Abstract
Viral and bacterial infections are often associated with deficiencies in macronutrients and micronutrients, including the essential trace element selenium. In selenium deficiency, benign strains of Coxsackie and influenza viruses can mutate to highly pathogenic strains. Dietary supplementation to provide adequate or supranutritional selenium supply has been proposed to confer health benefits for patients suffering from some viral diseases, most notably with respect to HIV and influenza A virus (IAV) infections. In addition, selenium-containing multimicronutrient supplements improved several clinical and lifestyle variables in patients coinfected with HIV and Mycobacterium tuberculosis. Selenium status may affect the function of cells of both adaptive and innate immunity. Supranutritional selenium promotes proliferation and favors differentiation of naive CD4-positive T lymphocytes toward T helper 1 cells, thus supporting the acute cellular immune response, whereas excessive activation of the immune system and ensuing host tissue damage are counteracted through directing macrophages toward the M2 phenotype. This review provides an up-to-date overview on selenium in infectious diseases caused by viruses (e.g., HIV, IAV, hepatitis C virus, poliovirus, West Nile virus) and bacteria (e.g., M. tuberculosis, Helicobacter pylori). Data from epidemiologic studies and intervention trials, with selenium alone or in combination with other micronutrients, and animal experiments are discussed against the background of dietary selenium requirements to alter immune functions.
Keywords: micronutrient, supplementation, selenoprotein, AIDS, immunity
Introduction
The debate on the influence of nutrition on public health led to a consensus definition of “sustainable diets” as “diets with low environmental impacts that contribute to food and nutrition security and to healthy lives for present and future generations” (1). However, it was stated that the present industrial agriculture and food-processing procedures promote consumption of low-nutrient and energy-rich foods, with negative impacts on a sufficient and balanced supply of the macro- and micronutrients required for human health (1). It has been estimated that 2 billion people worldwide are currently suffering from micronutrient deficiencies (1). The European Micronutrient Recommendations Aligned Network of Excellence (EURRECA)6 has prioritized 5 micronutrients for human nutrition: folic acid, vitamin B-12, iodine, iron, and zinc (2). In addition, EURRECA identified the following 5 relevant health outcomes for adequate and/or supranutritional intake of the essential trace element and micronutrient selenium, mainly with respect to human subpopulations or patient groups: 1) cognition (for elderly individuals aged >50 y), 2) viral load and onset of AIDS (for patients infected with HIV), 3) immune functions, 4) fertility (for men), and 5) cancer (particularly for persons at risk of prostate cancer) (2).
Selenium acts mainly through selenoproteins, many of which are antioxidant selenoenzymes such as glutathione peroxidases (GPxs) and thioredoxin reductases (TrxRs) (3). Selenoproteins contain the amino acid selenocysteine (Sec), which is incorporated cotranslationally during protein synthesis after conversion of O-phosphoseryl-transfer RNA (O-phosphoseryl-tRNA)[Ser]Sec into selenocysteyl-tRNA[Ser]Sec (4). Cancer and immune dysfunction have been associated with modest selenium deficiency and altered expression and single nucleotide polymorphisms of some selenoproteins (4–8). Cells of the immune system express most of the 25 genes encoding human selenoproteins, with the GPx isoenzymes GPx1 and GPx4 showing the highest expression levels in both T lymphocytes and macrophages (7).
The impact of selenium on health and disease, dietary selenium requirements for support of cognitive and immune functions and prevention of cancer, as well as underlying molecular mechanisms were discussed recently (4–9). In addition to selenium, several trace elements (e.g., copper, iron, zinc) and vitamins (e.g., vitamins A, C, and E) may modulate the susceptibility of hosts to pathogens and the immune defense against microbes (10). In this regard, we recently discussed the use of selenium, zinc, vitamins C and E, and natural products with antioxidant activity to support the treatment of infections with the intestinal protozoan parasite Eimeria (11). The present review focuses on the use of selenium to strengthen the immune response against viral and bacterial pathogens and to counteract oxidative cellular damage associated with infection and inflammation on the basis of the above-mentioned priority health topics—AIDS and immunity—as identified by the EURRECA team (2). The outcomes of intervention trials with selenium compounds given alone or, more often, in combination with other micronutrients are listed in Table 1, and are discussed against the mechanistic background of selenium and selenoproteins acting on proliferation, differentiation, and function of immune cells.
TABLE 1.
Intervention trials with selenium given alone or in combination with other micronutrients as discussed in the text1
| Pathogen, tested dose, and selenium compound | Additional micronutrients | Health effects (supplementation group vs. placebo group) | Reference |
| IAV vaccination | |||
| 100 μg Se/d as selenium sulfide | Zinc | Higher antibody titers after IAV vaccination; lower numbers of respiratory tract infections | (43) |
| Poliovirus vaccination | |||
| 50 or 100 μg Se/d as sodium selenite | None | Better viral clearance after poliovirus vaccination because of improved T cell immunity | (64) |
| Hepatitis C virus infection | |||
| 200 μg Se/d as selenomethionine | Vitamin C, vitamin E | No beneficial effects on viral load or liver damage | (63) |
| HIV infection | |||
| 200 μg Se/d as Se-yeast | None | Suppressed progression in viral load and increase in CD4+ T cells if serum selenium increased >26 μg/L | (54) |
| 200 μg Se/d as selenomethionine | None | No effect on maternal viral load, T cell count, or mortality but less diarrhea morbidity; improved child survival | (55, 56) |
| 400 μg Se/d (not specified) | Multivitamin and multimineral mix | No effect on viral load and T cell count but higher survival rate | (57) |
| 200 μg Se/d (not specified) | Vitamin B complex, vitamin C, vitamin E | Slower disease progression (delayed decline in CD4+ T cells, lower morbidity) | (58) |
| HIV/Mycobacterium tuberculosis coinfection | |||
| 100 μg Se/d (not specified) | Multivitamin mix | Marginal decrease in mortality, lower risk of TB recurrence after therapy | (75) |
| 200 μg Se/d as selenomethionine | Multivitamin mix, copper, zinc | Augmented increase in weight gain after TB therapy, marginal decrease in mortality | (77) |
| 200 μg Se/d as sodium selenite | Multivitamin mix, copper, zinc | Augmented increase in weight gain after TB therapy, enhanced hand-grip strength | (79) |
| 200 μg Se/d (not specified) | Multivitamin and multimineral mix | Enhanced hand-grip strength | (80) |
| 200 μg Se/d as sodium selenite | Multivitamin mix, copper, zinc | Improved bioavailability of the TB drug rifampicin | (81) |
IAV, influenza A virus; Se, selenium; TB, tuberculosis.
