Abstract
Objective
B cells have been shown to play an important role in the pathogenesis of rheumatoid arthritis and juvenile idiopathic arthritis (JIA). Current treatments include the disease-modifying antirheumatic drugs methotrexate (MTX) and tumor necrosis factor α inhibition with etanercept. This study was undertaken to determine how these drugs influence the B cell compartment in patients with JIA.
Methods
B cell subpopulations and follicular helper T (Tfh) cells in the peripheral blood of JIA patients were investigated by multicolor flow cytometry. Serum immunoglobulin and BAFF levels were determined by enzyme-linked immunosorbent assay.
Results
There was a significant decrease in transitional B cells and significantly lower serum immunoglobulin levels in patients receiving MTX than in untreated patients and those receiving etanercept. In contrast, etanercept treatment had no effect on most of the B cell subpopulations, but resulted in significantly lower BAFF levels and increased numbers of Tfh cells. Thus, our findings indicate an unexpected and previously unknown direct effect of low-dose MTX on B cells, whereas etanercept had a more indirect influence.
Conclusion
Our results contribute to a better understanding of the potency of MTX in autoantibody-mediated autoimmune disease and present a possible mechanism of prevention of the development of drug-induced antibodies to biologic agents. The finding that MTX and etanercept affect the B cell compartment differently supports the notion that combination therapy with etanercept and MTX is more effective than monotherapy.
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease in children younger than 16 years of age, and is characterized by joint inflammation of longer than 6 weeks' duration that cannot be explained by other causes, most importantly, systemic autoimmunity, infection, or trauma. Several factors are thought to contribute to the pathogenesis of JIA, including genetic, environmental, and immunologic factors. With respect to the immune system, different cell types of the innate and adaptive immune system, as well as various chemokines and inflammatory cytokines, are involved in the pathogenesis of JIA (1–6).
The frequent detection of autoantibodies, most importantly antinuclear antibodies (ANAs) in JIA and anti–cyclic citrullinated peptide antibodies in rheumatoid arthritis (RA), indicates that a disturbed B cell tolerance contributes to the pathogenesis of these diseases. This view is further supported by the effectiveness of therapeutic B cell depletion in several autoimmune disorders (7–9). Previously, it has been shown that defects in both central and peripheral B cell tolerance can result in increased numbers of autoreactive B cells in RA patients, thus promoting the development of the disease (10).
Depending on the severity of the disease, treatment of JIA comprises the administration of nonsteroidal antiinflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs, most importantly methotrexate (MTX) and biologic agents, including tumor necrosis factor α (TNFα) inhibition using etanercept (11).
MTX has long been used as a cytostatic drug to treat malignancies. More recently, although its mechanism of action is mostly unknown, low-dose MTX has been shown to be an effective antiinflammatory drug in managing the progression of autoimmune diseases such as RA and JIA (12).
Etanercept is a soluble TNFα inhibitor and is efficiently used for the treatment of polyarticular RA and JIA (13,14). TNFα is a pleiotropic proinflammatory cytokine secreted by different cell types and has effects on both innate and adaptive immune cells (15). It has been found to play an important role in the development and progression of several autoimmune diseases (16–18). Because both B cells and TNFα are important in the pathogenesis of RA and JIA, we aimed to determine how current treatment strategies influence B cells. In the present study, we therefore investigated the effect of MTX and etanercept on the B cell compartment in patients with JIA.
PATIENTS AND METHODS
Patients
JIA patients were recruited from the Pediatric Rheumatology clinics at Hannover Medical School and Professor Hess Children's Hospital (Bremen, Germany). The study was conducted in compliance with the Declaration of Helsinki, and approval was obtained from the local ethics committee. All patients fulfilled the International League of Associations for Rheumatology Durban criteria (19). Samples were collected after informed consent was obtained from the patients' parents or legal guardians. Patient characteristics are shown in Table1. Information on the drug doses administered, the routes of administration, and the duration of treatment are shown in Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38736/abstract. Briefly, the dosage of MTX was 15 mg/m2/week administered subcutaneously or orally for at least 6 months. Etanercept was administered subcutaneously in 1 or 2 dosages of 0.8 mg/kg/week. The dosages for particular NSAIDs were 15–20 mg/kg/day for naproxen, 2–3 mg/kg/day for diclofenac, and 2–3 mg/kg/day for indomethacin.
Table 1.
Baseline characteristics and clinical features of the JIA patients*
| No treatment | NSAIDs | MTX | Etanercept | Etanercept + MTX | |
|---|---|---|---|---|---|
| Flow cytometric analysis | |||||
| No. of patients | 28 | 47 | 17 | 12 | 6 |
| JIA subset, no. of patients | |||||
| Oligoarticular JIA | 23 | 33 | 12 | 6 | 1 |
| Psoriatic arthritis | 1 | 2 | 0 | 0 | 0 |
| Polyarticular JIA (seropositive) | 0 | 1 | 0 | 0 | 2 |
| Polyarticular JIA (seronegative) | 1 | 2 | 5 | 5 | 1 |
| Systemic-onset JIA | 0 | 0 | 0 | 1 | 0 |
| Enthesitis-related arthritis | 3 | 9 | 0 | 0 | 2 |
| Age at sampling, mean ± SD years | 11.9 ± 3.6 | 11.3 ± 3.6 | 11.0 ± 2.6 | 11.4 ± 3.2 | 14 ± 1.3 |
| Sex, % female | 64 | 57 | 65 | 58 | 83 |
| ANA, no. positive/no. tested | 24/28 | 28/44 | 10/14 | 5/7 | 3/6 |
| CRP, mean ± SD mg/liter | 1.7 ± 1.7 | 1.5 ± 1.8 | 1.6 ± 1.1 | 3.0 ± 2.6 | 0.8 ± 0.1 |
| ESR, mean ± SD mm/hour | 8.8 ± 6.4 | 11.5 ± 6.4 | 8.2 ± 5.0 | 6.4 ± 4.0 | 7.0 ± 2.0 |
| Immunoglobulin analysis | |||||
| No. of patients | 36 | 64 | 22 | 17 | 9 |
| Age at sampling, mean ± SD years | 11.4 ± 3.5 | 11.8 ± 3.4 | 11.0 ± 2.6 | 11.2 ± 3.2 | 11.7 ± 4.2 |
JIA = juvenile idiopathic arthritis; NSAIDs = nonsteroidal antiinflammatory drugs; MTX = methotrexate; ANA = antinuclear antibody; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
Cell isolation and serum handling
Peripheral blood mononuclear cells (PBMCs) were isolated from the heparinized blood of JIA patients or from buffy coat cells of anonymous healthy donors (Red Cross) by density-gradient centrifugation using Ficoll (Biocoll; Biochrom). Residual red blood cells were lysed with ammonium citrate solution for 2 minutes. Total PBMCs were stored in freezing media (90% fetal calf serum [Biochrom] and 10% DMSO [Sigma]) in liquid nitrogen until further use. Serum was isolated and stored at −80°C.
