Abstract
Objective To describe patterns of treatment adherence to early maintenance phase therapy for acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL). Methods Using an objective observational method (electronic monitoring), adherence was examined for 139 patients aged 7–19 years diagnosed with ALL or LBL across 6 centers. Results The mean adherence percentage was 86.2%. Adherence rates declined over the 1-month of follow-up to 83%. 3 linear trajectories of 6-mercaptopurine adherence were identified: (1) exemplary adherence (n = 99): Averaging nearly 100%; (2) deteriorating (n = 23): Adherence decreased from 100 to 60%; and (3) chronically poor adherence (n = 9): Averaging 40%. Conclusions Adherence promotion interventions might be tailored to subgroups of patients who demonstrated problematic patterns of treatment adherence that could place them at risk for relapse. This research demonstrates the importance of using objective real-time measures of medication adherence for measuring and documenting adherence patterns.
Keywords: adherence, cancer and oncology, research design and methods, statistical applications
Treatment advances have greatly improved survival rates for pediatric cancer from 58% in the late 1970s to 89% in the early 2000s. In fact, 95% of pediatric patients will reach disease remission and 80% of patients aged 1–18 years are expected to have long-term event-free survival (National Cancer Institute, 2008). Still, pediatric cancer remains the leading cause of disease-related death for children and adolescents in the United States (National Cancer Institute, 2008). Similarities in morphology, genetics, and immunophenotypes between lymphoblastic lymphoma (LBL) and acute lymphoblastic leukemia (ALL) indicate that ALL and LBL be considered as part of a spectrum of malignant lymphoproliferative disorders (Reddy & Perkins, 2004) that require similar treatment protocols (Weiss, Bindl, Picozzi, Link, & Warnke, 1986).
Maintenance therapy is vital for relapse prevention in youth with ALL and LBL and has been shown to increase survival rates (Pritchard, Butow, Stevens, & Duley, 2006). However, the complex course of maintenance therapy can be difficult for children, adolescents, and parents to manage effectively. For example, 6-mercaptopurine (6MP) is a critical component of maintenance treatment for ALL/LBL that is taken daily, on an empty stomach, typically at bedtime (Bhatia et al., 2012). Despite the importance of taking medications as prescribed, some studies have shown that as many as ≥50% of pediatric cancer patients do not adhere to their maintenance treatment regimen (Kondryn, Edmondson, Hill, & Eden, 2010). Nonadherence may contribute to preventable morbidity and mortality in pediatric patients (Festa, Tamaroff, Chasalow, & Lanzkowsky, 1992; Lau, Matsui, Greensberg, & Koren, 1998; Tebbi, 2006). In a recent Children’s Oncology Group (COG) study of 327 patients with ALL, even relatively low nonadherence rates of <95% for 6MP treatment during maintenance therapy were associated with an increased risk of relapse (Bhatia et al., 2012). Such findings heighten the need for objective and precise measurement of medication adherence during all phases of maintenance therapy.
More than 20 published studies have reported data on treatment adherence in ALL and related conditions (Festa et al., 1992; Kennard et al., 2004; Kondryn et al., 2010; Lau et al., 1998). However, significant methodological problems, such as the absence of objective measures of medication adherence in recent treatment protocols, small sample sizes, and the exclusive use of self-reported adherence, limit the scientific and clinical utility of most available studies. Most studies were published more than a decade ago and are not applicable to current treatment for ALL/LBL given the substantial changes in treatment protocols that have been implemented by the COG. With only four exceptions (Bhatia et al., 2012; Kato et al., 1992; Lennard, Welch, & Lilleyman, 1995), sample sizes have been small (<60) and limited to a single institution, which limits the generalizability of study findings as well as the ability to identify subgroups of patients based on patterns of treatment adherence. Finally, most studies of pediatric patients with ALL/LBL have used physician-, parent-, and/or patient-reported adherence measures. However, it is well established that such reports tend to overestimate adherence levels (Lau et al., 1998; Riekert & Rand, 2002) and cannot validly identify dosing patterns that are relevant targets for adherence promotion.
The primary measure of medication adherence in previous studies of pediatric chronic illness, including pediatric cancer, has been based on a percentage of doses taken as prescribed. However, this measure does not capture clinically relevant patterns of adherence including the timing of medication taking and provides misleading results when used as the sole measure of adherence. For example, a patient who demonstrates a significant gap in adherence (e.g., missing 5 consecutive days of medication) would have exactly the same adherence percentage as a patient who missed medication sporadically for 5 days over a comparable monitored period. Such discrepant patterns of adherence are likely to have a different impact on health outcomes, including the therapeutic action of a medication that has a half-life of several days. The timing of medication doses may be particularly important for chemotherapy agents given it is recommended that they be taken around the same time each day to avoid over- or under-dosing (Pritchard et al., 2006; Relling, 1999; Ruddy, Mayer, & Partridge, 2009). For this reason, measuring the timing between doses (i.e., interdose intervals: IDIs) based on objective observational measures (Kenna, Labbé, Barrett, & Pfister, 2005) is a potentially important variable that has not been examined in previous research.
