Abstract
During the past several years, there has been enormous progress in the understanding of the causative factors that initiate neuronal damage in various neurodegenerative diseases, including Alzheimer disease, Parkinson disease, multiple sclerosis, amyotrophic lateral sclerosis, and Huntington disease. Preventing neuronal damage and neuronal death will have a huge clinical benefit. However, despite major advances in causative factors that trigger these neurodegenerative diseases, to date there have been no therapies available that benefit patients who suffer from these diseases. Because most neurodegenerative diseases are late-onset and remain asymptomatic for most of the phases, the therapies initiated in advanced stages of the disease have limited value to patients. It may be possible to prevent or halt the disease progression to a great extent if therapies start at the initial stage of the disease. Such therapies may restore neuronal function by reducing or even eliminating the primary stressor. Flavonoids are key compounds for the development of a new generation of therapeutic agents that are clinically effective in treating neurodegenerative diseases. Regular consumption of flavonoids has been associated with a reduced risk of neurodegenerative diseases. In addition to their antioxidant properties, these polyphenolic compounds exhibit neuroprotective properties by their interaction with cellular signaling pathways followed by transcription and translation that mediate cell function under both normal and pathologic conditions. This review focuses on human intervention studies as well as animal studies on the role of various flavonoids in the prevention of neurodegenerative diseases.
Keywords: flavonoids, bioactive compounds, neurodegenerative diseases, neuroprotection, mitochondria, oxidative stress, antioxidant, cellular signaling, cognitive functions
Introduction
Neurodegeneration is the slow and progressive loss of neuronal cells in specified regions of the brain and is the main pathologic feature of various neurodegenerative diseases such as Alzheimer disease (AD)3, Parkinson disease (PD), multiple sclerosis (MS), Huntington disease (HD), and amyotrophic lateral sclerosis (ALS) (1, 2). The main causes of neurodegeneration in these diseases, along with normal brain aging, are several cellular and molecular events such as oxidative stress, impaired mitochondrial function, deposition of aggregated proteins, neuroinflammation, and activation of apoptotic factors (2). Despite enormous progress in understanding the pathogenesis of neurodegenerative diseases, the treatment of most of those conditions are still obscure. Because, in most cases, neurodegenerative diseases begin very early in life and symptoms appear very late, early diagnosis and appropriate therapeutic intervention are necessary to stop the progression of disease and suffering of patients. Flavonoids are bioactive components that are derived from fruit and vegetables. Since ancient times, flavonoid- and nonflavonoid-rich nutraceuticals have been used as food supplements in improving cognitive function and in prevention of neurodegenerative diseases in humans (3). Flavonoid-enriched extracts should be given strong consideration as novel therapies to prevent neurodegenerative diseases because of their potential beneficial effects on human health. These flavonoids may be able to target multiple sites in the brain and prevent neurodegenerative diseases. In this review, we emphasize the protective and preventive functions of flavonoids in neurodegenerative diseases by modulation of neurosignaling pathways.
Flavonoids: Their Classification, Sources, and Brain Penetration
Flavonoids are a group of diverse, low-molecular-weight plant polyphenolic compounds. They possess unique biological properties that may be responsible for many health benefits. Flavonoids are natural products widely distributed in the plant kingdom, and >6000 types of flavonoids currently have been identified. Flavonoids may be divided into 6 subclasses on the basis of their structural variations. These subclasses include the following: 1) flavonols, 2) flavanols, 3) isoflavones, 4) anthocyanidins, 5) flavanones, and 6) flavones (4). The subclasses, their representative flavonoids, and their common food sources are described in Table 1.
TABLE 1.
Main groups of flavonoids, their representative flavonoids, and common sources
| Groups | Flavonoids | Common sources |
| Flavonols | Rutin | Leeks, onions, broccoli, kale, apples, cherries, berries, tea, red wine |
| Quercetin | ||
| Kaempherol | ||
| Myricetin | ||
| Flavanols | Catechin | Green tea, red wine, chocolate, apples |
| Epicatechin | ||
| Epigallocatechin | ||
| EGCG1 | ||
| Isoflavones | Genistein | Legumes, soybeans, soy products |
| Daidzein | ||
| Glycetin | ||
| Formanantine | ||
| Anthocyanidins | Cyanidin | Red wine, berry fruits, cherries, grapes |
| Malvidin | ||
| Pelargonidin | ||
| Delphinidin | ||
| Flavanones | Hesperetin | Citrus fruits, tomatoes |
| Naringenin | ||
| Isoxanthohumol | ||
| Taxifolin | ||
| Flavones | Apigenin | Parsley, celery |
| Luteolin |
EGCG, epigallocatechin gallate.
To understand whether flavonoids and their metabolic derivatives are capable of direct neuroprotective action, it is critical to establish if they are able to access the central nervous system. However, there is little information available on their ability to cross the blood-brain barrier (BBB) to access the central nervous system. In a model of brain endothelial cells, several flavonoids and their conjugated metabolites were found to penetrate the BBB (5). In particular, hesperetin and naringenin, and their in vivo metabolites, along with cyanidin-3-rutinoside and pelargonidin-3-glucoside were shown to cross the BBB (6). In most experiments, flavonoids are directed into the brain either by intravenous or by oral administration. Naringenin, when administered by intravenous route, was shown to enter the brain of animals (7). Similarly, epicatechin (8), anthocyanins (9), and epigallocatechin gallate (EGCG) (10) through oral administration were also found to enter the brain. Thus, these results show that flavonoids are able to cross the BBB and localize in the brain, suggesting that they are important candidates for direct neuroprotective and neuromodulatory actions.