Flow cytometric analysis
Immunophenotypic characterization of isolated PBMCs was performed using multicolor flow cytometry. PBMCs from healthy donors were used as an internal control for possible day-to-day variation experiments. Each sample was divided into 3 different staining panels (see Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38736/abstract). Briefly, 1 × 106 PBMCs were incubated with 5% FcR blocking reagent (normal mouse serum; Invitrogen) in FACS buffer (phosphate buffered saline, 2% bovine serum albumin [Roth]). Subsequently, specific antibody combinations were added and incubated on ice for 30 minutes. After washing, PBMCs were stained with Live/Dead staining dye according to the recommendations of the manufacturer (Invitrogen). Samples were acquired on an LSRII or FACSCanto II flow cytometer (both from BD Biosciences) and analyzed using FlowJo software (Tree Star). Gating strategies and phenotypes of analyzed B and T cell subpopulations are shown in Supplementary Figure 1 and Supplementary Table 3 (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38736/abstract).
Enzyme-linked immunosorbent assay (ELISA)
For determination of BAFF and APRIL levels in the sera of JIA patients, we used a Quantikine ELISA, a Human BAFF/BLys/TNFSF13B Immunoassay for BAFF, and a Human APRIL/TNFSF13 DuoSet for APRIL according to the recommendations of the manufacturer (R&D Systems). Each serum sample was tested in duplicate. Specific absorbance was measured at 450 nm and 560 nm, and optical density was quantified by a GloMax Reader (Promega). Analysis was performed with GraphPad Prism software.
Statistical analysis
Statistical differences were determined using an unpaired 2-tailed t-test for independent samples using GraphPad Prism software. Values are shown as the mean ± SEM. P values less than 0.05 were considered significant.
RESULTS
Patient characteristics
For flow cytometric analysis, 110 patients with JIA were included in the study. Patients with elevated systemic inflammatory parameters (either C-reactive protein level >10 mg/liter or erythrocyte sedimentation rate >25 mm/hour) were excluded to avoid any effect of inflammatory cytokines and other mediators on B cells. Consequently, patients included in the study had inactive disease or were in remission while receiving treatment, except for some patients with low disease activity in the NSAID and no treatment groups. Patients were grouped based on therapy regimen as receiving no treatment, NSAIDs, MTX, etanercept, or etanercept and MTX combined. As shown in Table1, no significant differences existed between the groups with respect to age or inflammatory parameters.
Effect of MTX treatment on early peripheral B cell developmental stages
Analysis of B cell subpopulations from the peripheral blood of JIA patients revealed no difference between treatment groups in the relative frequency of CD19+ B cells. However, there was a slight, but significant decrease in absolute numbers of CD19+ B cells in patients treated with MTX (mean ± SEM 181 ± 97/μl) compared to those treated with etanercept (mean ± SEM 272 ± 109/μl) (Figure 1A). Subsequently, the numbers of CD27− transitional and mature naive B cells were determined. We found a significant decrease in both relative frequency and absolute number of transitional B cells in patients treated with MTX (5 ± 3%; 9 ± 5/μl) compared to untreated patients (9 ± 4%; 21 ± 23/μl) and those treated with etanercept (10 ± 9%; 24 ± 17/μl). In addition, there was a trend toward lower relative numbers of transitional B cells in the MTX group (5 ± 3%) compared to the NSAID group (7 ± 4%), which did not reach statistical significance (P = 0.0597). Interestingly, MTX treatment also resulted in a decrease in transitional B cells when given in combination with etanercept (7 ± 6/μl) compared to etanercept treatment alone (24 ± 17/μl) (Figure 1C).
Figure 1.