Previous studies have also suggested that there is considerable within-sample heterogeneity in patterns of adherence to pediatric cancer treatment (Kennard et al., 2004; Kondryn et al., 2010; Lau et al., 1998; Ruddy et al., 2009). Moreover, research in other pediatric chronic conditions has identified clinically relevant subgroups who demonstrate different patterns of adherence (Modi, Rausch, & Glauser, 2011). However, to our knowledge, subgroups that demonstrate different adherence patterns based on objective observational measures have not been previously identified in pediatric ALL and LBL but have important implications for tailoring and targeting adherence promotion interventions. For example, individuals who demonstrate either chronic or declining patterns of adherence that are associated with risk for relapse are prime candidates for more intensive adherence promotion intervention. Bhatia et al. (2012) identified a progressive increase in risk for relapse in a large cohort of children with ALL: As adherence to treatment decreased, especially below a level of 95%, risk for relapse increased. However, subgroups of children with different trajectories of adherence/nonadherence or variable IDIs were not assessed.
The present study addressed salient methodological limitations of previous studies of oral medication adherence in maintenance phase therapy for ALL and LBL by studying a cohort of 139 pediatric patients across multiple sites enrolled in treatment protocols used by COG (2007–2012) that were currently in maintenance treatment. The primary objective of this study was to describe patterns of adherence to 6MP treatment, including heterogeneity in trajectories of adherence patterns and IDIs based on electronic monitoring, which is an objective observational method recognized as a valid adherence measure (Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008). One medication (6MP) was selected as the primary medication to monitor for adherence because (1) substantial data have demonstrated its efficacy and importance in preventing relapse (Bhatia et al., 2012); and (2) it is prescribed daily over the course of maintenance therapy. A second objective of the study was to describe the interrelationships between adherence measures (e.g., overall adherence percentage, adherence subgroups, medication gaps, and IDIs) and demographic correlates of adherence.
Methods
Participants and Procedure
Participants were 139 children and adolescents aged 7–19 years diagnosed with ALL or LBL and their primary caregivers who were followed at six centers in different regions of the United States that specialize in pediatric cancer treatment, including site 1 (n = 18 patients, 12.9%), site 2 (n = 33 patients, 23.7%), site 3 (n = 28, 20.1%), site 4 (n = 33, 23.7%), site 5 (n = 22, 15.8%), and site 6 (n = 5, 3.6). Demographic and medical characteristics of the baseline sample are provided in Table I. Institutional Review Boards at each site approved the study. Data were collected as part of an ongoing 15-month longitudinal study of a family-centered problem-solving intervention to promote medication adherence for pediatric cancer, which is still in process. The age range of 7–19 years was selected to maximize child/adolescent participation in problem-solving with parents, which was the primary focus of the family-centered intervention. The present study focused on measurement of adherence patterns based on electronic monitoring during the first month of study enrollment before the intervention.
Table I.
Demographic and Medical Characteristics of Baseline Sample (N = 139)
| Child’s/adolescent’s age at baseline (years), M ± SD (range) | 12.29 years ± 3.44 (7–19.1 years) |
|---|---|
| Type of cancer diagnosis | |
| ALL, n (%) | 133 (95.7) |
| LBL, n (%) | 6 (4.3) |
| Duration of cancer diagnosis at baseline (years), M ± SD (range) | 1.29 years ± 0.35 (0.68–2.27 years) |
| Child’s gender | |
| Male, n (%) | 94 (67.6) |
| Female, n (%) | 45 (32.4) |
| Child’s ethnicity/race | |
| Non-Hispanic, Caucasian, n (%) | 75 (54.0) |
| Non-Hispanic, other, n (%) | 15 (10.9) |
| Hispanic, n (%) | 49 (33.9) |
| Ethnicity unknown/not reported, n (%) | 2 (1.4) |
| Primary caregiver age (years), M ± SD (range) | 40.66 years ± 7.31 (24–59 years) |
| Caregiver relationship who participated in baseline visit | |
| Biological mother | 126 (90.6) |
| Biological father | 12 (8.6) |
| Stepfather | 1 (0.7) |
| Primary caregiver’s marital status | |
| Married, n (%) | 96 (69.1) |
| Not married, n (%) | 43 (30.9) |
| Highest level of education completed by primary caregiver | |
| No high school diploma, n (%) | 26 (18.7) |
| High school diploma or G.E.D., n (%) | 32 (23.0) |
| College courses/vocational/trade school/associate's degree, n (%) | 45 (32.4) |
| Bachelor’s/master's/professional degree (MD, PhD, JD), n (%) | 36 (25.9) |
| Household composition | |
| One-caregiver household | 45 (32.4) |
| Two-caregiver household | 94 (67.6) |
| Total annual household income before taxes, median | $49,000–$72,999 |
| <$18,745 | 36 (25.9) |
| $18,745–$32,874 | 18 (12.9) |
| $32,875–$48,999 | 13 (9.4) |
| $49,000–$72,999 | 20 (14.4) |
| $73,000–$126,500 | 31 (22.3) |
| >$126,500 | 17 (12.2) |
Note. M = mean, SD = standard deviation.