Flavonoids: treatments in neurodegenerative diseases
Neurodegenerative diseases are characterized by different structural and pathologic conditions; thus, a variety of targets and more efficient methods are required for their treatment. Flavonoids exert beneficial effects on the body by affecting multiple cell systems. Flavonoids can modulate the activity of various metabolic pathways to reduce cognitive decline and neuronal dysfunction (11) and are thought to delay or even prevent the onset of neurodegenerative diseases at their effective doses or concentrations (Table 2). Here, we discuss the role of various flavonoids and their specific doses in the prevention or treatment of various neurodegenerative diseases.
TABLE 2.
Various flavonoids and their specific doses or concentrations in the prevention or protection of neuronal cells against neurodegenerative diseases
| Neurodegenerative diseases | Flavonoids | Doses | Reference |
| Alzheimer disease | Myricetin | 0.1–1 μM | (14) |
| Morin | 0.1–1 μM; 1 μM, 10 μM | (14, 29) | |
| Rutin | 0.1–1 μM | (15) | |
| Quercetin | 0.1–1 μM | (15) | |
| Fisetin | 25 mg/kg | (16) | |
| Ginkgo biloba extract | 10–100 μg/mL | (20) | |
| Genistein | 40 μM | (22) | |
| Kaempferol | 50 μM | (23) | |
| Apigenin | 50 μM | (23) | |
| Anthocyanin | 100 μM | (24) | |
| Glycitein | 100 μg/mL | (26) | |
| Parkinson disease | Quercetin | 50, 100, and 200 mg/kg body weight | (37) |
| Green tea catechin | 0.5 and 1 mg/kg | (40) | |
| Acacetin | 10 mg/kg per day | (41) | |
| Rutin | 25 mg/kg body weight | (43) | |
| EGCG1 | 2 and 10 mg/kg; 200 μM, 5 mg/kg | (40, 42) | |
| Genistein | 10 mg/kg | (44) | |
| Tangeretin | 10 and 20 mg/kg | (48) | |
| Huntington disease | EGCG | ∼1 μM; 10, 20 and 40 mg/kg | (54, 58) |
| Naringin | 80 mg/kg body weight | (55) | |
| Quercetin | 25 mg/kg body weight | (57) | |
| Eriodictyol | 100 μM | (59) | |
| Amyotrophic lateral sclerosis | EGCG | >2.9 μg/g body weight; 10 mg/kg | (61, 62) |
| Genistein | 16 mg/kg | (63) | |
| Multiple sclerosis | EGCG | 10 μg/mL | (67) |
| Luteolin | 20–80 μM | (68) | |
| Quercetin | 20–80 μM | (68) | |
| Fisetin | 20–80 μM | (68) |
EGCG, epigallocatechin gallate.
AD.
AD is a late-onset, progressive, age-dependent neurodegenerative disease that is mainly characterized by decline in memory, impairment of cognitive function, and irreversible loss of neurons, mainly in the cortex and hippocampus regions of brain (12). Neuropathologically, AD is measured by the presence of amyloid plaques, neuritic plaques, and neurofibrillary tangles. These plaques are mainly formed because of accumulation of amyloid β (Aβ) and abnormal concentrations of tau protein; thus, both of these proteins can be considered to be important biomarkers in the pathology of AD (13).
Recently, naturally occurring flavonoids were marked as potential candidates for the prevention and treatment of AD. As discussed earlier, AD is characterized by the presence of amyloid plaques, neuritic plaques, and neurofibrillary tangles, which are mainly formed due to accumulation of Aβ and abnormal tau concentrations. Thus, flavonoids that inhibit the formation of these plaque-forming factors can be used for the prevention of AD. Myricetin (0.1–1 μM) (14), rutin (0.1–1 μM) (15), and fisetin (25 mg/kg) (16) inhibited the formation and aggregation of Aβ fibrils at their effective concentrations. Cognitive function and learning ability in rats were found to be improved by long-term administration of EGCG and a preparation of green tea catechins (17). Green tea flavonoids can also protect against Aβ-induced cytotoxicity in primary rat cortical neurons (18). The neurotoxicity of Aβ is also increased by the presence of acetylcholinesterase. Thus, flavonoids present in green and white tea extracts can be used as acetylcholinesterase inhibitors in the treatment of AD (19). The neuroprotective potential of flavonoids in AD is shown not only in Aβ-induced neuronal death models but also in oxidative stress–induced neuronal death. Studies showed that flavonoids present in Ginkgo biloba extract (10–100 μg/mL) were able to protect hippocampal cells against Aβ peptides or oxidative stress–induced toxicities (20). Moreover, the neuroprotective effects of flavonoids such as EGCG (21) and genistein (40 μM) (22) were shown to be effective via scavenging of reactive oxygen species induced by Aβ. In addition, Aβ-mediated increases in reactive oxygen species production can be substantially lowered by preincubation of neuronal cells with 50 μM kaempferol and apigenin (23) and 100 μM anthocyanin in neuro-2a (N2a) neuroblastoma cells (24). Furthermore, oxidative stress was found to be lowered by rutin in SH-SY5Y neuroblastoma cells along with a reduction in malondialdehyde and glutathione disulfide formation (25). However, Aβ toxicity can also be reduced by the inhibition of Aβ deposition via the antioxidative activity of the soy isoflavone glycitein (100 μg/mL) (26). Apigenin ameliorates AD-associated learning and memory impairment via inhibiting Aβ deposition and decreasing insoluble Aβ concentrations, inhibiting oxidative stress, and improving the antioxidative enzyme activity of superoxide dismutase (SOD) and glutathione peroxidase (27). Another major plaque-forming factor in AD, abnormal concentrations of tau protein, have been shown to be neutralized by grape flavonoids (28). In addition, morin (1 and 10 μM) was also found to prevent neuronal apoptosis against tau hyperphosphorylation (29). In addition to flavonoids, nonflavonoid