNA analysis, and what sort of bioinformatics pipeline and computational approach yields the most consistent and informative results. The speed, adequacy, and usefulness of these studies will likely depend greatly on strategic, collaborative, interdisciplinary research among lactation physiologists, clinicians, milk chemists, nutritionists, microbiologists, anthropologists, and biostatisticians.
Conclusions
The overarching question posed in this article was “Is human milk a probiotic food?” Clearly, if one were to rely solely on the current WHO/FAO definition of a probiotic food, the answer would be “no.” This conclusion is based on the reality that “dosages” of bacterial taxa present in milk are currently unknown and undoubtedly variable. In addition, health outcomes have not been clearly delineated for the infant and in fact might also be relevant to the mother. However, if one were to consider the original concept proffered by Mechnikov, human milk would necessarily be considered a probiotic food. Indeed, feeding this microbe-rich fluid to infants has been shown to substantially improve both short- and long-term health as well as decrease mortality, especially in the most at-risk infants (57). Until recently, however, the involvement of milk-borne microbes was not considered one of the possible mechanisms for this effect because of the state of knowledge (or lack thereof) in this regard.
In light of this paradigm shift, we propose that the more “back-to-basics” approach be reconsidered in terms of using the word probiotic to describe human milk. In fact, because of the co-occurrence of both prebiotic compounds (oligosaccharides and growth-promoting FAs) and live bacteria, human milk might even be considered a synbiotic food. Now that the scientific community has begun to come to terms with the concept that human milk contains myriad, viable commensal and/or symbiotic bacteria, understanding the nature of these bacteria and how they affect infant health should provide fundamental information as to “optimal” early bacterial exposure in various situations. Indeed, understanding the probiotic nature of mother’s milk (arguably the only food uniquely designed to nourish humans) may be an important step in understanding how all probiotic foods affect health and disease throughout the life cycle. Indeed, mother’s milk may turn out to be Mother Nature’s archetypal probiotic (and perhaps even synbiotic) food.
Acknowledgments
Both authors read and approved the final version of the manuscript.
Footnotes
Lactic acid bacteria are gram-positive, non–spore-forming cocci, coccobacilli, or rods that ferment glucose primarily to lactic acid, or to lactic acid, carbon dioxide, and ethanol. All lactic acid bacteria grow anaerobically, but unlike most anaerobes, they can also grow in the presence of oxygen. The term lactic acid bacteria is traditionally reserved for genera in the order Lactobacillales, which includes Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, and Streptococcus, in addition to Carnobacterium, Enterococcus, Oenococcus, Tetragenococcus, Vagococcus, and Weisella. Lactic acid bacteria are among the most important groups of microorganisms used in food fermentations (27).
References
- 1.Nobelprize.org. The Nobel Prize in Physiology or Medicine 1908 [cited 2014 Aug 18]. Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1908/.
- 2.Gordon S. Elie Metchnikoff: father of natural immunity. Eur J Immunol 2008;38:3257–64. [DOI] [PubMed] [Google Scholar]
- 3.Mackowiak PA. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. 2013;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton-Miller JM, Gibson GR, Bruck W. Some insights into the derivation and early uses of the word 'probiotic’. Br J Nutr 2003;90:845. [DOI] [PubMed] [Google Scholar]
- 5.Fuller R. History and development of probiotics. In: Fuller R, editor. Probiotics. London: Chapman and Hall; 1992. p. 1–8. [Google Scholar]
- 6.Metchnikoff E. The prolongation of life. London: Heinemann; 1907. [Google Scholar]
- 7.Rettger LF, Cheplin HA. A treatise on the transformation of the intestinal flora with special reference to the implantation of Bacillus acidophilus. New Haven (CT): Yale University Press; 1921. [Google Scholar]
- 8.Rettger LF, Levy MN, Weinstein L, Weiss JE. Lactobacillus acidophilus and its therapeutic application. New Haven (CT): Yale University Press; 1935. [Google Scholar]
- 9.Nuttal GHF, Thierfelder H. Animal life without bacteria in the digestive tract. Z Physiol Chem 1895;21:102–21 (in German). [Google Scholar]
- 10.Bohnhoff N, Drake BL, Muller CP. Effect of streptomycin on susceptibility of the intestinal tract to experimental salmonella infection. Proc Soc Exp Biol Med 1954;86:132–7. [DOI] [PubMed] [Google Scholar]
- 11.Freter R. Experimental enteric shigella and vibrio infection in mice and guinea pigs. J Exp Med 1956;104:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Microbial ecology in states of health and disease: workshop summary. Washington: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 13.Lilly DM, Stillwell RH. Probiotics: growth promoting factors produced by microorganisms. Science 1965;147:747–8. [DOI] [PubMed] [Google Scholar]
- 14.Sperti GS. Probiotics. West Point (CT): Avi Publishing Company; 1971.
