Abstract
Nutritional epidemiology has recently been criticized on several fronts, including the inability to measure diet accurately, and for its reliance on observational studies to address etiologic questions. In addition, several recent meta-analyses with serious methodologic flaws have arrived at erroneous or misleading conclusions, reigniting controversy over formerly settled debates. All of this has raised questions regarding the ability of nutritional epidemiologic studies to inform policy. These criticisms, to a large degree, stem from a misunderstanding of the methodologic issues of the field and the inappropriate use of the drug trial paradigm in nutrition research. The exposure of interest in nutritional epidemiology is human diet, which is a complex system of interacting components that cumulatively affect health. Consequently, nutritional epidemiology constantly faces a unique set of challenges and continually develops specific methodologies to address these. Misunderstanding these issues can lead to the nonconstructive and sometimes naive criticisms we see today. This article aims to clarify common misunderstandings of nutritional epidemiology, address challenges to the field, and discuss the utility of nutritional science in guiding policy by focusing on 5 broad questions commonly asked of the field.
Keywords: dietary assessment, food policy, meta-analysis, nutritional epidemiology, randomized controlled trials, prospective cohort studies
Introduction
Epidemiology has long had its share of skeptics, with Taubes’ 1995 article being the most well-known (1). However, more recent commentaries have attacked nutritional epidemiology on several fronts. Ioannidis (2) criticizes the observational nature of epidemiologic studies and small trials, stating that “definitive solutions won’t come from another million observational papers or small randomized trials.” He refers to an article by Archer et al. (3), which calls into question the validity of data from the NHANES and suggests that “the ability to estimate population trends in caloric intake and generate empirically supported public policy relevant to diet-health relations from US nutritional surveillance is extremely limited.” Furthermore, questionably designed and executed meta-analyses have disseminated conflicting messages about nutrition and health, such as the conclusion that being overweight lowers the risk of all-cause mortality (4) and that replacing saturated fat with polyunsaturated fats has no substantial impact on cardiovascular risk (5). Such conclusions are not only confusing but also dangerous because they can be perceived as misleading messages, or can lead to the communication of misleading messages to the public by popular media and the consequent adoption of unhealthy practices by the population at large. For instance, after the publication of the latter meta-analysis, New York Times columnist Mark Bittman told his readers that they “can go back to eating butter” (6).
Many authors have suggested that large randomized controlled trials (RCTs)6 are the only solution to circumventing the problems in observational research. In reality, RCTs are far from being the panacea in the study of diet and chronic disease, and the results of such trials can be misleading. A key reason is that the exposure of interest in nutritional epidemiology—dietary intake—is complex, with interactions and synergies across different dietary components, which can be difficult to study with use of a linear drug trial approach. A complex behavioral exposure such as diet also makes other aspects important in pharmacologic RCTs, such as high compliance and blinding, difficult and infeasible in most dietary intervention trials. Consequently, nutritional epidemiology has design and analysis issues unique to the field, and understanding the details of nutritional epidemiologic studies requires a deep knowledge of nutritional science and its methodologic background.
The purpose of this article is to clarify common misunderstandings of nutritional epidemiology, address the challenges to the field, and discuss the utility of nutritional science in guiding policy. In particular, we address 5 broad questions that have been commonly raised about nutritional epidemiologic studies.
Can We Reliably Measure Dietary Intakes in Individuals and Populations?
Measuring diet in free-living populations is challenging because individual diets are complex exposures with innumerable and sometimes poorly characterized components that are consumed in varying amounts and combinations by different individuals. Dietary variables are rarely dichotomous; often, but not always, the entire population is “exposed” to some degree. Diet is also a time-varying exposure, with individual dietary habits and food composition changing over time. It is not surprising, then, that most dietary assessment methods have a component of error, which could be random day-to-day, diurnal, and seasonal variation in an individual’s diet over time, or because of systematic mechanisms, such as omission of foods when collecting data. Nonetheless, several techniques have been developed to ascertain dietary intake from free-living populations, and these methods have shown good validity with use of multiple criteria. Although each assessment method comes with its own set of limitations, strengths unique to each method make it appropriate for use in specific applications (7–10).
Multiple-week diet records, which require participants to record everything they eat or drink over the course of several weeks, are regarded as the gold standard for ascertaining dietary information because, unlike other methods, they do not rely on memory. The high participant burden and cost of keeping diet records has limited their use in large-scale epidemiologic studies; however, their ability to accurately ascertain detailed dietary information makes them useful in validation studies of other diet assessment methods, and in monitoring compliance in trials. Another limitation of diet records is that the process of recording can change an individual’s diet, rendering the data atypical of usual intake, although estimated intakes from diet records have been found to correlate reasonably well with those from multiple 24-h recalls (11). Repeated 24-h recalls involve a respondent reporting all foods consumed in the previous 24 h or calendar day to a trained interviewer in person or over the phone. Although reliance on the participant’s memory leaves room for measurement error, a skilled interviewer can produce highly detailed and useful nutritional data comparable to a diet record (11, 12). This method has been widely employed in dietary intervention trials. It is also used in national surveys to monitor trends in nutritional intake.
A potential source of error common to these methods is in the estimation of nutrients with use of food composition tables. The nutrient content of a food varies with season, location of production, growing conditions, storage, processing, and cooking techniques, and many of these factors are unaccounted for in food composition tables. The degree to which this is problematic differs from nutrient to nutrient. Although for some nutrients, such as dietary FAs, it is reasonable to assume that these variations do not substantially affect calculated intakes, for others, such as selenium, the variation can result in calculated intakes that are substantially different from true intakes (7). In general, however, this source of error does not substantially compromise the ability to rank individuals with respect to nutrient intake so as to evaluate associations with health outcomes (7, 13). Nevertheless, estimating nutrient composition from food intake data is a challenge, especially given the changing food landscape, and it is crucial that we continue to improve the accuracy of food composition databases.
When participants provide biological specimens, researchers can additionally measure intake by assaying biomarkers. Examples of biomarkers include doubly labeled water (DLW) (for total energy intake), urinary nitrogen (for protein intake), 24-h urinary sodium and potassium, blood lipid profiles, serum and plasma folate, and selenium and other trace minerals in toenails. Biomarkers allow for objective measurement of intake without any bias because of self-reporting. The limitations of biomarkers, however, have prevented their wider use. In particular, many foods and nutrients lack sensitive or specific biomarkers, their assessment always includes error from multiple sources, they may not be indicators of individual long-term intake, and obtaining and testing for biomarkers is expensive and burdensome. Thus, use of biomarkers to investigate nutrient-disease relations has been mostly confined to nested case-control studies and small trials. Biomarkers are also useful in assessing the validity of less-expensive, self-reported assessments of diet, such as FFQs.
An FFQ consists of a structured food list and a frequency response section on which the participant indicates his/her usual frequency of intake of each food over a certain period of time in the past, usually 1 y. This is the most common choice for measuring intake in large observational studies owing to its ease of use, low participant burden, and ability to capture usual long-term dietary intake. These features make possible repeated assessments over time, which is important to capture longer term variation in diets. Table 1 presents a comparative summary of the advantages, disadvantages, and applications of these dietary assessment methods.
TABLE 1.