polyphenols also show neuroprotective effects in various neurodegenerative diseases including AD. Resveratrol extracted from grapes was found to reduce hippocampal neurodegeneration and learning impairment in an inducible p25 transgenic mouse model of AD (30). Curcumin, a nonflavonoid polyphenol found abundantly in Curcuma longa (turmeric), plays a beneficial role in patients with AD and in animal models of neurodegenerative disease. Curcumin was found to protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from Aβ-induced toxicity through its antioxidant activity (31). Phenolic acids constitute another family of nutraceuticals that protect the brain. These include rosmarinic and carnosic acid, which are found in rosemary and are also effective in protecting neuronal function. Rosmarinic acid was shown to protect memory impairment associated with Aβ neurotoxicity in mouse models of AD (32). Carnosic acid was also found to protect the hippocampus against Aβ-induced neurodegeneration in AD (33). Nonflavonoid organosulfur nutraceuticals, such as allicin (abundantly present in garlic), also play neuroprotective roles in a variety of neurodegenerative disease models. Garlic extract was found to protect against Aβ-induced neurocytotoxicity and inhibits caspase-3 activity, the executioner protease of the apoptotic cascade (34). These studies showed that flavonoids and other natural compounds have the ability to reduce oxidative stress and Aβ and tau toxicity and to inhibit apoptosis, thus showing therapeutic potential for prevention of or treatment for AD.
PD.
PD is the second most common progressive neurodegenerative disease and is characterized by slowness of movement (bradykinesia), resting tremors, rigidity, and postural instability. Along with these symptoms, individuals with PD also show autonomic, cognitive, and psychiatric disturbances. PD is caused by persistent degeneration of dopamine-producing neurons that project from the substantia nigra pars compacta region to the striatum, which controls voluntary movement (35). The pathogenesis of PD is characterized by the misfolding and aggregation of proteins (36) along with mitochondrial dysfunction and successive oxidative stress. Thus, in PD, agents such as flavonoids that can target oxidative stress and mitochondrial dysfunction can be prime candidates for neuroprotection.
Studies showed that flavonoids such as quercetin (50, 100, and 200 mg/kg body weight) markedly improved the motor balance and coordination in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; a parkinsonism-inducing neurotoxin)–treated mice, and significant increases were observed in the activities of various antioxidants such as glutathione peroxidase, SOD, and Na-K ATPase (37). In neurons, the administration of quercetin not only attenuated microglia activation (a precursor of PD pathogenesis) but also suppressed cell death (38). Studies have demonstrated that the consumption of green and black tea had beneficial effects in reducing the risk of PD (39). In another study, green tea extract (0.5 and 1 mg/kg) or isolated EGCG (2 and 10 mg/kg) prevented striatal dopamine depletion and loss of dopaminergic neuron in the substantia nigra of mice chronically treated with MPTP (40). In a recent study, acacetin (5,7-dihydroxy-4′-methoxyflavone), a constituent of a flavone naturally present in plants, also inhibited the degeneration of dopaminergic neurons and depletion of dopamine concentrations induced by MPTP toxicity in the substantia nigra and striatum of the brain at a dose of 10 mg/kg per day (41). PD caused by 6-hydroxydopamine (6-OHDA) was also shown to be prevented by EGCG (200 μM) in PC12 cells (42) or by rutin (25 mg/kg body weight) in male Wistar rats (43). 6-OHDA–induced toxicity and rotational behavior in lesioned rats were also found to be attenuated by genistein (10 mg/kg) (44). Luteolin (6–13 μM) increased cell survival and mitochondrial ATP levels in 3-hydroxykynurenine and 6-OHDA–induced neurotoxicity in human SH-SY5Y neuroblastoma cells and improved mitochondrial function (45). In another study, 1-methyl-4-phenylpyridinium ion [(MPP)+]-induced toxicity in mixed ventral mesencephalic cultures was significantly attenuated by quercetin in PD (46). Flavonoid-rich Ginkgo biloba extract also was found to protect against dopaminergic neurons in an animal model of PD (47). Furthermore, the citrus flavonoids, such as tangeretin (10 mg/kg per day for 28 d and 20 mg/kg per day for 4 d), were observed to maintain nigrostriatal integrity against 6-OHDA–induced neurotoxicity (48). Certain nonflavonoid nutraceuticals also revealed neuroprotective potential in PD models. Resveratrol functioned as an antioxidant and protected against 6-OHDA–induced toxicity in a rat model of PD (49). In MPTP-induced neurodegeneration, curcumin is neuroprotective and was shown to prevent glutathione depletion and lipid peroxidation in the nigrostriatal tract in mice (50). Rosmarinic acid was effective in protecting SH-SY5Y human neuroblastoma cells against hydrogen peroxide–induced oxidative stress and cell death (51). Nonflavonoid organosulfur nutraceutical compounds such as l-sulforaphane, which is abundantly found in broccoli and other cruciferous vegetables, were also shown to effectively protect against dopamine quinone–induced neuronal death (52). These results suggest that flavonoid and nonflavonoid nutraceuticals may serve as potential neuroprotective agents against the underlying pathology associated with PD.