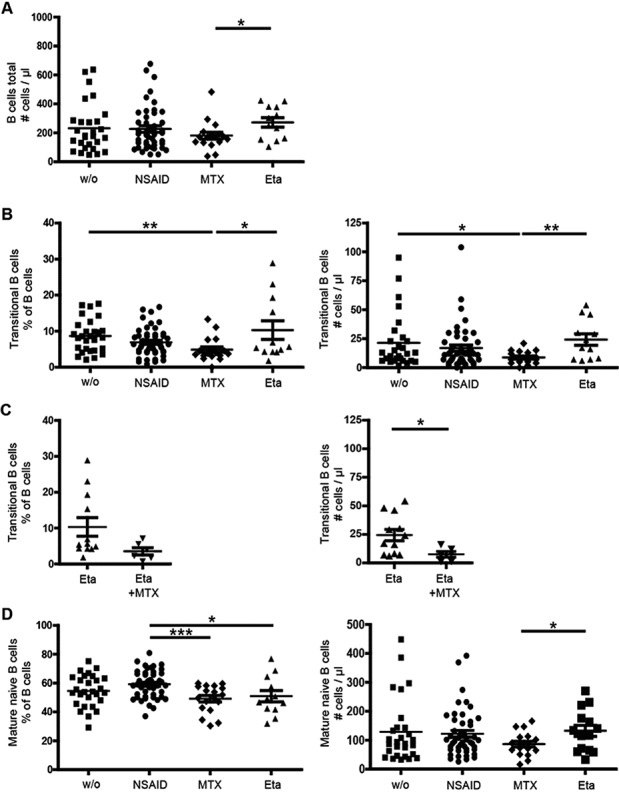
Effect of methotrexate (MTX) treatment on transitional and naive B cell numbers in patients with juvenile idiopathic arthritis (JIA) who were not treated (w/o) or were treated with nonsteroidal antiinflammatory drugs (NSAIDs), MTX, etanercept (Eta), or a combination of etanercept and MTX. A, Absolute B cell count per microliter. B and C, Percentage (left) and absolute number (right) of transitional B cells. D, Percentage (left) and absolute number (right) of mature naive B cells. Symbols represent individual patients; horizontal lines and error bars show the mean ± SEM. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001.
When analyzing mature naive B cells, we found significantly decreased relative numbers in patients treated with MTX (mean ± SEM 49 ± 9%) compared to patients treated with NSAIDs (59 ± 9%) and lower absolute cell counts in patients treated with MTX (87 ± 40/μl) than in those treated with etanercept (144 ± 78/μl) (Figure 1D). These results demonstrate that treatment with MTX affects early B cell developmental stages in the peripheral blood of JIA patients, most importantly transitional B cells.
Effect of MTX and etanercept on memory B cells
A previous study suggested that anti-TNFα therapy results in a decrease in memory B cells in adult RA patients (16). We therefore investigated the CD19+ CD27+ memory B cell compartment. Memory B cells were further subdivided into IgM+, IgG+, and IgA+ cells. Relative numbers of IgM+ memory B cells were significantly reduced in etanercept-treated patients (mean ± SEM 8 ± 7%) compared to all other treatment cohorts (mean ± SEM 14 ± 8% in untreated patients, 17 ± 7% in NSAID-treated patients, and 13 ± 6% in MTX-treated patients) (Figure 2A), whereas IgG+ memory B cells were significantly increased in patients treated with MTX (6 ± 3%) compared to patients treated with NSAIDs (4 ± 2%) and untreated patients (4 ± 2%) (Figure 2B). In addition, the ratio of IgM to IgG memory B cells was significantly lower in both MTX- and etanercept-treated patients compared to NSAID-treated patients and untreated patients (Figure 2C). However, no significant changes in absolute numbers of unswitched IgM+ or switched IgG+ and IgA+ memory B cell subsets were observed (Figure 2 and data not shown). Therefore, although absolute numbers of memory B cells did not change due to treatment with MTX or etanercept, relative numbers were influenced. This could suggest either increased class-switching or slightly lower generation of IgM+ memory B cells.
Figure 2.
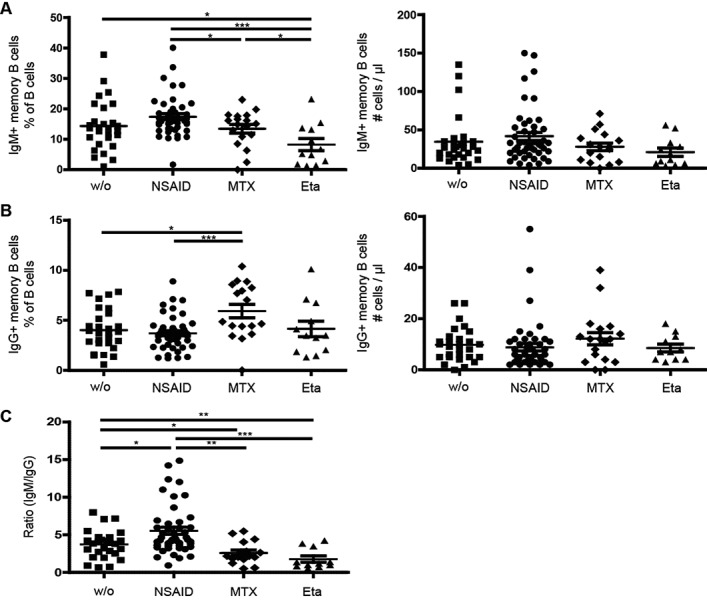
Lower percentages of memory B cells, but no change in absolute memory B cell counts, in patients with JIA treated with etanercept. A, Percentage (left) and absolute number (right) of IgM+ (unswitched) memory B cells in patients with JIA receiving the indicated treatments. B, Percentage (left) and absolute number (right) of IgG+ (switched) memory B cells in patients with JIA receiving the indicated treatments. C, Ratio of IgM to IgG memory B cells in patients with JIA receiving the indicated treatments. Symbols represent individual patients; horizontal lines and error bars show the mean ± SEM. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001. See Figure 1 for definitions.