Comparison of diet assessment methods
| Several day/week diet records | Multiple 24-h recalls | A single 24-h recall | Validated FFQ | Biomarkers | |
| Advantages | Provides accurate, detailed, open-ended data on dietary intake, with no reliance on memory, and direct computation of portion sizes. | Provides fairly accurate, detailed, open-ended data on dietary intake, without reliance on long-term memory. | Provides detailed, open-ended data on dietary intake, without reliance on long-term memory. | Provides time-integrated data that represents usual long-term intake. Can assess past dietary intake. | Provides an objective assessment of intake. Represents bioavailable dose, which is relevant when it is used in etiologic analyses. |
| Errors from omission, portion size estimation, and recall are least likely. | Has lower respondent burden and is less expensive than diet records, and works well in low-literacy contexts. | Has lower respondent burden and is less expensive than diet records and multiple recalls; works in low-literacy contexts. | The least expensive and most easily administered diet assessment method, with the lowest respondent burden. | May be available in retrospect (analysis of stored specimens). | |
| Disadvantages | Needs literate, motivated participants; participant burden is very high when done over several days. Could also alter usual eating habits. | There is scope for short-term recall error, omissions, and errors in portion size estimation. | There is scope for short-term recall error, omissions, and errors in portion size estimation. Has high random within-person error. | There is scope for long-term recall error. Omissions possible because of fixed-food list. FFQs need to be culture- and population-specific. |
Biomarker may not be sensitive to intake, may have low specificity, may not be time-integrated, may not represent usual long-term intake, and is subject to laboratory errors and other sources of bias. |
| Expensive and resource-intensive diet assessment method. Potential for errors in nutrient estimation from food composition tables. |
Has high interviewer burden and is more expensive than a single recall and FFQs. Potential for errors in nutrient estimation from food composition tables. |
Has high interviewer burden and is more expensive than FFQs. Potential for errors in nutrient estimation from food composition tables. |
Semi-quantitative. Potential for errors in nutrient estimation from food composition tables. |
Expensive and more invasive. Biomarkers are not available for many nutrients. | |
| Applications | Validation of other diet assessment methods. | Validation of other diet assessment methods. | National surveillance of mean population intake. | Association analyses in large epidemiologic studies. | Validation of other diet assessment methods. |
| Monitoring compliance in dietary intervention trials. | Monitoring compliance in dietary intervention trials. | Assessment of trends in dietary intake (earlier NHANES). | Assessing past dietary intake. | Association analyses in epidemiologic studies and monitoring compliance in intervention trials | |
| Assessment of trends in dietary intake (current NHANES). |
Thus, a collection of diverse diet assessment methods is available; their appropriate application, alone or in combination, allows for a reasonably comprehensive assessment of the diet of free-living populations. Nevertheless, recent critiques of these dietary assessment methods have called into question their utility in examining diet-disease relations and informing policy. A recent example is the article by Archer et al. (3), which criticizes the use of 24-h dietary recall data periodically collected in the NHANES. Archer et al. compared reported energy intake as assessed by the 24-h recalls with expected basal metabolic rate and concluded that recalled energy intake data were implausibly low and recommended that NHANES data be eliminated in considering public policy. This finding represents the danger of misunderstanding methodologic issues and making inferences with use of faulty logic. A recent article by Hébert et al. (13) comprehensively refutes the conclusions drawn from this study. The following section discusses key points from this article while providing an overview of measurement error assessment and correction in nutritional epidemiology.
Nutritional epidemiology has advanced considerably over the last 50 y with respect to understanding types and sources of measurement error in dietary intake data (7, 14). An insufficient appreciation of this can lead to erroneous conclusions like those of Archer et al. (3). Because of the considerable day-to-day variation in dietary intake among individuals, a single recall, as was used by Archer et al. in their analysis, will tend to capture extremes of dietary intake as opposed to usual current intake, increasing the likelihood that any individual’s single recall will be implausibly high or low. This random variation adds noise to the data, overestimating the variance, and flattening the distribution, thereby increasing the numbers of individuals in the extremes of the distribution. Thus, repeated 24-h recalls on nonconsecutive days are recommended to reduce within-person error. More epidemiologic studies that use 24-h recalls to assess diet now obtain multiple replicate measures on each participant, and starting in 2002, a second 24-h recall was introduced in the NHANES to address some of these issues (8).
However, as noted earlier, error in diet assessment need not be completely random. Systematic sources of variation include omission of foods consumed by individuals, errors in estimating portion sizes, and over- or under-reporting because of social approval (respond in certain ways to get social praise) or social desirability (respond in certain ways avoid social criticism) (15–17). All of these could have led to the under-reporting of energy intake observed by Archer et al. The underestimation of energy intake from self-reported data has long been known to nutrition researchers, and many strides in methodology have been made to reduce this source of measurement error (7, 18–22). Under-reporting because of omission or portion size estimation errors is unlikely to be differential with respect to determinants of the outcome of interest. In addition, there have been improvements in 24-h recall methodology that reduce these sources of error, such as the USDA 5-pass method (8), which is structured to minimize omission of foods and to help participants report accurate portion sizes by using visual aids. This 5-pass method, which was introduced into the NHANES starting in 2002, was found to agree reasonably well with actual intake assessed by direct observation (r = 0.57, P < 0.05) (23), as well as with energy intake as assessed by the DLW technique (r = 0.32 for males; r = 0.25 for females), which is considered the gold standard for energy intake assessment (provided body compartment masses such as fat mass and water mass are stable over time because DLW is a measure of energy expenditure), although the DLW technique has errors of its own (8). Another solution is to use an isocaloric statistical model in analysis, i.e., adjust for total energy intake. In analytic epidemiology we are generally less interested in the association of absolute energy/macronutrient intake with health outcomes, and more with how dietary composition relates to risk of disease because this is what is most modifiable by individuals or populations. Hence, adjusting for energy intake is standard practice in nutritional epidemiology. Adjusting for energy intake also diminishes extraneous sources of variation in dietary intake, and to some extent also reduces systematic sources of under- and over-reporting (7, 21, 22). Issues related to measurement error are not isolated to dietary assessment methods, but extend to assessment of most behavioral exposures and biomarkers, including physical activity (24, 25), with which Archer and colleagues, having worked considerably in the area of physical activity epidemiology, are familiar (26, 27).
Hence, although there is no perfect method, there is ample evidence that dietary measurements in national surveys have reasonably good reliability and validity. The conclusion of Archer et al. that dietary data with use of these methods cannot support public policy is misleading. National survey data such as the NHANES represent a small fraction of the totality of evidence on the basis of which national guidelines and public policy are made. A main purpose of using national survey data is to assess average population intakes and trends. For example, we recently examined trends in dietary quality from 1999 to 2010 in the US adult population among a nationally representative sample of 29,124 adults aged 20–85 y with use of the NHANES data (28). We found that better dietary quality, measured by the Alternate Healthy Eating Index, was associated with higher socioeconomic status, and the gap between the rich and the poor widened with time. These data underscore the importance of developing nutritional policies to improve diet quality and reduce health disparities.
Because of its low cost and low participant burden, self-administered computer-processed FFQs are the only option in most large cohort studies to assess usual dietary intakes. FFQs usually have lower random within-person variation than other dietary assessment methods because they are designed to assess average usual intake over the past year. For this reason, they are better equipped to assess long-term dietary intake, the exposure of etiologic interest for most diseases (7). Because of their reliance on memory, FFQs may suffer from greater measurement error relative to recalls and records if these methods are used for many days to reflect longer-term intakes (for certain nutrients, just a few days of diet records or recalls might be enough, provided the days are spread out over the entire reference period of the FFQ). Nevertheless, FFQs have been shown to have acceptable validity when compared to reference measures (29, 30), with typical correlation coefficients for individual nutrients or foods ranging from 0.4 to 0.7 (7). Adjustment for total energy intake, along with use of repeated FFQs in long-term prospective cohort studies, further improves these validity coefficients. Although extended dietary records are the most popular reference method, when biomarkers are available, triangulation methods can be used to obtain improved estimates of correlations of FFQ intake with true intake (31). These validity coefficients can be used to correct for measurement error in epidemiologic analyses, and the application of these measurement error correction methods is increasingly being extended to more complicated analyses (18–20). These techniques have allowed for valid inferences to be drawn from large cohort studies with use of FFQ data.
Despite these developments in reducing measurement error in dietary intake data, continued improvements in dietary assessment methodology and measurement error correction are needed to advance the field. Nevertheless, the considerable progress made over the past few decades, especially the use of repeated measures of diet over time, has enabled nutritional epidemiologists to reliably collect and use dietary information in both individuals and populations.
What Is the Role of Nutritional Epidemiology in Inferring Causality?