HD.
HD is a genetic autosomal disease. It is clinically characterized by psychiatric disturbances, involuntary movements, progressive cognitive impairment, choreoathetosis, dementia, and premature death. Similarly, HD is pathologically characterized by selective degeneration of deep layers of the cerebral cortex and γ-aminobutyric acid–producing striatal neurons, resulting in a progressive atrophy of the caudate and putamen nucleus and globus pallidus (53). The genetic defect responsible for HD is the expansion of a cytosine adenine guanine (CAG) trinucleotide repeat in the Huntingtin gene. Because there is ongoing active research into potential therapies for the treatment or prevention of HD, some flavonoids are considered as specific therapies for HD.
Studies showed that various types of flavonoids such as EGCG (∼1 μM) (54) exhibited potential for prevention or treatment of the pathogenesis of HD. In addition, naringin (80 mg/kg body weight per day) (55), hesperidin (56), and quercetin (25 mg/kg body weight) (57) administration were also found to be preventive in 3-nitropropionic acid–induced HD. In another study, EGCG (10, 20, and 40 mg/kg) significantly improved memory function and restored glutathione system functioning in 3-nitropropionic acid–induced HD (58). Flavonoids present in citrus fruit such as eriodictyol (100 μM) increased the concentrations of intracellular glutathione and thus can also be used in the prevention of HD (59). These studies suggest that flavonoids can be used as a potential therapeutic agents in the prevention and treatment of chemically induced HD. Thus, if used in effective concentrations, they could also aid in the prevention of HD during normal disease progression.
ALS.
ALS is a fatal motor neuron disease characterized by the selective degeneration of the anterior horn cells of the spinal cord and cortical motor neurons, leading to muscle weakness and paralysis. Approximately 10% of cases of ALS are familial and caused by mutations in the copper-zinc SOD type 1 gene (60), although the majority of cases are sporadic. Because ALS is a multifactorial disease, bioactive compounds such as flavonoids that target more than one aspect are needed to fight this devastating disease.
With regard to the role of flavonoids in ALS, very few studies have been reported. In one study, EGCG (>2.9 μg/g body weight) was found to protect motor neurons in wild-type and G93A SOD1 mutant mice from oxidative stress–induced cytotoxicity in an ALS mouse model (61). In another study, EGCG (10 mg/kg) significantly increased the number of motor neurons, reduced microglial activation and NF-κB concentration, and reduced the concentration of inducible NO synthase (iNOS) (62). In addition, genistein (16 mg/kg) was also investigated as a prophylactic agent against ALS (63). Thus, studies show that flavonoids protect against the pathogenesis of ALS.
MS.
MS is a chronic disease of the central nervous system characterized by neurodegeneration, demyelination, and astroglial proliferation (64), affecting both white and gray matter of neuronal cells. Clinically, MS is characterized by relapsing-remitting phenotypes and neuropathologic manifestations in which the patient experiences clinical attacks causing neurologic dysfunction including optic neuritis and transverse myelitis. Neuropathologically, MS can be characterized by inflammation, demyelination, and axonal degeneration (65).
MS is a neuroinflammatory disease, and flavonoids are naturally occurring immunomodulatory compounds that can limit demyelination, reduce neuroinflammation, and downregulate immune function. Studies have shown that flavonoids such as luteolin (66) and EGCG (10 μg/mL) (67) provide neuroprotection by reducing neuroinflammation and axonal damage. In addition, flavonoids such as luteolin, quercetin, and fisetin at concentrations of 20–80 μM are able to decrease the amount of myelin phagocytosed by macrophages and thus can be preventive of MS (68). In MS, IL-1 is mainly responsible for T cell activation and TNF-α is mainly responsible for demyelination (69). Quercetin was found to control the immune response via control of IL-1 and TNF-α peripheral blood mononuclear cells isolated from patients with MS (70). The potential of flavonoids in limiting demyelination, reducing neuroinflammation, and downregulating immune function makes them prominent therapeutic agents in age-related MS.
Molecular mechanisms of neuroprotection by flavonoids
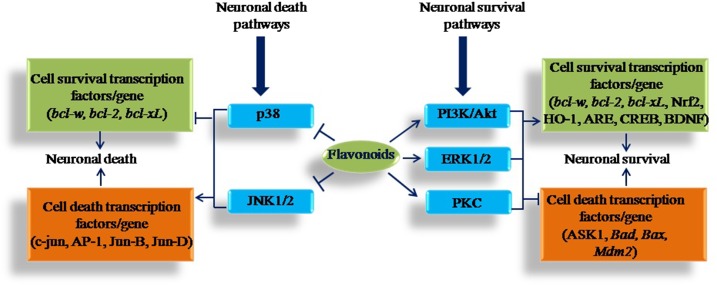
Flavonoids are known to provide neuroprotective effects by interacting with brain tissue at multiple sites. The neuroprotective action of dietary flavonoids includes their potential to protect neurons against oxidative stress and neuronal injury via their potential as antioxidants, an ability to suppress neuroinflammation, and the potential to modulate cell signaling pathways. Flavonoids are well-known antioxidants, and they may protect cell constituents against oxidative stress and therefore reduce the risk of neurodegenerative disease associated with oxidative stress. Flavonoids also protect neurons against some neurotoxic drugs whose toxicity is linked to the stimulation of oxidative stress. Various flavonoids, such as tangeretin (48), EGCG (42), genistein (44), and rutin (43), can attenuate 6-OHDA–induced neurotoxicity in PD. In addition, MPTP-induced neurotoxicity is also decreased by EGCG (40) and quercetin (37). Flavonoids interact not only through their antioxidant potential in protecting neurons but they also modulate various cell signaling pathways (3). It has become evident that flavonoids interact with critical neuronal intracellular signaling pathways and are able to exert neuroprotective actions (11). Flavonoids and their metabolites may exert modulatory actions in cells through actions at protein kinase and lipid kinase signaling pathways. Inhibitory or stimulatory actions at these signaling pathways strongly affect neuronal function by altering the phosphorylation state of target molecules and by modulating gene expression (71). Important cell survival signaling pathways include the following: phosphatidylinositol-3 kinase/Akt (PI3K/Akt), extracellular signal–regulated protein kinase (ERK), protein kinase C (PKC), and the cell death pathways p38 and c-Jun N-terminal kinase (JNK). Various flavonoids interact with these pathways and provide protection against neurodegeneration (Figure 1).
