Abstract
Objective The primary aim of the current study was to use new methods to examine 1-year quality of medication dosing (adherence) and continuation with medication treatment (persistence) rates to antiepileptic drugs (AEDs) in children with newly diagnosed epilepsy. Methods Medication-taking behaviors of AEDs were assessed using electronic monitors for 117 children with newly diagnosed epilepsy for the first year after diagnosis. Results Approximately 15% of participants were categorized as nonpersistent (i.e., failed to take medication for >15 consecutive days) 6 months after AED initiation, which increased to 26.6% of participants at 1 year. The majority of medication dosing events took place within a +/−2-hr interval as recommended. The group with lower socioeconomic status demonstrated more nonpersistence over time. Conclusion Examining adherence and persistence in medication taking behaviors may yield different types of data for clinical and research purposes.
Keywords: adherence, chronic illness, neurological disorders, research design and methods
Pediatric epilepsy affects approximately 325,000 children under the age of 15 years in the United States (Zupanc, 1996). The primary treatment regimen for pediatric epilepsy involves adhering to antiepileptic drug (AED) therapy to decrease the likelihood of future seizures. Broadly, adherence has been defined as “the extent to which a person’s behavior coincides with medical or health advice” (Haynes, 1979; Modi et al., 2012), and can include taking medications, following dietary regimens, and/or engaging in recommended healthy lifestyle behaviors. Despite significant treatment advances in pediatrics, improvements in medical technology to assess adherence behaviors (Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008), and increased focus on adherence in research and clinical practice, nonadherence rates across all pediatric chronic illness groups continue to be quite high, averaging 50–75% (Rapoff, 2010). Within pediatric epilepsy, approximately 60% of children exhibit nonadherence within the first 6 months of AED therapy (Modi, Rausch, & Glauser, 2011). Poor treatment adherence can result in increased hospitalizations and emergency room visits, increased mortality and morbidity (e.g., seizures; Bassili, Omar, Zaki, Abdel-Fattah, & Tognoni, 2002; Besli, Saltik, Erguven, Bulut, & Abul, 2010; Cramer, Glassman, & Rienzi, 2002), and higher healthcare costs (Cutler & Everett, 2010; DiMatteo, Giordani, Lepper, & Croghan, 2002; Rapoff, 2010).
New technology has resulted in improved clinical and research understanding of adherence behaviors in pediatric chronic illness populations (Modi et al., 2012; Quittner et al., 2008). Electronic adherence monitors (e.g., Medication Event Monitoring System [MEMS] TrackCaps), which are classified as an objective and empirically supported adherence measure (Quittner et al., 2008; Rapoff, 2010), allow for daily assessment of medication adherence in a real-time format and can be used to track regimen deviations and subsequently inform clinical recommendations (Shellmer & Zelikovsky, 2007). Electronic monitoring also allows for assessment of medication dosing histories with high temporal resolution that can be useful in ambulatory medical care (Blaschke, Osterberg, Vrijens, & Urquhart, 2011). Specifically, electronic monitors provide an abundance of data that can be used by both researchers and clinicians, such as overall medication-taking and timing, intermittent or temporal patterns of dose omissions, and dosing persistence (how long the patient actually continues to take the medication). Although these devices cannot detect whether the individual actually ingested the medication, they are a proxy of medication-taking behavior and are considered the current “gold-standard” for medication adherence measurement (Rapoff, 2010).
Statistics From Electronic Monitoring Reported in Pediatric Psychology Research
There is a continued need to advance the field of adherence research by using the wealth of data from electronic monitors to further elucidate the relationship between adherence behaviors and health outcomes. Recent literature has highlighted the need to examine trajectories and variations in adherence behaviors (Modi et al., 2012; Rohan et al., 2009; Wu, Aylward, & Steele, 2010). However, most studies using adherence electronic monitors use summary adherence statistics (e.g., mean adherence, total number of doses missed), which may mask clinically useful information. For example, although two patients prescribed an AED may have similar rates of overall adherence (e.g., 50% of doses taken during a 2-week period), their daily patterns of adherence as well as dose timing may be vastly different. These daily adherence patterns have the potential to significantly influence pharmacodynamics, resulting in subtherapeutic coverage of the AED for one patient (e.g., two consecutive missed doses) but not the other (e.g., two intermittent missed doses; Pellock, Smith, Cloyd, Uthman, & Wilder, 2004). Thus, it is important to appreciate daily variation in adherence and use methods that can objectively provide detailed information on the patient’s treatment behavior outside the clinical setting. This type of data would enable clinicians to identify specific periods where adherence is lower (e.g., weekdays vs. weekends, morning vs. evening), thereby aiding more effective and targeted intervention strategies.