Eligibility criteria were as follows: (1) diagnosis of ALL or LBL in remission with at least one cycle of the maintenance phase of therapy completed and 15 months of treatment remaining; (2) prescription of a daily dosage of 6MP oral medication; and (3) age 7–18 years at recruitment and fluent in English or Spanish. Participants were excluded (n = 7) if they were involved in foster care or did not have a primary caregiver available to participate (n = 2), had known plans to relocate (n = 5), were diagnosed with a comorbid chronic condition requiring burdensome treatments (e.g., cystic fibrosis), and/or were diagnosed with an intellectual disability or psychiatric condition that made it impossible to complete study procedures.
In accord with Health Insurance Portability and Accountability Act (HIPAA) guidelines, families were first contacted by their medical provider to obtain their permission to be approached about the study. If families agreed to be contacted, they were approached by study coordinators at each site to obtain parental permission/consent and assent for children/adolescents aged ≥11 years. Of the 171 patients and families approached to participate, 18.7% (n = 32) refused to participate owing to the following reasons: Too busy (n = 12), not interested (n = 19), or no transportation (n = 1). A comparison of patients and families who participated in the study with those who did not participate indicated no differences (p > .05) with respect to patients’ age and gender. However, more non-Hispanic, Caucasian patients and families (9.4%) refused participation compared with Hispanic (3.5%) and non-Hispanic, minority (5.8%) patients and families (p < .05).
Measures
Prescribed Medical Treatment
Prescribed medical treatment was comparable across all sites based on treatment protocols for ALL and LBL implemented by the COG. Medical charts were reviewed at baseline and 1-month follow-up using standardized forms to obtain information regarding prescribed treatment regimens, including medication type, dose, and timing of medication administration. Information regarding the prescribed treatment regimen was used to operationalize nonadherence (e.g., discrepancy between the prescribed daily dosage of 6MP compared with electronic monitoring data). The mean 6MP dosage per week was 92.42 mg (standard deviation [SD] = 39.19 mg, range: 25–200 mg).
Electronic Monitoring of 6MP Medication Adherence
An electronic monitoring device (i.e., the Medication Event Monitoring System [MEMS®] from the AARDEX Corporation, Palo Alto, CA) was used to monitor adherence to 6MP oral medication therapy for 1 month. The MEMS® system is similar to a prescription bottle, but contains a micro-electronic chip in the cap that registers dates/times when the bottle was opened and closed. Patients and their parents were instructed to take the 6MP only from the MEMS® bottle for the duration of the study, not to open the bottle unless the patient was taking a medication dose at that time, and to close the bottle immediately after removing the prescribed dose. A standardized form was used during each download to capture information regarding extra openings, refills, and periods of nonuse during the previous 1-month period. Adherence calculations did not include nonmonitored periods during which the patient was put on a medication hold owing to low lab values and/or other medical reasons (verified by chart review) and other periods in which the MEMS® cap and bottle were not used based on parent/patient reports (e.g., overnights, vacations, etc.).
Nine patients did not have electronic monitoring data available for the first month of study enrollment owing to not wanting to use the device and/or forgetting to use the device or lost devices. A subsample of patients (n = 39) requested to use the MEMS® cap along with their current medication administration system (e.g., pill box) to avoid disrupting their adherence. These patients and their parents were given a detailed description of how best to use the MEMS® cap with their current system such that each time 6MP medication was removed from the alternative medication management system (e.g., pill box), the parent/patient also opened the MEMS® cap to record the date/time medication was removed. Patients and their parents were asked during each MEMS® download about any issues they encountered with using the MEMS® in this way.
Operational Definitions of Primary Adherence Variables Derived From MEMS
Electronic monitoring provides an objective estimate of a range of adherence behaviors, including the average adherence percentage over a specified period, information regarding dose timing and IDIs, and number of consecutive days in which medication was not taken (Bhatia et al., 2012; Kenna et al., 2005; Lau et al., 1998; Pritchard et al., 2006; Quittner et al., 2008; Tebbi, 2006). Adherence percentages were defined as the number of times that doses of oral medication were taken as prescribed. IDIs were defined as the period in between two does (i.e., time lapsed between the current dose and the previous dose). Medication gaps were defined as the number of consecutive 24-hr periods in which medication was not taken.
Data Analytic Plan
Our data analytic plan used multiple methods for describing adherence to 6MP medication based on results obtained from electronic monitoring.
Description of Changes in Daily Adherence: Overall Sample
Unconditional growth curve models were used to examine changes in daily adherence rates during the first month of study enrollment. Individual growth curve models (i.e., level 1) measured changes in daily adherence rates over time for the entire sample at both the population level as well as for each individual patient enrolled in the study. Growth was summarized for the population and for each individual using two terms: Fitted intercept (i.e., baseline value) and fitted slope (i.e., rate of change over time) (Singer & Willet, 2003). Unconditional growth curve modeling was performed using SAS Proc Mixed. Restricted maximum likelihood estimations were used to avoid biased estimates of the variance components. Unstructured covariance matrices were used to allow variances and covariances to vary across time rather than to conform to a priori constraints (Singer & Willet, 2003).