Current Status of Knowledge
Dietary requirements and current recommendations for selenium intake
The selenium supply for humans varies greatly around the world, depending on the selenium content of the soil and, consequently, the accumulation of selenium in farm crops and animals, the bioavailability of selenium compounds from soil and diet, and the intake of additional selenium through dietary supplements. Selenium is taken up predominantly as selenomethionine, selenium-methylselenocysteine or γ-glutamyl-selenium-methylselenocysteine from vegetables and as selenocysteine from meat (8). A novel organic selenium compound, selenoneine, was discovered in sea fish (e.g., tuna, mackerel) (12). In addition to selenium-containing amino acids, dietary supplements may contain inorganic selenium compounds such as sodium selenite and selenate (8). Selenium intake is high in North America and in Japan but considerably lower in most European countries. In Europe, selenium intake averages 40 μg/d compared with 93 and 134 μg/d for men and women, respectively, in the United States (8). Dietary supplements can provide an additional 10–200 μg Se/d; supplement consumption is common in Europe and more so in the United States, where one-third of the population regularly ingests multivitamin/mineral supplements (13). Daily selenium intake may be as low as 7 μg and reach amounts as high as 4990 μg (8). However, such extreme values are associated with signs of overt selenium deficiency and toxicity, respectively, and were reported from regions in China with very low as well as with very high selenium content in the soil (4, 8). Two diseases associated with selenium deficiency, Keshan disease and Kashin-Beck disease, used to occur in rural areas of China with soil poor in selenium. Dietary supplementation with selenite is an effective measure for prevention, resulting in a steady decline of reported cases since its implementation in the 1970s (14). In Africa, the lowest selenium intakes were reported for sub-Saharan countries in East and Central Africa (15).
Currently, the recommended amounts for adequate selenium intake of adults range between 25 and 100 μg/d (2), with an average of 60 μg/d for men and 53 μg/d for women (8). The Tolerable Upper Intake Level is set at 300–450 μg Se/d (4). Thus, selenium has a relatively narrow safety range. Sporadic cases of selenium toxicity (selenosis) have been observed in humans and animals consuming plants grown in selenium-rich soil or after accidental ingestion of very high doses of selenium (4). Notably, selenium intake above the nutritional requirements might trigger adverse effects on human health even when it is below the Tolerable Upper Intake Level. Indeed, there is debate whether selenium is an additional risk factor in the pathogenesis of type 2 diabetes (16). On the other hand, the addition of selenium to fertilizers in food crop production has been proven to be safe in Finland, optimizing the selenium status of its formerly selenium-poor population since the start of the program in 1985 (17). Generally, individuals with low selenium status and/or increased needs because of a disease may benefit from additional selenium intake (8).
Several surrogate variables are in use to determine the selenium status of an individual and the success of dietary supplementation. Selenium content is measured in plasma/serum, hair, or toenails. Selenium in plasma is mainly present in 2 extracellular selenoproteins, GPx3 and selenoprotein P (Sepp1), which are considered to be suitable biomarkers of selenium status (4). Maximal activity of the selenoenzyme GPx3 in plasma is achieved at a daily intake of ∼70 μg Se and is associated with plasma concentrations of 90 μg Se/L (1.14 μM) (18), values that were used to set the reference for nutritional recommendations in the United States (4). Sepp1 concentrations in plasma respond to a wider range of selenium intakes and reach a plateau not before ∼105 μg Se/d (19). Optimization of plasma Sepp1 is associated with plasma concentrations of 124 μg Se/L (1.57 μM) (19) and prevention of some types of cancer (4, 6, 8).
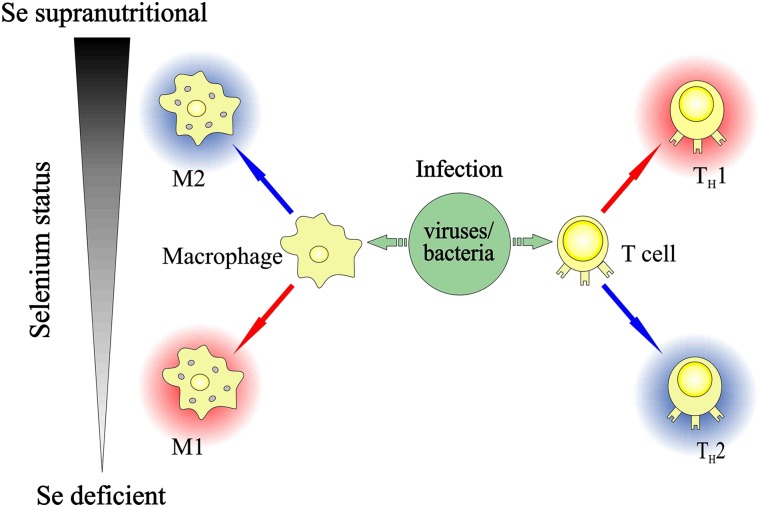
Supplementation of humans with 100 μg Se/d in the form of selenite resulted in increased gene expression of factors required for protein biosynthesis in isolated blood lymphocytes, pointing to a higher proliferation of lymphocytes at supranutritional selenium intakes (20). At adequate selenium intakes, the differentiation of naive CD4+ T cells is thought to be susceptible to the action of cytokines and antigen-presenting cells (7). As depicted in Figure 1, selenium deficiency may favor a T helper (Th) type 2 (Th2) phenotype, whereas supranutritional selenium shifts the Th1/Th2 balance via a higher reductive tone toward a Th1 phenotype (7, 21). Activated T cells isolated from mice fed a high-selenium (1 ppm Se) diet showed higher gene expression of the Th1 cytokines IL-2 and IFN-γ and less pronounced stimulation of the Th2 cytokine IL-4 compared with T cells obtained from selenium-deficient or selenium-adequate mice (21). The effect of selenium has been shown to depend on the mitogen used for activation of the T cells: selenite promoted T cell receptor– or concanavalin A–induced T cell proliferation and IL-2 production but failed to affect the T cell response to phytohemagglutinin in porcine splenocytes (22). On the other hand, selenium status influences the activation of macrophages in a somewhat converse manner. Selenite was shown to increase production of arachidonic acid–derived anti-inflammatory 15-deoxy-Δ(12,14)-prostaglandin J2 by upregulation of prostaglandin D2 synthase and to decrease production of the proinflammatory prostaglandin E2 (PGE2) in murine macrophages (23). Dietary selenium supplementation of patients with low selenium status might thus contribute to support the proinflammatory cellular (Th1-type) immune response against viral and bacterial pathogens, whereas excessive activation of the immune system and ensuing tissue damage are avoided through driving the differentiation of macrophages toward a more anti-inflammatory M2 phenotype (Figure 1). For a detailed elaboration of the action of selenium and individual selenoproteins in immunity, the reader is referred to the comprehensive review of Huang et al. (7).
FIGURE 1.