Medication-Taking Behaviors
New taxonomy and terminology have recently been developed via a multidisciplinary consensus group involved in the Ascertaining Barriers for Compliance Project (Blaschke et al., 2011; http://www.ABCproject.eu/). Specifically, medication-taking, or adherence, is a multidimensional and dynamic process composed of three behaviors: initiation, execution, and discontinuation (Blaschke et al., 2011). Initiation occurs when a patient takes the first dose of a prescribed medication; the lack of initiation to a prescribed medication regimen is termed nonacceptance. Next, execution is the comparison between the patient’s actual dosing and the prescribed dosing regimen (i.e., medication adherence). The quality of execution may be represented by a simple percentage calculated by total number of doses taken by the total number of prescribed doses over a delineated period. At a more detailed level, this may also include the time at which the individual actually takes the medication (i.e., dose timing). Dose timing is important because pharmacokinetics act as a significant source of variance in medication response for patients (Harter & Peck, 1991). Finally, discontinuation is when the patient stops taking the medication. The period between initiation of the treatment regimen and discontinuation is termed persistence and can be expressed as a continuous (i.e., number of days continuing therapy) or dichotomous (i.e., persistent vs. nonpersistent; Burnier, 2006; Cramer et al., 2008) variable. While there are various ways to operationalize the point of actual discontinuation using either prospective or retrospective assessments (e.g., nonrefill of pharmacy prescription), a prospective assessment approach can be more clinically useful and afford for proactive and timely intervention.
Clinical evidence has indicated that there are differing risk factors and clinical consequences for the quality of execution and persistence; however, treatment nonpersistence has not typically been examined (Barron, Bennett, & Feely, 2010; Tremlett et al., 2008). Taking into account nonpersistence may lead to a more accurate estimation of adherence and covariates associated with medication-taking behaviors. For example, Tremlett and colleagues (2008) found that divergent factors influenced missed doses (i.e., alcohol consumption) versus stopping long-term immunomodulatory drug therapy (i.e., lower education levels, previous relapses) in a population-based cohort of patients with multiple sclerosis. As a result, researchers have recommended that these two behaviors be measured and assessed separately (Cramer et al., 2008). Despite this recommendation, there are few empirical studies that have differentiated the various medication-taking behaviors (Vrijens, Vincze, Kristanto, Urquhart, & Burnier, 2008). Vrijens and colleagues (2008) examined electronic dosing histories of adult patients on once-a-day antihypertensive medications from an archived database of clinical studies. Based on the dosing histories of nearly 4,800 patients, 2% never engaged in the antihypertensive dosing regimen (nonacceptance), 65% remained persistent by day 200 of monitoring, almost half stopped taking their medication within 1 year (nonpersistence), and 50% missed a single day’s dose at a rate of one a month (12 a year). Furthermore, there was an inverse relationship between the quality of one’s daily execution of the dosing regimen and the likelihood that he or she would discontinue with treatment. Although informative to the overall literature on medication-taking behaviors, these studies are not generalizable to children for whom adherence behaviors are more complex (e.g., include parental involvement, BID/TID dosing regimen).
The purpose of the current study was to examine AED medication-taking behaviors in children with epilepsy in the first year of treatment after diagnosis, including the following: (1) nonacceptance, (2) quality of execution while engaged in dosing regimen (i.e., adherence), (3) nonpersistence (i.e., early discontinuation), and (4) dose-timing frequency. In addition, a secondary goal was to examine the association between demographic variables and persistence trajectories in children with epilepsy. Based on the adult literature (Blaschke et al., 2011; Tremlett et al., 2008; Vrijens et al., 2008), it was hypothesized that (1) approximately 2% of the study population would not accept the AED treatment; (2) overall adherence over a 1-year period would be about 75% and the overall group adherence trajectory would demonstrate a gradual decline over time; and (3) 66% and 50% of patients would remain persistent with AED treatment at 6 and 12 months, respectively. Given the lack of dose-timing data in the pediatric and adult literature, we made no a priori hypotheses regarding the percentage of scheduled medication doses taken on time. We also conducted an exploratory investigation to assess the association between demographic and medical variables (e.g., child age, parent marital status, child sex, seizure type, family socioeconomic status [SES]) with persistence trajectories.