Description of Changes in Daily Adherence: Analysis of Subgroups Based on Trajectories of Adherence
Growth curve modeling describes change over time at the individual level as well as for the population as a whole but does not provide information regarding the heterogeneity of the adherence patterns being modeled, including distinct specific subgroups that follow similar patterns over time (Nagin, 2005). For this reason, latent group-based trajectory modeling (LGTM) based on the SAS Trajectory (TRAJ) procedure was used to identify specific subgroups that demonstrated different adherence patterns of 6MP medication adherence. The normal distribution option in the TRAJ procedure was used to model adherence patterns across 1 month. Linear, cubic, and quadratic solutions with 2, 3, 4, 5, and 6 subgroups were examined. The adequacy of the final model was evaluated using statistical diagnostics recommended by Nagin (2005), including (1) identifying the model that had the “best” statistical fit based on the Bayesian Information Criterion; (2) subgroup proportions of at least 0.10 to ensure the group size was large enough to be of practical utility; (3) average posterior p > .7; and (4) odds correct classification >5 for all of the groups in the model.
Description of IDIs and Gaps in Adherence
The timing of daily medication, including the intervals between doses (i.e., IDI) as well as the number of consecutive days in which no medication was taken (i.e., medication gaps) were also measured.
Interrelationships Among Adherence Measures
Spearman and Pearson correlations described the relationship among the adherence measures and demographics. A between-subjects analysis of variance was used to examine whether there were mean differences between adherence trajectories (optimal adherence, deteriorating adherence, and chronic nonadherence) with respect to mean IDIs, medication gaps (total consecutive days without medication), and patient age. Post hoc comparisons were performed using Tukey’s Honestly Significant Difference test. Chi-square analyses were used to evaluate differences between the adherence subgroup trajectories and relevant demographic factors (patient gender, ethnicity/race, family structure [one- vs. two-parent homes], income, and maternal education).
Results
Description of Sample
The demographic characteristics of the sample (N = 139) are shown in Table I. The overwhelming majority of the patients (n = 133) were diagnosed with ALL. The average age of the sample was 12.3 years (SD = 3.4). The majority of patients were male (n = 94, 68%) and were of Caucasian ancestry (n = 75, 54%).
Description of Adherence Rates
The mean percentage of adherence defined as the number of doses taken compared with what was prescribed was 86.5% across 1 month of monitoring (SD = 22.4%, range = 0–110%). Mean IDI was 30 hr (SD = 22 hr, range = 21–242 hr), and mean consecutive 24-hr medication gaps (or the mean number of consecutive days 6MP medication was missed) was 3 days (SD = 6 days, range = 0–31 days).
To provide a context in which to consider the potential clinical relevance of our findings, we examined the average percentage of adherence in this sample based on Bhatia et al. (2012) who found that adherence rates <95% were associated with an increased risk for disease relapse. In the current sample, 44.3% (n = 58) of patients demonstrated adherence rates <95% across the first month of monitoring: 9.2% (n = 12) ranged from 90 to 95%, 4.6% (n = 6) ranged from 85 to 90%, and 30.5% (n = 40) were <85%. Adherence rates did not differ between Hispanic and non-Hispanic patients (χ2 = 4.8, p > .05).
Changes in Adherence Over Time
Individual Growth Curve Modeling
Unconditional growth curve modeling for the current sample (n = 131) indicated that medication adherence was 89.9% at baseline and significantly decreased over the first month of monitoring at a rate of −0.22% per day such that adherence estimates at the end of month 1 were 83% [F(1,111) = 7.48; p < .01].
Group-Based Trajectory Modeling
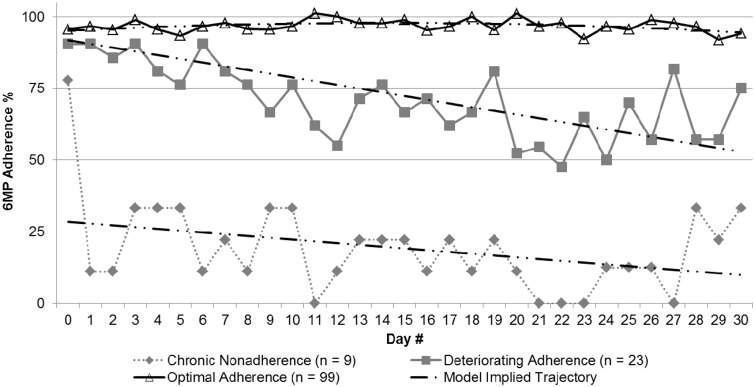
Based on LGTM, three linear trajectories of 6MP medication adherence over the first month of maintenance treatment had the best model fit (see Figure 1). The majority of patients (n = 99, 75.8%) demonstrated exemplary adherence rates during the first month of maintenance phase treatment: Starting at 100% and decreasing at a rate of only −0.01% per day. A second, much smaller group (n = 23, 17.1%) started at 100% adherence at the start of monitoring and decreased at a rate of −3.3% per day to an average of 60% adherence by the end of the first month. The third and smallest group (n = 9, 7.1%) never established an adequate pattern of adherence with adherence levels of only ≤40% across the first month with adherence decreasing at a rate of −5.6% per day. There was no difference between trajectory groups with respect to patient age [F (1,128) = 1.18, p > .05], Hispanic-ethnicity (χ2 = 4.9, p > .05), or whether the patient used the MEMS cap or used the MEMS as a proxy for an alternative medication administration system such as a pill box (χ2 = 4.0, p > .05).