Simplified scheme depicting the influence of dietary Se status on the immune response against pathogens, as summarized from references 7 and 21–23. Supranutritional selenium intake was shown to favor proliferation and differentiation of activated CD4-positive T cells toward TH1 cells, whereas macrophages are directed toward an M2 phenotype. Red and blue arrows indicate the shift toward a more proinflammatory and a more anti-inflammatory phenotype, respectively. Se, selenium; TH1, T helper 1; TH2, T helper 2.
Selenium and infections by RNA viruses
RNA viruses include well-known human pathogenic viruses such as HIV, hepatitis C virus (HCV), influenza A virus (IAV), and poliovirus as well as emerging viruses such as West Nile virus (WNV) and Ebola virus. Viral infection causes elevated generation of reactive oxygen species (ROS), both in the mitochondria of host cells and through an oxidative burst of phagocytes, whereas the biosynthesis of major antioxidant enzymes is downregulated in the infected cells (24, 25). Moreover, the production of reactive nitrogen species (RNS) is elevated through induction of the inducible NO synthase enzyme. Th1-related cytokines trigger ROS/RNS production in virus-infected host tissues (24, 25). Imbalance of ROS/RNS production and removal results in oxidative/nitrosative stress (26), which may enhance the replication of the virus as well as the mutation rate of the viral RNA genome, leading to enhanced damage to host tissues (24, 25).
Compared with the genome of DNA viruses, the genome of RNA viruses is more prone to genetic changes, because proofreading activity is lacking in RNA-dependent RNA polymerases required for its replication. RNA viruses exhibit the highest known mutation rates, with up to one mutation per genome per generation cycle (27). Landmark studies by Beck and Levander and colleagues (28–30) revealed that overt selenium deficiency increases the pathogenicity and severity of infections by benign or mildly virulent strains of Coxsackie and influenza viruses, giving rise to multiple changes in the viral RNA. Thus, selenium is not only important to boost Th1-type host immunity against viral infections (7) but, in addition, it appears to impede the evolution of more virulent strains of some viral pathogens (31, 32).
Dietary selenium supplementation of human populations at risk of modest selenium deficiency—as in many countries in Europe and Africa and some regions in East Asia—might serve as an inexpensive and widely available adjuvant therapy of viral infections. Intriguingly, beneficial effects of selenium have almost exclusively been reported for infections by RNA viruses, whereas information on selenium and DNA viruses remains scarce. A noteworthy exception is provided by an intervention trial in the Chinese Qidong province, a region with selenium-poor soil: selenium supplementation lowered the incidence of liver cancer associated with infection by hepatitis B virus, a DNA virus (33).
Coxsackie virus.
Keshan disease, an endemic cardiomyopathy named after an outbreak in the Keshan County, China, in 1935, used to occur in areas of China with very low selenium content in the soil. Affected patients were severely selenium-deficient, with blood selenium concentrations of <20 μg/L (0.25 μM) (14). Coxsackie B virus, a small nonenveloped, single-stranded RNA virus, has been suggested as an infectious cofactor of Keshan disease after its isolation from blood and tissues of patients (34). Infection with a noncardiovirulent strain of Coxsackie B virus (CVB3/0) caused heart damage similar to human pathology only in selenium-deficient mice, whereas mice fed a selenium-adequate diet (0.2 ppm Se as selenite) were protected. The selenium-deficient mice exhibited higher virus titers in the heart and decreased antigen-specific T cell responses than their selenium-adequate littermates (35). In selenium-deficient mice, the formerly benign CVB3/0 strain mutated to a pathogenic geno- and phenotype; 4 out of the 6 specific nucleotide exchanges in the viral RNA were located in the coding region of proteins and resulted in amino acid exchanges (28).
Biosynthesis of the selenoenzyme GPx1 undergoes a pronounced and rapid decline under selenium-deficient conditions, as reviewed in reference 36. Mice carrying a disrupted GPx1 gene (GPx1−/−) developed myocarditis after infection with the benign CVB3/0 strain. Most of the nucleotide exchanges in viruses isolated from diseased GPx1−/− mice were identical to those found in mutated viruses from selenium-deficient mice (37). This suggests that selenium protects from ROS-induced mutations of the viral RNA genome through the action of GPx1.
An insufficient supply of selenium and other micronutrients was also hypothesized to be linked to the emergence of an epidemic neuropathy in Cuba in the early 1990s. There was evidence for oxidative stress in the patients, and Coxsackie A9 virus or an antigenically related infectious agent was present in the cerebrospinal fluid of 84% of 125 selected patients (34, 38).
IAV.
The single-stranded RNA genome of this enveloped virus is more susceptible to mutations than the genome of influenza B and C virus. The rapid immune response after IAV infection has to be finely balanced, because too little inflammation may result in immune escape and increased replication of the virus, whereas excessive inflammation may cause collateral lung damage and increased mortality (39, 40). Biomarkers of oxidative damage and inflammation [e.g., plasma F2-isoprostanes, hydroxyeicosatetraenoic products (HETEs), 7β-hydroxy-cholesterol, 7-keto-cholesterol, γ-glutamyltransferase] have been measured in patients with IAV during acute infection and 3 mo after recovery (41). Patients with postinfectious fatigue had higher concentratios of F2-isoprostanes and HETEs than did individuals without fatigue, pointing to persistent oxidative stress even after recovery (41).
Concentrations of selenium and selenoenzymes (e.g., GPx, TrxR) in plasma and/or erythrocytes were found to be decreased in children infected with the highly pathogenic H1N1 subtype of IAV (42). Elderly participants of a French randomized controlled trial who had low plasma selenium concentrations at baseline and received a daily supplement of 100 μg Se and 20 mg Zn for 15–17 mo showed a better humoral response after IAV vaccination than did the individuals in the placebo group (43). Similar to the outcome of experiments with Coxsackie virus, selenium intake and selenium status affected the host immune response, the mutation rate of the viral genome, and the pathology in mice infected with the human influenza A/Bangkok/1/79 (H3N2) virus strain that usually causes only mild pneumonitis in mice (29, 30). Compared with mice fed a selenium-adequate diet, virus-infected selenium-deficient mice had more severe and persistent inflammatory infiltration of the lungs and exhibited increased induction of both Th1-related chemokines [e.g., monocyte chemoattractant protein (MCP-1), regulated upon activation, normal T cell expressed and secreted (RANTES)] and Th2-related cytokines (e.g., IL-4, IL-10) in the lymph nodes draining the lungs (29). Again, viruses isolated from selenium-deficient mice had nucleotide exchanges in their RNA that made them more virulent (30). When transgenic mice with impaired biosynthesis of selenoproteins were infected with the mild influenza A/Bangkok/1/79 virus strain, they showed delayed virus clearance but a similar severity of the lung pathology compared with wild-type mice with normal selenoprotein expression. The GPx activity in lungs of the transgenic mice was 82% lower than in selenium-adequate wild-type mice but slightly (12.5%) higher than in wild-type selenium-deficient mice, suggesting a threshold level of GPx activity required for antiviral protection (44).