Methods
Participants and Procedures
Study participants included children with epilepsy and their parents who were seen in the New Onset Seizure Clinic at a Midwestern children’s hospital. Eligibility criteria for study participation included: (a) new diagnosis of epilepsy, (b) child age between 2 and 12 years, (c) no significant parent-reported developmental delay (e.g., autism, Down syndrome), (d) no comorbid chronic illness (e.g., diabetes) requiring daily medications, and (e) written informed consent/assent. As a part of their routine clinical care, children were prescribed an AED by the treating epileptologist. AED changes (e.g., dose change, AED change) were made, as necessary, by the healthcare team throughout the course of treatment to optimize seizure freedom and minimize side effects.
Potential participants were approached for study participation during their first scheduled clinic visit (i.e., day of diagnosis). If children met eligibility criteria and agreed to participate, parental consent and child assent (children 11–12 years of age) were obtained. Next, parents completed a demographic questionnaire and received the MEMS TrackCap® to begin electronically monitoring adherence to their prescribed AED treatment. Research staff informed parents that the bottle monitored medication-taking; however, data from the MEMS TrackCaps were not shared with the healthcare team or parents/children. As part of routine clinical care, patients returned to clinic approximately 1 month after diagnosis and every 3 months thereafter (i.e., 4, 7, 10, 13 months after diagnosis). During each follow-up appointment, MEMS TrackCaps data were downloaded. Parents and children also completed questionnaires as part of the larger, longitudinal natural-history study of pediatric epilepsy adherence behaviors (National Institutes of Health-Eunice Kennedy Shriver National Institute of Child Health and Human Development). Participants received a $10 gift card for completion of the questionnaires and an additional $10 gift card for bringing back the MEMS TrackCaps. The study was approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board.
Measures
Background Information Form
Basic demographic information (e.g., child age, sex, caregiver occupation and education) was obtained at the initial clinic visit. A revised Duncan score, an occupation-based measure of SES, was calculated based on parent occupation (Mueller & Parcel, 1981; Stevens & Featherman, 1981). Scores range from 15 to 97, with higher scores representing greater occupational attainment. For two-caregiver households, the higher Duncan score was used, and served as a proxy for SES.
Medical History
Information regarding seizure type, seizure activity, and AEDs was obtained from medical chart review and parent interview at each clinic visit.
Adherence
The Medication Event Monitoring System (MEMS® 6 TrackCap; AARDEX Corporation, Union City, CA), an electronic monitoring system that measures daily adherence to oral medications, was used to monitor AED adherence. The MEMS TrackCap stores times and dates of bottle openings for approximately 36 months and data can be transferred to a Windows-based computer. Participants were given a MEMS TrackCap and bottle at the initial clinic visit and instructed to transfer their AED to the electronic device. Families were contacted 3 days after the clinic visit to ensure the medication had been transferred. Data from the MEMS TrackCap were downloaded at each follow-up clinic visit. Truncated adherence rates (i.e., maximum adherence rate of 100%) were used in analyses to reduce inflation as a result of overuse or extra openings that may have occurred owing to prescription refills.
Statistical and Data Analyses
There exist a number of varying operational definitions for the terms nonacceptance, adherence, and persistence. Below are the operational definitions used in the current study, which reflect a prospective approach to quantitatively examine medication-taking behaviors. Nonacceptance was defined as participants never initiating the prescribed AED regimen. Persistence was measured as the proportion of patients continuing therapy during the 12-month observation period without discontinuing use of the AED. Patients were considered to be nonpersistent if they failed to take their medication for >15 consecutive days. Consistent with traditional methods, daily execution or adherence was expressed as a percentage derived by dividing the total number of doses taken by the total number of prescribed doses on a given day. A less conservative approach was used to characterize daily adherence, where a participant was labeled as “nonadherent” when all prescribed MEMS cap openings were missed on any given day (e.g., patient prescribed to take morning and evening dose, yet no openings of electronic pill container occur) and “adherent” when at least a single MEMS cap opening occurred on a given day. Data were missing for 10 participants owing to withdrawal from the study and/or moving before the 1-year post-diagnosis date (4.6% of all data points). Data before these events were used in the data analyses. In addition, some participants had “nonmonitored” periods during the study in which data were not captured for a given reason (e.g., malfunctioning MEMS bottle, family on vacation). We chose to exclude these periods from the study, as they do not accurately represent persistence or adherence behaviors. Median duration for “non-monitored” periods was 12 days; 4.9% of all data points. Nonmonitored periods and missing data were not used in calculations for persistence and/or adherence (i.e., not counted against participant toward determining nonpersistence, denominators in calculations for adherence adjusted accordingly).