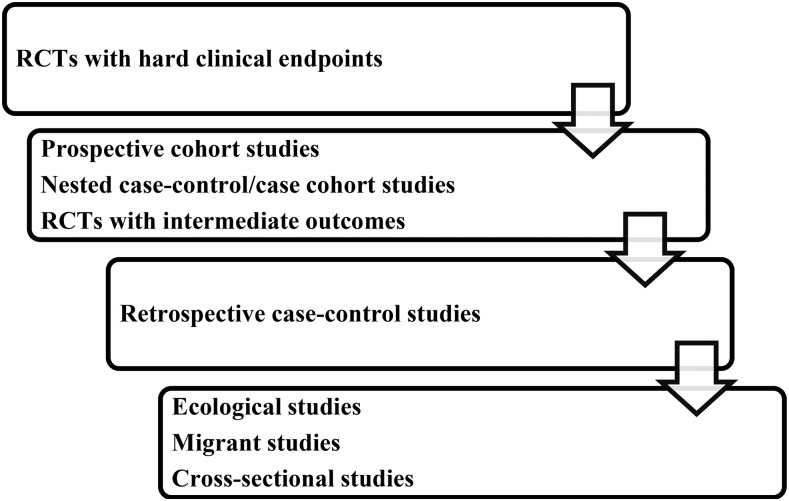
One of the main criticisms leveled against nutritional epidemiology is that it relies predominantly on observational data, which is deemed to be inferior to experimental data in determining causality. Figure 1 illustrates the typical hierarchy of evidence from various study designs. While randomized trials with hard endpoints occupy the highest position in this hierarchy, they are usually not the most appropriate or feasible study design to answer nutritional epidemiologic questions regarding long-term effects of specific foods or nutrients (unless they can be packaged in a pill).
FIGURE 1.
Hierarchy of study designs in nutritional epidemiology. RCT, randomized controlled trial.
In the absence of evidence from large RCTs on hard endpoints, nutritional epidemiologists typically rely on prospective cohort studies, the strongest observational study design in terms of minimizing bias and inferring causality. Being prospective in nature, they are less affected by several biases, such as reverse causation, recall bias, and selection bias, which commonly plague retrospective or cross-sectional study designs. Reverse causation describes the situation in which the outcome affects the exposure, rather than the other way around. This is a common concern with cross-sectional studies and retrospective case-control studies because they assess exposure and outcome at the same time. Prospective cohort studies can minimize the possibility of reverse causation by apparent disease because participants are followed forward in time, and these studies can examine the extent of reverse causation from subclinical disease by lagged analyses. They are superior to retrospective case-control studies because the issues of selection bias (controls not being representative of the underlying population that gave rise to cases) and recall bias (knowledge of disease status affecting recall of diet) can be largely minimized because prospective cohort studies begin with a disease-free population at baseline that is followed up to ascertain incident cases that develop over time. This is evident from the fact that spurious links between total energy intake and colon cancer (32), and fats and breast cancer (33), which were reported in case-control studies, have not been replicated in large prospective cohort studies.
A major challenge when working with any kind of observational data is confounding. A confounder is a variable that is associated with both the exposure and outcome but is not caused by either, and when unaccounted for, introduces bias into the exposure-disease relation. The main reason why randomized trials are considered superior in inferring causality is that randomly assigning participants to treatment groups nullifies all sources of measured and unmeasured confounding, provided the sample size is large enough. To account for this type of bias in an observational study design such as a prospective cohort study, researchers must rely on their content knowledge to identify and adjust for all relevant confounders. Once data have been collected on these variables, the investigator can statistically adjust for confounders in a regression model or restrict the data to a specific subgroup to minimize residual confounding. A well-conducted cohort study can simulate a randomized trial when the most relevant confounders are accounted for (34). Sensitivity analysis further strengthens results by delineating the magnitude of unmeasured confounding needed to completely neutralize an effect. Moreover, a prospective design allows for up-to-date tracking of confounders and diminishes the threat of residual confounding because updated information may reduce measurement error in assessment of confounders, and additional information on confounders can be collected when needed.
Although there are several ways in which confounding can be accounted for in prospective cohort studies, the critical assumption of “no unmeasured or residual confounding” that is needed to infer causality cannot be empirically verified in observational epidemiology (34). For this reason, prospective cohort studies are often seen as providing statistical associations but not causations. This can be a dangerous premise to blindly adhere to, especially when randomized trials of hard endpoints are not feasible and policy decisions have to be made based on existing evidence. In this scenario, the Hill criteria, published in 1965 by Sir Austin Bradford Hill, are useful in inferring causality from observational data and making timely policy decisions that could avert preventable morbidity and mortality in the population (35). In his classic paper, Hill outlined a checklist of several key conditions for establishing causality: strength, consistency, temporality, biological gradient (dose-response), plausibility, coherence, and experimental evidence. These criteria have been satisfied in several exposure-disease relations such as sugar-sweetened beverages (SSBs) and diabetes (36), whole grains and cardiovascular disease (CVD) (37), and trans fats and CVD (38), which has resulted in timely public health action to reduce the burden of these diseases in the United States.
Given the complex nature of the human diet, another way of inferring causality is to consider different types of exposure (i.e., dietary patterns, foods, nutrients, and biomarkers) simultaneously (39, 40). For example, adherence to the Mediterranean-style diet has been known to confer a cardioprotective effect in observational studies and RCTs (41). Staples of the Mediterranean-style diet such as fish, nuts, olive oil, fruits, and vegetables have been individually linked to lower risk of heart disease (42–44). Reviews of major nutrients abundant in these foods, such as unsaturated fats and polyphenols, have confirmed this finding as well (45, 46), as have studies of related biomarkers (47, 48). Such a convergence among studies provides convincing support for adoption of the Mediterranean-style diet in prevention of CVD.
In the study of nutrition and health, prospective cohort studies aren’t the only sources of data used when considering causality. Evidence from animal studies, mechanistic studies in humans, prospective cohort studies of hard endpoints, and randomized trials of intermediate outcomes are taken together to arrive at a consensus. The inference of causality is strengthened when these different types of studies provide consistent evidence. For example, a meta-analysis of randomized trials of trans fat intake has shown harmful effects of higher intake on intermediate endpoints, such as increased LDL cholesterol and reduced HDL cholesterol (49). Based on observational studies, there is a significant and robust association between higher intake of trans fat and increased risk of ischemic heart disease (IHD) (49). Taken together, these studies forge a strong case for the harmful effects of trans fat consumption on heart disease. The most important aspect of this approach is that it discourages viewing individual studies and measures of association in isolation and encourages interpreting them in context of the larger evidence base.
Well-conducted prospective cohort studies thus can be used to infer causality with a high degree of certainty when randomized trials of hard endpoints are impractical. Researchers can minimize bias from confounding and other sources of bias by relying on high-quality study design, careful statistical analysis and interpretation, and replications of the findings across different populations. The Hill criteria are a useful tool for establishing causality in the absence of large RCTs on hard endpoints, and corroborating data from multiple study types and populations can enhance the weight of evidence.
Is the Drug Trial Paradigm Relevant in Investigating Diet and Disease Relationships?
Some researchers consider RCTs as the be-all and end-all of causal inference (2). This sentiment may be appropriate in the pharmaceutical industry, but the drug trial paradigm cannot be readily translated for use in the nutritional sciences. Table 2 summarizes the differences between observational prospective cohort studies and RCTs, and Table 3 outlines the differences between RCTs in pharmaceutical and nutritional research. Unlike classic drug trials, RCTs of dietary interventions typically cannot be blinded, leading to the possibility that the effect of the intervention is due to knowledge of treatment assignment as opposed to the dietary component of the intervention. Dropout rates also tend to be higher in RCTs of nutritional interventions relative to those in drug trials, especially if the intervention is implemented for long periods or is very demanding or both. Dietary interventions to promote weight loss routinely have dropout rates of 30–40% even after just 1 y of follow-up (50, 51). Such dropout, when substantial, will reduce analytical power in the presence of random loss to follow-up. However, it can also introduce systematic bias in the effect estimate, usually in unpredictable directions, if the dropout is differential with respect to treatment and outcome (52). Another problem faced to a greater extent in nutritional RCTs relative to drug trials is noncompliance, i.e., insufficient adherence by participants to their assigned intervention. Such noncompliance may become severe in trials of longer duration. For example, over 8 y of follow-up in the Women’s Health Initiative (WHI), the most expensive human study ever conducted, most of the participants randomly assigned to the low-fat group were unable to achieve their fat reduction target of 20% (53). Moreover, during the trial, there were no differences between the low-fat and control groups in plasma concentrations of HDL cholesterol and triglycerides, which are known to change on low-fat diets (53). The WHI, hence, failed to test its original hypothesis, and its null findings were largely uninformative with respect to the causal effect of a low-fat dietary intervention. A similar fate afflicted the earlier Multiple Risk Factor Intervention Trial, in which reduction of serum cholesterol by dietary means failed to produce a substantial contrast between intervention and control groups and thus no significant difference in CVD was seen (54). Randomized trials of nutritional interventions, although free from confounding and selection bias at baseline, can suffer from similar biases postbaseline that we often observe in observational studies, especially when they are of long duration, complicating their interpretation and diminishing their utility above prospective cohort studies. An additional issue with trials that last for extended periods of time is that they risk becoming obsolete. During the early 1990s, the WHI was set up to test the prevailing theory that reduced fat intake would decrease breast cancer and CVD risk. However, during the course of the trial, clinical and epidemiologic evidence that had accumulated to support greater importance of types of fat over total fat substantially weakened the original rationale, especially with respect to CVD (55).