The impact of selenium on the outcome of the immune response appears to depend on the virulence of the applied IAV strain as well. Infection with influenza A/NWS/33 (H1N1) virus resulted in high (75%) mortality in selenium-deficient mice, whereas selenium supplementation up to 0.5 ppm (as selenite) dose-dependently lowered the mortality rate, accompanied by increased production of the Th1 cytokines IFN-γ and TNF-α (45). Another study that used an IAV strain that is highly pathogenic in mice yielded opposing results: 50% of the mice in the selenium-adequate group died 7 d postinfection with influenza A/Puerto Rico/8/34 virus, whereas there were no deaths in the selenium-deficient group. Virus-induced induction of Th1 cytokines did not differ between the 2 groups, but the selenium-deficient mice exhibited less pronounced induction of Th1-related chemokines and strong induction of the Th2 cytokine IL-4 (46).
Thus, the shift toward Th2 immunity under selenium-deficient conditions appears to limit or delay the host immune response. This may result either in a beneficial outcome with better recovery from infection with a highly virulent IAV strain due to less immunopathology or in an adverse outcome with immune escape and mutation of less virulent to more virulent pathogens. On the other hand, optimized expression/activity of GPx under selenium-adequate conditions may protect infected cells and tissues from IAV-induced oxidative damage and cell death. Cultured selenium-deficient differentiated bronchial epithelial cells showed more apoptotic cell death upon infection with IAV than did cells grown in selenium-adequate medium (47).
HIV.
Human HIV-1 and HIV-2 retroviruses are enveloped, single-stranded RNA viruses. Untreated HIV infection causes progressive failure of the immune system, resulting in AIDS. Antiretroviral therapy (ART) with a combined drug consisting of a protease inhibitor and 2 reverse transcriptase inhibitors has made HIV infection a manageable chronic disease. Despite this progress, ART is expensive, does not fully restore the immune system, and has considerable side effects (48). Deficiencies in micronutrients, including selenium, are widespread in HIV-infected individuals, most notably under resource-limited conditions, and their remedy can be a reliable, beneficial, and safe measure to support ART (49). Among HIV-infected Kenyan women with mean CD4+ T cell counts of 451/μL (reference value for healthy adults: 700–1100/μL), 11% were marginally selenium-deficient [serum Se <85 μg/L (1.07 μM)]. Marginal selenium deficiency was associated with increased shedding of HIV-infected cells in the genital tract (50). Serum selenium concentrations and GPx activity were substantially lower in hospitalized patients with AIDS than in asymptomatic HIV-infected patients and healthy subjects, and serum selenium concentrations were positively correlated with the CD4+ T cell count (51). Similarly, low plasma selenium concentrations were associated with accelerated HIV disease progression and an increased risk of mortality (52). The decrease in blood selenium was proposed to be related to the frequent occurrence of low serum albumin concentrations and an acute phase response among individuals with more advanced HIV-1 infection (53).
Intervention trials have been carried out with selenium alone or in combination with other trace elements and/or vitamins. Comparisons between the results of these trials are hampered because of differences in age, baseline nutrition status, and stage of HIV infection of the participants as well as differences in the protocols for dietary micronutrient supplementation. A review published in 2013 listed 12 trials, conducted in the United States, Europe, and Asia (2). In a US trial, HIV-positive men and women with relatively high selenium status [serum Se >100 μg/L (1.27 μM) at baseline] received 200 μg Se/d as selenium-enriched yeast for 9 mo. Suppression of the progression in viral load and an increase in blood CD4+ T cells were observed only in the subgroup of selenium-supplemented subjects who responded with an increase in their serum selenium concentrations to >26 μg/L (54). Selenium supplementation (200 μg Se/d as selenomethionine) of HIV-infected pregnant Tanzanian women during pregnancy and until 6 mo after delivery did not affect maternal viral load, CD4+ T cell count, or mortality but improved child survival (55); a secondary analysis of the data revealed lowered diarrhea morbidity in the selenium-supplemented participants (56). HIV-infected adults in Thailand who already had a very low CD4+ T cell count of <200/μL blood received a micronutrient mixture of vitamins and trace elements including 400 μg Se/d for 48 wk. Even though there was no impact on viral load and CD4+ T cell numbers, micronutrient supplementation enhanced the survival rate of the patients (57). More recently, HIV-infected adults from Botswana with a CD4+ T cell count >350/μL were supplemented for 2 y with either 200 μg Se/d, a vitamin mixture (B-vitamins, vitamins C and E) or vitamins plus selenium. Neither the vitamins nor selenium alone affected any of the clinical variables, whereas the combination of selenium and vitamins significantly lowered disease progression as measured by decline in CD4+ T cells and morbidity (58). Even though the data are still inconsistent and insufficient, there is some evidence that selenium supplementation may delay the progressive destruction of CD4+ T cells and the onset of AIDS and decrease the risk of comorbidities. This approach is particularly promising when selenium is given in combination with other micronutrients. Although there is currently no evidence for an influence of the host selenium status on the mutation rate of HIV, the selenoenzyme TrxR1 has been identified as a negative regulator of the HIV-encoded Tat protein that is required for virus replication (59). TrxR1 protein concentrations were decreased, together with GPx concentrations, in human Jurkat T cells upon infection with HIV (60).
HCV.
Infection with this enveloped, single-stranded RNA virus is a major cause of chronic liver disease, and there is a high prevalence of HCV coinfection among patients infected with HIV (61). Plasma selenium concentrations and GPx activity as well as selenium concentrations in erythrocytes were significantly lower in HCV-infected patients than in healthy controls, and selenium concentrations in both plasma and erythrocytes of patients were inversely correlated with HCV virus load (62). Moreover, HCV/HIV-coinfected patients had lower serum selenium concentrations than did HIV-infected patients without concomitant HCV infection (51). In a small intervention trial, Danish HCV-infected patients were supplemented for 6 mo with vitamins C and E together with 200 μg Se/d as selenomethionine. However, no beneficial effect with respect to viral load and liver damage was observed in the micronutrient group (63).
Poliovirus.
Poliovirus is a nonenveloped, single-stranded RNA virus. An intervention trial in healthy British adults with a modest selenium deficit [plasma Se <94 μg/L (1.2 μM)] investigated the effect of selenium supplementation on the immune reaction against a live attenuated poliovirus vaccine (64). Before vaccination, participants received 50 or 100 μg Se/d as selenite or a placebo for 15 wk. Poliovirus-induced lymphocyte proliferation as well as production of the Th1 cytokine IFN-γ and the Th2 cytokine IL-10 occurred faster in both selenium-supplemented groups than in the placebo group. Participants supplemented with 100 μg Se/d showed a significantly higher number of total T cells and Th cells after the virus challenge and a better virus clearance. Moreover, the mutation rate of the poliovirus was lowered in the selenium-supplemented subjects. Selenium supplementation augmented and accelerated the cellular antiviral immune response, but it did not shift the Th1/Th2 balance and it did not affect the humoral response (64).