Decreased serum BAFF levels in JIA patients treated with etanercept
BAFF is an important B cell survival factor and has been shown to be crucial for B cell development and differentiation in the periphery (20,21). Moreover, elevated BAFF levels are known to be associated with systemic autoimmunity (22). Thus, we assessed serum BAFF levels and the relative surface expression of the BAFF receptors BAFF-R and BCMA on CD19+ B cells. Surprisingly, we found significantly lower BAFF levels in anti-TNFα–treated patients compared to all other groups (mean ± SEM 831 ± 164 pg/ml in the etanercept-treated group, 1,039 ± 251 pg/ml in the untreated group, 1,012 ± 268 pg/ml in the NSAID-treated group, and 1,046 ± 291 pg/ml in the MTX-treated group) (Figure 3A). No differences were found in BAFF-R and BCMA expression on CD19+ B cells (data not shown).
Figure 3.
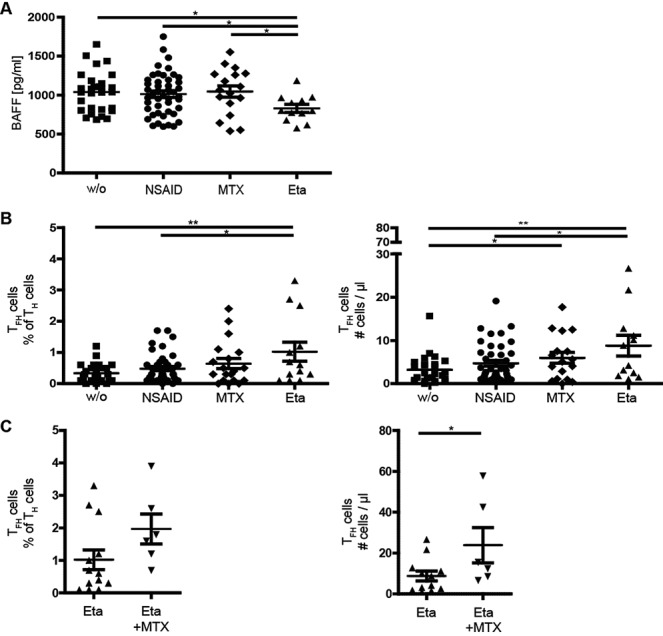
Indirect effect of etanercept on the B cell compartment in patients with JIA. A, Decreased serum BAFF levels in etanercept-treated patients with JIA. B and C, Percentage (left) and absolute number (right) of follicular helper T (Tfh) cells in patients with JIA receiving the indicated treatments. Anti–tumor necrosis factor α treatment caused an increase in Tfh cells (CD4+CxRC5+PD-1+). Symbols represent individual patients; horizontal lines show the mean ± SEM. ∗ = P < 0.05; ∗∗ = P < 0.01. See Figure 1 for other definitions.
Increased numbers of follicular helper T (Tfh) cells in patients treated with TNFα inhibition or MTX
Tfh cells represent a small subset of CD4+ helper T cells, which is known to be important during germinal center reaction for production of high-affinity, class-switched antibodies (23). As shown previously, Tfh cell numbers are increased in patients with autoimmune diseases, including systemic lupus erythematosus (SLE) and RA (24). Therefore, we also analyzed the effect of antirheumatic therapy on Tfh cells.
Our analysis revealed a significant increase in both frequency and absolute numbers of Tfh cells in etanercept-treated patients (mean ± SEM 1.0 ± 1.1%; 9 ± 8/μl) compared to untreated patients (0.3 ± 0.3%; 3 ± 3/μl). There was also a slight, significant increase in MTX-treated patients (0.6 ± 0.7%; 6 ± 5/μl) compared to untreated patients. Importantly, the highest numbers of Tfh cells were seen in patients receiving etanercept and MTX combined (2.0 ± 1.0%; 24 ± 19/μl), possibly reflecting an additive effect of both etanercept and MTX (Figure 3C). No association between the number of Tfh cells and other parameters, such as JIA subgroup, ANAs, appearance of uveitis, or response to treatment, was found.
Lower serum immunoglobulin levels in patients treated with MTX
Because our data demonstrated an impact of antirheumatic therapy on the B cell compartment in JIA patients, we investigated whether changes in the B cell compartment have functional consequences. We therefore assessed serum immunoglobulin (IgM, IgG, and IgA) levels. For this part of the study, additional patients were included by reviewing patient charts. A total of 148 patients were analyzed, as shown in Table1. Again, only patients with inflammatory parameters within the normal range were included, and all groups were age matched.
Patients receiving MTX had highly significantly lower serum levels of all 3 immunoglobulin isotypes than all other treatment groups (Figure 4). This was most pronounced for IgM but still significant for IgA and IgG. The combination of MTX and etanercept also resulted in a significant decrease in IgM levels, and a small, albeit not significant, reduction in IgG and IgA levels, compared to etanercept monotherapy. No effect on immunoglobulin levels was seen in JIA patients treated with etanercept alone compared to untreated patients and those treated with NSAIDs. Since antibody is known to be mostly produced by plasma or plasmablast cells (circulating plasma cells in the periphery), we further analyzed the effect of MTX and etanercept treatment on the circulating plasmablast/plasma cells defined as CD19+IgD−CD27++CD38++ cells. Our data revealed no significant difference in the absolute count of these cells among different treatment groups, with the exception of a slight increase in NSAID-treated patients compared to untreated patients (data not shown).
Figure 4.