In addition, dose-time intervals were examined for all recorded openings of the medication bottle (n = 117,871) across all participants for a 1-year duration. AEDs used in the current study were dosed every 12 hr, and patients were advised to take their AEDs within a +/−2-hr window (i.e., 10–14 hr in between dosings). Dose-timing frequency was examined for all medication events and summarized using descriptive statistics.
To determine the probability of persistence over a 1-year period, a nonparametric survival analysis using the LIFETEST procedure in SAS was performed. Nonparametric survival curves were of interest, given the exploratory nature of this study. These curves allowed us to examine persistence over time in a more flexible manner, for example, to determine if the rate of nonpersistence changes at a particular time point or is markedly different in different periods. In addition, we examined whether the persistence survival curve varied across child age, parent marital status, child sex, seizure type, family SES as measured by the Duncan score, and seizures (whether seizures were reported at the clinic visit closest to the 1-year anniversary after diagnosis). The survival curves were examined for each of these predictors separately using log-rank tests (Kalbfleisch & Prentice, 1980).
Results
Participants
Of the 129 potential participants, 124 agreed to participate (96.1% recruitment rate) in the longitudinal study. Of these, seven participants did not return to the epilepsy clinic after diagnosis and/or did not start medication (nonacceptance). The final sample to examine persistence and adherence trajectories included 117 participants. A summary of demographic characteristics of participants is presented in Table I.
Table I.
Participant Characteristics
| Variable | M (SD) or % (N) |
|---|---|
| Child age (years) | 7.2 ± 2.9 |
| Family Duncan scorea | 52.4 ± 20.4 |
| Sex: female | 39% (N = 46) |
| Child race/ethnicity | |
| White: Non-Hispanic | 74% (N = 87) |
| White: Hispanic | 2% (N = 2) |
| Black | 17% (N = 20) |
| Bi/Multiracial | 6% (N = 7) |
| Asian | 1% (N = 1) |
| Parent marital status | |
| Single | 20% (N = 23) |
| Married | 64% (N = 75) |
| Divorced/Separated/Widowed | 16% (N = 19) |
| Primary caregiver | |
| Mother | 85% (N = 99/100) |
| Father | 12% (N = 14) |
| Other | 3% (N = 3/4) |
| Initial antiepileptic drug | |
| Carbamazepine | 60.5% (N = 71) |
| Valproic acid | 39.5% (N = 46) |
| Seizure type | |
| Localization-related | 60% (N = 70) |
| Generalized | 24% (N = 28) |
| Unclassified | 16% (N = 19) |
Nonacceptance, Persistence, and Adherence Data
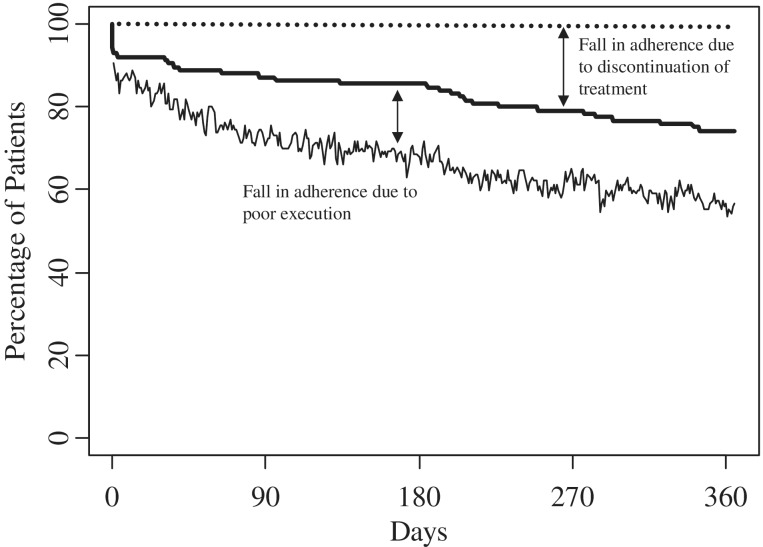
The mean adherence [(total number of openings/total number of prescribed openings) × 100] for the total sample was 86.09% (SD = 27.37%). The nonparametric survival curve for adherence and persistence over 1 year after diagnosis is illustrated in Figure 1. As illustrated, 5.6% (n = 7) of participants never started their medication regimen and/or did not accept the treatment (i.e., nonacceptance; note the initial drop-off in the persistence survival curve at day 0). After this initial drop, persistence gradually decreased over time and at day 180, 85.5% (n = 106) of those who agreed to participate persisted with treatment. Of those still engaged in the treatment regimen, 5.4% (n = 5; adherence denominator equaled 92 participants at day 180 owing to nonmonitored periods in 14 persistent participants) did not take any of their prescribed AED on day 180. Thus, we can say that 80.9% [i.e., (percent persistent; 85.5%) × (percent of those adherent; 94.6%) divided by 100 = 80.9%] of the participants comprising the original sample recorded a medication event on day 180 with their MEMS TrackCap. After 1 year, 73.4% (n = 91) of participants were still persistent with their AED regimen.