WNV.
This enveloped, single-stranded RNA virus originated from Uganda and had rapidly spread through parts of Africa, Asia, and Europe before its emergence in North America in 1999 (65). Selenium status and the impact of dietary selenium supplementation in WNV-infected humans have not been investigated yet, but there is in vitro and in vivo evidence for a beneficial role of selenium. Selenium-deficient monkey kidney epithelial cells were more prone to virus-induced apoptotic cell death than cells cultured in selenium-supplemented (50 nM selenite) medium (66). The selenium-inducible selenoprotein K (SelK), a selenoprotein that is relatively highly expressed in immune cells and located in the endoplasmic reticulum, appears to be involved in the protective action of selenium (67). Mice with targeted deletion of SelK exhibited decreased viral clearance in the periphery and elevated viral titers in the brain upon WNV infection. This resulted in markedly lowered survival of SelK knockout mice compared with their wild-type littermates. Elucidating the molecular mechanism, the authors found that SelK is required for effective Ca2+ flux during activation of immune cells (67).
Selenium and bacterial infections
Although adequate/supranutritional selenium was shown to support the immune response against some RNA viruses such as HIV and IAV, there is less evidence for beneficial effects of selenium in humans infected by bacterial pathogens. Currently, the most promising approach appears to be the addition of selenium compounds to multivitamin/mineral supplements that are given to patients infected with Mycobacterium tuberculosis in order to overcome the broad nutritional deficiencies and the weight loss often associated with tuberculosis. In particular, micronutrient supplementation of M. tuberculosis and HIV–coinfected patients has received attention.
M. tuberculosis.
The slow-growing gram-positive non–spore-forming bacteria of the M. tuberculosis complex cause tuberculosis, a major infectious disease that kills ∼2 million humans every year. M. tuberculosis induces a Th1-type immune response characterized by interplay of phagocytes (e.g., macrophages, dendritic cells) and CD4+ T cells, secretion of Th1 cytokines, and formation of pathogen-encapsulating granuloma in the lungs (68). Because of their impaired cell-mediated immune defense, HIV-infected persons are at particular risk of developing overt tuberculosis and to die of tuberculosis (68). Nutritional challenges in patients with tuberculosis include macro- and micronutrient malnutrition, nutrient malabsorption, and increased metabolic demands (69). Deficient or marginal status of vitamins (e.g., vitamins A, B complex, C, D, and E) and trace elements is frequently observed in patients with tuberculosis with and without HIV coinfection. This may increase the risk of M. tuberculosis infection progressing to overt tuberculosis and affect the outcome of antituberculosis chemotherapy or delay recovery (69, 70). The combination of tuberculosis, HIV coinfection, and malnutrition has been commonly denominated as “triple trouble” (69).
Four studies—2 each from Africa and from Asia—reported significantly decreased serum selenium concentrations in patients with tuberculosis compared with healthy controls (71–74). According to one study, both HIV-negative and HIV-positive patients with tuberculosis were similarly selenium-deficient (72). Two other studies measured lower serum selenium concentrations in patients with tuberculosis coinfected with HIV than in HIV-negative patients with tuberculosis (73, 74). The effect of anti-tuberculosis chemotherapy on serum selenium concentrations in patients with tuberculosis is controversial: one study observed an increase in serum selenium concentrations after therapy (73), whereas serum selenium concentrations did not respond to anti-tuberculosis therapy in another study (71).
Dietary supplementation of patients with tuberculosis with selenium alone does not appear to hold much promise. Instead, a number of intervention trials applied selenium compounds as part of multivitamin/mineral mixtures (75–81). Daily doses of selenium in the micronutrient formula were either 100 μg (75, 76) or 200 μg (77–81). The selenium status of the volunteers before and after the intervention was determined in 2 trials; both found an increase of serum selenium concentrations in the micronutrient-supplemented group (78, 80). Multimicronutrient supplementation of adult patients undergoing anti-tuberculosis chemotherapy improved different clinical and lifestyle variables, but the currently available data do not offer sufficient clues to judge whether individuals with or without HIV coinfection might benefit more. Participants supplemented with multimicronutrients exhibited, after anti-tuberculosis therapy, an augmented increase in weight gain, which was irrespective of HIV status in one trial (77) but confined to HIV-negative patients in another trial (79); a third trial did not observe any influence of micronutrients (75). Marginal decreases in mortality were reported either exclusively in HIV-positive or in HIV-negative patients with tuberculosis supplemented with micronutrients (75, 77). Enhanced hand-grip strength after micronutrient supplementation was reported in 2 trials (79, 80). In one trial, the risk of tuberculosis recurrence was lowered in the micronutrient-supplemented group (75). Two recent studies intended to elucidate molecular mechanisms underlying the effects of micronutrients in patients with tuberculosis. Interestingly, multimicronutrient supplementation improved the bioavailability of the first-line anti-tuberculosis drug rifampicin in HIV-positive patients with tuberculosis, probably through a better intestinal absorption of the drug (81). Another intervention trial investigated whether micronutrients improve the immune response during tuberculosis treatment. However, lymphocyte proliferation after challenge with T cell mitogen or mycobacterial antigens was not significantly altered in the micronutrient-supplemented group compared with the placebo group (76). In conclusion, the selenium status of patients (as well as their supply with vitamins and other trace elements) may be improved by taking oral multimicronutrient supplements during tuberculosis treatment, but a consistent benefit on clinical outcome and/or quality of life remains to be demonstrated (82).
Helicobacter pylori.
It has been estimated that this gram-negative, helix-shaped bacterium colonizes the upper gastrointestinal tract of >50% of the human population worldwide. Nontreated Helicobacter pylori infection is a major pathogenic factor for the development of gastritis, gastric cancer, and duodenal ulcer (83, 84). Micronutrient homeostasis is frequently impaired in H. pylori–infected individuals, probably through malabsorption, lowered gastric acid secretion, and atrophy of the gastric mucosa (85). A recent meta-analysis of epidemiologic data from 46 studies on vitamins and 10 studies on trace elements identified a significant association of H. pylori infection with lowered concentrations of ascorbic acid and cobalamin, whereas there was not enough evidence for trace elements including selenium because of the limited number of studies (85). Plasma selenium concentrations were not altered in H. pylori–infected patients and did not change after successful eradication of H. pylori. Interestingly, selenium concentrations were significantly elevated in antral biopsies of patients with H. pylori–associated gastritis and correlated with the degree of severity of gastric inflammation (86). The authors proposed that selenium concentrations may have been higher in the gastric mucosa of H. pylori–infected patients in response to elevated ROS generation (86). This might indicate elevated biosynthesis of selenoproteins in the inflamed gastric mucosa, because oxidative stress may result in selective upregulation of some selenoenzymes (e.g., GPx1, GPx4, TrxR1) involved in antioxidant defense (87). On the other hand, selenium concentrations of antral biopsies were significantly lower in the presence of precancerous gastric lesions in patients with H. pylori–associated chronic atrophic gastritis, which might suggest an eventual loss of the compensatory response (86). Currently, there is no consistent evidence whether dietary selenium supplementation could prevent gastric cancer. An earlier intervention trial in Linxian County, China, reported a significant decrease in gastric cancer mortality among participants supplemented for 5 y with a mixture of selenium, vitamin E, and β-carotene compared with the placebo group (88). However, combined supplementation with selenium plus vitamins C and E yielded only a slight, nonsignificant lower gastric cancer incidence and mortality, as recently published in a 15-y follow-up of the Shandong intervention trial carried out in Linqu County, China (89).