FIGURE 1.
The modulation of neuronal survival and death protein kinase pathways by flavonoids. The activation of PI3K/Akt, ERK1/2, and PKC pathways acts to stimulate neuronal survival through the induction of prosurvival or antiapoptotic genes and via inhibition of proapoptotic proteins. JNK and p38 are stress-activated pathways and cause neuronal death via activation of c-Jun and other AP-1 proteins; they lead to apoptosis and neuronal death. In addition, the inhibitory actions of flavonoids within the JNK and p38 pathways are likely to be neuroprotective in the presence of stress signals. However, flavonoids have neuroprotective and neuromodulatory properties and prevent neuronal function via inhibitory and stimulatory actions at these signaling pathways. AP-1, activated protein 1; ARE, antioxidant response element; ASK1, apoptosis signal-regulating kinase 1; Bad, Bcl-xL/Bcl-2–associated death promoter; Bax, BCL2-associated X protein; bcl-w, BCL2-like 2; bcl-xL, BCL2-like 1; bcl-2, B-cell CLL/lymphoma 2; BDNF, brain-derived neurotrophic factor; CREBP, cAMP response element-binding protein; ERK, extracellular signal-regulated protein kinase; HO-1, heme-oxygenase 1; JNK, c-Jun N-terminal kinase; Mdm2, murine double minute 2; Nrf2, NF-E2–related factor 2; PI3K/Akt, phosphatidylinositol-3 kinase/Akt; PKC, protein kinase C.
Flavonoids such as EGCG (72) and hesperetin (73), flavonoid-rich blueberry extract (74), and the flavonoid baicalein (75) were found to activate PI3K/Akt, ERK, and PKC pathways. The activation of pathways by these flavonoids provides beneficial effects on cell survival via upregulation of the antiapoptotic or prosurvival genes such as B-cell lymphoma 2 (Bcl2), BCL2-like 2 (Bclw), and BCL2-like 1 (BclxL) (72, 75); inhibition of the proapoptotic proteins (caspase 9 and caspase 3 activation); and inhibition of apoptosis signal-regulating kinase 1 (ASK1) (73) and nontranscriptional inhibition of BclxL/Bcl2-associated death promoter (Bad), BCL2-associated X protein (Bax), and murine double minute 2 (Mdm2) (72). Flavonoids also have a protective effect on cell survival through mechanisms that may involve activation of the cAMP response element-binding protein (CREBP) phosphorylation (74), increases in the amount of brain-derived neurotrophic factor (BDNF) (74), and increased transcriptional factor NF-E2–related factor 2 (Nrf2)/heme oxygenase 1 (HO-1) protein expression and enhanced antioxidant response element transcriptional activity (75). The stimulatory effects of all of these flavonoids may be preventive against neurodegeneration and protect brain function (Figure 1).
JNK and p38 are considered to be cell death pathways because they have been strongly linked to transcription-dependent apoptotic signaling (76) via the activation of c-Jun (77) and other activated protein 1 (AP-1) proteins, including JunB and JunD. Flavonoids have antiapoptotic action via the inhibition of JNK and p38 pathways (Figure 1). Flavonoids such as quercetin (78), epicatechin and 3′-O-methyl-epicatechin (79), and hesperetin and its structural counterparts isorhamnetin and isosakuranetin (80) were found to inhibit JNK activity. Quercetin, hesperetin, and its structural counterparts isorhamnetin and isosakuranetin may suppress JNK activity and apoptosis induced by hydrogen peroxide (78, 80). Epicatechin and 3′-O-methyl-epicatechin also protect neurons against oxidative damage via a mechanism involving the suppression of JNK and its downstream partners c-Jun and pro-caspase-3 (79). There are very few studies that suggest that flavonoids modulate neuronal signaling through the p38 pathway. The p38 pathway is inhibited by genistein (81) and cocoa extract and its main flavonoid epicatechin (82). Genistein protects the neurons from Aβ-induced cell death by preventing oxidative stress, which, in turn, inhibits the activation of p38, protecting neurons from cell death (81). Epicatechin is also an important flavonoid that protects against oxidative stress–induced neurodegeneration (82). However, the above mentioned flavonoids activate or suppress various cell signaling pathways and thus show an ability to induce morphologic changes that have a direct influence on memory performance and brain function.