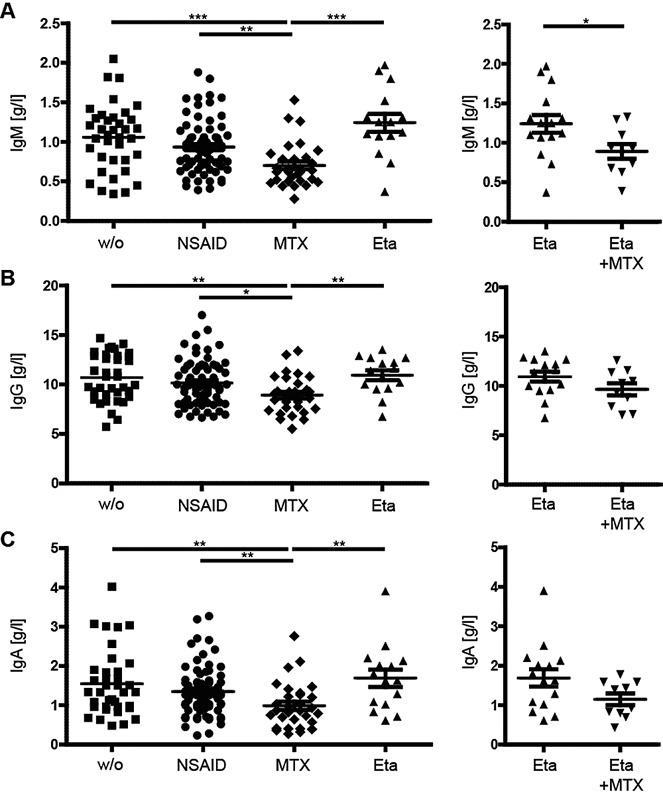
Decreased serum immunoglobulin levels in patients with JIA treated with MTX. A, IgM levels, B, IgG levels, and C, IgA levels in patients with JIA receiving the indicated treatments. Symbols represent individual patients; horizontal lines show the mean ± SEM. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001. See Figure 1 for definitions.
Overall, our data show a highly significant reduction in serum immunoglobulin level in all isotypes in patients with JIA treated with MTX, which most likely is not due to the reduced number of circulating plasmablast/plasma cells.
Effect of MTX on B cells independent of JIA subgroup
As shown in Table1, JIA subgroups were differently represented within treatment groups. In particular, there were fewer patients with oligoarticular disease in the MTX and etanercept groups than in the group of untreated patients. Therefore, to exclude the possibility that the observed effect of MTX was due to different JIA subgroups, we performed a separate analysis of patients with oligoarticular JIA, which was the largest patient group in our study. All patients had inactive disease or were in clinical remission while receiving treatment, based on the criteria defined by Wallace et al (25). The effect of MTX on transitional B cell subpopulations was also observed when only patients with oligoarticular JIA were included in the analysis (Figure 5A). With regard to mature naive B cells, we did not detect any significant differences in relative or absolute cell numbers between untreated patients with oligoarticular JIA and patients with oligoarticular JIA treated with MTX (Figure 5B). Immunoglobulin levels were all significantly decreased in MTX-treated patients compared to untreated patients (Figure 5C). Therefore, we conclude that the observed decrease in transitional B cells and immunoglobulin levels in patients receiving MTX therapy was not due to different JIA subgroups or the level of disease activity, based on an analysis of only patients with oligoarticular JIA.
Figure 5.
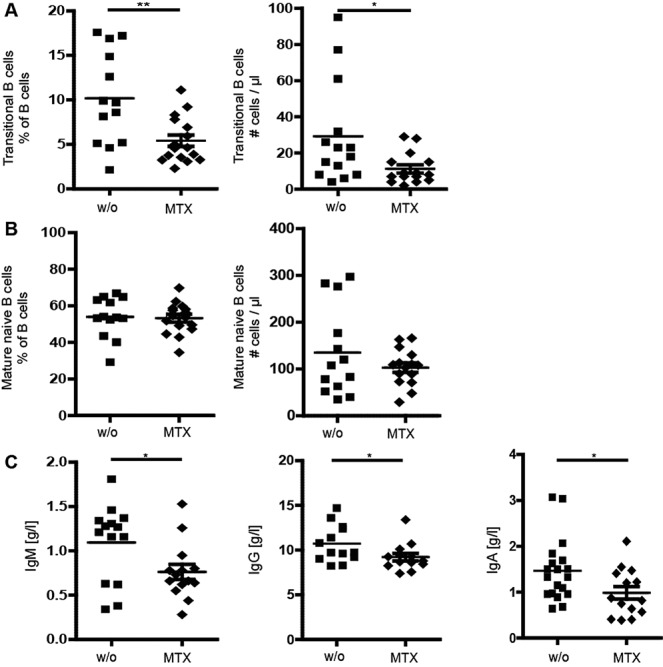
Effect of MTX on transitional B cells and immunoglobulin levels in the subgroup of patients with oligoarticular JIA. The effect of MTX was similar to that found when all subgroups of JIA patients were included in the analysis. A, Percentage (left) and absolute number (right) of transitional B cells in untreated patients and patients treated with MTX. B, Percentage (left) and absolute number (right) of mature naive B cells in untreated patients and patients treated with MTX. C, Serum Ig levels in untreated patients and patients treated with MTX. Symbols represent individual patients; horizontal lines show the mean ± SEM. ∗ = P < 0.05; ∗∗ = P < 0.01. See Figure 1 for definitions.
DISCUSSION
The importance of B cells in the pathogenesis of several autoimmune diseases is highlighted by the effectiveness of B cell depletion therapies (26,27). B cells can promote the development of autoimmunity not only by the production of autoantibodies and cytokines, but also by serving as antigen-presenting cells for autoreactive T cells (27). Previous studies have shown an impact of therapeutic TNFα inhibition on B cell subpopulations in adult patients with RA, most importantly a decrease in memory B cells (16). MTX has been demonstrated to lower total B cell numbers in peripheral blood (28,29), but no detailed analysis regarding the effect of low-dose MTX on B cell subpopulations has been reported so far. In addition, no such data exist with regard to JIA for either etanercept or MTX. In the present study, we therefore performed a detailed analysis of the effect of low-dose MTX and the TNFα inhibitor etanercept on the B cell compartment in patients with JIA. Our data demonstrate that MTX more directly affects the B cell compartment, as demonstrated by lower absolute numbers of transitional B cells and decreased immunoglobulin levels. In contrast, etanercept treatment results in decreased serum BAFF levels and elevated Tfh cells, thus more indirectly influencing B cells.