Figure 1.
Persistence (thick solid line) and adherence (thin solid line) percentages across 365 days for AEDs in a pediatric population. Dotted line represents perfect adherence/persistence.
Dosing Intervals
On average, about 5% of doses were omitted on a given day of treatment (range: 0–12.35%) for those still deemed persistent. A majority (61.53%, n = 72,529 of 117,871) of MEMS TrackCap openings occurred within the recommended time interval (i.e., +/−2-hr window, 10–14 hr), followed by 11.06% (n = 13,033) occurring between 6 and 10 hr, 9.98% (n = 11,766) occurring between 14 and 18 hr, and 7.58% (n = 8,940) occurring between 22 and 26 hr. A full list of frequencies of medication events occurring within defined 4-hr intervals and the associated percentage is presented in Table II.
Table II.
Dose-Timing Percentages
| Dose timing | Frequency of doses occurring within the dose-timing window | Percentage within the dose-timing range |
|---|---|---|
| 0–2 hr | 1,874 | 1.59% |
| 2–6 hr | 2,575 | 2.18% |
| 6–10 hr | 13,033 | 11.06% |
| 10–14 hr | 72,529 | 61.53% |
| 14–18 hr | 11,766 | 9.98% |
| 18–22 hr | 1,999 | 1.70% |
| 22–26 hr | 8,940 | 7.58% |
| 26–30 hr | 1,207 | 1.02% |
| 30–34 hr | 559 | 0.47% |
| 34–38 hr | 1,123 | 0.95% |
| 38–42 hr | 389 | 0.33% |
| 42–46 hr | 205 | 0.17% |
| 46–50 hr | 600 | 0.51% |
| 50+ hr | 1,072 | 0.91% |
Predictors of Persistence
Finally, we tested each of the demographic and medical variables (e.g., child age, parent marital status, child sex, seizure type, family SES) to determine if the nonparametric persistence survival curve was related to these variables (Table III). Notably, SES (p = .003) was the only significant predictor of the persistence survival curve. Figure 2 illustrates nonparametric persistent survival curves for the upper-third, middle-third, and lower-third of the family SES distribution, where higher family SES is generally associated with more persistence. In particular, (a) the upper-third family SES curve ends with a persistence rate of 93% after 1 year; (b) the middle-third family SES curve is relatively consistent with a persistence rate of 88% around 6 months, then declines more rapidly to a persistence rate of 74% after 1 year; and (c) the lower-third family SES curve declines relatively consistently throughout the entire year, ending with a persistence rate of 68%.
Table III.
Predictors of Persistence Survival Curves
| Variable | χ2 | p-value |
|---|---|---|
| Seizures | 1.50 | .22 |
| Seizure type | 0.57 | .45 |
| Child age | 0.17 | .68 |
| Child gender | 2.20 | .14 |
| Parent marital status | 2.73 | .10 |
| SES | 9.08 | .003 |
Note. Predictors of nonparametric persistence survival curve were examined separately.
Figure 2.
Persistence percentage across 365 days for AEDs in a pediatric population for upper-third (short dashed line), middle-third (long dashed line), and lower-third (solid line) SES groups.
Discussion
The current study examined daily electronically monitored AED medication-taking in children with epilepsy during the first year after diagnosis. This rich database allowed for an analysis of medication-taking behaviors, namely, quality of execution of daily AED (adherence), duration of continued treatment (persistence), and dose timing over the first year. A higher than expected percentage of participants never started their medication (5.6%), which may be owing to parental unacceptance of the initial diagnosis; however, it is difficult to fully ascertain reasons for not initiating the AED. With regard to persistence, by 6 months, 14.5% of participants had discontinued treatment and by 1 year, this percentage increased to 26.6% participants being classified as nonpersistent. Our findings are better than hypothesized based on previous studies (Caro, Speckmann, Sales, Raggio, & Jackson, 1999; Vrijens et al., 2008), which highlighted higher estimates of nonpersistence at both 6 months and 1 year (32–28% and 49–52%, respectively); however, these studies were conducted with adult patients on a once-a-day antihypertensive medication dosing regimen and used a varying definition for persistence (i.e., starting with day 365, nonpersistence was defined as occurring when during a 2-week period, the average adherence to the prescribed medication was <50%; B. Vrijens, personal communication). We recognize that there is no universally accepted definition of persistence, but believe that the operational definition used in the current study reflects a more prospective clinical approach to identifying those who discontinue treatment. For example, other traditional modalities such as pharmacy refills may not provide the more objective daily adherence behavior recorded with electronic monitoring (i.e., dosing errors; Vrijens et al., 2008). Furthermore, an operational definition of persistence that uses a backward-calculation to determine the point of nonpersistence does not allow for timely clinical intervention, as this categorization is done in a post hoc manner. Definitions of persistence may vary in future studies; yet, it is recommended that researchers consider a prospective approach based on the clinical guidelines of the condition under examination. By examining the time at which patients become nonpersistent, it may highlight important time points for clinical intervention (e.g., booster educational sessions on adherence to AEDs, referral adherence promotion interventions; Graves, Roberts, Rapoff, & Boyer, 2010; Kahana, Drotar, & Frazier, 2008).