Other bacteria.
In several animal models, selenium status was reported to affect the immune response after bacterial infections. Combined pretreatment with selenium and the antibiotic ciprofloxacin for 4 wk was more effective than ciprofloxacin alone to prevent the development of chronic bacterial prostatitis in rats after infusion of an Escherichia coli suspension into the prostatic urethra (90). Compared with mice fed an adequate-selenium diet, selenium-deficient mice showed a compromised response of the innate immune system after infection with the gram-positive bacterium Listeria monocytogenes (91). The innate as well as the humoral immune response was impaired in selenium-deficient sheep affected by foot root, an endemic disease of ruminants caused by infection of claws with the gram-negative bacterium Dichelobacter nodosus. Selenium supplementation did not prevent footrot but restored immune functions (92). In dairy cows, selenium deficiency has been associated with increased incidence and severity of intramammary infections by E. coli and Staphylococcus aureus; combined supplementation with selenium and vitamin E improved the intracellular bacterial killing in blood neutrophils (93).
Conclusions and Outlook
A balanced and sufficient supply of macro- and micronutrients is important to support host immune defense and resistance against pathogens. The habitual diet is often not sufficient to meet the increased demands for micronutrients in infectious diseases. Dietary multimicronutrient supplements containing selenium up to 200 μg/d have potential as safe, inexpensive, and widely available adjuvant therapy in viral infections (e.g., HIV, IAV) as well as in coinfections by HIV and M. tuberculosis to support the chemotherapy and/or to improve fitness and quality of life of the patients (Table 1). Because many of these patients experience broad nutritional deficiencies, multimicronutrient supplementation appears to be a more promising approach than the use of selenium alone. Dietary supplementation with selenium-containing multimicronutrients might also be useful to improve supportive care and to strengthen the immune system of patients suffering from newly emerging viral diseases, such as in the current epidemic of Ebola fever in West Africa. Populations in several countries most afflicted by past and current outbreaks of Ebola fever (e.g., Liberia, Guinea, Democratic Republic of Congo) exhibit a high risk of selenium deficiency, and strikingly, the lowest dietary selenium supply in Africa was reported from Liberia, with a daily intake of only 23 μg Se (15).
Gaps in our knowledge that need further research include the following:
More intervention trials should help to better define which patients with infectious diseases may benefit from additional selenium and what is the most suitable selenium compound and dose in dietary supplements.
A better understanding of interactions between selenium and other micronutrients will help to improve protocols for multimicronutrient supplementation.
The opposing effects of supranutritional selenium on differentiation and maturation of T cells and macrophages are somewhat puzzling and are expected to be more complex than depicted in the simplified scheme (Figure 1). The molecular mechanisms underlying the actions of selenium and selenoproteins on immune cells require further elucidation.
Similar doses of selenium were found to be effective both in infectious diseases and in cancer prevention. This is intriguing and deserves closer attention to the mechanistic links, because cancer and infectious diseases share similarities such as the involvement of ROS/RNS synthase, the host T cell response, and the activation of common signaling pathways that mediate and amplify inflammation (94). Supranutritional selenium increases the production of the proinflammatory cytokine IL-2 in CD4+ T cells activated by T cell receptor stimulation (7, 21, 22), and interestingly, IL-2 was used as the first effective immunotherapy in human cancer (95).
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ART, antiretroviral therapy; CVB3/0, Coxsackie B virus; EURRECA, European Micronutrient Recommendations Aligned Network of Excellence; GPx, glutathione peroxidase; HCV, hepatitis C virus; HETE, hydroxyeicosatetraenoic products; IAV, influenza A virus; MCP-1, monocyte chemoattractant protein 1; RANTES, regulated upon activation, normal T cell expressed and secreted; RNS, reactive nitrogen species; ROS, reactive oxygen species; Sec, selenocysteine; SelK, selenoprotein K; Sepp1, selenoprotein P; Th, T helper; tRNA, transfer RNA; TrxR, thioredoxin reductase; WNV, West Nile virus.
References
- 1.Johnston JL, Fanzo JC, Cogill B. Understanding sustainable diets: a descriptive analysis of the determinants and processes that influence diets and their impact on health, food security, and environmental sustainability. Adv Nutr 2014;5:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurst R, Collings R, Harvey LJ, King M, Hooper L, Bouwman J, Gurinovic M, Fairweather-Tait SJ. EURRECA—estimating selenium requirements for deriving dietary reference values. Crit Rev Food Sci Nutr 2013;53:1077–96. [DOI] [PubMed] [Google Scholar]
- 3.Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 2009;1790:1478–85. [DOI] [PubMed]
- 4.Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R. Selenium in human health and disease. Antioxid Redox Signal 2011;14:1337–83. [DOI] [PubMed] [Google Scholar]
- 5.McCann JC, Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J 2011;25:1793–814. [DOI] [PubMed] [Google Scholar]
- 6.Steinbrenner H, Speckmann B, Sies H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid Redox Signal 2013;19:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2012;16:705–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayman MP. Selenium and human health. Lancet 2012;379:1256–68. [DOI] [PubMed] [Google Scholar]
- 9.Steinbrenner H, Sies H. Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys 2013;536:152–7. [DOI] [PubMed] [Google Scholar]
- 10.Puertollano MA, Puertollano E, de Cienfuegos GA, de Pablo MA. Dietary antioxidants: immunity and host defense. Curr Top Med Chem 2011;11:1752–66. [DOI] [PubMed] [Google Scholar]
- 11.Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil M. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 2014;113:3547–56. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita Y, Yamashita M. Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J Biol Chem 2010;285:18134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr 2011;141:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr 1993;57(Suppl):259S–63S. [DOI] [PubMed] [Google Scholar]
- 15.Joy EJ, Ander EL, Young SD, Black CR, Watts MJ, Chilimba AD, Chilima B, Siyame EW, Kalimbira AA, Hurst R, et al. Dietary mineral supplies in Africa. Physiol Plant 2014;151:208–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbrenner H, Speckmann B, Pinto A, Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 2011;48:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alfthan G, Eurola M, Ekholm P, Venäläinen ER, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J Trace Elem Med Biol 2014 May 20 (Epub ahead of print; DOI: 10.1016/j.jtemb.2014.04.009). [DOI] [PubMed]