TABLE 2.
Prospective cohort studies vs. RCTs of foods and nutrients1
| Design criterion | Prospective cohort study | RCT |
| Study characteristics | ||
| Size | Variable, usually fairly large | Usually small |
| Follow-up time | Years and decades | Weeks, months, a couple of years |
| Dietary exposure | Naturally occurring distributions | Specific predefined intervention and control |
| Endpoint | Mortality, incident disease, intermediate outcomes | Disease prognosis and management, intermediate outcomes |
| Sources of bias | ||
| Confounding | Residual and/or unmeasured confounding possible | No confounding if groups are balanced |
| Selection bias | Possible because of differential dropout | Possible because of differential dropout |
| Measurement error | Possible because of error in diet assessment methods | Possible because of incomplete compliance |
| Generalizability | Potential for high generalizability | Usually fairly limited |
| Feasibility | Usually more feasible | Feasibility limited by costs and ethical constraints |
RCT, randomized controlled trial.
TABLE 3.
RCTs of nutrients or foods vs. drugs1
| Design criterion | RCTs of nutrients or foods | RCTs of drugs |
| Exposure | Complex, interacting network of foods/nutrients | Individual, isolated chemical compounds |
| Choice of control | Variable, dependent on prior studies, feasibility, and ethicality | Placebo |
| Endpoint | Disease prognosis and management, intermediate outcomes | Clinical events, adverse events |
| Dropout rate | Moderate to high depending on length and type of intervention | Low to moderate |
| Blinding | Not possible with foods and dietary patterns | Easy |
| Compliance | Often decreases substantially over long period of follow-up | High to moderate over follow-up |
RCT, randomized controlled trial.
The choice of a control group can also be more complicated in nutritional intervention trials relative to drug trials because the latter are usually placebo-controlled, which is rarely a possibility in the former because of ethical considerations. Thus, the intervention and control groups are differentiated in terms of the dose of a nutrient (“high” vs. “low”), and the definition of these doses is also usually determined by ethical constraints. For instance, it may not be ethically feasible to give a very low dose to, or introduce deficiency in, the control group. One way of circumventing this is to conduct a trial in a naturally deficient population by providing supplementation to the intervention group. Even in this instance, it is not ethically feasible to provide the control group with just a placebo, and a minimum level of supplementation in the control group is usually necessary. This could result in too narrow a contrast in nutrient intake between the control and intervention groups, undermining the trial’s ability to identify a true effect of the nutrient.
Another factor further complicating the choice of a control group is that nutrients and foods are not consumed in isolation, and decreasing the intake of one nutrient/food usually entails increasing the intake of another nutrient/food to make up the reduction in calories in isocaloric trials (e.g., a reduction in dietary fat is often accompanied by a compensatory increase in carbohydrate intake or vice versa). Thus, the choice of comparison group can influence the effect observed of a dietary intervention, further complicating the interpretation of dietary intervention trials.
The utility of the drug trial paradigm in nutritional epidemiology is further diminished by the fact that the human diet is a complex system, not amenable to the reductionist approach of isolating individual nutrients or compounds (56). Although drugs usually target a single system/pathway in the pathology of disease, nutrients are usually pan-systemic, influencing multiple systems and affecting disease risk through multiple pathways. Unlike drugs, which are designed to have large and targeted effects on individual pathways that play out in relatively short periods of time, individual nutrients usually have modest effects that interact with and aggregate across multiple nutrients and systems over long periods of time to cumulatively affect disease risk. In drug trials, other drugs with known interactions are excluded or controlled for in some manner; in nutritional intervention trials, synergies and antagonisms with other nutrients and drugs are usually not given adequate consideration. High-dose vitamin and antioxidant trials mimic drug trials that examine the effect of isolated compounds, but these findings have been largely negative (57), which could be explained in part by the inappropriate use of the linear drug trial paradigm, which fails to take into account the complex, interconnected nature of the dietary “exposome” (58).
Well-conducted RCTs and observational studies that answer similar questions tend to find consistent results (59). However, when RCTs contradict the findings of observational studies, there is a tendency for the academic community to believe that RCTs conclusively refute the hypotheses generated by such observational studies. Although this is a possibility, an equally, and sometimes more likely, possibility is that the RCT and observational studies are answering very different questions. For example, an observational study might answer the question “are some persons in a population at increased risk because of low intake of nutrient X?,” whereas an RCT might answer the question “does adding more nutrient X to the whole population reduce risk?” In this example, the latter would not necessarily refute the former. In fields like nutritional and chronic disease epidemiology, RCTs typically cannot answer the questions that observational cohorts do answer. RCTs tend to have several exclusion criteria, and with diseases like CVD as the outcome, tend to be carried out in high-risk populations. Observational studies tend to be more inclusive and hence more representative of the general population. Additionally, although observational studies can evaluate any exposure contrast that is observed, RCTs usually examine a narrow exposure distribution that is feasible within ethical and practical constraints.
Owing to these limitations, most randomized trials are small (<200 subjects) and test for intermediate endpoints. One exception is Prevención con Dieta Mediterránea (60). In this landmark trial, the investigators tested for primary CVD events in 7447 men and women. After a median follow-up of 4.8 y, a Mediterranean diet was superior to a low-fat diet in reducing CVD incidence. It should be noted that the Prevención con Dieta Mediterránea was built on prior observational evidence from both ecologic and prospective cohort data that supported the benefits of the Mediterranean diet. Furthermore, the investigators provided free foods to its participants to ensure high compliance, a creative but often infeasible strategy in large nutritional intervention trials. Thus, it is important to remember that although large, well-designed RCTs are desirable when feasible, the field of nutritional epidemiology cannot solely depend on them, especially when testing individual components of a dietary pattern. A large dietary intervention study, carried out with use of considerable funds, and over many years, tends to address only one question and only one exposure contrast within that question. Hence, the question that it answers needs to be carefully thought through because not examining the most etiologically relevant range of the exposure distribution will lead to potentially misleading results. Arriving at the right question to pose with use of an RCT needs valid observational data. The two, thus, go hand-in-hand, with their strengths and limitations complementing each other.
For these reasons, the drug trial paradigm is usually not appropriate for investigating diet and disease relations and, when attempted, can lead to misleading results. Instead, a holistic research approach is likely to be more informative (61). A reductionist view that one compound brings about one physiologic effect ignores the intricacies of physiologic interactions. Foods cannot be treated as drugs, and a broader biological perspective will be necessary to effectively address nutritional questions in the future. Finally, nutritional epidemiology is not the only field where RCTs can be misleading. Although few would now question the benefits of smoking cessation, RCTs examining this issue have found no effect on mortality, probably because of recidivism and insufficient follow-up time (62). This is sobering considering that smoking is one of the most powerful risk factors known, and diet and other behaviorally related risk factors such as physical activity tend to have more subtle effects on disease outcomes.
Can We Trust the Findings from Meta-Analyses and Systematic Reviews of Nutritional Epidemiologic Studies?