Figure 1.
Trajectories of 6MP medication adherence across 1 month.
IDIs and Gaps in 6MP Adherence Over Time
The standard prescriptive recommendation is to take 6MP medication within 24 hr of the previous dose. The average IDI for 6MP medication when examining all patient doses in our sample (N = 3,020) was above the recommendation: 27 hr. There was considerable intrasample variation: SD = 19 hr; range 5–678 hr. The majority of 6MP doses (77%) were taken between 22 and 26 hr after the previous dose. A small percentage (8.4%) of medication doses were taken <22 hr after the prior dose. Similarly, 14.4% of medication doses were taken 26–60+ hr after the previous dose was given.
With respect to significant medication gaps of more than a day: 35% of the sample did not miss any doses, 32.5% missed only one consecutive dose, and 13.8% missed two consecutive doses. A subgroup of patients (18%) had gaps in 6MP medication of 3–10 consecutive days.
Interrelations Among Adherence Measures
Pearson and Spearman correlations describing the relationships between the various adherence measurements and demographic characteristics are provided in Table II. As shown in Table II, measures of adherence were highly intercorrelated for the most part. Average adherence percentage correlated highly with mean IDIs (r = −.87): The higher the adherence levels, the less time between medication doses. Moreover, the adherence percentage also correlated with gaps in adherence (r = −.89): Higher adherence was associated with fewer medication gaps. Finally, adherence group trajectory membership was correlated with the adherence percentage (r = −.24), mean IDIs (r = .38), and medication gaps (r = .17).
Table II.
Interrelationships for Baseline Demographics and 6MP Medication Adherence Data (N = 130)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 = Mean adherence | 1.00 | ||||||||||
| 2 = Mean IDI | −0.87** | 1.00 | |||||||||
| 3 = Total medication gaps | −0.89** | 0.77** | 1.00 | ||||||||
| 4 = Adherence group | −0.91** | 0.81** | 0.79** | 1.00 | |||||||
| 5 = Adherence trajectory group membership | −0.24** | 0.31** | 0.17* | 0.35** | 1.00 | ||||||
| 6 = Patient age | −0.10 | 0.07 | 0.09 | 0.14 | 0.07 | 1.00 | |||||
| 7 = Patient gender | 0.01 | −0.02 | 0.04 | 0.00 | 0.08 | 0.15 | 1.00 | ||||
| 8 = Patient ethnicity/race | −0.03 | 0.03 | −0.02 | 0.01 | −0.17 | 0.10 | 2.00 | 1.00 | |||
| 9 = Family structure (one vs. two parent) | 0.16 | −0.16 | −0.12 | −0.15 | −0.15 | −0.06 | −0.08 | −0.28** | 1.00 | ||
| 10 = Family income | 0.06 | −0.07 | −0.06 | −0.01 | −0.03 | −0.13 | −0.07 | −0.58** | 0.43** | 1.00 | |
| 11 = Maternal education | −0.12 | 0.11 | 0.04 | 0.17 | 0.09 | −0.08 | 0.12 | −0.56** | 0.14 | 0.67** | 1.00 |
Note. SD = standard deviation.
**p < .01; *p < .05.
Significant differences were identified between the adherence trajectory subgroups (chronic nonadherence, deteriorating adherence, and optimal adherence) with respect to average monthly adherence rates [F (2, 124) = 501.9, p < .01], medication gaps [F (2, 128) = 162.7, p < .01], and average IDIs [F (2, 122) = 59.9, p < .01]. The optimal adherence subgroup demonstrated significantly higher mean adherence rates across 1 month (M = 96.8%, SD = 5.4%) and had fewer medication gaps (M = 0.69, SD = 0.79). The deteriorating adherence and chronic nonadherence subgroups demonstrated lower mean adherence rates (deteriorating: 70.1 ± 11%; chronic: 19.8 ± 13.9%) and more medication gaps (deteriorating: 3.7 ± 3; chronic: 18.7 ± 10). The chronic nonadherence subgroup demonstrated a much higher IDI between 6MP medication doses (M = 93 hr, SD = 70 hr) compared with the optimal (M = 25 hr, SD = 2 hr) and deteriorating adherence subgroups (33 hr, SD = 7 hr).