Related to the quality of execution, dosing histories, measured via the MEMS TrackCaps, revealed that a majority of patients took their AED within a 12-hr time interval (±2 hr). Dosing within this time frame is optimal to minimize AED side effects. Despite the evidence that the majority of events occurred within the recommended window, nearly 24% of dosing events occurred beyond the recommended guidelines (i.e., >14 hr). These lapses in medication-taking may reflect periods in which nonadherence may be more likely (e.g., weekends vs. weekdays). Although it is quite clear that C. Everett Koop’s widely quoted epigram “drugs don’t work in patients who don’t take them” holds true (Osterberg & Blaschke, 2005), Blaschke et al. (2011) added that medications “work erratically in patients who take them erratically” (p. 276). This corollary underscores the importance of establishing a routine regarding taking medications, and is an area in which pediatric psychologists can be particularly helpful to children and their families within medical subspecialty clinics.
Although several variables were used to examine predictors of persistence, lower SES was identified as the only significant predictor. This finding is consistent with previous studies that have demonstrated a strong relationship between SES and adherence for children and adolescents with a chronic illness, including epilepsy (Modi et al., 2011; Modi, Morita, & Glauser, 2008). It is plausible that families with lower SES may have competing demands and limited resources that make it difficult to buy expensive AEDs. Overall, the findings highlight that healthcare teams need to recognize and work with families if finances are a barrier to adherence (e.g., changing to generic vs. brand-name AEDs, financial assistance programs, etc.). We recognize that our list of potential predictors was not exhaustive, and other studies in pediatric epilepsy have identified additional factors associated with nonadherence using a validated medication self-management tool (Modi, Monahan, Daniels, & Glauser, 2010). Patient factors such as forgetting, difficulty swallowing pills, and beliefs about how well medication will work to treat seizures can play a role in both the initiation of medication use (acceptance) as well as persistence (e.g., child discontinues use of medication after experiencing seizure). These factors need to be further examined in future studies.
Limitations and Future Directions
Although the examination of adherence and persistent rates in children using electronic monitoring is novel to the field of pediatric psychology, there are some limitations to the current study. First, we used a less conservative method to categorize daily adherence, potentially biasing the adherence trajectory over the first year. Specifically, use of a more conservative approach (e.g., all-or-none approach) might have resulted in the adherence trajectory being shifted downward (i.e., greater percentage of participants being categorized as nonadherent on a day-to-day basis). In addition, differing dosing regimens may lead to varying results in other studies. For example, participants in the current study were on a primarily BID dosing regimen; thus, daily adherence percentages could be 0, 50, or 100%, whereas a once-a-day dosing regimen is limited to an all-or-none categorical approach. Second, the current analysis did not include other measures of adherence such as self-report, pill counts, or prescription refill information; however, a previous study with this population demonstrated a significant correlation between electronically monitored and parent-reported adherence 1 week before clinic visit (rho = 0.46, p < 0.001; Modi, Guilfoyle, Morita, & Glauser, 2011). Notably, self-reported adherence was inflated compared with electronic monitoring. Electronic monitoring is only a proxy assessment of medication-taking behaviors and other methods also exist (i.e., self-report, blood assays); however, all methods have their limitations, and electronic monitoring is considered the current “gold standard”, despite high costs limiting its clinical utility. While it is plausible that a participant could open the bottle with the MEMS TrackCap, yet not actually ingest the AED, previous studies have demonstrated limited differences between medication events (i.e., opening the medication bottle) and actual medication dosing (i.e., taking the medication after opening the medication bottle; Girard, Sheiner, Kastrissios, & Blaschke, 1996; Rubio, Cox, & Weintraub, 1992; Vrijens et al., 2005). Additionally, we capped the data regarding medication-taking behaviors, limiting our understanding of medication overuse, which is an area that warrants further study. An area for future research is to use a multimethod approach to assess medication-taking behaviors (e.g., composite of electronic monitoring, self-report, and blood assays). Finally, the sample of children with epilepsy represented a restricted age range (2–12 years) and uncomplicated epilepsies (e.g., no major comorbid medical or developmental disorders). Future studies need to examine medication-taking behaviors in adolescents and children with comorbidities.