Systematic reviews and meta-analyses have been instrumental in advancing the field and informing policy. Because meta-analyses and systematic reviews synthesize aggregate data, they are regarded by some to be the most authoritative form of available evidence (63, 64). Although they can be extremely useful in summarizing relevant literature, they are not immune to limitations. Publication bias, the tendency for null findings to remain unpublished, is a common difficulty in such analyses, although Egger’s test (65) and trim-and-fill techniques (66) can be used to detect and correct for bias. Substantial between-study heterogeneity (e.g., different exposures, outcomes, study populations, etc.) presents another major obstacle to pooling and interpreting effect estimates, although the presence of statistical heterogeneity does not necessarily invalidate the pooled results. Several methods have been devised to deal with this problem. First, if heterogeneity (e.g., varying methods for outcome ascertainment across studies) is so severe such that a statistical synthesis of effect estimates is meaningless, then a systematic review and qualitative description of study results is more appropriate. Second, one may choose to subset studies by specific characteristics (e.g., cohorts consisting only of women and only of men) to explore sources of heterogeneity. Third, one can use a random-effects model rather than a fixed-effects model, which assumes that reported effect estimates do not converge on the same value (67). However, this solution introduces another complication. Random-effects models tend to give more weight to and thus exaggerate the importance of smaller studies, leading to potentially distorted pooled-effect estimates and wider CIs. Lastly, a meta-analysis is only as good as the studies it pools together. If the methodologic quality of included studies is low, the meta-analysis can result in a misleading pooled effect estimate. Thus, it is important to give adequate consideration to the quality of included studies and either exclude lower-quality studies from the analysis or report pooled effects separately for higher-quality studies, and discuss the impact of study quality on the pooled results.
One must exercise care when interpreting a systematic review or meta-analysis in nutritional epidemiology. The number of systematic reviews and meta-analyses has exploded in recent years. A search on PubMed for “systematic review” and “meta-analysis” retrieves 4374 hits published from 2000 to 2004, 11,597 hits from 2005 to 2009, and 21,420 hits from 2010 to 2014. The rush to fill in gaps in the literature has also led to sloppy reviews that would benefit from a more thorough evaluation. Furthermore, because a review does not require original data, anyone could potentially write one; unlike the conduct of original studies, for which the qualifications of the investigators and the research plan are carefully vetted in the funding process. Too often, articles appear to have been pushed to publication by authors who do not fully appreciate the complexity of the subject. Lack of content knowledge and insufficient understanding of underlying biological mechanisms can lead to incomplete literature searches, flawed analytic methods, and misleading conclusions.
Some of the methodologic problems were evident in a recent meta-analysis of BMI and mortality, which concluded that being overweight lowers the risk of all-cause mortality than being normal weight (4). However, this finding is at least partly explained by reverse causation because individuals with existing or even preclinical chronic diseases such as cancer and neurodegenerative diseases often experience weight loss long before death (68, 69). Excluding sick individuals (70) or deaths from the first several years of follow-up (71) yields reasonably unbiased estimates that confirm a BMI range of 22–24.9 kg/m2 as having the lowest rates of all-cause mortality. The BMI-mortality analyses were also severely distorted by smoking, which is strongly associated with death but lower body weight (72), and restriction of analysis to never-smokers shifts the point of lowest mortality to the normal weight range (73).
Another example is a recent meta-analysis that concluded that types of fat including saturated or polyunsaturated fats have no substantial impact on heart disease, a conclusion stemming in part from an inadequate understanding of nutritional epidemiologic methods (5). It has been long known in clinical nutrition and nutritional epidemiology that, when examining the effects of macronutrients in an isocaloric manner, one must be cognizant of which other macronutrient is replacing the one in question. In the example of saturated fat, assuming diets are isocaloric, a diet high in saturated fats must be replacing another nutrient to maintain the same energy intake. At the population level, most of an individual’s energy is derived from carbohydrates (74, 75), which implies that those who consume high amounts of saturated fats are most likely replacing carbohydrates. Substituting carbohydrates for saturated fats, however, does not substantially alter the risk of heart disease (76). Refined carbohydrates, in particular, may be more harmful than saturated fats in terms of metabolic effects (77). However, substituting polyunsaturated fats for saturated fats substantially decreases the incidence of heart disease (76). The conclusion, therefore, should not be that saturated fat has no impact on heart disease, but that saturated fat is not substantially better or worse than carbohydrates at reducing heart disease.
Thus, although systematic reviews and meta-analyses are useful tools for summarizing a large body of evidence, it is important to recognize their limitations, give adequate consideration to sources of heterogeneity or bias, and consider their conclusions in the context of other relevant literature.
What Is the Role of Nutritional Epidemiologic Studies in Developing Policies?
In quality rating the strength of evidence, national organizations such as the American Diabetes Association, AHA, American College of Cardiology, US Preventive Services Task Force, and FDA have used well-defined grading systems to assign the weight of evidence to particular study types (78–81). Properly conducted RCTs with disease endpoints occupy the top tier of evidence in most scales, and prospective cohort studies reside one level below RCTs. In the absence of large RCTs with disease endpoints, evidence from prospective cohort studies in conjunction with smaller RCTs with intermediate endpoints is often considered in substantiating nutritional claims or establishing policies. For instance, the USDA/US Department of Health and Human Services Dietary Guideline Advisory Committee has used evidence from prospective cohort studies extensively, in addition to evidence from RCTs, to evaluate the relations between specific dietary factors and chronic disease risk (82), which forms one of the foundations for making dietary recommendations for the US population. Three examples of epidemiologic data affecting policy change are discussed below.
Folate was first identified as an important nutrient in preventing neural tube defects (NTDs), such as anencephaly and spina bifida, based on observational data from the United Kingdom in the 1970s (83). Subsequent case-control studies in the 1980s suggested that women who supplemented with folate before pregnancy had a reduced risk of giving birth to infants with NTDs than those who did not (84–87). Only 1 prospective cohort study was conducted at this time, and the results also supported a strong protective effect of periconceptual folate supplementation (88). Observational studies examining blood folate concentrations (89) and randomized trials of folate supplementation (90, 91) confirmed this association. Calls began for government action with commentators proposing several strategies including the promotion of increased fruit and vegetable consumption, recommendation of supplementation to reproductive-aged women, and fortification (92–95). In 1996, the FDA authorized the enrichment of grain products with folate at the rate of 0.14 mg per 100-g flour, with mandatory compliance by 1998. Fortification had a dramatic effect, leading to a 19% reduction in incident NTDs in just 5 y (96).
Numerous reports have linked SSBs, which include soda, sports drinks, and sweetened juices, with obesity (97, 98), type 2 diabetes (99), and heart disease (100). In addition, 2 large and rigorously conducted RCTs provided convincing evidence that decreasing consumption of SSBs substantially reduces excess weight gain and obesity in children and adolescents (101, 102). Calls have been made for policies to reduce consumption of SSBs. In the United States, one popular proposal has been an excise tax on SSBs. Brownell and colleagues (103) estimate that an increase of 1 cent per ounce would lead to a minimum intake reduction of 10%. Such a tax would not only discourage unhealthy diet, it would also generate revenue and offset health care costs incurred by chronic diseases associated with refined sugar intake (104). In 2013 Mexico approved a 1 peso per liter tax on SSBs (105), and sales volumes for SSBs fell by 5% in early 2014 (106). That same year, Mayor Michael Bloomberg led efforts to instate a size limit of 16 oz. on soft drinks sold in New York City. The proposal was passed by the city but was later struck down by the state supreme court (107). Other cities such as San Francisco have proposed excise taxes on SSBs, but opposition from soda and sugar lobbyists remains stiff. Nevertheless, certain developments, such as the removal of SSBs from most schools in the United States (108) and a ban on their sale in all public buildings in Boston (109), reflect major steps in reducing consumption of SSBs.
A highly successful example of nutritional epidemiology affecting policy is that of trans FAs (TFAs). TFAs were first developed to stabilize vegetable fat at room temperature and quickly became popular in food manufacturing processes in the early 20th century. Ancel Keys speculated that TFAs were associated with heart disease in the 1950s (110, 111), but it wasn’t until the 1990s that experimental evidence suggested that TFAs both increase LDL cholesterol and decrease HDL cholesterol (112). Subsequent epidemiologic evidence from the Nurses’ Health Study corroborated this link (113). As more evidence mounted, scientists called for a reduction of TFAs in foods (114), and in 2003, the FDA approved a proposal for manufacturers to list TFAs in the nutrition facts label of foods (115). In 2006 a meta-analysis of observational studies estimated that replacing 2% of energy from carbohydrates with the same amount of energy from TFAs would result in a 29% (95% CI: 11%, 49%) increase in IHD risk (116). A follow-up review combined both observational and experimental findings and provided convincing evidence of a causal link between TFAs and IHD (38). New York City and other city and state governments have banned use of trans fats in restaurants and schools (117). It is now estimated that ∼72% of trans fats have been removed from the US food supply (118), and as expected, blood lipids have improved in both children and adults (119). In a recent analysis within New York State, rates of IHD declined faster in municipalities that banned trans fat than in those that did not, and it is estimated that this prevented between 518 and 1037 deaths from IHD per year in New York State (120). It is estimated that a nationwide trans fat ban, by removing the remaining partially hydrogenated fats, would prevent between 6480 and 12,960 deaths from IHD annually in the United States (120). In 2013 the FDA took preliminary steps to phase out TFAs altogether by deeming them as not generally recognized as safe (121).