Relationship of Demographic Characteristics to Adherence Measures
As shown in Table II, none of the demographic characteristics were related to adherence measures (average frequency, IDI, or gaps). Chi-square analyses indicted that there were no differences between the different adherence subgroup trajectories and relevant demographic factors such as patient gender, ethnicity/race, age, family structure (one- vs. two-parent homes), family income, and maternal education.
Discussion
To our knowledge, our study is the first study to identify distinct adherence trajectories of 6MP medication adherence during maintenance phase treatment for pediatric ALL/LBL using an objective observational measure of treatment adherence. The overall percentage of treatment adherence (86.5%) at the initial baseline assessment (i.e., day 1 of monitoring) was higher than that reported in most previous studies of children and adolescents with cancer, most of which have not used objective measures (Kondryn et al., 2010). However, it should be noted that the overall rate of 6MP medication adherence for the sample as a whole demonstrated a significant level of deterioration over the first month to 83% adherence at the end of the initial 1-month monitoring period. Moreover, the significant intrasample variation in the levels of adherence to 6MP medication has potential clinical relevance. For example, while more than half of the sample (55.7%) demonstrated levels of adherence >95% level noted by Bhatia et al. (2012) to be a protective factor for relapse, a substantial percentage (44.3%) had overall adherence levels suggesting an increased risk for relapse (<95% adherence) (Bhatia et al., 2012). Moreover, analyses of trajectories of adherence indicated that two subgroups totaling 24% that either never established an adequate level of adherence or had adherence rates that dropped significantly over the month. Assuming these adherence trajectories persist over time, patients in these subgroups would be prime candidates for more intensive, empirically supported adherence promotion interventions (e.g., behavioral and problem-solving approaches) (Kondryn et al., 2010).
It should be noted that the adherence rates reported in our study (86.5%) are lower than those presented by Bhatia et al’s. (2012) study, who reported mean adherence rates of 95% across the first month that decreased to 90% after 6 months of monitoring. The lower adherence rates observed here could be attributed to our older patient sample (mean age of 12.3 years) compared with the median age of 6 years in Bhatia et al. (2012). Moreover, in contrast to Bhatia et al. (2012), we did not note significant differences in adherence rates between Hispanic versus non-Hispanic patients. In contrast to Bhatia et al. (2012), which was powered to detect differences in ethnicity given its primary aim, our study may not have had sufficient power to detect differences between Hispanic and non-Hispanic patients. Moreover, the relatively short monitoring period of 1 month in our study compared with Bhatia et al. (2012) who monitored patients across 6 months may have limited our ability to detect differences.
To our knowledge, our study is the first to report data concerning IDIs, medication gaps (i.e., the number of consecutive days in which no medication was taken), and trajectories of adherence to 6MP treatment for ALL/LBL. 6MP medication is prescribed to be given once a day, ideally at the same time each day, and typically at bedtime unless otherwise instructed by a health care provider. Although the majority of the 6MP medication doses (77%) were taken within 22–26 hr, nearly one fifth of the sample had larger medication gaps of 3–10+ consecutive days, which could limit the therapeutic efficacy of the 6MP medication. The half-life of the metabolites of 6-MP is approximately 5 days following the medication dose (Derijks et al., 2004; Fishman & Mrozek-Orlowsk, 1999). Consequently, patterns of IDI that reflect highly variable dose timing and/or prolonged clinically significant gaps in IDI might also be targeted for more intensive adherence promotion intervention. Our unique finding that alternative measures of treatment adherence based on objective observational methods (e.g., adherence percentages, IDI, and trajectories) were significantly correlated has implications for assessment and intervention. Because measures of nonadherence including adherence percentages, IDI, and trajectories cluster together, targeting specific nonadherence behaviors in any of several domains may be effective.
Several limitations should be considered when interpreting our findings. First, the study focused on 30 days of maintenance treatment. Short-term findings may or may not reflect longer-term patterns. Nevertheless, studies with other pediatric chronic illness populations have shown stability in subgroups of trajectories of adherence over 1 year (Modi et al., 2011; Rohan et al., 2010). Furthermore, research on adherence with pediatric ALL (Bhatia et al., 2012) and other chronic pediatric conditions (Modi et al., 2011; Rohan et al., 2010) indicates that rates of adherence deteriorate over the course of treatment. The present data may therefore reflect the most optimal period of adherence for this sample overall. Because participants knew that their adherence was being monitored and the MEMS Caps were novel, our findings may be influenced by patient and family reactivity, which could inflate levels of adherence. On the other hand, two subgroups of children and adolescents demonstrated problematic levels of adherence from the outset of the study that were found to be associated with risk for relapse in previous research (Bhatia et al., 2012) and the sample as a whole demonstrated significantly lower adherence over the course of time than in Bhatia et al. (2012), which lends credence to our findings. It should also be noted that 6MP was only one of several medications that comprise the treatment protocol for ALL or LBL. We do not know if adherence to 6MP generalizes to other medications. Finally, this study was restricted to enrolling school-age children and adolescents given our focus on testing the efficacy of a family-centered intervention involving the active participation of the child or adolescent.