- 18.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr 1999;70:896–903. [DOI] [PubMed] [Google Scholar]
- 19.Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, Broadley MR, Motley AK, Fairweather-Tait SJ. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2010;91:923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagmantidis V, Meplan C, van Schothorst EM, Keijer J, Hesketh JE. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am J Clin Nutr 2008;87:181–9. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR. Dietary selenium modulates activation and differentiation of CD4 + T cells in mice through a mechanism involving cellular free thiols. J Nutr 2010;140:1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren F, Chen X, Hesketh J, Gan F, Huang K. Selenium promotes T-cell response to TCR-stimulation and ConA, but not PHA in primary porcine splenocytes. PLoS ONE 2012;7:e35375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi UH, Kaushal N, Ravindra KC, Hegde S, Nelson SM, Narayan V, Vunta H, Paulson RF, Prabhu KS. Selenoprotein-dependent upregulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) γ. J Biol Chem 2011;286:27471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molteni CG, Principi N, Esposito S. Reactive oxygen and nitrogen species during viral infections. Free Radic Res 2014;48:1163–9. [DOI] [PubMed] [Google Scholar]
- 25.Reshi ML, Su YC, Hong JR. RNA viruses: ROS-mediated cell death. Int J Cell Biol 2014;2014:467452. [DOI] [PMC free article] [PubMed]
- 26.Sies H. Biochemistry of oxidative stress. Angew Chem Int Ed Engl 1986;25:1058–71. [Google Scholar]
- 27.Lauring AS, Frydman J, Andino R. The role of mutational robustness in RNA virus evolution. Nat Rev Microbiol 2013;11:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med 1995;1:433–6. [DOI] [PubMed] [Google Scholar]
- 29.Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J 2001;15:1481–3. [PubMed] [Google Scholar]
- 30.Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA, Beck MA. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J 2001;15:1846–8. [PubMed] [Google Scholar]
- 31.Louria DB. Undernutrition can affect the invading microorganism. Clin Infect Dis 2007;45:470–4. [DOI] [PubMed] [Google Scholar]
- 32.Harthill M. Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol Trace Elem Res 2011;143:1325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res 1997;56:117–24. [DOI] [PubMed] [Google Scholar]
- 34.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr 2003;133(Suppl):1463S–7S. [DOI] [PubMed] [Google Scholar]
- 35.Beck MA, Kolbeck PC, Rohr LH, Shi Q, Morris VC, Levander OA. Benign human enterovirus becomes virulent in selenium-deficient mice. J Med Virol 1994;43:166–70. [DOI] [PubMed] [Google Scholar]
- 36.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 2013;1830:3289–303. [DOI] [PubMed]
- 37.Beck MA, Esworthy RS, Ho YS, Chu FF. Glutathione peroxidase protects mice from viral-induced myocarditis. FASEB J 1998;12:1143–9. [DOI] [PubMed] [Google Scholar]
- 38.Más P, Pelegrino JL, Guzmán MG, Comellas MM, Resik S, Alvarez M, Rodríguez R, Muné M, Capó V, Balmaseda A, et al. Viral isolation from cases of epidemic neuropathy in Cuba. Arch Pathol Lab Med 1997;121:825–33. [PubMed] [Google Scholar]
- 39.Bot A, Smith KA, von Herrath M. Molecular and cellular control of T1/T2 immunity at the interface between antimicrobial defense and immune pathology. DNA Cell Biol 2004;23:341–50. [DOI] [PubMed] [Google Scholar]
- 40.Berri F, Lê VB, Jandrot-Perrus M, Lina B, Riteau B. Switch from protective to adverse inflammation during influenza: viral determinants and hemostasis are caught as culprits. Cell Mol Life Sci 2014;71:885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng MP, Lee JC, Loke WM, Yeo LL, Quek AM, Lim EC, Halliwell B, Seet RC. Does influenza A infection increase oxidative damage? Antioxid Redox Signal 2014;21:1025–31. [DOI] [PubMed] [Google Scholar]
- 42.Erkekoğlu P, Asçõ A, Ceyhan M, Kõzõlgün M, Schweizer U, Atas C, Kara A, Koçer Giray B. Selenium levels, selenoenzyme activities and oxidant/antioxidant parameters in H1N1-infected children. Turk J Pediatr 2013;55:271–82. [PubMed] [Google Scholar]
- 43.Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P, Preziosi P, Arnaud J, Manuguerra JC, Herchberg S. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN.VIT.AOX. Geriatric Network. Arch Intern Med 1999;159:748–54. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan PA, Zhong N, Carlson BA, Perella CM, Hatfield DL, Beck MA. Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J Nutr 2007;137:1466–71. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Sun L, Nan Y, Zhu LY. Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice. Biol Trace Elem Res 2011;141:254–61. [DOI] [PubMed] [Google Scholar]
- 46.Li W, Beck MA. Selenium deficiency induced an altered immune response and increased survival following influenza A/Puerto Rico/8/34 infection. Exp Biol Med (Maywood) 2007;232:412–9. [PubMed] [Google Scholar]
- 47.Jaspers I, Zhang W, Brighton LE, Carson JL, Styblo M, Beck MA. Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic Biol Med 2007;42:1826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barré-Sinoussi F, Ross AL, Delfraissy JF. Past, present and future: 30 years of HIV research. Nat Rev Microbiol 2013;11:877–83. [DOI] [PubMed] [Google Scholar]
- 49.Irlam JH, Siegfried N, Visser ME, Rollins NC. Micronutrient supplementation for children with HIV infection. Cochrane Database Syst Rev 2013;10:CD010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baeten JM, Mostad SB, Hughes MP, Overbaugh J, Bankson DD, Mandaliya K, Ndinya-Achola JO, Bwayo JJ, Kreiss JK. Selenium deficiency is associated with shedding of HIV-1–infected cells in the female genital tract. J Acquir Immune Defic Syndr 2001;26:360–4. [DOI] [PubMed] [Google Scholar]
- 51.Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Barton S, Lemoch H, Sudhop T, Hoch J, Stockinger K, Spengler U, et al. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr 1997;51:266–72. [DOI] [PubMed] [Google Scholar]
- 52.Kupka R, Msamanga GI, Spiegelman D, Morris S, Mugusi F, Hunter DJ, Fawzi WW. Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania. J Nutr 2004;134:2556–60. [DOI] [PubMed] [Google Scholar]
- 53.Drain PK, Baeten JM, Overbaugh J, Wener MH, Bankson DD, Lavreys L, Mandaliya K, Ndinya-Achola JO, McClelland RS. Low serum albumin and the acute phase response predict low serum selenium in HIV-1 infected women. BMC Infect Dis 2006;6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med 2007;167:148–54. [DOI] [PubMed] [Google Scholar]