Neuroinflammatory processes are assumed to play a critical role in the development of various neurodegenerative diseases (83) in which glial cells play a significant role. Activated glial cells (microglia and astrocytes) lead to the production of cytokines and other inflammatory mediators that may contribute to the apoptotic cell death of neurons (84). Various flavonoids, such as those in flavonoid-rich blueberry extracts (85), luteolin (86), kaempferol (87), wogonin, bacalein (88), EGCG (89), and quercetin (90, 91), were found to inhibit production of proinflammatory mediators such as NO, TNF-α, IL-1β, and iNOS expression, cyclooxygenase-2 expression, NADPH oxidase activation, and reactive oxygen species production. All of these effects of flavonoids appear via their ability to directly modulate the cell signaling pathways (3): for example, the inhibition of cell signaling cascades, such as p38 and ERK, via flavonoids that control both iNOS and TNF-α expression in activated glial cells (92). In addition, fisetin inhibited p38 phosphorylation (93) and luteolin inhibited IL-6 production via the inhibition of the JNK pathway (94). Flavonoids were also found to prevent transcription factor activation; for example, quercetin attenuated neuroinflammation via mechanisms that involved downregulation of iNOS gene transcription by inhibition of NF-κB, which is responsible for iNOS transcription (90). Thus, all of these molecular mechanisms suggest that flavonoids might have therapeutic potential in maintaining optimal neuronal function and neurocognitive performance in various neurodegenerative diseases.
Conclusions
Dietary supplementation of flavonoids has shown several neuroprotective actions in the brain, including prevention of toxicity against various neurotoxins, decreasing neuroinflammation and oxidative stress, and the ability to enhance memory and improve cognitive function. These beneficial effects on brain function are modulated by mechanisms that involve the interaction with key neuronal signaling process leading to expression of neuronal survival and differentiation genes and the suppression of genes responsible for neurodegeneration. Thus, regular dietary supplementation of flavonoid-rich foods at an appropriate stage of neurodegeneration holds promise as a natural remedy to halt many neurodegenerative diseases and to improve cognitive and other brain functions. In view of the recent evidence on the role of flavonoids in the activation of many transcription factors and cell survival signaling pathways in the brain, it is hoped that, in the future, these compounds may represent one of a new generation of bioactive drugs for improving brain function in various neurodegenerative diseases.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: Aβ, amyloid β; AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; AP-1, activated protein 1; ASK1, apoptosis signal-regulating kinase-1; Bad, BclxL/Bcl2-associated death promoter; Bax, BCL2-associated X protein; BBB, blood-brain barrier; Bcl2, B-cell CLL/lymphoma 2; Bclw, BCL2-like 2; Bcl-xL, BCL2-like 1; BDNF, brain-derived neurotrophic factor; CREBP, cAMP response element binding protein; EGCG, epigallocatechin gallate; ERK, extracellular signal-regulated protein kinase; HD, Huntington disease; HO-1, heme oxygenase-1; iNOS, inducible NO synthase; JNK, c-Jun N-terminal kinase; Mdm2, murine double minute 2; MPTP, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MS, multiple sclerosis; Nrf2, NF-E2–related factor 2; PD, Parkinson disease; PI3K/Akt, phosphatidylinositol-3 kinase/Akt; PKC, protein kinase C; SOD, superoxide dismutase; 6-OHDA, 6-hydroxydopamine.
References
- 1.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology 2010;129:154–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellinger KA. Cell death mechanisms in neurodegeneration. J Cell Mol Med 2001;5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer JPE. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr 2007;2:257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47. [DOI] [PubMed] [Google Scholar]
- 5.Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem 2003;85:180–92. [DOI] [PubMed] [Google Scholar]
- 6.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med 2004;36:592–604. [DOI] [PubMed] [Google Scholar]
- 7.Peng HW, Cheng FC, Huang YT, Chen CF, Tsai TH. Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 1998;714:369–74. [DOI] [PubMed] [Google Scholar]
- 8.Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med 2002;33:1693–702. [DOI] [PubMed] [Google Scholar]
- 9.Talavéra S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, Rémésy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem 2005;53:3902–8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Rusciano D, Osborne NN. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res 2008;1198:141–52. [DOI] [PubMed] [Google Scholar]
- 11.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 2008;3:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 2008;14:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon 2010;56:484–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem 2003;87:172–81. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez-Aliaga K, Bermejo-Bescós P, Benedí J, Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci 2011;89:939–45. [DOI] [PubMed] [Google Scholar]
- 16.Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, Quehenberger O, Maher P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer's disease transgenic mice. Aging Cell 2014;13:379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr 2006;136:1043–7. [DOI] [PubMed] [Google Scholar]
- 18.Qin XY, Cheng Y, Yu LC. Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci Lett 2012;513:170–3. [DOI] [PubMed] [Google Scholar]
- 19.Okello EJ, Leylabi R, McDougall GJ. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct 2012;3:651–61. [DOI] [PubMed] [Google Scholar]