Several directions for future research are needed to address the above limitations. First, it would be important to determine whether our findings related to rates of adherence, adherence trajectories, IDI, and gaps in adherence that reflect longer-term patterns of adherence. Second, it would be useful to determine the relationship of behavioral measures of treatment adherence (e.g., electronic monitoring with MEMS), to other objective measures of adherence (e.g., the serum metabolites of 6MP), which can assess the therapeutic action of 6MP and may be less vulnerable to participant reactivity than electronic monitoring alone (Traore et al., 2006). Finally, future studies should examine adherence patterns of younger patients diagnosed with ALL.
The approach to measurement of adherence to oral treatment in ALL and LBL reported here can be applied to any orally administered medication. Moreover, the statistical methods presented here that describe adherence for the sample as a whole, patterns of dosing frequency and timing, and subgroups with different trajectories of adherence can be readily applied to other chronic illness populations. Finally, our findings suggest that implementing measurement of adherence via electronic monitoring early and throughout the course of medical treatment could provide an effective method to allocate resources for adherence promotion to those who need it most (e.g., children with chronically problematic trajectories of adherence and gaps in the timing of doses that predict problematic clinical outcomes). We appreciate that electronic monitoring of patient adherence can pose technical challenges (e.g., lost monitors, families forgetting to bring monitors in for downloads, use of pill boxes, etc.) (Ingerski, Hente, Modi, & Hommel, 2011). In addition, objective measures of medication adherence are not typically used in ongoing clinical management of adherence with some exceptions (Cortina, Somers, Rohan, & Drotar, 2013). Nevertheless, implementing objective observational measures of treatment adherence in the management of pediatric cancer and other chronic illness populations will provide real-time information regarding adherence patterns over time, including average adherence percentages, description of medication timing between doses, therapeutic efficacy, medication gaps, etc. The development and implementation of observational measures of adherence in routine care for pediatric cancer provides an important method to tailor and target interventions for adherence promotion that hold the promise of preventing disease relapse, which is extraordinarily costly from an economic and human vantage point (Bleyer, 2011; Brown & Yabroff, 2006).
Acknowledgments
The efforts of children, adolescents, mothers, fathers, and health care providers who gave their time and energy to this work are gratefully acknowledged. Data collection and data management of this study was facilitated by a talented group of research assistants and fellows: Leanue Bolo, Megan DeRosier, Matthew Maley, Megan Miller, Claire Peterson, Katharina March, Octavio Zavala, Brenda Quinonez, Karla Castillo, Joanna Cohen, Leela Jackson, Dailyn Martinez, Gabriela Reed, Jaime Crowley, Lauren Smith, Chelsea Howe, Alina Vaisleib, Jennifer Ing, and Daphne Papadopoulos. Finally, we greatly appreciated the assistance of several undergraduate student research assistants who assisted with the processing and cleaning of data.
Funding
National Cancer Institute at the National Institutes of Health (1F31CA168307 to J.M.R., 1R01CA119162 to D.D.); National Center for Research Resources (UL1RR024134 to M.A.); the National Center for Advancing Translational Sciences (UL1TR000003 to M.A.).
Conflicts of interest: None declared.
References
- Bhatia S, Landier W, Shangguan M, Hageman L, Schaible A N, Carter A R, Hanby C L, Leisenring W, Yasui Y, Kornegay N M, Mascarenhas L, Ritchey A K, Casillas J N, Dickens D S, Meza J, Carroll W L, Relling M V, Wong F L. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: A report from the children's oncology group. Journal of Clinical Oncology. 2012;30:2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer A. Latest estimates of survival rates of the 24 most common cancers in adolescent and young adult Americans. Journal of Adolescent and Young Adult Oncology. 2011;1:37–42. doi: 10.1089/jayao.2010.0005. [DOI] [PubMed] [Google Scholar]
- Brown M, Yabroff K R. Economic impact of cancer in the United States. In: Schottenfeld D, Fraumeni J F, editors. Cancer epidemiology and prevention. New York, NY: Oxford University Press, Inc; 2006. pp. 202–216. [Google Scholar]
- Cortina S, Somers M, Rohan J M, Drotar D. Clinical effectiveness of comprehensive psychological intervention for nonadherence to medical treatment: A case series. Journal of Pediatric Psychology. 2013;38:649–663. doi: 10.1093/jpepsy/jss175. doi:10.1093/jpepsy/jss175. [DOI] [PubMed] [Google Scholar]
- Derijks L J, Gilissen L P, Engels L G, Bos L P, Bus P J, Lohman J J, Curvers W L, Van Deventer S J, Hommes D W, Hooymans P M. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: Implications for therapy. Therapeutic Drug Monitoring. 2004;26:311–318. doi: 10.1097/00007691-200406000-00016. [DOI] [PubMed] [Google Scholar]