- 15.Parker RB. Probiotics, the other half of the antibiotic story. Anim Nutr Health. 1974;29:4–8. [Google Scholar]
- 16.Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989;66:365–78. [PubMed] [Google Scholar]
- 17.Fuller R. Probiotics: their development and use. In: Fuller R, Heidt PJ, Rusch V, van der Waaij D, editors. Old Herborn University Seminar Monograph. 8. Probiotics: prospects of use in opportunistic infections. Herborn-Dill (Germany): Institute for Microbiology and Biochemistry; 1995. p. 1–8.
- 18.FAO/WHO. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. October 2001 [cited 2014 Aug 17]. Available from: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
- 19.Osterman KL, Rahm V-A. Lactation mastitis: bacterial cultivation of breast milk, symptoms, treatment, and outcomes. J Hum Lact 2000;16:297–302. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen AC, Hansen KB, Moller BR. Leukocyte counts and microbiological cultivation in the diagnosis of puerperal mastitis. Am J Obstet Gynecol 1983;146:938–41. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen AC, Espersen T, Maigaard S. Course and treatment of milk stasis, noninfectious inflammation of the breast, and infectious mastitis in nursing women. Am J Obstet Gynecol 1984;149:492–5. [DOI] [PubMed] [Google Scholar]
- 22.Kvist LJ. Toward a clarification of the concept of mastitis as used in empirical studies of breast inflammation during lactation. J Hum Lact 2010;26:53–9. [DOI] [PubMed] [Google Scholar]
- 23.Kvist LJ, Wilde Larsson B, Hall-Lord ML, Steen A, Schalén C. The role of bacteria in lactational mastitis and some considerations of the use of antibiotic treatment. Int Breastfeed J 2008;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fetherston C. Mastitis in lactating women: physiology or pathology? Breastfeed Rev 2001;9:5–12. [PubMed] [Google Scholar]
- 25.Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J, Fernández L, Rodríguez JM. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 2003;143:754–8. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez J. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr 2015;6:779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todar K. Todar’s online textbook of bacteriology [cited 2014 Aug 22]. Available from: http://textbookofbacteriology.net/lactics.html.
- 28.Collado MC, Delgado S, Maldonado A, Rodríguez JM. Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett Appl Microbiol 2009;48:523–8. [DOI] [PubMed] [Google Scholar]
- 29.Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013;69:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Jeurink PV, van Bergenhenegouwen J, Jiménez E, Knippels LM, Fernández L, Garssen J, Knol J, Rodríguez JM, Martín R. Human milk: a source of more life than we imagine. Benef Microbes 2013;4:17–30. [DOI] [PubMed] [Google Scholar]
- 31.Fernández L, Langa S, Martín V, Jiménez E, Martín R, Rodríguez JM. The microbiota of human milk in healthy women. Cell Mol Biol (Noisy-le-Grand) 2013;59:31–42. [PubMed] [Google Scholar]
- 32.Arroyo R, Martín V, Maldonado A, Jiménez E, Fernández L, Rodríguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin Infect Dis 2010;50:1551–8. [DOI] [PubMed] [Google Scholar]
- 33.Hunt KM, Foster JA, Forney LJ, Schutte UME, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011;6:e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Mastitis Council. Laboratory and field handbook on bovine mastitis. Fort Atkinson (WI): WD Hoard & Sons; 1987.