To our knowledge, this is the first study examining adherence and persistence trajectories in a pediatric population. Ultimately, suboptimal adherence and failure to persist with medications are important determinants of drug response/failure and can have a substantial negative impact on associated healthcare expenditures, a point highlighted in the recent Institute of Medicine report (IOM, 2012). Examining adherence and persistence in assessing medication adherence for chronic illness populations may yield different types of data for clinical and research purposes. This differentiation requires an understanding of the taxonomy of medication-taking behaviors as well as asking appropriate questions during the clinical visit to further understand a patient’s adherence with the treatment regimen. For example, merely asking a patient “Did you take your medication?” may lead to social desirability bias and limited insight into medication-taking behaviors (i.e., limited to yes/no response and summary information). Conversely, asking questions such as “How many times over the past 2 weeks did you miss taking a dose of your medication?” or “What times do you have trouble remembering to take your medication?” may elucidate more detailed information on the patient’s dosing patterns and routine, appear less confrontational to the patient, and identify periods in which nonadherence is more likely (e.g., two doses missed occurred on weekends). Erratic dosing patterns can contribute to patient-reported side effects; thus, understanding a patient’s dosing patterns from electronic monitoring data may aid a clinician’s decision on potential medication changes, as truly understanding the effectiveness of a particular medication for an individual patient is dependent on his or her adherence to the regimen.
Acknowledgments
We would like to extend our deepest appreciation to the children with epilepsy and their families who participated in this study. We thank Julie Koumoutsos, Elizabeth Painter, Julie Adcock, and Shanna Guilfoyle for recruiting participants and collecting their data. We also thank the healthcare team involved in the medical and psychosocial care of children with new-onset epilepsy who facilitated the current research.
Conflicts of interest: This research was funded by a grant from the National Institutes of Health- Eunice Kennedy Shriver National Institute of Child Health and Human Development to the senior author (K23-HD057333).
References
- Barron T I, Bennett K, Feely J. A competing risks prescription refill model of compliance and persistence. Value in Health. 2010;13:796–804. doi: 10.1111/j.1524-4733.2010.00741.x. [DOI] [PubMed] [Google Scholar]
- Bassili A, Omar T, Zaki A, Abdel-Fattah M, Tognoni G. Pattern of diagnostic and therapeutic care of childhood epilepsy in Alexandria, Egypt. International Journal of Quality Health Care. 2002;14:277–284. doi: 10.1093/intqhc/14.4.277. [DOI] [PubMed] [Google Scholar]
- Besli G E, Saltik S, Erguven M, Bulut O, Abul M H. Status epilepticus in children: Causes, clinical features and short-term outcome. Pediatrics International: Official Journal of the Japan Pediatric Society. 2010;52:749–753. doi: 10.1111/j.1442-200X.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- Blaschke T F, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: Insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annual Review of Pharmacology and Toxicology. 2011;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. American Journal of Hypertension. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Caro J J, Speckmann J L, Salas M, Raggio G, Jackson J D. Effect of initial drug choice on persistence with antihypertensive therapy: The importance of actual practice data. Canadian Medical Association Journal. 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- Cramer J A, Glassman M, Rienzi B A. The relationship between medication compliance and seizures. Epilepsy Behavior. 2002;3:338–342. doi: 10.1016/s1525-5050(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Cramer J, Roy A, Burrell A, Fairchild C J, Fuldeore M J, Ollendorf D A, Wong P K. Medication compliance and persistence: Terminology and definitions. Value in Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- Cutler D M, Everett W. Thinking outside the pillbox: Medication adherence as a priority for health care reform. New England Journal of Medicine. 2010;362:1553–1555. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- DiMatteo M R, Giordani P J, Lepper H S, Croghan T W. Patient adherence and medical treatment outcomes: A meta-analysis. Medical Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Girard P, Sheiner L B, Kastrissios H, Blaschke T F. Do we need full compliance data for population pharmacokinetics analysis? Journal of Pharmacokinetics and Biopharmacology. 1996;24:265–282. doi: 10.1007/BF02353671. [DOI] [PubMed] [Google Scholar]