These 3 examples recount changes in public policies in the face of mounting evidence from multiple lines of research, and well-conducted observational studies have played a major role in shaping the policies. However, research is a slow and evolving process, and consensus is hard to achieve within a short timeframe. For substances that have shown potential harmful effects, even in the absence of conclusive evidence, public health experts have sometimes appealed to the precautionary principle to address the concern. The principle states that when only limited evidence is available, a substance is presumed to be harmful and the burden of proof falls upon the scientific community or the industry to demonstrate that the substance is safe. Observational data are especially important in this regard because they often present the first reports of negative health outcomes associated with certain dietary or other environmental exposures. They are also usually the first to show lack of hypothesized harm, e.g., in the cases of total fat in the diet and ω-6 FA intake, and thus can play a key role in resolving ongoing controversies (45, 122).
Conclusions
Nutritional epidemiology is far from being a perfect science, but with a thorough understanding of the discipline, valuable insights on diet and health outcomes can be obtained from free-living populations. Use of repeated measures and validated FFQs is critical for the assessment of long-term dietary intake. Although measurement error is a key problem, careful study design and meticulously developed dietary assessment tools coupled with specific biomarkers and informed statistical analysis can reduce its impact. Well-conducted RCTs can eliminate confounding and selection bias at baseline and are hence considered the highest level of evidence to infer causality; however, the linear drug trial paradigm cannot be directly translated into nutritional research, in which change in one dietary component is typically accompanied by compensatory change in another component, and good compliance is often difficult to achieve in long-term studies. Thus, prospective cohort studies, which are considered the strongest observational study design when well designed, are an irreplaceable component of nutritional research. Evidence from several types of studies, in particular prospective cohort studies of hard clinical endpoints and intervention trials of intermediate outcomes, in totality can be used to infer causality and inform policy. Summarizing this evidence in systematic reviews and meta-analyses can be especially helpful in understanding a vast evidence base and informing policy. However, meta-analyses and systematic reviews should be conducted with caution and interpreted in light of the broader context of the field.
Although the future of nutritional epidemiology is bright, with large ongoing international cohorts around the world [e.g., EPIC (123) and UK Biobank (124)] collecting detailed data on diet and lifestyle, which will help advance nutritional epidemiologic methods and contribute to the understanding of diet and disease relations, it also faces many challenges. The scientific community must consider several emerging issues. First, diets are evolving, and researchers will have to adapt to the changing food, societal, and cultural landscapes by developing new approaches to assessing dietary changes in large and diverse populations. There is an increasing need to adopt the socioecologic model to address the widening food quality gap between the rich and the poor and to reduce health disparities that are associated with obesity and poor diet quality (125, 126). Second, the globalization of the food systems and chronic diseases signifies the need for greater focus on low and middle income countries, where nutrition and epidemiologic transitions are occurring at an unprecedented pace, in parallel with rapid economic development and urbanization. Third, the environmental impact of food consumption and production should be considered when formulating dietary guidelines and agricultural policies. Fourth, there is a need to incorporate a life course approach into nutritional epidemiology (127), studying the effects of nutrition over the entire lifecycle, from gestation to adult life, which would go beyond the prevailing practice of initiating studies in midlife and carrying out randomized trials for extremely short durations.
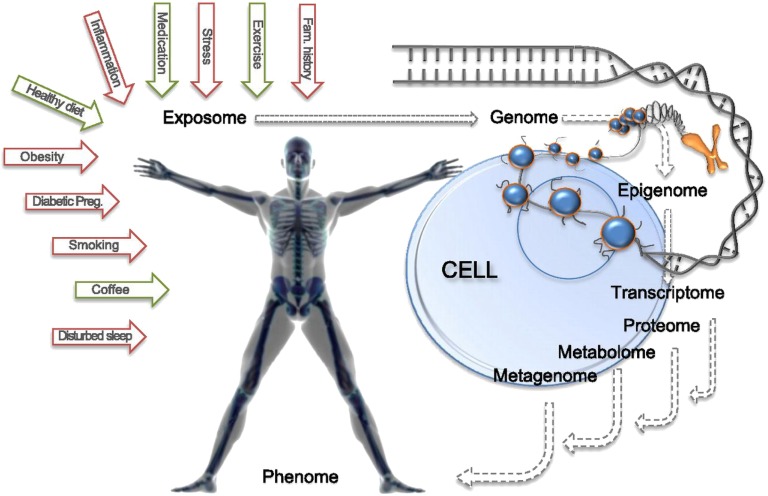
Lastly, nutritional epidemiology can benefit considerably from the incorporation of recent developments in “omics” technology, which although having its own challenges, holds promise for advancing the field (128–130). “Omics” technology refers to a collection of high-throughput methods for assessing a large number of genomic, epigenomic, transcriptomic, proteomic, and metabolomic traits from biological specimens. The integration of such technology into traditional nutritional epidemiology, or adopting a “systems epidemiology” approach (see Figure 2), can strengthen study designs and provide additional insights on mechanistic pathways, which the traditional epidemiologic approach is often unable to do. All of these areas require an interdisciplinary approach in which nutritional epidemiologic studies will continue to play an indispensable role.
FIGURE 2.
A future direction in nutritional epidemiologic research: a systems epidemiology approach to the discovery of interactions between the exposome (all nongenetic elements to which we are exposed) and the quantifiable elements of the human physiome [Reproduced with permission from Franks et al. (130)].
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DLW, doubly labeled water; IHD, ischemic heart disease; NTD, neural tube defect; RCT, randomized controlled trial; SSB, sugar-sweetened beverage; TFA, trans FA; WHI, Women’s Health Initiative.
References
- 1.Taubes G. Epidemiology faces its limits. Science 1995;269:164–9. [DOI] [PubMed] [Google Scholar]
- 2.Ioannidis JP. Implausible results in human nutrition research. BMJ 2013;347:f6698. [DOI] [PubMed] [Google Scholar]
- 3.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS ONE 2013;8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 6.Bittman M. Butter is back. The New York Times 2014 March 25.