- 20.Bastianetto S, Ramassamy C, Doré S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci 2000;12:1882–90. [DOI] [PubMed] [Google Scholar]
- 21.Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH, Park J, Park CW, Suh SI. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci 2001;70:603–14. [DOI] [PubMed] [Google Scholar]
- 22.Zeng H, Chen Q, Zhao B. Genistein ameliorates beta-amyloid peptide (25–35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med 2004;36:180–8. [DOI] [PubMed] [Google Scholar]
- 23.Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem 2001;276:5287–95. [DOI] [PubMed] [Google Scholar]
- 24.Liu LL, Sheng BY, Yan YF, Gong K, Ma T, Zhao NM, Zhang XF, Gong YD. Protective effect of anthocyanin against the oxidative stress in neuroblastoma N2a cells. Prog Biochem Biophys 2010;37:779–85. [Google Scholar]
- 25.Wang SW, Wang YJ, Su YJ, Zhou WW, Yang SG, Zhang R, Zhao M, Li YN, Zhang ZP, Zhan DW, et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 2012;33:482–90. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez-Zepeda A, Santell R, Wu Z, Brown M, Wu Y, Khan I, Link CD, Zhao B, Luo Y. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci 2005;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Wang JL, Liu R, Li XX, Li JF, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer's disease mouse model. Molecules 2013;18:9949–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ksiezak-Reding H, Ho L, Santa-Maria I, Diaz-Ruiz C, Wang J, Pasinetti GM. Ultrastructural alterations of Alzheimer's disease paired helical filaments by grape seed-derived polyphenols. Neurobiol Aging 2012;33:1427–39. [DOI] [PubMed] [Google Scholar]
- 29.Gong EJ, Park HR, Kim ME, Piao S, Lee E, Jo DG, Chung HY, Ha NC, Mattson MP, Lee J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β. Neurobiol Dis 2011;44:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 2007;26:3169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci Lett 2001;303:57–61. [DOI] [PubMed] [Google Scholar]
- 32.Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25–35). Behav Brain Res 2007;180:139–45. [DOI] [PubMed] [Google Scholar]
- 33.Azad N, Rasoolijazi H, Joghataie MT, Soleimani S. Neuroprotective effects of carnosic Acid in an experimental model of Alzheimer’s disease in rats. Cell J. 2011;13:39–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson R, McNeil B, Taylor C, Holl G, Ruff D, Gwebu ET. Effect of aged garlic extract on caspase-3 activity, in vitro. Nutr Neurosci 2002;5:287–90. [DOI] [PubMed] [Google Scholar]
- 35.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron 2003;39:889–909. [DOI] [PubMed] [Google Scholar]
- 36.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 2008;65:1272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv C, Hong T, Yang Z, Zhang Y, Wang L, Dong M, Zhao J, Mu J, Meng Y. Effect of quercetin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson's disease. Evid Based Complement Alternat Med 2012;2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bournival J, Plouffe M, Renaud J, Provencher C, Martinoli MG. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid Med Cell Longev 2012;2012:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol 2002;155:732–8. [DOI] [PubMed] [Google Scholar]
- 40.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem 2001;78:1073–82. [DOI] [PubMed] [Google Scholar]
- 41.Kim HG, Ju MS, Ha SK, Lee H, Lee H, Kim SY, Oh MS. Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Biol Pharm Bull 2012;35:1287–94. [DOI] [PubMed] [Google Scholar]
- 42.Nie G, Cao Y, Zhao B. Protective effects of green tea polyphenols and their major component, (-)-epigallocatechin-3-gallate (EGCG), on 6-hydroxydopamine-induced apoptosis in PC12 cells. Redox Rep 2002;7:171–7. [DOI] [PubMed] [Google Scholar]
- 43.Khan MM, Raza SS, Javed H, Ahmad A, Khan A, Islam F, Safhi MM, Islam F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson's disease. Neurotox Res 2012;22:1–15. [DOI] [PubMed] [Google Scholar]
- 44.Baluchnejadmojarad T, Roghani M, Nadoushan MR, Bagheri M. Neuroprotective effect of genistein in 6-hydroxydopamine hemi-parkinsonian rat model. Phytother Res 2009;23:132–5. [DOI] [PubMed] [Google Scholar]
- 45.Wszelaki N, Melzig MF. Additive protective effects of luteolin and pyruvate against 6-hydroxydopamine and 3-hydroxykynurenine induced neurotoxicity in SH-SY5Y cells. Pharmacol & Pharm 2013;4:369–76. [Google Scholar]
- 46.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem Pharmacol 2005;69:339–45. [DOI] [PubMed] [Google Scholar]
- 47.Rojas P, Montes P, Rojas C, Serrano-García N, Rojas-Castañeda JC. Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson's disease: therapeutic perspectives. Nutrition 2012;28:1081–8. [DOI] [PubMed] [Google Scholar]
- 48.Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinsonaposs disease. Neuroreport 2001;12:3871–5. [DOI] [PubMed]
- 49.Khan MM, Ahmad A, Ishrat T, Khan MB, Hoda MN, Khuwaja G, Raza SS, Khan A, Javed H, Vaibhav K, et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res 2010;1328:139–51. [DOI] [PubMed] [Google Scholar]
- 50.Rajeswari A. Curcumin protects mouse brain from oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Eur Rev Med Pharmacol Sci 2006;10:157–61. [PubMed] [Google Scholar]
- 51.Lee HJ, Cho HS, Park E, Kim S, Lee SY, Kim CS, Kim DK, Kim SJ, Chun HS. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008;250:109–15. [DOI] [PubMed] [Google Scholar]
- 52.Han JM, Lee YJ, Lee SY, Kim EM, Moon Y, Kim HW, Hwang O. Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther 2007;321:249–56. [DOI] [PubMed] [Google Scholar]
- 53.Davies S, Ramsden DB. Huntington's disease. Mol Pathol 2001;54:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum Mol Genet 2006;15:2743–51. [DOI] [PubMed] [Google Scholar]
- 55.Gopinath K, Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012;227:134–43. [DOI] [PubMed] [Google Scholar]