- Graves M M, Roberts M C, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology. 2010;35: 368–382. doi: 10.1093/jpepsy/jsp072. [DOI] [PubMed] [Google Scholar]
- Harter J G, Peck C C. Chronobiology: Suggestions for integrating it into drug development. Annals of the New York Academy of Science. 1991;618:563–571. doi: 10.1111/j.1749-6632.1991.tb27276.x. [DOI] [PubMed] [Google Scholar]
- Haynes R B. Introduction. In: Taylor D W, Sackett D C, editors. Compliance in health care. Baltimore, MD: The Johns Hopkins University Press; 1979. pp. 1–7. [Google Scholar]
- IOM (Institute of Medicine) Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academics Press; 2012. [PubMed] [Google Scholar]
- Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology. 2008;33:590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch J D, Prentice R L. The statistical analysis of failure time data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- Modi A C, Guilfoyle S M, Morita D A, Glauser T A. Development and reliability of a correction factor for parent-reported adherence to pediatric antiepileptic drug therapy. Epilepsia. 2011;52:370–376. doi: 10.1111/j.1528-1167.2010.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A C, Monahan S, Daniels D, Glauser T A. Development and validation of the Pediatric Epilepsy Medication Self-Management Questionnaire. Epilepsy and Behavior. 2010;18:94–99. doi: 10.1016/j.yebeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A C, Morita D A, Glauser T A. One-month adherence in children with new-onset epilepsy: White coat compliance does not occur. Pediatrics. 2008;121:e961–e966. doi: 10.1542/peds.2007-1690. [DOI] [PubMed] [Google Scholar]
- Modi A C, Pai A L, Hommel K A, Hood K K, Cortina S, Hilliard M E, Guilfoyle S M, Gray WN, Drotar D. Pediatric self-management: A framework for research, practice, and policy. Pediatrics. 2012;129:e473–485. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A C, Rausch J R, Glauser T A. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305:1669–1676. doi: 10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C W, Parcel T L. Measures of socioeconomic status: Alternatives and recommendations. Child Development. 1981;52:13–20. [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Pellock J M, Smith M C, Cloyd J C, Uthman B, Wilder B J. Extended-release formulations: Simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behavior. 2004;5:301–307. doi: 10.1016/j.yebeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Quittner A L, Modi A C, Lemanek K L, Ievers-Landis C E, Rapoff M A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:916–936. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoff M A. Adherence to pediatric medical regimens. 2nd ed. New York, NY: Springer; 2010. [Google Scholar]
- Rohan J, Drotar D, McNally K, Schluchter M, Riekert K, Vavrek P, Kercsmar C. Adherence to pediatric asthma treatment in economically disadvantaged African-American children and adolescents: An application of growth curve analysis. Journal of Pediatric Psychology. 2009;35:394–404. doi: 10.1093/jpepsy/jsp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A, Cox C, Weintraub M. Prediction of diltiazem plasma concentration curves from limited measurements using compliance data. Clinical Pharmacokinetics. 1992;22:238–246. doi: 10.2165/00003088-199222030-00006. [DOI] [PubMed] [Google Scholar]
- Shellmer D, Zelikovsky N. The challenges of using Medication Event Monitoring Technology with pediatric transplant patients. Pediatric Transplantation. 2007;11:422–428. doi: 10.1111/j.1399-3046.2007.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G, Featherman D L. A revised socioeconomic index of occupational status. Social Science Research. 1981;10:364–395. [Google Scholar]
- Tremlett H, Van der Mei I, Pittas F, Blizzard L, Dwyer T, Ponson A L. Adherence to the immunomodulatory drugs for multiple sclerosis: Contrasting factors affect stopping drug and missed doses. Pharmacoepidemiology and Drug Safety. 2008;17:565–576. doi: 10.1002/pds.1593. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data as input to individually parameterized pharmacokinetic models. Journal of Clinical Pharmacology. 2005;45:461–467. doi: 10.1177/0091270004274433. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: Longitudinal study of electronically compiled dosing histories. British Medical Journal. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y P, Aylward B S, Steele R G. The influence of internalizing symptoms on trajectories of medication adherence among pediatric renal and liver transplant recipients. Journal of Pediatric Psychology. 2010;35:1016–1027. doi: 10.1093/jpepsy/jsq014. [DOI] [PubMed] [Google Scholar]
- Zupanc M L. Update on epilepsy in pediatric patients. Mayo Clinic Proceedings. 1996;71:899–916. doi: 10.4065/71.9.899. [DOI] [PubMed] [Google Scholar]