- 7.Willett W. Nutritional epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 8.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The U.S. Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 9.Bingham SACA, Cole TJ, Welch A, Runswick SA, Black AE, Thurnham D, Bates C, Khaw KT, Key TJ, Day NE. Methods for data collection at an individual level. In: Cameron M, Staverene WV, editors. Manual on methodology for food consumption studies. New York: Oxford University Press; 1988. p. 53–106. [Google Scholar]
- 10.Pekkarinen M. Methodology in the collection of food consumption data. World Rev Nutr Diet 1970;12:145–71. [DOI] [PubMed] [Google Scholar]
- 11.Hebert JR, Hurley TG, Chiriboga DE, Barone J. A comparison of selected nutrient intakes derived from three diet assessment methods used in a low-fat maintenance trial. Public Health Nutr 1998;1:207–14. [DOI] [PubMed] [Google Scholar]
- 12.De Keyzer W, Huybrechts I, De Vriendt V, Vandevijvere S, Slimani N, Van Oyen H, De Henauw S. Repeated 24-hour recalls versus dietary records for estimating nutrient intakes in a national food consumption survey. Food Nutr Res 2011;55:doi: 10.3402/fnr.v55i0.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hébert JR, Hurley TG, Steck SE, Miller DR, Tabung FK, Peterson KE, Kushi LH, Frongillo EA. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv Nutr 2014;5:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guolo A. Robust techniques for measurement error correction: a review. Stat Methods Med Res 2008;17:555–80. [DOI] [PubMed] [Google Scholar]
- 15.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol 1995;24:389–98. [DOI] [PubMed] [Google Scholar]
- 16.Hebert JR, Ma Y, Clemow L, Ockene IS, Saperia G, Stanek EJ, 3rd, Merriam PA, Ockene JK. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol 1997;146:1046–55. [DOI] [PubMed] [Google Scholar]
- 17.Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L. Systematic errors in middle-aged women's estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol 2002;12:577–86. [DOI] [PubMed] [Google Scholar]
- 18.Preis SR, Spiegelman D, Zhao BB, Moshfegh A, Baer DJ, Willett WC. Application of a repeat-measure biomarker measurement error model to 2 validation studies: examination of the effect of within-person variation in biomarker measurements. Am J Epidemiol 2011;173:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for measurement error: the case of multiple covariates measured with error. Am J Epidemiol 1990;132:734–45. [DOI] [PubMed] [Google Scholar]
- 20.Spiegelman D, Zhao B, Kim J. Correlated errors in biased surrogates: study designs and methods for measurement error correction. Stat Med 2005;24:1657–82. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Stampfer MJ, Underwood BA, Speizer FE, Rosner B, Hennekens CH. Validation of a dietary questionnaire with plasma carotenoid and alpha-tocopherol levels. Am J Clin Nutr 1983;38:631–9. [DOI] [PubMed] [Google Scholar]
- 22.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr 1992;122:1792–801. [DOI] [PubMed] [Google Scholar]
- 23.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc 2004;104:595–603. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari P, Friedenreich C, Matthews CE. The role of measurement error in estimating levels of physical activity. Am J Epidemiol 2007;166:832–40. [DOI] [PubMed] [Google Scholar]
- 25.Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, Hebert JR. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol 2005;161:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archer E, Hand GA, Hebert JR, Lau EY, Wang X, Shook RP, Fayad R, Lavie CJ, Blair SN. Validation of a novel protocol for calculating estimated energy requirements and average daily physical activity ratio for the US population: 2005–2006. Mayo Clin Proc 2013;88:1398–407. [DOI] [PubMed] [Google Scholar]
- 27.Archer E, Shook RP, Thomas DM, Church TS, Katzmarzyk PT, Hebert JR, McIver KL, Hand GA, Lavie CJ, Blair SN. 45-Year trends in women's use of time and household management energy expenditure. PLoS ONE 2013;8:e56620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med 2014;174:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires—a review. Public Health Nutr 2002;5:567–87. [DOI] [PubMed] [Google Scholar]
- 30.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 31.Kaaks RJ. Biochemical markers as additional measurements in studies of the accuracy of dietary questionnaire measurements: conceptual issues. Am J Clin Nutr 1997;65(Suppl 4):1232S–9S. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E, Goldin B. The role of fat, fatty acids, and total energy intake in the etiology of human colon cancer. Am J Clin Nutr 1997;66(Suppl 6):1564S–71S. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E, Stampfer MJ, Colditz GA, Manson JE, Rosner BA, Longnecker M, Speizer FE, Willett WC. A comparison of prospective and retrospective assessments of diet in the study of breast cancer. Am J Epidemiol 1993;137:502–11. [DOI] [PubMed] [Google Scholar]
- 34.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006;60:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep 2012;12:195–203. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs DR, Jr, Gallaher DD. Whole grain intake and cardiovascular disease: a review. Curr Atheroscler Rep 2004;6:415–23. [DOI] [PubMed] [Google Scholar]
- 38.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 2009;63(Suppl 2):S5–21. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs DR, Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr 2009;89:1543S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs DR., Jr What comes first: the food or the nutrient? Executive summary of a symposium. J Nutr 2014;144(Suppl 4):543S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, Stampfer MJ. Nut consumption and risk of coronary heart disease: a review of epidemiologic evidence. Curr Atheroscler Rep 1999;1:204–9. [DOI] [PubMed] [Google Scholar]
- 43.Katan MB, Zock PL, Mensink RP. Dietary oils, serum lipoproteins, and coronary heart disease. Am J Clin Nutr 1995;61(Suppl 6):1368S–73S. [DOI] [PubMed] [Google Scholar]
- 44.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 2006;136:2588–93. [DOI] [PubMed] [Google Scholar]
- 45.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 2001;20:5–19. [DOI] [PubMed] [Google Scholar]
- 46.Quiñones M, Miguel M, Aleixandre A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol Res 2013;68:125–31. [DOI] [PubMed] [Google Scholar]
- 47.Bredsdorff L, Obel T, Dethlefsen C, Tjonneland A, Schmidt EB, Rasmussen SE, Overvad K. Urinary flavonoid excretion and risk of acute coronary syndrome in a nested case-control study. Am J Clin Nutr 2013;98:209–16. [DOI] [PubMed] [Google Scholar]
- 48.Joensen AM, Overvad K, Dethlefsen C, Johnsen SP, Tjonneland A, Rasmussen LH, Schmidt EB. Marine n-3 polyunsaturated fatty acids in adipose tissue and the risk of acute coronary syndrome. Circulation 2011;124:1232–8. [DOI] [PubMed] [Google Scholar]
- 49.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009;63(Suppl 2):S22–33. [DOI] [PubMed] [Google Scholar]
- 50.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53. [DOI] [PubMed] [Google Scholar]
- 51.Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 2004;140:778–85. [DOI] [PubMed] [Google Scholar]
- 52.Rubin DB. Inference and missing data. Biometrika 1976;63:581–92. [Google Scholar]
- 53.Willett WC. The WHI joins MRFIT: a revealing look beneath the covers. Am J Clin Nutr 2010;91:829–30. [DOI] [PubMed] [Google Scholar]
- 54.Willett WC, Stampfer MJ. Dietary fat and cancer: another view. Cancer Causes Control 1990;1:103–9. [Google Scholar]
- 55.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–78. [DOI] [PubMed] [Google Scholar]
- 56.Blumberg J, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH. Evidence-based criteria in the nutritional context. Nutr Rev 2010;68:478–84. [DOI] [PubMed] [Google Scholar]
- 57.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol 2008;101:14D–9D. [DOI] [PubMed] [Google Scholar]
- 58.Wiseman MJ, Jackson AA. Interpreting nutrition research – more complex than apparent plausibility. Electronic response to Ioannidis JP, Implausible results in human nutrition research. BMJ 2013;347:f6698. [DOI] [PubMed] [Google Scholar]
- 59.Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014;4:MR000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 61.Fardet A, Rock E. Toward a new philosophy of preventive nutrition: from a reductionist to a holistic paradigm to improve nutritional recommendations. Adv Nutr 2014;5:430–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rose G, Hamilton PJ. A randomised controlled trial of the effect on middle-aged men of advice to stop smoking. J Epidemiol Community Health 1978;32:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenhalgh T. Getting your bearings: what is this paper about? How to read a paper: the basics of evidence-based medicine. London: BMJ Books; 2014. p. 41. [Google Scholar]
- 64.Guyatt GH, Sackett DL, Sinclair JC, Hayward R, Cook DJ, Cook RJ. Users’ guides to the medical literature. IX. A method for grading health care recommendations. Evidence-Based Medicine Working Group. JAMA 1995;274:1800–4. [DOI] [PubMed] [Google Scholar]
- 65.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 67.Cooper HM, Hedges LV, Valentine JC. editors. The handbook of research synthesis and meta-analysis. 2nd ed New York: Russell Sage Foundation; 2009. [Google Scholar]
- 68.Andres R, Muller DC, Sorkin JD. Long-term effects of change in body weight on all-cause mortality. A review. Ann Intern Med 1993;119:737–43. [DOI] [PubMed] [Google Scholar]
- 69.Lee IM, Paffenbarger RS., Jr Is weight loss hazardous? Nutr Rev 1996;54:S116–24. [DOI] [PubMed] [Google Scholar]
- 70.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manson JE, Stampfer MJ, Hennekens CH, Willett WC. Body weight and longevity. A reassessment. JAMA 1987;257:353–8. [PubMed] [Google Scholar]
- 73.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med 1995;333:677–85. [DOI] [PubMed] [Google Scholar]
- 74.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr 2011;93:836–43. [DOI] [PubMed] [Google Scholar]
- 75.Vergnaud AC, Norat T, Mouw T, Romaguera D, May AM, Bueno-de-Mesquita HB, van der AD, Agudo A, Wareham N, Khaw KT, et al. Macronutrient composition of the diet and prospective weight change in participants of the EPIC-PANACEA study. PLoS ONE 2013;8:e57300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9. [DOI] [PubMed] [Google Scholar]
- 77.Hu FB. Are refined carbohydrates worse than saturated fat? Am J Clin Nutr 2010;91:1541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Introduction. Diabetes Care 2008;31(Suppl 1):S1–2. [DOI] [PubMed] [Google Scholar]
- 79.Silber S. A new and rapid scoring system to assess the scientific evidence from clinical trials. J Interv Cardiol 2006;19:485–92. [DOI] [PubMed] [Google Scholar]
- 80.U.S. Preventive Services Task Force [Internet]. Grade definitions [cited 2014 Aug 30]. Available from: http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm#post.