- 55.Kupka R, Mugusi F, Aboud S, Msamanga GI, Finkelstein JL, Spiegelman D, Fawzi WW. Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: effects on maternal and child outcomes. Am J Clin Nutr 2008;87:1802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kupka R, Mugusi F, Aboud S, Hertzmark E, Spiegelman D, Fawzi WW. Effect of selenium supplements on hemoglobin concentration and morbidity among HIV-1-infected Tanzanian women. Clin Infect Dis 2009;48:1475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, Chaisilwattana P, Suthipinittharm P, Shetty P, Jaffar S. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS 2003;17:2461–9. [DOI] [PubMed] [Google Scholar]
- 58.Baum MK, Campa A, Lai S, Sales Martinez S, Tsalaile L, Burns P, Farahani M, Li Y, van Widenfelt E, Page JB, et al. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical trial. JAMA 2013;310:2154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalantari P, Narayan V, Natarajan SK, Muralidhar K, Gandhi UH, Vunta H, Henderson AJ, Prabhu KS. Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J Biol Chem 2008;283:33183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gladyshev VN, Stadtman TC, Hatfield DL, Jeang KT. Levels of major selenoproteins in T cells decrease during HIV infection and low molecular mass selenium compounds increase. Proc Natl Acad Sci USA 1999;96:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health 2008;8:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ko WS, Guo CH, Yeh MS, Lin LY, Hsu GS, Chen PC, Luo MC, Lin CY. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J Gastroenterol 2005;11:4697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groenbaek K, Friis H, Hansen M, Ring-Larsen H, Krarup HB. The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: a randomized trial among chronic hepatitis C virus-infected patients. Eur J Gastroenterol Hepatol 2006;18:985–9. [DOI] [PubMed] [Google Scholar]
- 64.Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 2004;80:154–62. [DOI] [PubMed] [Google Scholar]
- 65.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004;10:S98–109. [DOI] [PubMed] [Google Scholar]
- 66.Verma S, Molina Y, Lo YY, Cropp B, Nakano C, Yanagihara R, Nerurkar VR. In vitro effects of selenium deficiency on West Nile virus replication and cytopathogenicity. Virol J 2008;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 2011;186:2127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol 2004;22:599–623. [DOI] [PubMed] [Google Scholar]
- 69.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev 2003;61:81–90. [DOI] [PubMed] [Google Scholar]
- 70.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004;8:286–98. [PubMed] [Google Scholar]
- 71.Ciftci TU, Ciftci B, Yis O, Guney Y, Bilgihan A, Ogretensoy M. Changes in serum selenium, copper, zinc levels and Cu/Zn ratio in patients with pulmonary tuberculosis during therapy. Biol Trace Elem Res 2003;95:65–71. [DOI] [PubMed] [Google Scholar]
- 72.van Lettow M, Harries AD, Kumwenda JJ, Zijlstra EE, Clark TD, Taha TE, Semba RD. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis 2004;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kassu A, Yabutani T, Mahmud ZH, Mohammad A, Nguyen N, Huong BT, Hailemariam G, Diro E, Ayele B, Wondmikun Y, et al. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur J Clin Nutr 2006;60:580–6. [DOI] [PubMed] [Google Scholar]
- 74.Ramakrishnan K, Shenbagarathai R, Kavitha K, Thirumalaikolundusubramanian P, Rathinasabapati R. Selenium levels in persons with HIV/tuberculosis in India, Madurai City. Clin Lab 2012;58:165–8. [PubMed] [Google Scholar]
- 75.Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, Meydani SN, Fawzi WW. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis 2008;197:1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawai K, Meydani SN, Urassa W, Wu D, Mugusi FM, Saathoff E, Bosch RJ, Villamor E, Spiegelman D, Fawzi WW. Micronutrient supplementation and T cell-mediated immune responses in patients with tuberculosis in Tanzania. Epidemiol Infect 2014;142:1505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr 2006;95:762–70. [DOI] [PubMed] [Google Scholar]
- 78.Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology 2008;13:294–8. [DOI] [PubMed] [Google Scholar]
- 79.PrayGod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, Jensen L, Jensen AV, Grewal HM, Magnussen P, et al. Daily multi-micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV-uninfected but not HIV-infected patients in Mwanza, Tanzania. J Nutr 2011;141:685–91. [DOI] [PubMed] [Google Scholar]
- 80.Rudolph M, Kroll F, Beery M, Marinda E, Sobiecki JF, Douglas G, Orr G. A pilot study assessing the impact of a fortified supplementary food on the health and well-being of crèche children and adult TB patients in South Africa. PLoS ONE 2013;8:e55544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeremiah K, Denti P, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, Castel S, Wiesner L, Hagen CM, Christiansen M, et al. Nutritional supplementation increases rifampin exposure among tuberculosis patients coinfected with HIV. Antimicrob Agents Chemother 2014;58:3468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2011;11:CD006086. [DOI] [PubMed] [Google Scholar]
- 83.Marshall BJ, Goodwin CS, Warren JR, Murray R, Blincow ED, Blackbourn SJ, Phillips M, Waters TE, Sanderson CR. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet 1988;2:1437–42. [DOI] [PubMed] [Google Scholar]
- 84.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]
- 85.Lahner E, Persechino S, Annibale B. Micronutrients (other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter 2012;17:1–15. [DOI] [PubMed] [Google Scholar]
- 86.Ustündağ Y, Boyacioğlu S, Haberal A, Demirhan B, Bilezikçi B. Plasma and gastric tissue selenium levels in patients with Helicobacter pylori infection. J Clin Gastroenterol 2001;32:405–8. [DOI] [PubMed] [Google Scholar]
- 87.Touat-Hamici Z, Legrain Y, Bulteau AL, Chavatte L. Selective up-regulation of human selenoproteins in response to oxidative stress. J Biol Chem 2014;289:14750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 1993;85:1483–92. [DOI] [PubMed] [Google Scholar]
- 89.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim HW, Ha US, Woo JC, Kim SJ, Yoon BI, Lee SJ, Cho YH. Preventive effect of selenium on chronic bacterial prostatitis. J Infect Chemother 2012;18:30–4. [DOI] [PubMed] [Google Scholar]
- 91.Wang C, Wang H, Luo J, Hu Y, Wei L, Duan M, He H. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol 2009;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall JA, Vorachek WR, Stewart WC, Gorman ME, Mosher WD, Pirelli GJ, Bobe G. Selenium supplementation restores innate and humoral immune responses in footrot-affected sheep. PLoS ONE 2013;8:e82572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith KL, Hogan JS, Weiss WP. Dietary vitamin E and selenium affect mastitis and milk quality. J Anim Sci 1997;75:1659–65. [DOI] [PubMed] [Google Scholar]
- 94.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med 2014;371:380–3. [DOI] [PubMed] [Google Scholar]
- 95.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]