Generally, high doses of MTX are used for cytostatic therapy to treat malignancies, whereas low-dose MTX is used to treat patients with autoimmune disease due to its antiinflammatory effect, which is mainly mediated by an increase in adenosine (30). Low-dose MTX has been shown to reduce overall B and T cell numbers (28,29) and T cell proliferation, increase T cell apoptosis, alter the expression levels of cellular adhesion molecules, and influence the production of cytokines. Our data demonstrate that MTX specifically affects early developmental B cell stages and possibly B cell differentiation.
Consistent with our results, in a recent study lower transitional B cells were also observed in MTX-treated compared to etanercept-treated RA patients (16). In addition to those data, we found lower transitional B cell numbers in MTX-treated compared to untreated JIA patients, whereas no differences were oberserved in etanercept-treated compared to untreated patients. We thus propose that MTX treatment affects the transitional B cell compartment, whereas etanercept does not. Notably, while we analyzed only peripheral B cell subsets due to availability, MTX may already affect bone marrow B cell subsets.
In addition to a decrease in transitional B cells, our data demonstrate lower IgM, IgA, and IgG levels in the serum of MTX-treated JIA patients. A decrease in immunoglobulins after treatment with MTX has been described previously in a longitudinal retrospective study of pediatric rheumatology patients (31). Since immunoglobulin levels are often elevated in chronic inflammatory disease, this decrease could also reflect an improvement in chronic inflammation in patients receiving MTX treatment. However, a cross-sectional analysis confirmed that immunoglobulin levels were indeed decreased in patients treated with MTX compared to untreated patients, and this is specific to MTX since we found no decrease in immunoglobulin levels in JIA patients receiving etanercept. Moreover, addition of MTX to etanercept also resulted in decreased immunoglobulin levels compared to etanercept monotherapy.
Thus, our data indicate an unexpected and previously unknown effect of low-dose MTX on B cells that can contribute to understanding the efficiency of this drug in the treatment of chronic arthritis and other autoimmune diseases. The transitional B cell stage has been described as an important peripheral tolerance checkpoint during B cell development (32), in contrast to the central tolerance checkpoint in the bone marrow. At these checkpoints, the B cell receptor is negatively selected for autoreactive self-antigens, and as a consequence, B cells bearing autoreactive receptors are either deleted, undergo receptor editing, or are rendered anergic. Consistent with the idea that MTX impacts this tolerance checkpoint, MTX has been shown to be most effective in JIA patients who are positive for ANAs (33), in particular those with uveitis. This points to a B cell–mediated effect, although ANA titers in JIA patients are generally not altered by antirheumatic treatment and, consistent with this observation, we did not observe any effect of MTX on ANA titers in our study (data not shown).
Most interestingly, MTX has recently been shown to reduce the immunogenicity of biologic agents, such as adalimumab, in RA patients by decreasing antidrug antibodies in a dose-dependent manner (34,35), thereby providing a new aspect of the effects of MTX treatment. Our finding of MTX impacting the B cell tolerance checkpoint and decreasing immunoglobulin production, thus influencing the B cell repertoire, may present a possible explanation for the mechanism of this observation.
In contrast to MTX-treated JIA patients, we did not observe a clear direct effect on the B cell subpopulations analyzed in etanercept-treated patients. Although we detected lower relative frequencies of IgM+ memory B cells and a decreased ratio of IgM to IgG memory B cells in etanercept-treated JIA patients, no significant differences were observed when comparing absolute numbers of these cells. The result is consistent with the findings of Anolik et al (16), which showed a difference only when comparing percentages but not absolute numbers.
Our results demonstrate a more indirect effect of etanercept on the B cell compartment, with decreased serum BAFF levels and increased numbers of Tfh cells. BAFF is an important B cell survival factor in the periphery, triggering B cell survival, differentiation, proliferation, and antibody production. Elevated BAFF levels are associated with autoimmunity, most importantly Sjögren's syndrome and SLE (36–38), and recently, therapeutic BAFF inhibition has been approved by the Food and Drug Administration for the treatment of SLE. Elevated BAFF levels have also been shown in active RA, whereas with respect to JIA, one study found higher BAFF levels (39), and another group did not observe elevated BAFF levels in these patients (40). In all of those studies, higher BAFF levels were associated with higher disease activity.
Several studies consistently demonstrated that BAFF synthesis can be induced by proinflammatory cytokines, including TNFα, in fibroblast-like synoviocytes (41–43) or neutrophils infiltrating the rheumatoid joint (44). With respect to therapeutic TNFα inhibition, La et al showed decreased BAFF levels after treatment with TNFα antagonists in patients with RA who had a good response to treatment (45), consistent with our results. Our study shows this effect was specific to etanercept, as we did not find lower BAFF levels in MTX-treated JIA patients, and it was independent of disease activity. In summary, our finding of a reduction in serum BAFF levels in patients treated with etanercept, as compared to other treatment groups, might reflect a previously unknown additional effect of TNFα inhibition. The mechanism of this effect is yet to be investigated. This is an interesting observation since therapeutic BAFF inhibition is currently being tested for the treatment of RA (46).