- 81.U.S. FDA [Internet]. Guidance for industry: evidence-based review system for the scientific evaluation of health claims—final [updated 2014 Jul 9; cited 2014 Aug 30]. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm073332.htm#system.
- 82.Spahn JM, Lyon JM, Altman JM, Blum-Kemelor DM, Essery EV, Fungwe TV, Macneil PC, McGrane MM, Obbagy JE, Wong YP. The systematic review methodology used to support the 2010 Dietary Guidelines Advisory Committee. J Am Diet Assoc 2011;111:520–3. [DOI] [PubMed] [Google Scholar]
- 83.Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, Harris R, Read AP, Fielding DW. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet 1980;1:339–40. [DOI] [PubMed] [Google Scholar]
- 84.Bower C, Stanley FJ. Dietary folate as a risk factor for neural-tube defects: evidence from a case-control study in Western Australia. Med J Aust 1989;150:613–9. [DOI] [PubMed] [Google Scholar]
- 85.Mulinare J, Cordero JF, Erickson JD, Berry RJ. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 1988;260:3141–5. [PubMed] [Google Scholar]
- 86.Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology 1995;6:219–26. [DOI] [PubMed] [Google Scholar]
- 87.Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993;269:1257–61. [PubMed] [Google Scholar]
- 88.Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ, Willett W. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989;262:2847–52. [DOI] [PubMed] [Google Scholar]
- 89.Wald NJ, Hackshaw AD, Stone R, Sourial NA. Blood folic acid and vitamin B12 in relation to neural tube defects. Br J Obstet Gynaecol 1996;103:319–24. [DOI] [PubMed] [Google Scholar]
- 90.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338:131–7. [PubMed] [Google Scholar]
- 91.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5. [DOI] [PubMed] [Google Scholar]
- 92.Beresford SA. How do we get enough folic acid to prevent some neural tube defects? Am J Public Health 1994;84:348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rayburn WF, Stanley JR, Garrett ME. Periconceptional folate intake and neural tube defects. J Am Coll Nutr 1996;15:121–5. [DOI] [PubMed] [Google Scholar]
- 94. Wald NJ. Folic acid and neural tube defects: the current evidence and implications for prevention. Ciba Found Symp 1994;181:192–208. [DOI] [PubMed]
- 95.Willett WC. Folic acid and neural tube defect: can't we come to closure? Am J Public Health 1992;82:666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001;285:2981–6. [DOI] [PubMed] [Google Scholar]
- 97.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 2007;97:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis 2014;234:11–6. [DOI] [PubMed] [Google Scholar]
- 101.Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–406. [DOI] [PubMed] [Google Scholar]
- 103.Brownell KD, Farley T, Willett WC, Popkin BM, Chaloupka FJ, Thompson JW, Ludwig DS. The public health and economic benefits of taxing sugar-sweetened beverages. N Engl J Med 2009;361:1599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brownell KD, Frieden TR. Ounces of prevention—the public policy case for taxes on sugared beverages. N Engl J Med 2009;360:1805–8. [DOI] [PubMed] [Google Scholar]
- 105.University of North Carolina at Chapel Hill Food Research Program [Internet]. Evaluation of 2014 SSB & non-essential foods taxes in Mexico [cited 2014 Aug 30]. Available from: http://uncfoodresearchprogram.web.unc.edu/projects/mexico-tax-eval/.
- 106.Guthrie A. Mexico soda tax dents Coke bottler’s sales. The Wall Street Journal 2014 Feb 26.
- 107.Bloomberg [Internet]. New York soda size limit statute barred by state judge [updated 2013 Mar 11; cited 2014 Aug 30]. Available from: http://www.bloomberg.com/news/2013-03-11/new-york-city-soda-size-limitations-barred-by-state-court-judge.html.
- 108.CDC. Competitive foods and beverages in U.S. schools: a state policy analysis. Atlanta, GA: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 109.CityofBoston.gov [Internet press release]. Mayor Menino issues order to end sugary drink sales on city property [updated 2011 Apr 7; cited 2014 Aug 30]. Available from: http://www.cityofboston.gov/news/default.aspx?id=5051.
- 110.Anderson JT, Grande F, Keys A. Essential fatty acids, degree of unsaturation, and effect of corn (maize) oil on the serum-cholesterol level in man. Lancet 1957;272:66–8. [DOI] [PubMed] [Google Scholar]
- 111.Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957;273:959–66. [DOI] [PubMed] [Google Scholar]
- 112.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1990;323:439–45. [DOI] [PubMed] [Google Scholar]
- 113.Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, Sampson LA, Hennekens CH. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993;341:581–5. [DOI] [PubMed] [Google Scholar]
- 114.Willett WC, Ascherio A. Trans fatty acids: are the effects only marginal? Am J Public Health 1994;84:722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.U.S. FDA [Internet]. Trans fat now listed with saturated fat and cholesterol [updated 2013 Dec 17; cited 2014 Aug 30]. Available from: http://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm274590.htm.
- 116.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601–13. [DOI] [PubMed] [Google Scholar]
- 117.Mello MM. New York City's war on fat. N Engl J Med 2009;360:2015–20. [DOI] [PubMed] [Google Scholar]
- 118.Dietz WH, Scanlon KS. Eliminating the use of partially hydrogenated oil in food production and preparation. JAMA 2012;308:143–4. [DOI] [PubMed] [Google Scholar]
- 119.Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA 2012;308:1545–54. [DOI] [PubMed] [Google Scholar]
- 120.Restrepo B, Rieger M. Trans fat and cardiovascular disease mortality: evidence from bans in restaurants in New York. Italy: European University Institute, Max Weber Programme; 2014. [DOI] [PubMed] [Google Scholar]
- 121.Federal Register [Internet]. Washington (DC): National Archives and Records Administration; 2013 [2014 Aug 19]. Available from: https://www.federalregister.gov/articles/2013/11/08/2013-26854/tentative-determination-regarding-partially-hydrogenated-oils-request-for-comments-and-for.
- 122.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7. [DOI] [PubMed] [Google Scholar]
- 123.International Agency for Research on Cancer [Internet]. EPIC Study [cited 2014 Aug 29]. Available from: http://epic.iarc.fr/.
- 124.UK Biobank [Internet] [cited 2014 Aug 29]. Available from: http://www.ukbiobank.ac.uk/.
- 125.Sallis JF, Owen N, Fisher EB. Ecological models of health behavior. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: theory, research, and practice. San Francisco, CA: Jossey-Bass; 2008. p. 465–86. [Google Scholar]
- 126.Brug J, Oenema A, Ferreira I. Theory, evidence and intervention mapping to improve behavior nutrition and physical activity interventions. Int J Behav Nutr Phys Act 2005;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 2002;31:285–93. [PubMed] [Google Scholar]
- 128.Hu FB. Metabolic profiling of diabetes: from black-box epidemiology to systems epidemiology. Clin Chem 2011;57:1224–6. [DOI] [PubMed] [Google Scholar]
- 129.Cornelis MC, Hu FB. Systems epidemiology: a new direction in nutrition and metabolic disease research. Curr Nutr Rep 2013;2:225–35. [DOI] [PMC free article] [PubMed]
- 130.Franks PW, Pearson E, Florez JC. Gene-environment and gene-treatment interactions in type 2 diabetes: progress, pitfalls, and prospects. Diabetes Care 2013;36:1413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]