- 56.Menze ET, Tadros MG, Abdel-Tawab AM, Khalifa AE. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology 2012;33:1265–75. [DOI] [PubMed] [Google Scholar]
- 57.Sandhir R, Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntingtonaposs disease. Biochim Biophys Acta 2013;1832:421–30. [DOI] [PubMed]
- 58.Kumar P, Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem Toxicol 2009;47:2522–30. [DOI] [PubMed] [Google Scholar]
- 59.Johnson J, Maher P, Hanneken A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci 2009;50:2398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutation in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993;362:59–62. [DOI] [PubMed] [Google Scholar]
- 61.Koh SH, Lee SM, Kim HY, Lee KY, Lee YJ, Kim HT, Kim J, Kim MH, Hwang MS, Song C, et al. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci Lett 2006;395:103–7. [DOI] [PubMed] [Google Scholar]
- 62.Xu Z, Chen S, Li X, Luo G, Li L, Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res 2006;31:1263–9. [DOI] [PubMed] [Google Scholar]
- 63.Trieu VN, Uckun FM. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem Biophys Res Commun 1999;258:685–8. [DOI] [PubMed] [Google Scholar]
- 64.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol 1992;10:153–87. [DOI] [PubMed] [Google Scholar]
- 65.Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol 2005;252:3–9. [DOI] [PubMed] [Google Scholar]
- 66.Hendriks JJ, Alblas J, van der Pol SM, van Tol EA, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med 2004;200:1667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herges K, Millward JM, Hentschel N, Infante-Duarte C, Aktas O, Zipp F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS ONE 2011;6:e25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendriks JJ, de Vries HE, van der Pol SM, van den Berg TK, van Tol EA, Dijkstra CD. Flavonoids inhibit myelin phagocytosis by macrophages; a structure-activity relationship study. Biochem Pharmacol 2003;65:877–85. [DOI] [PubMed] [Google Scholar]
- 69.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol 1991;32:67–74. [DOI] [PubMed] [Google Scholar]
- 70.Sternberg Z, Chadha K, Lieberman A, Hojnacki D, Drake A, Zamboni P, Rocco P, Grazioli E, Weinstock-Guttman B, Munschauer F. Quercetin and interferon-beta modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J Neuroimmunol 2008;205:142–7. [DOI] [PubMed] [Google Scholar]
- 71.Mansuri ML, Parihar P, Solanki I, Parihar MS. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014;9:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 2002;277:30574–80. [DOI] [PubMed] [Google Scholar]
- 73.Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem 2007;103:1355–67. [DOI] [PubMed] [Google Scholar]
- 74.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med 2008;45:295–305. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Z, Cui W, Li G, Yuan S, Xu D, Hoi MP, Lin Z, Dou J, Han Y, Lee SM. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signalling pathways. J Agric Food Chem 2012;60:8171–82. [DOI] [PubMed] [Google Scholar]
- 76.Mielke K, Herdegen T. JNK and p38 stresskinases—degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol 2000;61:45–60. [DOI] [PubMed] [Google Scholar]
- 77.Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet 1999;21:326–9. [DOI] [PubMed] [Google Scholar]
- 78.Ishikawa Y, Kitamura M. Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int 2000;58:1078–87. [DOI] [PubMed] [Google Scholar]
- 79.Schroeter H, Spencer JP, Rice-Evans C, Williams RJ. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J 2001;358:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hwang SL, Yen GC. Modulation of Akt, JNK, and p38 activation is involved in citrus flavonoid-mediated cytoprotection of PC12 cells challenged by hydrogen peroxide. J Agric Food Chem 2009;57:2576–82. [DOI] [PubMed] [Google Scholar]
- 81.Vallés SL, Borrás C, Gambini J, Furriol J, Ortega A, Sastre J, Pallardó FV, Viña J. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell 2008;7:112–8. [DOI] [PubMed] [Google Scholar]
- 82.Ramiro-Puig E, Casadesús G, Lee HG, Zhu X, McShea A, Perry G, Pérez-Cano FJ, Smith MA, Castell M. Neuroprotective effect of cocoa flavonoids on in vitro oxidative stress. Eur J Nutr 2009;48:54–61. [DOI] [PubMed] [Google Scholar]
- 83.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:741–9. [DOI] [PubMed] [Google Scholar]
- 84.Kozuka N, Itofusa R, Kudo Y, Morita M. Lipopolysaccharide and proinflammatory cytokines require different astrocyte states to induce nitric oxide production. J Neurosci Res 2005;82:717–28. [DOI] [PubMed] [Google Scholar]
- 85.Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res 2007;85:1010–7. [DOI] [PubMed] [Google Scholar]
- 86.Zhu LH, Bi W, Qi RB, Wang HD, Wang ZG, Zeng Q, Zhao YR, Lu DX. Luteolin reduces primary hippocampal neurons death induced by neuroinflammation. Neurol Res 2011;33:927–34. [DOI] [PubMed] [Google Scholar]
- 87.Park SE, Sapkota K, Kim S, Kim H, Kim SJ. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br J Pharmacol 2011;164:1008–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J 2003;17:1943–4. [DOI] [PubMed] [Google Scholar]
- 89.Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res 2004;78:723–31. [DOI] [PubMed] [Google Scholar]
- 90.Chen JC, Ho FM, Pei-Dawn Lee Chao, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung Wu, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 2005;521:9–20. [DOI] [PubMed] [Google Scholar]
- 91.Kao TK, Ou YC, Raung SL, Lai CY, Liao SL, Chen CJ. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci 2010;86:315–21. [DOI] [PubMed] [Google Scholar]
- 92.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 1998;18:1633–41. [DOI] [PMC free article] [PubMed]
- 93.Zheng LT, Ock J, Kwon BM, Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int Immunopharmacol 2008;8:484–94. [DOI] [PubMed] [Google Scholar]
- 94.Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 2008;105:7534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]