The present study further shows that etanercept therapy results in a significant increase in Tfh cell numbers. In addition, MTX treatment led to a slight increase in Tfh cell numbers, whereas the highest increase was observed in patients treated with MTX and etanercept combined. Tfh cells are crucial for the generation of high-affinity antigen-specific and class-switched antibodies (47). This is also true with respect to autoantibody production, and thus, Tfh cells are important in driving systemic autoimmunity (48). Consistent with this idea, their numbers were shown to be increased in SLE patients (49). Thus, the observed increase in Tfh cell numbers in patients treated with etanercept is seemingly in contrast with the well-known therapeutic effect in autoimmune diseases. However, an increase in autoantibody production following anti-TNFα therapy and the development of SLE-like disease has been repeatedly described for different biologic agents, including infliximab, etanercept, and adalimumab. So far, the pathophysiologic mechanism for this finding is not known. An increase in Tfh cells may contribute to this side effect.
In summary, our study demonstrates that MTX has a more direct influence on the B cell compartment, whereas etanercept acts more indirectly. The findings contribute to a better understanding of the effectiveness of MTX in autoantibody-mediated autoimmune disease. Notably, the recently described effect of MTX in preventing drug-induced antibodies to biologic agents might be explained by the impact of MTX on early developmental B cell stages and immunoglobulin production demonstrated in this study. Because MTX and etanercept affect the B cell compartment differently, the data support the assumption that combination therapy with etanercept and MTX might be more effective to treat autoimmune diseases than monotherapy, which is currently a subject of controversy in both RA and JIA (14,50).
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Meyer-Bahlburg had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Quách, Weller-Heinemann, Huppertz, Meyer-Bahlburg.
Acquisition of data. Glaesener, Quách, Onken, Weller-Heinemann, Dressler, Huppertz, Thon, Meyer-Bahlburg.
Analysis and interpretation of data. Glaesener, Quách, Meyer-Bahlburg.
ROLE OF THE STUDY SPONSOR
Pfizer had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Pfizer.
Acknowledgments
The authors would like to thank Silvia Tödter (Hannover Medical School) and Claudia Tiedemann (Professor Hess Children's Hospital) for collecting the patient samples. We would also like to thank all of the patients who participated in the study and their families.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Figure 1. Gating of analyzed B cell subsets. A, Transitional B cells (CD19+CD27−CD24++ CD38++IgD+) and mature naïve B cells (CD19+CD27−CD24+CD38+IgD++). B, Memory B cell subpopulations (IgM+ [unswitched memory B cells]: CD19+CD27+IgM+;IgG+ or IgA+ [switched memory B cells]: CD19+CD27+IgG+ or IgA+). C, TFH cells (CD4+CD8−CxCR5+PD−1+).
REFERENCES
- Wiegering V, Girschick HJ, Morbach H. B-cell pathology in juvenile idiopathic arthritis. Arthritis. 2010;2010:759868. doi: 10.1155/2010/759868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattorno M, Prigione I, Morandi F, Gregorio A, Chiesa S, Ferlito F, et al. Phenotypic and functional characterisation of CCR7+ and CCR7− CD4+ memory T cells homing to the joints in juvenile idiopathic arthritis. Arthritis Res Ther. 2005;7:R256–67. doi: 10.1186/ar1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Wang CT, Gershwin ME, Chiang BL. The pathogenesis of oligoarticular/polyarticular vs systemic juvenile idiopathic arthritis. Autoimmun Rev. 2011;10:482–9. doi: 10.1016/j.autrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Omoyinmi E, Hamaoui R, Pesenacker A, Nistala K, Moncrieffe H, Ursu S, et al. Th1 and Th17 cell subpopulations are enriched in the peripheral blood of patients with systemic juvenile idiopathic arthritis. Rheumatology (Oxford) 2012;51:1881–6. doi: 10.1093/rheumatology/kes162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakken BJ, Albani S. Using biology of disease to understand and guide therapy of JIA. Best Pract Res Clin Rheumatol. 2009;23:599–608. doi: 10.1016/j.berh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Lee SK, Vinuesa CG. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Curr Opin Immunol. 2012;24:658–64. doi: 10.1016/j.coi.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Alexeeva EI, Valieva SI, Bzarova TM, Semikina EL, Isaeva KB, Lisitsyn AO, et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin Rheumatol. 2011;30:1163–72. doi: 10.1007/s10067-011-1720-7. [DOI] [PubMed] [Google Scholar]
- Braun-Moscovici Y, Butbul-Aviel Y, Guralnik L, Toledano K, Markovits D, Rozin A, et al. Rituximab: rescue therapy in life-threatening complications or refractory autoimmune diseases: a single center experience. Rheumatol Int. 2013;33:1495–504. doi: 10.1007/s00296-012-2587-x. [DOI] [PubMed] [Google Scholar]
- Nakou M, Katsikas G, Sidiropoulos P, Bertsias G, Papadimitraki E, Raptopoulou A, et al. Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis Res Ther. 2009;11:R131. doi: 10.1186/ar2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–67. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA. 2005;294:1671–84. doi: 10.1001/jama.294.13.1671. [DOI] [PubMed] [Google Scholar]
- Mello SB, Tavares ER, Bulgarelli A, Bonfa E, Maranhao RC. Intra-articular methotrexate associated to lipid nanoemulsions: anti-inflammatory effect upon antigen-induced arthritis. Int J Nanomedicine. 2013;8:443–9. doi: 10.2147/IJN.S29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Pediatric Rheumatology Collaborative Study Group. Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342:763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun. 2010;11:1–26. doi: 10.1159/000289195. [DOI] [PubMed] [Google Scholar]