- Festa R S, Tamaroff M H, Chasalow F, Lanzkowsky P. Therapeutic adherence to oral medication regimens by adolescents with cancer. I. Laboratory assessment. The Journal of Pediatrics. 1992;120:807–811. doi: 10.1016/s0022-3476(05)80256-2. [DOI] [PubMed] [Google Scholar]
- Fishman M, Mrozek-Orlowsk M. Cancer chemotherapy guidelines and recommendations for practice. Pittsburgh, PA: Oncology Nursing Press; 1999. [Google Scholar]
- Ingerski L M, Hente E A, Modi A C, Hommel K A. Electronic measurement of medication adherence in pediatric chronic illness: A review of measures. Journal of Pediatrics. 2011;159:528–534. doi: 10.1016/j.jpeds.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Matsushita T, Uchida H, Egi S, Yokoyama T, Mohri K. Rectal bioavailability of 6-mercaptopurine in children with acute lymphoblastic leukaemia: Partial avoidance of “first-pass” metabolism. European Journal of Clinical Pharmacology. 1992;42:619–622. doi: 10.1007/BF00265925. [DOI] [PubMed] [Google Scholar]
- Kenna L A, Labbé L, Barrett J S, Pfister M. Modeling and simulation of adherence: Approaches and applications in therapeutics. The AAPS Journal. 2005;7:E390–E407. doi: 10.1208/aapsj070240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard B D, Stewart S M, Olvera R, Bawdon R E, OhAilin A, Lewis C P, Winick N J. Nonadherence in adolescent oncology patients: Preliminary data on psychological risk factos and relationships to outcome. Journal of Clinical Psychology in Medical Settings. 2004;11:31–39. [Google Scholar]
- Kondryn H J, Edmondson C L, Hill J W, Eden T O B. Treatment non-adherence in teenage and young adult patients with cancer. Lancet Oncology. 2010;12:100–108. doi: 10.1016/S1470-2045(10)70069-3. [DOI] [PubMed] [Google Scholar]
- Lau R C W, Matsui D, Greensberg M, Koren G. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Medical and Pediatric Oncology. 1998;30:85–90. doi: 10.1002/(sici)1096-911x(199802)30:2<85::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Lennard L, Welch J, Lilleyman J. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: A possible indicator of non-compliance? British Journal of Cancer. 1995;72:1004. doi: 10.1038/bjc.1995.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A C, Rausch J R, Glauser T A. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. Journal of the Americal Medical Association. 2011;305:1669–1676. doi: 10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D S. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- National Cancer Institute. 2008. National Cancer Institute: Factsheet: Childhood Cancers. National Institutes of Health. Retrieved from http://www.cancer.gov/cancertopics/factsheet/sites-types/childhood/print. [Google Scholar]
- Pritchard M T, Butow P N, Stevens M M, Duley J A. Understanding medication adherence in pediatric acute lymphoblastic leukemia: A review. Journal of Pediatric Hematology/Oncology. 2006;28:816–823. doi: 10.1097/01.mph.0000243666.79303.45. [DOI] [PubMed] [Google Scholar]
- Quittner A L, Modi A C, Lemanek K L, Ievers-Landis C E, Rapoff M A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:916–936; discussion 937–938. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K S, Perkins S L. Advances in the diagnostic approach to childhood lymphoblastic malignant neoplasms. American Journal of Clinical Pathology. 2004;122:S3. doi: 10.1309/MQP7PTW7RQPJLDL4. [DOI] [PubMed] [Google Scholar]
- Relling M V, Hancock M L, Boyett J M, Pui C H, Evans W E. Prognostic importnace of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817–2823. [PubMed] [Google Scholar]
- Riekert K A, Rand C. Electronic monitoring of adherence: When is high-tech the best? Journal of Clinical Psychology in Medical Settings. 2002;9:25–34. [Google Scholar]
- Rohan J, Drotar D, McNally K, Schluchter M, Riekert K, Vavrek P, Schmidt A, Redline S, Kercsmar C. Adherence to pediatric asthma treatment in economically disadvantaged African-American children and adolescents: An application of growth curve analysis. Journal of Pediatric Psychology. 2010;35:394–404. doi: 10.1093/jpepsy/jsp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. A Cancer Journal for Clinicians. 2009;59:56–64. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- Singer J D, Willet J B. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford Press; 2003. [Google Scholar]
- Tebbi C K. Treatment compliance in childhood and adolescence. Cancer. 2006;71:3441–3449. doi: 10.1002/1097-0142(19930515)71:10+<3441::aid-cncr2820711751>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Traore F, O'Riordan M A, Myers C, Groth K, Hoff A, Angiolillo A, Rheingold S, Drotar D, Kodish E. How low is too low? Use of cluster analysis to define low levels of mercaptopurine metabolites. Pediatric Blood and Cancer. 2006;46:187–192. doi: 10.1002/pbc.20518. [DOI] [PubMed] [Google Scholar]
- Weiss L M, Bindl J M, Picozzi V J, Link M P, Warnke R A. Lymphoblastic lymphoma: An immunophenotype study of 26 cases with comparison to T cell acute lymphoblastic leukemia. Blood. 1986;67:474–478. [PubMed] [Google Scholar]