- 35.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012;96:544–51. [DOI] [PubMed] [Google Scholar]
- 36.Jost T, Lacroix C, Braegger C, Chassard C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr 2013;110:1253–62. [DOI] [PubMed] [Google Scholar]
- 37.Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol 2014;16:2891–904. [DOI] [PubMed] [Google Scholar]
- 38.Ward TL, Hosid S, Ioshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC Microbiol 2013;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khodayar-Pardo P, Mira-Pascual L, Collado MC, Martinez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol 2014;34:599–605. [DOI] [PubMed] [Google Scholar]
- 40.Tušar T, Žerdoner K, Bogovič Matijašić B, Paveljšek D, Benedik E, Brantanič B, Fidler N, Rogelj I. Cultivable bacteria from milk from Slovenian breastfeeding mothers. Food Technol Biotechnol 2014;52:242–7. [Google Scholar]
- 41.González R, Maldonado A, Martín V, Mandomando I, Fumadó V, Metzner KJ, Sacoor C, Fernández L, Macete E, Alonso PL, et al. Breast milk and gut microbiota in African mothers and infants from an area of high HIV prevalence. PLoS ONE 2013;8:e80299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albesharat R, Ehrmann MA, Korakli M, Yazaji S, Vogel RF. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol 2011;34:148–55. [DOI] [PubMed] [Google Scholar]
- 43.Callon C, Duthoit F, Delbès C, Ferrand M, Le Frileux Y, De Crémoux R, Montel M-C. Stability of microbial communities in goat milk during a lactation year: molecular approaches. Syst Appl Microbiol 2007;30:547–60. [DOI] [PubMed] [Google Scholar]
- 44.Oikonomou G, Machado VS, Sinntisteban C, Schukken YH, Bicalho RC. Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomic 16S rDNA. PLoS ONE 2012;7:e47671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oikonomou G, Bicalho ML, Meira E, Rossi RE, Foditsch C, Machado VS, Teixeira AGV, Santisteban C, Schukken YH, Bicalho RC. Microbiota of cow’s milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS ONE 2014;9:e85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quigley L, O’Sullivan O, Stanton C, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. The complex microbiota of raw milk. FEMS Microbiol Rev 2013;37:664–98. [DOI] [PubMed] [Google Scholar]
- 47.Collado MC, Laitinen K, Salminen S, Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr Res 2012;72:77–85. [DOI] [PubMed] [Google Scholar]
- 48.Soto A, Martín V, Jiménez E, Mader I, Rodríguez JM, Fernández L. Lactobacilli and Bifidobacteria in human breast milk: influence of antibiotherapy and other host and clinical factors. J Pediatr Gastroenterol Nutr. 2014;59:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivares M, Albrecht S, De Palma G, Ferrer MD, Castillejo G, Schols HA, Sanz Y. Human milk composition differs in healthy mothers and mothers with celiac disease. Eur J Nutr 2014. (Epub 2014 Apr 4; DOI: 10.1007/s00394-014-0692-1). [DOI] [PubMed] [Google Scholar]
- 50.Grönlund MM, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S, Isolauri E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy 2007;37:1764–72. [DOI] [PubMed] [Google Scholar]
- 51.Urbaniak C, McMillan A, Angelini M, Gloor GB, Sumarah M, Burton JP, Reid G. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome 2014;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bode L. Human milk oligosaccharides: every baby needs a sugar mom. Glycobiology 2012;22:1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Niekerk E, Autran CA, Nel DG, Kirsten GF, Blaauw R, Bode L. Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J Nutr 2014;144:1227–33. [DOI] [PubMed] [Google Scholar]
- 54.Hunt KM, Preuss J, Nissan C, Davlin CA, Williams JE, Shafii B, Richardson AD, McGuire MK, Bode L, McGuire MA. Human milk oligosaccharides promote the growth of staphylococci. Appl Environ Microbiol 2012;78:4763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelsey JA, Bayles KW, Shafii B, McGuire MA. Fatty acids and monoacylglycerols inhibit growth of Staphylococcus aureus. Lipids 2006;41:951–61. [DOI] [PubMed] [Google Scholar]
- 56.Heikkilä MP, Saris PE. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol 2003;95:471–8. [DOI] [PubMed] [Google Scholar]
- 57.U.S. Department of Health and Human Services. The Surgeon General’s call to action to support breastfeeding. Washington: U.S. Department of Health and Human Services, Office of the Surgeon General; 2011.
- 58.Solís G, de Los Reyes-Gavilan CG, Fernández N, Margolles A, Gueimonde M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 2010;16:307–10. [DOI] [PubMed] [Google Scholar]