- Anolik JH, Ravikumar R, Barnard J, Owen T, Almudevar A, Milner EC, et al. Anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180:688–92. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- Souto-Carneiro MM, Mahadevan V, Takada K, Fritsch-Stork R, Nanki T, Brown M, et al. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res Ther. 2009;11:R84. doi: 10.1186/ar2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, Kwon BS. Targeting TNF superfamily members for therapeutic intervention in rheumatoid arthritis. Cytokine. 2012;57:305–12. doi: 10.1016/j.cyto.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol. 2010;185:4570–81. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–36. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–47. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CA, Ruperto N, Giannini E Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology International Trials Organization; Pediatric Rheumatology Collaborative Study Group. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4. [PubMed] [Google Scholar]
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–68. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm I. Decrease of B-cells and autoantibodies after low-dose methotrexate. Biomed Pharmacother. 2003;57:278–81. doi: 10.1016/s0753-3322(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Wascher TC, Hermann J, Brezinschek HP, Brezinschek R, Wilders-Truschnig M, Rainer F, et al. Cell-type specific response of peripheral blood lymphocytes to methotrexate in the treatment of rheumatoid arthritis. Clin Investig. 1994;72:535–40. doi: 10.1007/BF00207484. [DOI] [PubMed] [Google Scholar]
- Stamp LK, Hazlett J, Roberts RL, Frampton C, Highton J, Hessian PA. Adenosine receptor expression in rheumatoid synovium: a basis for methotrexate action. Arthritis Res Ther. 2012;14:R138. doi: 10.1186/ar3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackham OJ, Sills JA, Davidson JE. Immunoglobulin levels in methotrexate treated paediatric rheumatology patients. Arch Dis Child. 2002;87:147–8. doi: 10.1136/adc.87.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tussiwand R, Bosco N, Ceredig R, Rolink AG. Tolerance checkpoints in B-cell development: Johnny B good. Eur J Immunol. 2009;39:2317–24. doi: 10.1002/eji.200939633. [DOI] [PubMed] [Google Scholar]
- Vilca I, Munitis PG, Pistorio A, Ravelli A, Buoncompagni A, Bica B, et al. Predictors of poor response to methotrexate in polyarticular-course juvenile idiopathic arthritis: analysis of the PRINTO methotrexate trial. Ann Rheum Dis. 2010;69:1479–83. doi: 10.1136/ard.2009.120840. [DOI] [PubMed] [Google Scholar]
- Krieckaert CL, Nurmohamed MT, Wolbink GJ. Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann Rheum Dis. 2012;71:1914–5. doi: 10.1136/annrheumdis-2012-201544. [DOI] [PubMed] [Google Scholar]
- Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases. Clin Rheumatol. 2013;32:1429–35. doi: 10.1007/s10067-013-2336-x. [DOI] [PubMed] [Google Scholar]
- Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune– based rheumatic diseases. Arthritis Rheum. 2001;44:1313–9. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Roschke V, Baker KP, Wang Z, Alarcon GS, Fessler BJ, et al. A role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- Gheita TA, Bassyouni IH, Emad Y, el-Din AM, Abdel-Rasheed E, Hussein H. Elevated BAFF (BLyS) and APRIL in Juvenile idiopathic arthritis patients: relation to clinical manifestations and disease activity. Joint Bone Spine. 2012;79:285–90. doi: 10.1016/j.jbspin.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Hong SD, Reiff A, Yang HT, Migone TS, Ward CD, Marzan K, et al. B lymphocyte stimulator expression in pediatric systemic lupus erythematosus and juvenile idiopathic arthritis patients. Arthritis Rheum. 2009;60:3400–9. doi: 10.1002/art.24902. [DOI] [PubMed] [Google Scholar]
- Alsaleh G, Messer L, Semaan N, Boulanger N, Gottenberg JE, Sibilia J, et al. BAFF synthesis by rheumatoid synoviocytes is positively controlled by α5β1 integrin stimulation and is negatively regulated by tumor necrosis factor α and Toll-like receptor ligands. Arthritis Rheum. 2007;56:3202–14. doi: 10.1002/art.22915. [DOI] [PubMed] [Google Scholar]
- Lee GH, Lee J, Lee JW, Choi WS, Moon EY. B cell activating factor-dependent expression of vascular endothelial growth factor in MH7A human synoviocytes stimulated with tumor necrosis factor-α. Int Immunopharmacol. 2013;17:142–7. doi: 10.1016/j.intimp.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Ohata J, Zvaifler NJ, Nishio M, Boyle DL, Kalled SL, Carson DA, et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol. 2005;174:864–70. doi: 10.4049/jimmunol.174.2.864. [DOI] [PubMed] [Google Scholar]
- Assi LK, Wong SH, Ludwig A, Raza K, Gordon C, Salmon M, et al. Tumor necrosis factor α activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 2007;56:1776–86. doi: 10.1002/art.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La DT, Collins CE, Yang HT, Migone TS, Stohl W. B lymphocyte stimulator expression in patients with rheumatoid arthritis treated with tumour necrosis factor a antagonists: differential effects between good and poor clinical responders. Ann Rheum Dis. 2008;67:1132–8. doi: 10.1136/ard.2007.079954. [DOI] [PubMed] [Google Scholar]
- Stohl W, Merrill JT, McKay JD, Lisse JR, Zhong ZJ, Freimuth WW, et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo- controlled, dose-ranging study. J Rheumatol. 2013;40:579–89. doi: 10.3899/jrheum.120886. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly—TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–26. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis. 2009;68:519–25. doi: 10.1136/ard.2007.087593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Figure 1. Gating of analyzed B cell subsets. A, Transitional B cells (CD19+CD27−CD24++ CD38++IgD+) and mature naïve B cells (CD19+CD27−CD24+CD38+IgD++). B, Memory B cell subpopulations (IgM+ [unswitched memory B cells]: CD19+CD27+IgM+;IgG+ or IgA+ [switched memory B cells]: CD19+CD27+IgG+ or IgA+). C, TFH cells (CD4+CD8−CxCR5+PD−1+).


